Abstract
Background
UNAIDS recently defined the 90-90-90 target as a way to end the HIV epidemic. However, the proportion of virological success following antiretroviral therapy (ART) may not be as high as the anticipated 90%, and may in fact be highly heterogeneous. We aimed to describe the proportion of virological success in sub-Saharan Africa and to identify factors associated with the proportion of virological success.
Methods
We performed a systematic review and meta-analysis focusing on the proportion of patients in sub-Saharan Africa who demonstrate virological success at 12 and 24 months since ART initiation, as well as at 6 and 36 months, where possible. Programme factors associated with the proportion of virological success were identified using meta-regression. Analyses were conducted using both on-treatment (OT) and intention-to-treat (ITT) approaches.
Results
Eighty-five articles were included in the meta-analysis, corresponding to 125 independent study populations. Using an on-treatment approach, the proportions (95% confidence interval (CI)) of virological success at 12 (n = 64) and at 24 (n = 32) months since ART initiation were 87.7% (81.3–91.0) and 83.7% (79.8–87.6), respectively. Univariate analysis indicated that the proportion of virological success was not different by study design. Multivariate analysis at 24 months showed that the proportion of virological success was significantly larger in studies conducted in public sector sites than in other sites (p = 0.045). Using an ITT approach, the proportions (95% CI) of virological success at 12 (n = 50) and at 24 (n = 20) months were 65.4% (61.8–69.1) and 56.8% (51.3–62.4), respectively. At 12 months, multivariate analysis showed that the proportion of success was significantly lower in cohort studies than in trials (63.0% vs. 71.1%; p = 0.017). At 24 months, univariate analysis demonstrated that the proportion of success was also lower in cohorts.
Discussion
Regardless of the time following ART initiation, and of the threshold, proportions of virological success were highly variable. Evidence from this review suggests that the new international target of 90% of patients controlled is not yet being achieved, and that in order to improve the virological outcome, efforts should be made to improve retention in care.
Introduction
At the end of 2015, the World Health Organization (WHO) estimated that about 36.7 million people worldwide were living with HIV, with Sub-Saharan Africa the most affected region in the world with 70% of the HIV burden [1].
The 6th Millennium Development Goal called for an unprecedented mobilization to halt and reverse the AIDS epidemic. UNAIDS also set the 90-90-90 target to help end the HIV epidemics (90% of HIV+ diagnosed, 90% of HIV+ treated, 90% of people on treatment achieving supressed viral load). As a result, by the end of 2015, the number of patients receiving antiretroviral therapy (ART) was estimated at more than 15.8 million, an 85% increase since 2010 [2]. This exceeded the WHO goal to provide HIV treatment to 15 million people by the end of 2015 [3]. However, this increase, as well as the recent WHO “treat-all” recommendation [4, 5], challenge the means to evaluate the effectiveness of ART, especially the long-term effectiveness in resource-limited settings.
To enable rapid deployment of ART, many countries have used the WHO public health approach [5], built on the experience of pilot programmes [6], which takes into account the constraints and weaknesses of health systems in low and middle income countries (LMICs): large numbers of patients, limited availability of drugs, and a lack of biological platforms. This approach is characterized by the standardization of 1st and 2nd line ART, the simplification of decision trees and monitoring, the standardization of biological monitoring and the decentralization of care.
In terms of treatment, the preferred choice for adults is a 1st line ART regimen consisting of a backbone of two nucleoside reverse transcriptase inhibitors (NRTI) (tenofovir (TDF) and lamuvidine (3TC) or emtricitabine (FTC)) and one non-nucleoside reverse transcriptase inhibitors (NNRTI) (efavirenz (EFV)) in one daily fixed dose combination. Zidovudine (AZT) may be an alternative to TDF and nevirapine (NVP) may be an alternative to EFV. Since 2010, stavudine (d4T) was replaced by TDF due to its toxicity [7].
The current WHO guidelines for HIV care [4] recommend viral load monitoring at 6 months since ART initiation, at 12 months and then every 12 months. The switch to 2nd line ART is recommended if the confirmed viral load exceeds the threshold of 1000 copies/mL. Although still not widely available in routine care, viral load monitoring is preferred to CD4 count monitoring for the follow-up of HIV patients.
There are numerous studies presenting virological outcomes in patients on ART in sub-Saharan Africa. However the definition of the outcome varies across studies, making the results of individual studies difficult to understand.
Previous systematic reviews of virological outcomes for patients on ART have focused on sub-Saharan Africa and on levels of acquired resistance to antiretroviral drugs [8–10]. In LMICs, summary estimates of viral suppression at different thresholds, including an intention to treat analysis are lacking. Although reviews have been published on virological outcomes, to our knowledge, no meta-analysis on recent data is currently available. This is important given the recent increase in the number of patients now receiving ART.
It is important to evaluate individual sites and programs in terms of virological outcomes. However, the pooling of data from several studies in a meta-analysis can be used to inform countries in the development of wider policy and public health actions to address the burden of HIV/AIDS and to enable WHO to generate international recommendations.
Current WHO guidelines recommend cross-sectional studies to monitor virological efficacy and resistance to ART [11], or the use of cohort studies to assess one of eight early warning indicators of HIV drug resistance: viral load suppression 12 months after ART initiation [12]. However, these two approaches may lead to very different outcomes.
This review and meta-analysis aim to provide updated data on proportions of virological success in adults on ART in sub-Saharan Africa. The objectives were to estimate the proportions of virological success at time points following the beginning of ART, to compare proportions of virological success between different study designs (clinical trials, cohort studies and cross-sectional studies) and to identify factors explaining the heterogeneity between the reported proportions of virological success.
Methods
We performed a systematic review and meta-analysis in accordance with the Centre for Reviews and Dissemination guidelines [13] and standards of reporting for systematic reviews (PRISMA) [14] (S1 Checklist).
Search strategy
PubMed, EmBase, Scopus, Web Of Science and Cochrane library were searched for all clinical trials, longitudinal cohort studies and cross-sectional studies on proportions of virological success in adults on ART in sub-Saharan Africa, published in any language between January 1, 2009 and September 30, 2014. We restricted the search to the last 5 years to provide estimates that reflect contemporary care. The search instruction was: (((((HIV OR AIDS[Title/Abstract]))) OR HIV/AIDS[Title/Abstract]) OR ((hiv OR AIDS OR HIV/AIDS[MeSH Terms])))) AND (Africa[Title/Abstract] OR Africa[MeSH Terms]) AND ((((antiretroviral OR ART OR HAART[MeSH Terms]))) OR ((antiretroviral OR ART OR HAART[Title/Abstract])))) AND (((virological OR virologic[MeSH Terms])) OR (virological OR virologic[Title/Abstract]))) AND (("2009/01/01"[PDat]: "2014/05/24"[PDat])). We also manually searched the references of relevant articles to identify studies that might have been missed.
The outcomes of interest were the proportions of patients demonstrating virological success at 6, 12, 24 and 36 months since ART initiation, using on-treatment (OT) and/or intention-to-treat (ITT) analysis.
Study selection
We considered studies conducted in sub-Saharan Africa that reported virological outcomes for at least one of the time points of interest, strictly in HIV-1 infected treatment-naïve adults (with the definition of the start of adulthood varying between studies: from 14 to 20 years) on 1st line ART, corresponding to the WHO recommendations at that time in Africa.
Studies that did not report virological outcomes at a specific time point or that only included HIV-2 infected populations, paediatric populations, patients on 2nd line ART, or in which more than 20% of patients received non-conventional ART regimen, were not included. Studies reporting virological outcomes on less than 20 patients were also excluded, as such small samples were considered unlikely to provide accurate information and were likely subject to significant biases.
All articles were independently reviewed by two of the authors (FT and YM). The titles and abstracts of all identified articles were reviewed to determine the eligibility of full text papers for inclusion. The remaining full-length articles were retrieved and read independently by the two authors to determine whether to include them in the meta-analysis. Results were compared and discrepancies in opinion between authors as to whether studies should be included were resolved by discussion.
Data extraction
To extract relevant data from the selected articles, a standardized collection form was prepared. Data extraction and validity assessment were carried out independently and in duplicate by two of the authors (FT and YM), and any discrepancies resolved by discussion. The authors contacted the corresponding authors of individual studies to retrieve any missing data. The completed forms and resulting electronic database were checked by a third author (AC) for data accuracy and quality.
For clinical trials, each study arm was considered as an independent study and outcomes were reported independently [15–17]. For longitudinal cohort studies and cross-sectional studies, results which were reported by groups were also regarded as independent studies and outcomes were reported independently.
For all studies, we recorded the threshold at which virological success had been defined, the number of patients evaluated, and the number/proportion of patients demonstrating virological success, enabling OT analysis. For ITT analysis, death, loss to follow-up (LTFU), and the switching to second-line ART prior to the virological evaluation were considered as virological failures, while patients who withdrew from the study or who transferred out of the study prior to the virological evaluation were not considered. The ITT result was not included if the proportion of patients not considered for unknown reasons exceeded 40%.
The quality of the studies included in this meta-analysis was assessed based on the STROBE (Strengthening the reporting of observational studies in epidemiology) checklist [18]. Availability of information regarding the description of the study design, the setting, eligibility criteria, details on virological evaluation, sample size calculation, number of participants at each stage of the study, the reasons for non-participation at each stage of the study, the presentation of baseline characteristics of study participants, reporting of the number of patients in virological success and discussion of the limitations of the study were assessed for all studies eligible for inclusion in the meta-analysis.
Statistical analysis
The overall proportion of patients demonstrating virological success was estimated using a random effect model, following the method of DerSimonian and Laird [19], without weight, and using the metan command in Stata (Stata Corp, College Station, Texas, USA). Heterogeneity was indicated if the Cochran test was significant at the level of 0.05 and/or if the I-square statistic exceeded 0.50.
The proportion of patients demonstrating virological success was estimated as the number of patients in virological success divided by the number of patients with a viral load measurement (OT analysis), or as the number of patients in virological success divided by the number of patients who should have reached the evaluation time point minus the number of transfers/withdrawal before the evaluation time point (ITT analysis). Substitution to 2nd line ART before the evaluation time-point was regarded as virological failure. The standard deviation for all proportions was obtained using a Gaussian approximation.
Due to the variability in reporting of the virological thresholds, virological success at <50 copies/mL included all results at 20, 40 and 50 copies/mL thresholds. In the same way, reporting of results at 100, 150, 200, 400 and 500 copies/mL thresholds were combined and considered as virological success at 400 copies/mL. Virological failure was defined as >1000 copies/mL, in accordance with the latest WHO recommendations [4]. We also assessed the proportion of virological failure at 5000 copies/mL, which corresponds to the 2010 WHO recommendations [7].
The effect of the following factors on the level of virological success was investigated: beginning of enrolment (i.e. ART initiation) (≤2005, ≥2006), end of enrolment (≤2005, 2006–2009, ≥2010), study design, study region [20], type of site (public vs. other), number of sites, decentralized setting (if the authors mentioned “decentralized”, “tertiary level”, “district level” or “rural” in the method section), general HIV population vs. specific population (i.e. HBV or HCV co-infected patients, TB-infected patients, or Kaposi-diagnosed patients), and tracking of LTFU patients (as reported in the study).
We wanted to evaluate whether previous viral load monitoring affected the level of virological success at the time of evaluation. Hence, we defined a binary indicator of previous routine virological evaluation and we included details of viral load monitoring if performed routinely as part of the study.
The effect of factors describing the population at baseline were also investigated once categorised based on the median across all studies: proportion of women, age, proportion of patients in WHO stage 3 or 4, median CD4 level, median viral load level, first line ART (EFV or NVP-based). The effect of all these factors was investigated using meta-regression and p-values were estimated through a permutation test. To ensure that convergence of the permutation test was achieved, we verified that estimates obtained using 10,000, 30,000 and 50,000 iterations were similar. Factors which presented a p-value <0.20 in univariate analysis were considered in the multivariate model. Using a backward stepwise procedure, we identified factors which remained independently and statistically significantly associated with the outcome. To assess the effect of publication bias, funnel plots representing the proportion of patients in a study in virological success and the sample size of the study were examined [21].
For the ITT analysis, we further distinguished two groups within the general population: studies which enrolled all patients without a minimum follow-up duration and studies which enrolled patients with a minimum follow-up of 6 months. This consideration was important as it has been shown that mortality is particularly high within the first 6 months following ART initiation [22, 23].
Results
Selection of studies
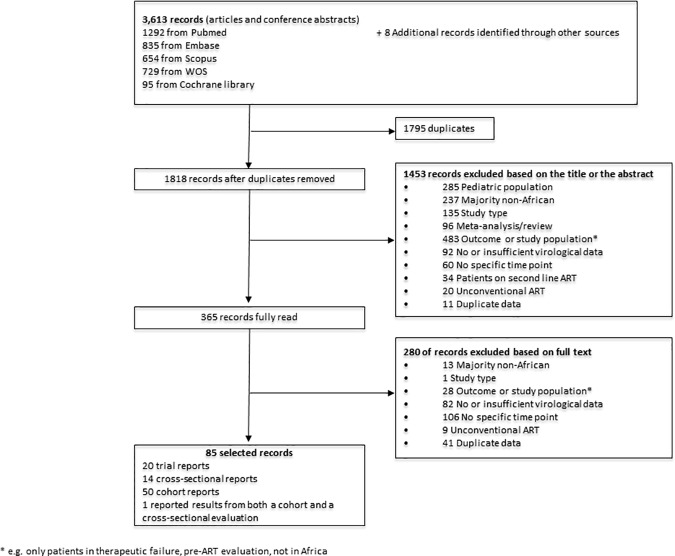
We identified 3613 articles reporting on proportions of virological success following ART initiation. After removing duplicate publications and clearly irrelevant publications, we retrieved 365 articles which were reviewed independently for eligibility. 85 articles identified in the original search contributed to the meta-analysis: 20 articles on clinical trials, 50 articles on cohort studies, 14 articles on cross-sectional studies, and one article reported results from both a cohort and a cross-sectional evaluation [24]. Fig 1 indicates the process of study selection.
Fig 1. Flow chart of study selection.
To evaluate the quality of the 85 articles considered in this meta-analysis, we selected the items from the STROBE statement that we considered relevant (S1 Table).
Characteristics of studies
Of the 20 articles on clinical trials included in the review and meta-analysis, two articles reported on the same trial, but reported results at different time points following ART initiation [25, 26]. Among these 19 clinical trials, 8 had a single arm [25, 27–33], 10 had two arms [34–43], and another one had two arms but reported only aggregated results from the two arms [44]. This gave 29 independent study populations. Most trials compared care conditions, and the treatment received in the different study arms were therefore comparable, although some trials considered compared ART regimen.
Of the 15 articles on cross-sectional studies, one was conducted across 5 countries at two different time points in each country [45], three were conducted in one country at two different time points [46–48], two were conducted in one country at one time point but among two different groups [49, 50], two were conducted in one country and at three different time points [51, 52], and the remaining seven were conducted at a single time point [24, 53–58]. This gave 33 distinct study populations.
Of the 51 articles on cohort studies, three articles were based on the same cohort but reported results at different time points [59–61], and two other articles reported on a same cohort [62, 63]. Of the 48 independent cohorts, 31 reported results from the overall cohort [24, 62, 64–92], while 17 reported results from two different groups within the cohort [59, 93–108], although for two of these studies, only one group was considered in our meta-analysis due to sample size restriction [100, 107]. This gave 63 distinct populations.
This meta-analysis is therefore based on 125 distinct populations, which accounted for a total of 156,798 patients, with individual study sizes ranging from 23 to 47,285 patients.
Across the 125 populations, the median proportion of women was 65.7% and individual studies ranged from 0 to 100% proportion of women. The populations were quite homogenous in terms of age, with the median/mean age in individual studies ranging from 29 to 42 years, and a median age across studies of 36 years. In all but one of the studies, ART initiation followed the WHO guidelines [109]. This study only enrolled patients with CD4 count >350 cells/mm3 [30]. The median proportion of patients across all studies classified as stage 3–4 according to WHO guidelines was 66.3%. The mean/median CD4 level at ART initiation in individual studies ranged from 33 to 569 cells/mm3 and the median across all studies was 134 cells/mm3. Baseline viral load was available for 58 (46.4%) of the study populations. The median viral load level at baseline in individual studies ranged from 4.0 to 6.2 log copies/mL, and across all studies the median was 5.1 log copies/mL. As we only selected studies in which the first line ART fulfilled WHO recommendations, all patients had initiated an ART regimen that contained either 3TC or FTC. For exposure to NNRTI, the median (IQR) proportion of patients across all studies receiving EFV and NVP was 34.5% (1.1–62.5) and 60.0% (31.6–95.0), respectively.
These 125 study populations enabled the evaluation of the proportion of virological success at 6 months (n = 54), 12 months (n = 84), 24 months (n = 41) and 36 months (n = 15) after ART initiation.
Of the 125 study populations, the proportion of virological success was available for using an on-treatment approach in 119 (95.2%). Using an intention-to-treat (ITT) approach, results on the proportion of virological success could be obtained from 76 (60.8%) study populations.
Virological success at 12 months
The results of 84 evaluations of virological success at 12 months after ART initiation were considered: 22 from clinical trials, 47 from cohort studies, and 15 from cross-sectional studies.
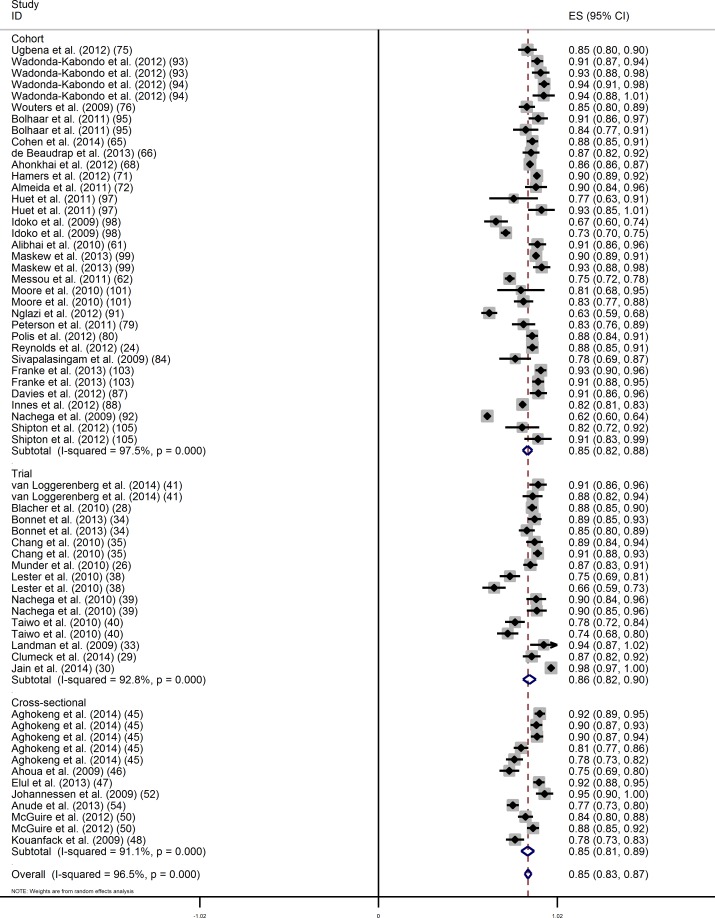
Considering OT results only, 64 evaluations of virological success were available at the threshold of 400 copies/mL and the overall proportion (95% confidence interval (CI)) of patients in virological success was 85.3% (83.3–87.3) (Fig 2). The Cochran test (p<0.001) and the I-square statistic (96.6%) indicated large between-study heterogeneity.
Fig 2. On treatment analysis of the proportion of virological success (400 copies/mL) at 12 months of ART by study design.
In univariate analysis, we did not find evidence that the proportions differed by study design (Table 1). The proportions (95% CI) of patients in virological success in clinical trials, cohort studies and cross-sectional studies were 86.1% (82.3–89.8), 85.1% (82.3–87.8) and 85.1% (81.3–88.9), respectively. The proportion of virological success was significantly higher in studies that were set in the public sector, that were in decentralised settings, and that had a higher proportion of patients on EFV-based ART (Table 1). On the other hand, the proportion of virological success was lower in studies in the general population with >6 months of follow-up, compared to studies without this restriction. When combining all factors in a multivariate model, no factor remained independently associated with the proportion of virological failure.
Table 1. Virological success (<400 copies/ml) at 12 months–on-treatment analysis.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| analysis | analysis | |||||
| Variables | Categories | N | Rate (95% CI) | (p) | Full model (p) | Reduced model (p) |
| Study design | Cohort | 35 | 85.1 [83.2–87.8] | Ref | ||
| Trial | 17 | 86.1 [82.3–89.8] | 0.90 | |||
| Cross sectional | 12 | 85.1 [81.3–88.9] | 0.99 | |||
| Beginning of enrolment | ≤2005 | 34 | 84.4 [81.5–87.4] | Ref | ||
| ≥2006 | 30 | 86.3 [83.8–88.8] | 0.36 | |||
| End of enrolment | ≤2005 | 11 | 85.6 [82.2–88.9] | Ref | ||
| 2006–2009 | 45 | 84.5 [82.2–86.9] | 0.92 | |||
| ≥2010 | 7 | 89.6 [84.9–94.3] | 0.46 | |||
| Region | Western Africa | 16 | 81.5 [77.4–85.5] | 0.11 | ||
| Eastern Africa | 25 | 87.3 [84.8–89.8] | Ref | |||
| Central Africa | 3 | 85.3 [78.2–92.4] | 0.99 | |||
| Southern Africa | 16 | 86.6 [84.1–89.1] | 0.99 | |||
| Several region | 2 | 76.2 [0.48–1.00] | 0.18 | |||
| Type of site | Public sector | 51 | 86.4 [84.5–88.3] | Ref | Ref | Ref |
| Other | 10 | 80.2 [73.2–87.3] | 0.04 | 0.76 | 0.04 | |
| Number of sites | 1 | 35 | 85.1 [81.9–88.3] | Ref | ||
| ≥2 | 20 | 85.9 [83.4–88.5] | 0.76 | |||
| Decentralised setting | No | 39 | 83.9 [80.8–86.9] | Ref | Ref | |
| Yes | 15 | 89.6 [85.7–93.5] | 0.04 | 0.35 | ||
| Both | 7 | 82.4 [78.4–86.5] | 0.79 | 0.30 | ||
| Population selected | General | 44 | 85.9 [84.3–87.5] | Ref | Ref | |
| General, with FU >6 months | 7 | 77.5 [68.9–86.1] | 0.01 | 0.43 | ||
| Specific | 7 | 89.0 [86.5–91.5] | 0.44 | 0.61 | ||
| VL monitoring before evaluation | No | 31 | 85.5 [82.1–88.8] | Ref | ||
| Yes | 33 | 85.2 [82.7–87.6] | 0.86 | |||
| Tracking of LTFU patients | No | 49 | 85.4 [83.0–87.9] | Ref | ||
| Yes | 15 | 85.0 [81.1–8.8] | 0.85 | |||
| Median age at ART initiation (years) | ≤36 | 32 | 84.7 [82.4–87.0] | Ref | ||
| >36 | 26 | 85.8 [81.4–90.2] | 0.60 | |||
| Proportion of women | ≤65 | 34 | 86.8 [83.7–89.9] | Ref | ||
| >65 | 30 | 83.7 [81.3–86.1} | 0.14 | |||
| Proportion of patients in WHO stage 3–4 | ≤69 | 25 | 84.4 [82.0–86.8] | Ref | ||
| >69 | 18 | 87.9 [85.3–90.6] | 0.23 | |||
| Median CD4 at ART initiation (cells/mm3) | ≤135 | 30 | 85.9 [84.1–87.7] | Ref | ||
| >135 | 27 | 84.4 [79.7–89.1] | 0.84 | |||
| Median VL at ART initiation (log copies/mL) | ≤5.2 | 14 | 81.6 [77.3–85.8] | Ref | ||
| >5.2 | 22 | 87.5 [84.8–90.1] | 0.09 | |||
| Proportion of EFV exposed patients | ≤40 | 22 | 82.0 [77.2–86.9] | Ref | Ref | |
| >40 | 20 | 87.4 [84.8–90.0] | 0.04 | 0.99 | ||
| Proportion of NVP exposed patients | ≤60 | 23 | 85.2 [81.4–89.0] | Ref | ||
| >60 | 19 | 83.1 [79.9–86.4] | 0.41 | |||
Using an OT approach, 19 evaluations of virological success were available at 40 copies/mL threshold, 22 evaluations of virological success at 1000 copies/mL threshold and 12 evaluations of virological success at 5000 copies/mL threshold. The overall proportions (95% CI) of virological success at these thresholds were 76.3% (71.6–81.0), 87.0% (84.6–89.5) and 90.8% (87.9–93.7), respectively (S1 Fig).
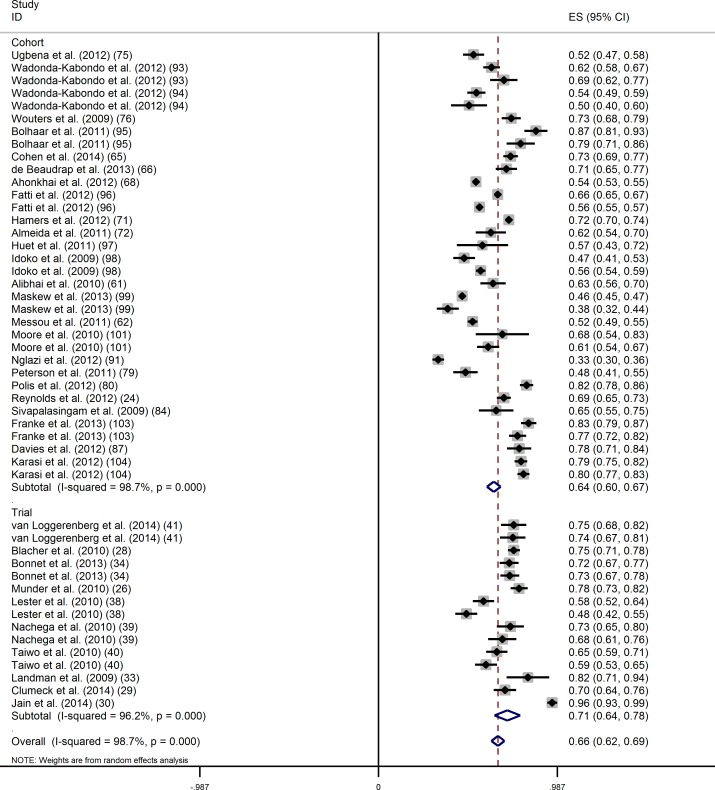
Using an ITT approach, 50 evaluations were considered at the threshold of 400 copies/mL, and the overall proportion of virological success (95% CI) was 65.9% [62.3–69.5] (Fig 3). Again, the Cochran test (p<0.001) and the I-square statistic (98.7%) indicated large between-study heterogeneity. In univariate analysis, the proportion of virological success (95% CI) was significantly lower in cohort studies than in clinical trials (63.6% (59.8–67.5) versus 71.1% (64.0–78.2);p = 0.04) (Table 2). The proportion of virological success was significantly higher in studies that enrolled patients who had initiated ART more recently (≥2006), and in studies with a higher median age at ART initiation. In multivariate analysis, the proportion of virological success remained significantly lower in cohort studies.
Fig 3. Intention-to-treat analysis of the proportion of virological success (400 copies/mL) at 12 months of ART by study design.
Table 2. Rate of virological success (<400 copies/ml) at 12 months–intention to treat analysis.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| analysis | analysis | |||||
| Variables | Categories | N | Rate (95% CI) | (p) | Full model (p) | Reduced model (p) |
| Study design | Cohort | 35 | 63.6 [59.8–67.5] | Ref | Ref | Ref |
| Trial | 15 | 71.1 [64.0–78.2] | 0.037 | 0.07 | 0.039 | |
| Beginning of enrolment | ≤2005 | 28 | 62.1 [58.2–66.1] | Ref | Ref | |
| ≥2006 | 22 | 69.4 [63.9–74.9] | 0.031 | 0.82 | ||
| End of enrolment | ≤2005 | 7 | 70.6 [65.5–75.7] | 0.22 | ||
| 2006–2009 | 35 | 62.1 [57.9–66.2] | Ref | |||
| ≥2010 | 7 | 74.4 [64.3–84.5] | 0.05 | |||
| Region | Western Africa | 10 | 58.4 [53.5–63.2] | 0.09 | ||
| Eastern Africa | 20 | 68.6 [62.8–74.3] | Ref | |||
| Central Africa | 1 | 69.9 [63.6–76.1] | 1.00 | |||
| Southern Africa | 16 | 62.8 [57.8–67.8] | 0.32 | |||
| Several region | 1 | 72.0 [70.3–73.8] | 0.99 | |||
| Type of site | Public sector | 37 | 65.5 [59.8–71.2] | Ref | ||
| Other | 8 | 62.4 [57.1–67.7] | 0.57 | |||
| Number of sites | 1 | 26 | 67.1 [59.8–74.3] | Ref | ||
| ≥2 | 16 | 63.1 [55.6–70.6] | 0.36 | |||
| Decentralised setting | No | 27 | 66.4 [60.3–70.4] | Ref | ||
| Yes | 11 | 68.8 [54.5–83.1] | 0.80 | |||
| Both | 7 | 58.4 [52.9–63.9] | 0.25 | |||
| Population selected | General | 33 | 65.1 [60.7–69.4] | Ref | ||
| General, with FU >6 months | 5 | 59.8 [51.8–67.8] | 0.64 | |||
| Specific | 6 | 66.6 [54.3–78.9] | 0.96 | |||
| VL monitoring before evaluation | No | 18 | 64.4 [60.4–68.5] | Ref | ||
| Yes | 32 | 66.0 [60.0–72.1] | 0.69 | |||
| Tracking of LTFU patients | No | 36 | 66.5 [61.2–71.8] | Ref | ||
| Yes | 14 | 62.7 [57.1–68.4] | 0.40 | |||
| Median age at ART initiation (years) | ≤36 | 25 | 59.9 [55.3–64.4] | Ref | Ref | Ref |
| >36 | 18 | 68.4 [64.2–72.5] | 0.029 | 0.13 | 0.10 | |
| % women | ≤65 | 21 | 64.7 [58.3–71.1] | Ref | ||
| >65 | 27 | 64.9 [60.7–59.2] | 0.94 | |||
| % WHO stage 3–4 | ≤65 | 15 | 62.4 [53.5–71.2] | Ref | ||
| >65 | 16 | 68.8 [63.3–74.3] | 0.82 | |||
| Median CD4 level | ≤130 | 26 | 63.0 [59.1–66.9] | Ref | ||
| >130 | 19 | 67.5 [61.3–73.6] | 0.21 | |||
| Median VL level | ≤5.2 | 12 | 61.3 [53.9–68.8] | Ref | ||
| >5.2 | 18 | 67.2 [61.5–72.9] | 0.22 | |||
| % with EFV | ≤40 | 15 | 64.6 [58.6–70.6] | Ref | ||
| >40 | 15 | 65.1 [58.9–71.4] | 0.92 | |||
| % with NVP | ≤60 | 16 | 64.3 [58.3–70.2] | Ref | ||
| >60 | 14 | 65.6 [59.7–71.5] | 0.80 | |||
Using an ITT approach, 15 evaluations of virological success were available at 40 copies/mL threshold, 12 evaluations of virological success were available at 1000 copies/mL and 3 evaluations of virological success were available at 5000 copies/mL threshold. The overall proportions (95% CI) of virological success at these thresholds were 54.9% (40.9–69.0), 66.5% (60.8–72.2) and 76.9% (71.2–82.6), respectively (S1 Fig).
Virological success at 24 months
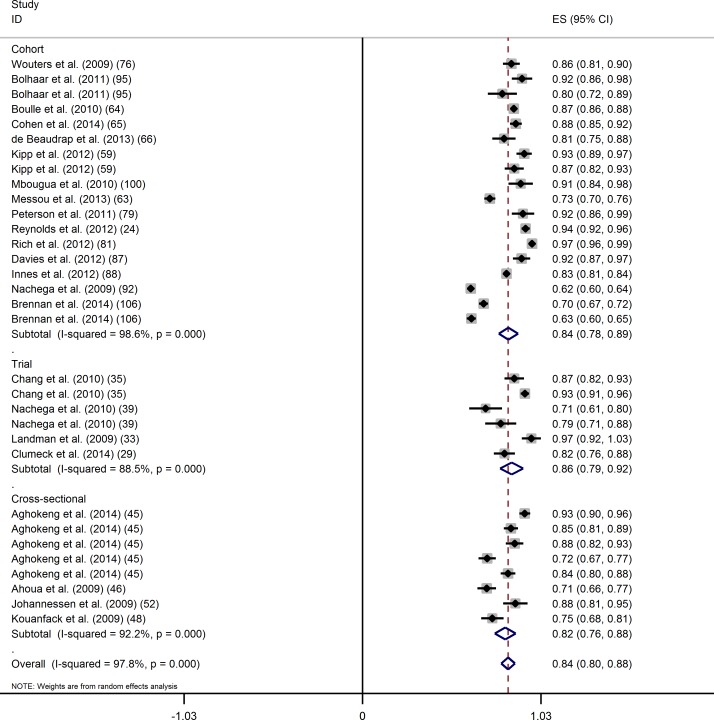
Using an OT approach, 32 evaluations of virological success at 24 months after ART initiation at the threshold of 400 copies/mL were available. The overall proportion of virological success (95% CI) was 83.7% (79.8–87.6) (Fig 4). The Cochran test (p<0.001) and the I-square statistic (97.8%) indicated large between-study heterogeneity.
Fig 4. On treatment analysis of the proportion of virological success (400 copies/mL) at 24 months of ART by study design.
In univariate analysis, we did not find evidence that the proportions differed by study design (Table 3). The proportions of virological success (95% CI) in clinical trials, cohort studies and cross-sectional studies were 85.8% (79.1–92.4), 83.9% (78.3–89.5) and 82.1% (76.1–88.1), respectively. The proportion of virological success was found to be significantly higher in public sector sites compared to other sites, in studies with younger populations, and in studies with a higher proportion of women. The proportion of virological success also tended to be higher in studies with lower viral load at ART initiation. When combining all factors in a multivariate model, the only factor that remained significantly associated with a higher proportion of virological success was when the study was set in the public sector.
Table 3. Rate of virological success (<400 copies/ml) at 24 months–on-treatment analysis.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| analysis | analysis | |||||
| Variables | Categories | N | Rate (95% CI) | (p) | Full model (p) | Reduced model (p) |
| Study design | Cohort | 18 | 83.9 [78.3–89.5] | Ref | ||
| Trial | 6 | 85.8 [79.1–92.4] | 0.88 | |||
| Cross-sectional | 8 | 82.1 [76.1–88.1] | 0.94 | |||
| Beginning of enrolment initiation | ≤2005 | 21 | 85.3 [80.6–89.9] | Ref | ||
| ≥2006 | 11 | 80.8 [73.9–87.7] | 0.21 | |||
| End of enrolment | ≤2005 | 6 | 85.0 [76.3–93.8] | 1.00 | ||
| 2006–2009 | 20 | 85.0 [80.3–89.7] | Ref | |||
| ≥2010 | 6 | 78.3 [67.8–88.9] | 0.26 | |||
| Region | Western Africa | 8 | 85.0 [78.1–91.9] | 0.64 | ||
| Eastern Africa | 8 | 89.4 [84.9–93.9] | Ref | |||
| Southern Africa | 11 | 81.0 [75.3–86.6] | 0.11 | |||
| Central Africa | 4 | 83.2 [77.2–89.1] | 0.51 | |||
| Several region | 1 | 62.3 [60.3–74.3] | 0.010 | |||
| Type of site | Public sector | 28 | 85.4 [81.6–89.3] | Ref | Ref | Ref |
| Other | 3 | 68.2 [60.9–75.5] | 0.005 | 0.030 | 0.008 | |
| Number of sites | 1 | 18 | 83.9 [78.0–89.8] | Ref | ||
| ≥2 | 8 | 82.3 [77.2–87.3] | 0.70 | |||
| Decentralized area | No | 24 | 82.5 [78.1–86.9] | Ref | ||
| Yes | 7 | 88.5 [83.7–93.3] | 0.21 | |||
| Population selected | General | 24 | 84.1 [79.8–88.4] | Ref | ||
| General, with FU >6 months | 5 | 77.8 [67.5–88.1] | 0.14 | |||
| VL monitoring before evaluation | No | 11 | 82.6 [73.2–92.0] | Ref | ||
| Yes | 21 | 84.3 [80.2–88.3] | 0.67 | |||
| Tracking of LTFU patients | No | 24 | 82.2 [77.4–86.9] | Ref | ||
| Yes | 8 | 88.2 [82.7–93.8] | 0.13 | |||
| Median age at ART initiation (years) | ≤36 | 13 | 87.7 [83.3–92.2] | Ref | Ref | |
| >36 | 15 | 80.3 [73.7–87.0] | 0.044 | 0.18 | ||
| Proportion of women | ≤65 | 12 | 79.1 [72.1–86.1] | Ref | Ref | Ref |
| >65 | 20 | 86.5 [83.4–89.7] | 0.020 | 0.29 | 0.09 | |
| Proportion of patients in WHO stage 3–4 | ≤69 | 11 | 83.3 [76.8–89.9] | Ref | ||
| >69 | 11 | 86.7 [82.2–91.2] | 0.45 | |||
| Median CD4 at ART initiation (cells/mm3) | ≤135 | 18 | 82.6 [78.1–87.1] | Ref | ||
| >135 | 12 | 85.8 [77.5–94.0] | 0.41 | |||
| Median VL at ART initiation (log copies/mL) | ≤5.2 | 5 | 91.7 [87.8–95.7] | Ref | Ref | |
| >5.2 | 8 | 83.0 [75.5–90.5] | 0.09 | 0.24 | ||
| Proportion of EFV exposed patients | ≤40 | 13 | 82.5 [74.7–90.4] | Ref | ||
| >40 | 10 | 82.6 [75.6–89.7] | 0.98 | |||
| Proportion of NVP exposed patients | ≤60 | 13 | 79.7 [73.0–86.5] | Ref | ||
| >60 | 10 | 86.4 [81.3–91.6] | 0.12 | |||
Using an OT approach, 7 evaluations of virological success were available at 40 copies/mL threshold, 14 evaluations of virological success were available at 1000 copies/mL threshold and 8 evaluations of virological success were available at 5000 copies/mL. The overall proportions (95% CI) of virological success at these thresholds were 83.1% (75.5–90.6), 84.1% (79.2–88.9) and 91.1% (87.3–94.9), respectively (S1 Fig).
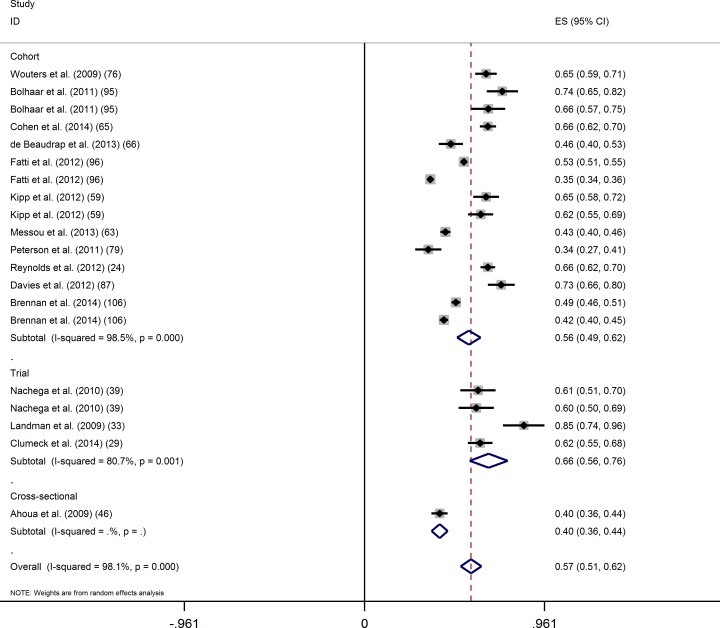
Using an ITT approach, 20 evaluations of virological success at 24 months after ART initiation at the threshold of 400 copies/mL were considered. The overall proportion of virological success (95% CI) was 56.8% (51.3–62.4) (Fig 5). Again, the Cochran test (p<0.001) and the I-square statistic (98.2%) indicated large between-study heterogeneity. In univariate analysis, the proportion of virological success tended to be higher in clinical trials than in cohort studies (Table 4). The proportion of virological success was significantly higher in studies set in the public sector, and in studies which did not track LTFU patients.
Fig 5. Intention-to-treat analysis of the proportion of virological success (400 copies/mL) at 24 months of ART by study design.
Table 4. Rate of virological success (<400 copies/mL) at 24 months–intention-to-treat analysis.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| analysis | analysis | |||||
| Variable | categories | N | Rate (95% CI) | (p) | Full model (p) | Reduced model (p) |
| Study design | Cohort | 16 | 54.6 [48.6–60.6] | Ref | Ref | |
| Trial | 4 | 66.2 [56.1–76.4] | 0.14 | 0.88 | ||
| Beginning of enrolment | ≤2005 | 14 | 58.5 [50.2–66.7] | Ref | ||
| ≥2006 | 6 | 53.0 [46.9–59.2] | 0.52 | |||
| End of enrolment | ≤2005 | 4 | 58.7 [41.9–75.6] | 0.92 | ||
| 2006–2009 | 12 | 60.7 [53.0–68.3] | Ref | |||
| ≥2010 | 4 | 44.8 [35.4–54.3] | 0.24 | |||
| Region | Western Africa | 4 | 51.2 [37.6–64.8] | 0.84 | ||
| Eastern Africa | 4 | 58.1 [44.3–72.0] | Ref | |||
| Southern Africa | 11 | 58.0 [50.5–65.5] | 0.99 | |||
| Central Africa | 1 | 61.7 [55.1–68.3] | 0.99 | |||
| Several region | - | - | - | |||
| Type of site | Public sector | 17 | 59.4 [53.7–65.2] | Ref | Ref | Ref |
| Other | 3 | 42.8 [29.4–56.2] | 0.05 | 0.43 | 0.29 | |
| Number of sites | 1 | 14 | 59.7 [52.9–66.5] | Ref | ||
| ≥2 | 3 | 50.1 [39.3–60.8] | 0.32 | |||
| Decentralized area | No | 14 | 57.1 [50.9–63.4] | Ref | ||
| Yes | 3 | 62.4 [57.6–67.2] | 0.86 | |||
| Both | 2 | 44.1 [26.5–61.5] | 0.39 | |||
| Population selected | General | 15 | 55.3 [48.9–61.7] | Ref | ||
| General, with FU >6 months | 3 | 51.2 [37.9–64.6] | 0.62 | |||
| Specific | - | - | - | |||
| VL monitoring before evaluation | No | 4 | 48.0 [35.8–60.3] | Ref | ||
| Yes | 16 | 59.1 [53.2–65.0] | 0.15 | |||
| Tracking of LTFU patients | No | 13 | 61.6 [54.8–68.5] | Ref | Ref | Ref |
| Yes | 7 | 48.2 [39.6–56.8] | 0.048 | 0.46 | 0.22 | |
| Median age at ART initiation | ≤36 years | 9 | 54.6 [45.4–63.8] | Ref | ||
| >36 years | 9 | 56.1 [49.4–62.9] | 0.78 | |||
Using an ITT approach, 8 evaluations of virological success at 24 months after ART initiation were available at 40 copies/mL threshold and 6 evaluations of virological success were available at 1000 copies/mL threshold. The overall proportions of virological success (95% CI) were 57.6% (49.6–65.5) and 47.0% (36.1–58.0), respectively. Only one evaluation was performed at the threshold of 5000 copies/mL. This indicated a 67.3% proportion of virological success (95% CI: 63.4–71.2) [24] (S1 Fig).
Virological success at M6
Using an OT approach, 13 evaluations of virological success at 6 months after ART initiation were available at the threshold of 40 copies/mL, 44 evaluations of virological success were available at the threshold of 400 copies/mL, 11 evaluations of virological success were available at the threshold of 1000 copies/mL, and 3 evaluations of virological success were available at the threshold of 5000 copies/mL. The overall proportions (95% CI) of virological success at these thresholds were 71.7% (62.8–80.7), 83.8% (81.0–86.6), 85.5% (81.7–89.3), and 92.7% (87.6–97.8), respectively.
Using an ITT approach, 7 evaluations of virological success at 6 months after ART initiation were available at the threshold of 40 copies/mL, 31 evaluations of virological success were available at the threshold of 400 copies/mL, 10 evaluations of virological success were available at the threshold of 1000 copies/mL and 2 evaluations of virological success were available at the threshold of 5000 copies/mL. The overall proportions (95% CI) of virological success at these thresholds were 65.0% (48.4–81.6), 68.5% (62.8–74.2), 70.9% (62.7–79.2) and 87.1% (83.6–90.5), respectively (S1 Fig).
Virological success at M36
Using an OT approach, 12 evaluations of virological success at 36 months after ART initiation were available at the threshold of 400 copies/mL, 4 evaluations of virological success were available at the threshold of 1000 copies/mL, and 3 evaluations of virological success were available at the threshold of 5000 copies/mL. The overall proportions (95% CI) of virological success at these thresholds were 85.8% (82.2–89.3), 81.7% (77.9–85.5) and 92.8% (89.4–96.2), respectively.
Using an ITT approach, 5 evaluations of virological success at 36 months after ART initiation were available at the threshold of 400 copies/mL, and 2 evaluations provided proportions of virological success at the thresholds of 1000 copies/mL. The overall proportions (95% CI) of virological success at these thresholds were 37.8% (22.7–52.9) and 31.6% (0.03–59.8) (S1 Fig), respectively. Only one evaluation was performed at the threshold of 5000 copies/mL. This indicated a 63.3% proportion of virological success (95% CI: 59.3–67.3) [24] (S1 Fig).
Evaluation of publication bias
The funnel plots of the individual studies showed an asymmetry, with smaller studies reporting higher proportions of virological success more often (S2 Fig). Applying the method proposed by Eger and colleagues, the presence of publication bias was evidenced in the ITT analysis at 24 months (p-value for the intercept: 0.018), but not in any other analysis.
Discussion
In this meta-analysis, we reported the proportion of patients in virological success while on first-line ART in sub-Saharan Africa, based on 85 recently published articles that led to 125 independent study populations comprised of 156,798 individuals. To our knowledge, this is the most recent and largest meta-analysis to compare virological success at specific time points in sub-Saharan Africa.
We did not observe an improvement in the proportion of virological success as compared to previous meta-analyses [8–10, 15, 17]. Our meta-analysis however is novel in the way in which we restricted our search to studies implemented in sub-Saharan Africa, and for the fact that it is not largely based on clinical trials. As compared to previously published meta-analyses which were not restricted by geographic location, and largely based on the results of clinical trials [15, 17], we believe that our meta-analysis is more representative of real-life treatment and care settings in sub-Saharan Africa–the region with the highest HIV burden.
In all the studies considered in this meta-analysis, the most common threshold for the evaluation of virological success was 400 copies/mL. Using this threshold and an OT approach, (e.g. only considering those still in care and on ART at the time of evaluation), the proportion of virological success was around 85%. Interestingly, this proportion of virological success remained stable regardless of the time point between M6 and M36. This result is likely due to the fact that only those who remain alive and in care are considered, which induces a healthy survivor bias–a bias which has been reported previously [17].
Several of the studies included in the meta-analysis also reported virological success at the threshold of 1000 copies/mL. This is the threshold for virological failure as defined by the latest WHO recommendations [110]. Using an OT approach, increasing the threshold from 400 to 1000 copies/mL did not increase the proportion of virological success by a significant amount, suggesting that few patients exhibit intermediate replicative levels. From a virological point of view, defining success at the threshold of 400 or 1000 copies/mL raises the potential for acquisition of drug resistance at a low level of replication [111, 112]. From a public health perspective, our results suggest that defining virological success at the threshold of 1000 or 5000 copies/mL is not identical, which is in accordance with the 2013 WHO recommendations in which the threshold defining virological success was lowered from 5000 to 1000 copies/mL [4]. Our results indicate that broadening access to viral load monitoring for patients under ART, especially through use of tools such as dried blood spots (which allows for a threshold of 1000 copies/mL, but not any lower) [113–116] should be more of a priority than lowering the threshold defining virological success. These findings support the WHO guidelines, in which virological failure remains at the threshold of 1000 copies/mL.
The results of our meta-analysis on the proportions of virological success at 6, 12, 24 and 36 months after ART initiation defined at the thresholds of 400 and 1000 copies/mL are comparable to the results from another meta-analysis performed in resource limited settings [17], despite this meta-analysis aggregating studies irrespective of the threshold.
Of the 125 study populations considered, only 28 (22.4%) directly reported the proportion of patients in virological success using an ITT approach. To better estimate the proportions of success, we used results when reported using an ITT approach, but also searched the articles for the number of deaths, loss to follow-up, switches to 2nd line ART, transfers/withdrawals, and for the number of patients who did not have a virological evaluation. This enabled us to estimate the proportion of patients in virological success using an ITT approach in an additional 38 studies.
Regardless of the time point following ART initiation and the threshold used to define virological success, results using an ITT approach were lower than those using an OT approach. OT results evaluate ART efficacy for those still on ART, while ITT results evaluate the effectiveness of the programmes delivering ART, as those who died or were lost to follow-up are considered as failures, and these numbers increase with time. The difference in estimated proportions between the two approaches was substantial and as large as 15% at 6 months after ART initiation, reaching 20% at 12 months, and 25% at 24 months. This difference can be explained by death and patients LTFU, but also by the number of patients who did not have a virological evaluation performed. Studies in which the proportion of non-evaluated patients was >40% [96, 99, 100, 117, 118] were not considered in the ITT analysis, as we sought “systematic” virological evaluation, and it is possible that a lack of viral load monitoring was not random.
We wanted to investigate whether clinical trials, cohort studies, and cross-sectional studies provide similar quality information on proportions of virological success.
The fact that the proportions of virological success, using an OT approach, compare well across the various study designs indicates the effectiveness of ART in Africa. It also seems to indicate that patients in routine care are as adherent to ART as those enrolled in trials, even though the latter group may be more sensitised to this issue.
Cross-sectional studies, if based on a representative sample of the targeted population, have fewer logistical and financial constraints, allowing results to be obtained more quickly than cohort studies. Such studies are of special interest for national HIV treatment programmes, as they provide valuable data for the planning of future activities. They are also recommended by WHO for the surveillance of resistance in patients on ART [119].
On the other hand, using an ITT approach, the proportion of virological success was significantly higher in randomised trials than in cohorts at 12 months after ART initiation, and tended to be higher at 24 months. This could be explained by the selection of healthier participants, but also by more frequent monitoring of patients, more systematic clinical and biological examinations and by reinforced clinical teams in trials as compared to routine practice. All of these factors may have reduced attrition in clinical trials, and may explain the higher proportion of virological success as compared to cohort studies. Indeed, a relatively high mortality in the first 6 to 12 months after ART initiation and substantial loss to follow-up have been widely reported in resource limited settings [22, 23, 120, 121]. These results highlight the need to strengthen retention in care in these settings.
Unexpectedly, in ITT analysis there was no evidence of an effect of tracking of LTFU patients on the proportions of virological success at 12 months after ART initiation, and at 24 months the proportion of virological success was even lower in studies that tracked LTFU patients than in studies that did not. However, this result is likely influenced by two studies that implemented tracking, but which had very low proportions of virological success. In one of these studies, there was a very large (32%) proportion of deaths [79]. The other study was a multicentre nation-wide study in South Africa that reported particularly low ITT results [96]. This likely illustrates the heterogeneity in the definition of tracking of LTFU patients which ranges from limited telephone contact efforts to more intensive home visits. Despite this meta-analysis not finding a strong link between the tracking of LTFU patients and virological success, such initiatives should be more widely implemented as a means to maintain patients in care and to prevent the acquisition of resistance.
The proportion of patients in virological success in OT analysis was found to be larger in public health centres compared to other sites (faith-based, NGO supported or with a mixed recruitment). Public centres may have been selected based on their capacities to participate in such studies (e.g. sufficient number of medical staff, easy access to a biological laboratory), but these are not necessarily representative of all health centres in the country. Faith-based and NGO supported centres may treat lower socio-economic populations and data from these centres may be under-reported, leading to a publication bias. Motivations to publish may differ between centres, while the funnel plots used in this meta-analysis indicated that some small study bias may be present.
Some limitations of this meta-analysis should be noted. Studies considered were heterogeneous in their design but also in the populations they considered. Most studies were based on the general population with limited selection criteria, but some were restricted to specific populations, such as patients co-infected with hepatitis or tuberculosis, or women who had previously been exposed to ART for PMTCT, etc. Studies on these specific populations had a limited sample size, and were therefore thought to contribute to the heterogeneity among estimations. However, the effect of these specific populations was investigated and was not found to be significant.
The studies also differed in terms of their definition of virological success, partly due to the variability of the techniques used to measure viral load, which do not all have the same thresholds. To control for this variability, we performed estimations of virological success at various thresholds. In only a small number of studies was virological success based on the result of two viral load measurements. This may also have contributed to the heterogeneity of our results. However, the limited number of such studies meant that this effect on the meta-analysis could not be evaluated.
To explain the heterogeneity of virological success across various study populations, programme characteristics were investigated (e.g. tracking of LTFU patients, type of site, setting). However, no specific characteristics were identified. This may be explained by characteristics not being mentioned in the individual studies, resulting in the pooling of studies in the meta-analysis that were not in fact similar.
By definition, only studies with virological evaluation were considered in our meta-analysis. Many programs in Africa, where no viral load monitoring is available have thus not been considered in our meta-analysis. However, we do not anticipate that this is a source of bias in our results. Indeed, some of the studies we considered were virological evaluations at a specific time point, without routine viral load monitoring. This was particularly true for cross-sectional evaluations, nested in cohort studies, and we did not find evidence that results from cross-sectional evaluations differed from the other studies.
In conclusion, this is the largest, most up-to-date meta-analysis on proportions of virological success at specific time points after ART initiation and at multiple thresholds, based on both OT and ITT analysis and the first comparison between clinical trials, cohort studies, and cross-sectional studies. Evidence from this review suggests that the current international target to have 90% of patients on ART in virological success will require intensive strategies to improve adherence to ART, and retention in care. It is also important to improve access to viral load monitoring in order to detect those at risk of virological failure, as early as possible, offering them effective ART.
Supporting information
(DOCX)
Estimation of the rate of virological success based on different threshold (in copies/mL) on on-treatment (full box) and intention-to treat (empty box) at: A) at 6 months of ART, B) 12 months of ART, c) 24 months of ART, and D) 36 months of ART.
(DOCX)
A) OT analysis at 12 months; B) ITT analysis at 12 months; C) OT analysis at 24 months; D) ITT analysis at 24 months.
(DOCX)
(DOCX)
Acknowledgments
We would to thank Ms Sandrine Royer-Devaux for her support in the article search and Ms Rebecca Grant and Ms Anna Louise Funk for their thorough proof reading and editing.
Data Availability
Relevant data can be found in Figshare, as follows: https://figshare.com/s/942c5e220b758357b703; DOI: 10.6084/m9.figshare.4811500.
Funding Statement
No funding.
References
- 1.WHO. Fact sheet N°360. Available at http://www.who.int/mediacentre/factsheets/fs360/en/ (Last accessed on August 16 2016). 2015.
- 2.UNAIDS. AIDS by the numbers 2015. Avaialble at: http://www.unaids.org/sites/default/files/media_asset/AIDS_by_the_numbers_2015_en.pdf last accessed August 16 2016. 2015.
- 3.WHO. Global update on the Health sector response to HIV, 2014. Available at http://apps.who.int/iris/bitstream/10665/128494/1/9789241507585_eng.pdf (Last accessed on August 16 2016). 2014.
- 4.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Recommendations for a public health approach—Second edition. Available at http://www.who.int/hiv/pub/arv/arv-2016/en/ (Last accessed on August 17 2016). 2016. [PubMed]
- 5.WHO. Consolidated Guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommandation for a public Health Approch. June 2013. Available at http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1 (Last accessed on August 17 2016). 2013.
- 6.Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368(9534):505–10. doi: 10.1016/S0140-6736(06)69158-7 [DOI] [PubMed] [Google Scholar]
- 7.WHO. Antiretroviral therapy for HIV infection in Adults and Adolescents. Recommendations for a public health approach. 2010 revision. Available at http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf (Last accessed on August 17 2016). 2010. [PubMed]
- 8.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. The Lancet infectious diseases. 2010;10(3):155–66. Epub 2010/02/27. PubMed Central PMCID: PMC Pubmed. doi: 10.1016/S1473-3099(09)70328-7 [DOI] [PubMed] [Google Scholar]
- 9.Gupta RK, Hill A, Sawyer AW, Cozzi-Lepri A, von Wyl V, Yerly S, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. The Lancet infectious diseases. 2009;9(7):409–17. Epub 2009/06/27. PubMed Central PMCID: PMC Pubmed. doi: 10.1016/S1473-3099(09)70136-7 [DOI] [PubMed] [Google Scholar]
- 10.Stadeli KM, Richman DD. Rates of emergence of HIV drug resistance in resource-limited settings: a systematic review. Antiviral therapy. 2013;18(1):115–23. Epub 2012/10/12. PubMed Central PMCID: PMC Pubmed. doi: 10.3851/IMP2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Surveillance of HIV drug resistance in adults initiating antiretroviral therapy (pre-treatment HIV drug resistance) Concept note. Available at http://apps.who.int/iris/bitstream/10665/112802/1/9789241507196_eng.pdf?ua=1 (Last accessed on August 17 2016). 2014.
- 12.WHO. Global report on early warning indicators of HIV drug resistance. Available at http://www.who.int/hiv/pub/drugresistance/ewi-hivdr-2016/en/ (Last accessed on August 17 2016). 2016.
- 13.Centre for Reviews and Dissemination. Systematic Reviews: CRD’s guidance for undertaking reviews in health care. Available at http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf (Last accessed on August 17 2016). 2009.
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535 PubMed Central PMCID: PMC2714657. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee FJ, Amin J, Carr A. Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks' follow-up. PloS one. 2014;9(5):e97482 PubMed Central PMCID: PMCPMC4022522. doi: 10.1371/journal.pone.0097482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madec Y, Leroy S, Rey-Cuille MA, Huber F, Calmy A. Persistent Difficulties in Switching to Second-Line ART in Sub-Saharan Africa—A Systematic Review and Meta-Analysis. PloS one. 2013;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boender TS, Sigaloff KC, McMahon JH, Kiertiburanakul S, Jordan MR, Barcarolo J, et al. Long-term Virological Outcomes of First-Line Antiretroviral Therapy for HIV-1 in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;61(9):1453–61. PubMed Central PMCID: PMC4599392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 20.Liao LJ, Xing H, Su B, Wang Z, Ruan YH, Wang X, et al. Impact of HIV drug resistance on virologic and immunologic failure and mortality in a cohort of patients on antiretroviral therapy in China. AIDS (London, England). 2013;27(11):1815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34. PubMed Central PMCID: PMCPMC2127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49(6):965–72. doi: 10.1086/605500 [DOI] [PubMed] [Google Scholar]
- 23.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–908. PubMed Central PMCID: PMC3816249. doi: 10.1097/QAD.0b013e32830007cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds SJ, Sendagire H, Newell K, Castelnuovo B, Nankya I, Kamya M, et al. Virologic versus immunologic monitoring and the rate of accumulated genotypic resistance to first-line antiretroviral drugs in Uganda. BMC infectious diseases. 2012;12:381 Epub 2012/12/29. PubMed Central PMCID: PMCPMC3548731 Pubmed. doi: 10.1186/1471-2334-12-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodall RL, Dunn DT, Pattery T, van Cauwenberge A, Nkurunziza P, Awio P, et al. Phenotypic and genotypic analyses to guide selection of reverse transcriptase inhibitors in second-line HIV therapy following extended virological failure in Uganda. The Journal of antimicrobial chemotherapy. 2014. Epub 2014/03/19. PubMed Central PMCID: PMC Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munderi P, Walker AS, Kityo C, Babiker AG, Ssali F, Reid A, et al. Nevirapine/zidovudine/lamivudine has superior immunological and virological responses not reflected in clinical outcomes in a 48-week randomized comparison with abacavir/zidovudine/lamivudine in HIV-infected Ugandan adults with low CD4 cell counts. HIV medicine. 2010;11(5):334–44. Epub 2010/02/09. PubMed Central PMCID: PMC Pubmed. doi: 10.1111/j.1468-1293.2009.00786.x [DOI] [PubMed] [Google Scholar]
- 27.Abdissa A, Yilma D, Fonager J, Audelin AM, Christensen LH, Olsen MF, et al. Drug resistance in HIV patients with virological failure or slow virological response to antiretroviral therapy in Ethiopia. BMC infectious diseases. 2014;14:181 Epub 2014/04/09. PubMed Central PMCID: PMC Pubmed. doi: 10.1186/1471-2334-14-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blacher RJ, Muiruri P, Njobvu L, Mutsotso W, Potter D, Ong'ech J, et al. How late is too late? Timeliness to scheduled visits as an antiretroviral therapy adherence measure in Nairobi, Kenya and Lusaka, Zambia. AIDS care. 2010;22(11):1323–31. Epub 2010/08/17. PubMed Central PMCID: PMC Pubmed. doi: 10.1080/09540121003692235 [DOI] [PubMed] [Google Scholar]
- 29.Clumeck N, Mwamba C, Kabeya K, Matanda S, Vaira D, Necsoi C, et al. First-line antiretroviral therapy with nevirapine versus lopinavir-ritonavir based regimens in a resource-limited setting. AIDS (London, England). 2014;28(8):1143–53. Epub 2014/07/17. PubMed Central PMCID: PMCPMC4004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain V, Byonanebye DM, Amanyire G, Kwarisiima D, Black D, Kabami J, et al. Successful antiretroviral therapy delivery and retention in care among asymptomatic individuals with high CD4+ T-cell counts at least 350 cells/mul in Rural Uganda. AIDS (London, England). 2014. Epub 2014/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rositch AF, Gravitt PE, Tobian AA, Newell K, Quinn TC, Serwadda D, et al. Frequent detection of HPV before and after initiation of antiretroviral therapy among HIV/HSV-2 co-infected women in Uganda. PloS one. 2013;8(1):e55383 Epub 2013/02/06. PubMed Central PMCID: PMCPMC3558485 Pubmed. doi: 10.1371/journal.pone.0055383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semvua HH, Mtabho CM, Fillekes Q, van den Boogaard J, Kisonga RM, Mleoh L, et al. Efavirenz, tenofovir and emtricitabine combined with first-line tuberculosis treatment in tuberculosis-HIV-coinfected Tanzanian patients: a pharmacokinetic and safety study. Antiviral therapy. 2013;18(1):105–13. doi: 10.3851/IMP2413 [DOI] [PubMed] [Google Scholar]
- 33.Landman R, Poupard M, Diallo M, Ngom Gueye NF, Diakhate N, Ndiaye B, et al. Tenofovir-emtricitabine-efavirenz in HIV-I-infected adults in Senegal: a 96-week pilot trial in treatment-naive patients. Journal of the International Association of Physicians in AIDS Care (Chicago, Ill: 2002). 2009;8(6):379–84. [DOI] [PubMed] [Google Scholar]
- 34.Bonnet M, Bhatt N, Baudin E, Silva C, Michon C, Taburet AM, et al. Nevirapine versus efavirenz for patients co-infected with HIV and tuberculosis: a randomised non-inferiority trial. The Lancet infectious diseases. 2013;13(4):303–12. Epub 2013/02/26. PubMed Central PMCID: PMC Pubmed. doi: 10.1016/S1473-3099(13)70007-0 [DOI] [PubMed] [Google Scholar]
- 35.Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PloS one. 2010;5(6):e10923 Epub 2010/06/10. PubMed Central PMCID: PMCPMC2880005 Pubmed. doi: 10.1371/journal.pone.0010923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung MH, Richardson BA, Tapia K, Benki-Nugent S, Kiarie JN, Simoni JM, et al. A randomized controlled trial comparing the effects of counseling and alarm device on HAART adherence and virologic outcomes. PLoS medicine. 2011;8(3):e1000422 Epub 2011/03/11. PubMed Central PMCID: PMCPMC3046986 Pubmed. doi: 10.1371/journal.pmed.1000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurent C, Kouanfack C, Laborde-Balen G, Aghokeng AF, Mbougua JB, Boyer S, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. The Lancet infectious diseases. 2011;11(11):825–33. Epub 2011/08/13. PubMed Central PMCID: PMC Pubmed. doi: 10.1016/S1473-3099(11)70168-2 [DOI] [PubMed] [Google Scholar]
- 38.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–45. Epub 2010/11/13. PubMed Central PMCID: PMC Pubmed. doi: 10.1016/S0140-6736(10)61997-6 [DOI] [PubMed] [Google Scholar]
- 39.Nachega JB, Chaisson RE, Goliath R, Efron A, Chaudhary MA, Ram M, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS (London, England). 2010;24(9):1273–80. Epub 2010/05/11. PubMed Central PMCID: PMCPMC2888722 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taiwo BO, Idoko JA, Welty LJ, Otoh I, Job G, Iyaji PG, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. Journal of acquired immune deficiency syndromes (1999). 2010;54(1):85–92. Epub 2010/04/27. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 41.van Loggerenberg F, Grant AD, Naidoo K, Murrman M, Gengiah S, Gengiah TN, et al. Individualised Motivational Counselling to Enhance Adherence to Antiretroviral Therapy is not Superior to Didactic Counselling in South African Patients: Findings of the CAPRISA 058 Randomised Controlled Trial. AIDS and behavior. 2014. Epub 2014/04/04. PubMed Central PMCID: PMC Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landman R, Koulla-Shiro S, Sow PS, Ngolle M, Diallo MB, Guèye NFN, et al. Evaluation of four tenofovir-containing regimens as first-line treatments in Cameroon and Senegal: The ANRS 12115 DAYANA trial. Antiviral therapy. 2014;19(1):51–9. doi: 10.3851/IMP2675 [DOI] [PubMed] [Google Scholar]
- 43.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. The New England journal of medicine. 2011;365(16):1492–501. PubMed Central PMCID: PMC3233684. doi: 10.1056/NEJMoa1014181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. Journal of acquired immune deficiency syndromes (1999). 2012;60(2):150–7. PubMed Central PMCID: PMC3360837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aghokeng AF, Monleau M, Eymard-Duvernay S, Dagnra A, Kania D, Ngo-Giang-Huong N, et al. Extraordinary heterogeneity of virological outcomes in patients receiving highly antiretroviral therapy and monitored with the World Health Organization public health approach in sub-saharan Africa and southeast Asia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(1):99–109. Epub 2013/10/01. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 46.Ahoua L, Guenther G, Pinoges L, Anguzu P, Chaix ML, Le Tiec C, et al. Risk factors for virological failure and subtherapeutic antiretroviral drug concentrations in HIV-positive adults treated in rural northwestern Uganda. BMC infectious diseases. 2009;9:81 Epub 2009/06/06. PubMed Central PMCID: PMCPMC2701435 Pubmed. doi: 10.1186/1471-2334-9-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elul B, Basinga P, Nuwagaba-Biribonwoha H, Saito S, Horowitz D, Nash D, et al. High levels of adherence and viral suppression in a nationally representative sample of HIV-infected adults on antiretroviral therapy for 6, 12 and 18 months in Rwanda. PloS one. 2013;8(1):e53586 Epub 2013/01/18. PubMed Central PMCID: PMCPMC3541229 Pubmed. doi: 10.1371/journal.pone.0053586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kouanfack C, Montavon C, Laurent C, Aghokeng A, Kenfack A, Bourgeois A, et al. Low levels of antiretroviral-resistant HIV infection in a routine clinic in Cameroon that uses the World Health Organization (WHO) public health approach to monitor antiretroviral treatment and adequacy with the WHO recommendation for second-line treatment. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48(9):1318–22. Epub 2009/03/27. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann CJ, Charalambous S, Fielding KL, Innes C, Chaisson RE, Grant AD, et al. HIV suppression with stavudine 30 mg versus 40 mg in adults over 60 kg on antiretroviral therapy in South Africa. AIDS (London, England). 2009;23(13):1784–6. Epub 2009/06/06. PubMed Central PMCID: PMCPMC2776829 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGuire M, Pinoges L, Kanapathipillai R, Munyenyembe T, Huckabee M, Makombe S, et al. Treatment initiation, program attrition and patient treatment outcomes associated with scale-up and decentralization of HIV care in rural Malawi. PloS one. 2012;7(10):e38044 Epub 2012/10/19. PubMed Central PMCID: PMCPMC3471893 Pubmed. doi: 10.1371/journal.pone.0038044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taieb F, Aghokeng AF, Eymard-Duvernay S, Chia JE, Einterz E, Mpoudi-Ngole E, et al. Challenges of Antiretroviral Treatment Monitoring in Rural and Remote-Access Regions in Africa. AIDS research and human retroviruses. 2014;30(7):623–5. doi: 10.1089/AID.2014.0035 [DOI] [PubMed] [Google Scholar]
- 52.Johannessen A, Naman E, Kivuyo SL, Kasubi MJ, Holberg-Petersen M, Matee MI, et al. Virological efficacy and emergence of drug resistance in adults on antiretroviral treatment in rural Tanzania. BMC infectious diseases. 2009;9:108 Epub 2009/07/09. PubMed Central PMCID: PMCPMC2713244 Pubmed. doi: 10.1186/1471-2334-9-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aghokeng AF, Kouanfack C, Eymard-Duvernay S, Butel C, Edoul GE, Laurent C, et al. Virological outcome and patterns of HIV-1 drug resistance in patients with 36 months' antiretroviral therapy experience in Cameroon. Journal of the International AIDS Society. 2013;16:18004 Epub 2013/02/05. PubMed Central PMCID: PMCPMC3562358 Pubmed. doi: 10.7448/IAS.16.1.18004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anude CJ, Eze E, Onyegbutulem HC, Charurat M, Etiebet MA, Ajayi S, et al. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC infectious diseases. 2013;13:113 Epub 2013/03/05. PubMed Central PMCID: PMCPMC3599241 Pubmed. doi: 10.1186/1471-2334-13-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chabikuli NO, Datonye DO, Nachega J, Ansong D. Adherence to antiretroviral therapy, virologic failure and workload at the Rustenburg Provincial Hospital. South African Family Practice. 2010;52(4):350–5. [Google Scholar]
- 56.Dagnra AY, Vidal N, Mensah A, Patassi A, Aho K, Salou M, et al. High prevalence of HIV-1 drug resistance among patients on first-line antiretroviral treatment in Lome, Togo. Journal of the International AIDS Society. 2011;14:30 Epub 2011/06/15. PubMed Central PMCID: PMCPMC3125306 Pubmed. doi: 10.1186/1758-2652-14-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dube NM, Tint KS, Summers RS. Early Warning Indicators for HIV Drug Resistance in Adults in South Africa at 2 Pilot Sites, 2008–2010. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(11):1607–14. Epub 2014/03/04. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 58.Hong SY, Jerger L, Jonas A, Badi A, Cohen S, Nachega JB, et al. Medication possession ratio associated with short-term virologic response in individuals initiating antiretroviral therapy in Namibia. PloS one. 2013;8(2):e56307 Epub 2013/03/20. PubMed Central PMCID: PMCPMC3585291 Pubmed. doi: 10.1371/journal.pone.0056307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kipp W, Konde-Lule J, Saunders LD, Alibhai A, Houston S, Rubaale T, et al. Antiretroviral treatment for HIV in rural Uganda: two-year treatment outcomes of a prospective health centre/community-based and hospital-based cohort. PloS one. 2012;7(7):e40902 Epub 2012/07/21. PubMed Central PMCID: PMCPMC3398945 Pubmed. doi: 10.1371/journal.pone.0040902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kipp W, Konde-Lule J, Saunders LD, Alibhai A, Houston S, Rubaale T, et al. Results of a community-based antiretroviral treatment program for HIV-1 infection in Western Uganda. Current HIV research. 2010;8(2):179–85. Epub 2010/02/19. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 61.Alibhai A, Martin LJ, Kipp W, Konde-Lule J, Saunders LD, Rubaale T, et al. Quality of life of HIV patients in a rural area of western Uganda: impact of a community-based antiretroviral treatment program. Current HIV research. 2010;8(5):370–8. Epub 2010/04/01. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 62.Messou E, Chaix ML, Gabillard D, Minga A, Losina E, Yapo V, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Cote d'Ivoire. Journal of acquired immune deficiency syndromes (1999). 2011;56(4):356–64. Epub 2010/12/31. PubMed Central PMCID: PMCPMC3050083 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Messou E, Chaix ML, Gabillard D, Yapo V, Toni TD, Minga A, et al. Increasing rate of TAMs and etravirine resistance in HIV-1-infected adults between 12 and 24 months of treatment: the VOLTART cohort study in Cote d'Ivoire, West Africa. Journal of acquired immune deficiency syndromes (1999). 2013;64(2):211–9. Epub 2013/06/26. PubMed Central PMCID: PMCPMC3834582 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS (London, England). 2010;24(4):563–72. Epub 2010/01/09. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 65.Cohen GM, Werner L, Gengiah S, Naidoo K. Role of Education in HIV Clinical Outcomes in a Tuberculosis Endemic Setting. Journal of the International Association of Providers of AIDS Care. 2013. Epub 2013/05/28. PubMed Central PMCID: PMCPMC3888482 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Beaudrap P, Thiam M, Diouf A, Toure-Kane C, Ngom-Gueye NF, Vidal N, et al. Risk of virological failure and drug resistance during first and second-line antiretroviral therapy in a 10-year cohort in Senegal: results from the ANRS 1215 cohort. Journal of acquired immune deficiency syndromes (1999). 2013;62(4):381–7. Epub 2012/11/03. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 67.De Luca A, Marazzi MC, Mancinelli S, Ceffa S, Altan AM, Buonomo E, et al. Prognostic value of virological and immunological responses after 6 months of antiretroviral treatment in adults with HIV-1 infection in sub-Saharan Africa. Journal of acquired immune deficiency syndromes (1999). 2012;59(3):236–44. Epub 2012/02/14. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 68.Ahonkhai AA, Noubary F, Munro A, Stark R, Wilke M, Freedberg KA, et al. Not all are lost: interrupted laboratory monitoring, early death, and loss to follow-up (LTFU) in a large South African treatment program. PloS one. 2012;7(3):e32993 Epub 2012/03/20. PubMed Central PMCID: PMCPMC3299719 Pubmed. doi: 10.1371/journal.pone.0032993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Khatib Z, Ekstrom AM, Coovadia A, Abrams EJ, Petzold M, Katzenstein D, et al. Adherence and virologic suppression during the first 24 weeks on antiretroviral therapy among women in Johannesburg, South Africa—a prospective cohort study. BMC public health. 2011;11:88 Epub 2011/02/10. PubMed Central PMCID: PMCPMC3046911 Pubmed. doi: 10.1186/1471-2458-11-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham SM, Masese L, Gitau R, Jalalian-Lechak Z, Richardson BA, Peshu N, et al. Antiretroviral adherence and development of drug resistance are the strongest predictors of genital HIV-1 shedding among women initiating treatment. The Journal of infectious diseases. 2010;202(10):1538–42. Epub 2010/10/07. PubMed Central PMCID: PMCPMC2957525 Pubmed. doi: 10.1086/656790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamers RL, Sigaloff KC, Wensing AM, Wallis CL, Kityo C, Siwale M, et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54(11):1660–9. Epub 2012/04/05. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 72.Almeida JM, Letang E, Nhampossa T, Ayala E, David C, Menendez C, et al. Rapid suppression of HIV-RNA is associated with improved control of immune activation in Mozambican adults initiating antiretroviral therapy with low CD4 counts. AIDS research and human retroviruses. 2011;27(7):705–11. Epub 2010/11/26. PubMed Central PMCID: PMC Pubmed. doi: 10.1089/AID.2010.0200 [DOI] [PubMed] [Google Scholar]
- 73.Jong E, Louw S, van Gorp EC, Meijers JC, ten Cate H, Jacobson BF. The effect of initiating combined antiretroviral therapy on endothelial cell activation and coagulation markers in South African HIV-infected individuals. Thrombosis and haemostasis. 2010;104(6):1228–34. Epub 2010/10/05. PubMed Central PMCID: PMC Pubmed. doi: 10.1160/TH10-04-0233 [DOI] [PubMed] [Google Scholar]
- 74.Tiba F, Nauwelaers F, Traore S, Coulibaly B, Ouedraogo T, Compaore A, et al. Immune Reconstitution During the First Year of Antiretroviral Therapy of HIV-1-Infected Adults in Rural Burkina Faso. The open AIDS journal. 2012;6:16–25. Epub 2012/03/22. PubMed Central PMCID: PMCPMC3308207 Pubmed. doi: 10.2174/1874613601206010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ugbena R, Aberle-Grasse J, Diallo K, Bassey O, Jelpe T, Rottinghaus E, et al. Virological response and HIV drug resistance 12 months after antiretroviral therapy initiation at 2 clinics in Nigeria. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54 Suppl 4:S375–80. Epub 2012/05/11. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 76.Wouters E, Van Damme W, Van Loon F, van Rensburg D, Meulemans H. Public-sector ART in the Free State Province, South Africa: community support as an important determinant of outcome. Social science & medicine (1982). 2009;69(8):1177–85. Epub 2009/08/21. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 77.Koyalta D, Charpentier C, Beassamda J, Rey E, Si-Mohamed A, Djemadji-Oudjeil N, et al. High frequency of antiretroviral drug resistance among HIV-infected adults receiving first-line highly active antiretroviral therapy in N'Djamena, Chad. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;49(1):155–9. Epub 2009/06/02. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 78.Mutevedzi PC, Lessells RJ, Heller T, Barnighausen T, Cooke GS, Newell ML. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bulletin of the World Health Organization. 2010;88(8):593–600. Epub 2010/08/04. PubMed Central PMCID: PMCPMC2908968 Pubmed. doi: 10.2471/BLT.09.069419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peterson I, Togun O, de Silva T, Oko F, Rowland-Jones S, Jaye A, et al. Mortality and immunovirological outcomes on antiretroviral therapy in HIV-1 and HIV-2-infected individuals in the Gambia. AIDS (London, England). 2011;25(17):2167–75. Epub 2011/09/02. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 80.Polis CB, Nakigozi G, Ssempijja V, Makumbi FE, Boaz I, Reynolds SJ, et al. Effect of injectable contraceptive use on response to antiretroviral therapy among women in Rakai, Uganda. Contraception. 2012;86(6):725–30. Epub 2012/06/22. PubMed Central PMCID: PMCPMC3449005 Pubmed. doi: 10.1016/j.contraception.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rich ML, Miller AC, Niyigena P, Franke MF, Niyonzima JB, Socci A, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. Journal of acquired immune deficiency syndromes (1999). 2012;59(3):e35–42. Epub 2011/12/14. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 82.Rougemont M, Stoll BE, Elia N, Ngang P. Antiretroviral treatment adherence and its determinants in Sub-Saharan Africa: a prospective study at Yaounde Central Hospital, Cameroon. AIDS research and therapy. 2009;6:21 Epub 2009/10/14. PubMed Central PMCID: PMCPMC2770068 Pubmed. doi: 10.1186/1742-6405-6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rusine J, Asiimwe-Kateera B, van de Wijgert J, Boer KR, Mukantwali E, Karita E, et al. Low primary and secondary HIV drug-resistance after 12 months of antiretroviral therapy in human immune-deficiency virus type 1 (HIV-1)-infected individuals from Kigali, Rwanda. PloS one. 2013;8(8):e64345 Epub 2013/08/21. PubMed Central PMCID: PMCPMC3741294 Pubmed. doi: 10.1371/journal.pone.0064345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sivapalasingam S, Wangechi B, Marshed F, Laverty M, Essajee S, Holzman RS, et al. Monitoring virologic responses to antiretroviral therapy in HIV-infected adults in Kenya: evaluation of a low-cost viral load assay. PloS one. 2009;4(8):e6828 Epub 2009/08/29. PubMed Central PMCID: PMCPMC2730572 Pubmed. doi: 10.1371/journal.pone.0006828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sunpath H, Edwin C, Chelin N, Nadesan S, Maharaj R, Moosa Y, et al. Operationalizing early antiretroviral therapy in HIV-infected in-patients with opportunistic infections including tuberculosis. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16(7):917–23. Epub 2012/06/13. PubMed Central PMCID: PMC Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Billong SC AA, Fokam J et al. Monitoring of HIV-1 drug resistance among patients on first-line antiretroviral therapy in Cameroon: discrepency between low levels of resistance and poor programatic performance? IAS conference. 2012.
- 87.Davies NE, Karstaedt AS. Antiretroviral outcomes in South African prisoners: a retrospective cohort analysis. PloS one. 2012;7(3):e33309 PubMed Central PMCID: PMC3310000. doi: 10.1371/journal.pone.0033309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Innes C, Hamilton R, Hoffmann CJ, Hippner P, Fielding K, Grant AD, et al. A novel HIV treatment model using private practitioners in South Africa. Sexually transmitted infections. 2012;88(2):136–40. PubMed Central PMCID: PMC3724420. doi: 10.1136/sextrans-2011-050194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Billong SC, Fokam J, Aghokeng AF, Milenge P, Kembou E, Abessouguie I, et al. Population-based monitoring of emerging HIV-1 drug resistance on antiretroviral therapy and associated factors in a sentinel site in Cameroon: low levels of resistance but poor programmatic performance. PloS one. 2013;8(8):e72680 Epub 2013/08/31. PubMed Central PMCID: PMCPMC3753336. doi: 10.1371/journal.pone.0072680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aoudjane S, Chaponda M, Gonzalez Del Castillo AA, O'Connor J, Noguera M, Beloukas A, et al. Hepatitis B virus (HBV) sub-genotype A1 infection is characterized by high replication levels and rapid emergence of drug resistance in HIV-positive adults receiving first-line antiretroviral therapy (ART) in Malawi. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014. Epub 2014/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nglazi MD, Kranzer K, Holele P, Kaplan R, Mark D, Jaspan H, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC infectious diseases. 2012;12:21 Epub 2012/01/26. PubMed Central PMCID: PMCPMC3295677 Pubmed. doi: 10.1186/1471-2334-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. Journal of acquired immune deficiency syndromes (1999). 2009;51(1):65–71. Epub 2009/03/14. PubMed Central PMCID: PMCPMC2674125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wadonda-Kabondo N, Bennett D, van Oosterhout JJ, Moyo K, Hosseinipour M, Devos J, et al. Prevalence of HIV drug resistance before and 1 year after treatment initiation in 4 sites in the Malawi antiretroviral treatment program. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54 Suppl 4:S362–8. Epub 2012/05/11. PubMed Central PMCID: PMCPMC3338306 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wadonda-Kabondo N, Hedt BL, van Oosterhout JJ, Moyo K, Limbambala E, Bello G, et al. A retrospective survey of HIV drug resistance among patients 1 year after initiation of antiretroviral therapy at 4 clinics in Malawi. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54 Suppl 4:S355–61. Epub 2012/05/11. PubMed Central PMCID: PMCPMC3338311 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bolhaar MG, Karstaedt AS. Efavirenz-based combination antiretroviral therapy after peripartum single-dose nevirapine. International journal of STD & AIDS. 2011;22(1):38–42. Epub 2011/03/03. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 96.Fatti G, Meintjes G, Shea J, Eley B, Grimwood A. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. Journal of acquired immune deficiency syndromes (1999). 2012;61(4):e50–8. Epub 2012/07/31. PubMed Central PMCID: PMC Pubmed. [DOI] [PubMed] [Google Scholar]
- 97.Huet C, Ouedraogo A, Konate I, Traore I, Rouet F, Kabore A, et al. Long-term virological, immunological and mortality outcomes in a cohort of HIV-infected female sex workers treated with highly active antiretroviral therapy in Africa. BMC public health. 2011;11:700 Epub 2011/09/16. PubMed Central PMCID: PMCPMC3191514 Pubmed. doi: 10.1186/1471-2458-11-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Idoko J, Meloni S, Muazu M, Nimzing L, Badung B, Hawkins C, et al. Impact of hepatitis B virus infection on human immunodeficiency virus response to antiretroviral therapy in Nigeria. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;49(8):1268–73. Epub 2009/09/24. PubMed Central PMCID: PMCPMC2753765 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maskew M, Fox MP, van Cutsem G, Chu K, Macphail P, Boulle A, et al. Treatment response and mortality among patients starting antiretroviral therapy with and without Kaposi sarcoma: a cohort study. PloS one. 2013;8(6):e64392 Epub 2013/06/12. PubMed Central PMCID: PMCPMC3673971 Pubmed. doi: 10.1371/journal.pone.0064392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mbougua JB, Laurent C, Kouanfack C, Bourgeois A, Ciaffi L, Calmy A, et al. Hepatotoxicity and effectiveness of a Nevirapine-based antiretroviral therapy in HIV-infected patients with or without viral hepatitis B or C infection in Cameroon. BMC public health. 2010;10:105 Epub 2010/03/03. PubMed Central PMCID: PMCPMC2841671 Pubmed. doi: 10.1186/1471-2458-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moore E, Beadsworth MB, Chaponda M, Mhango B, Faragher B, Njala J, et al. Favourable one-year ART outcomes in adult Malawians with hepatitis B and C co-infection. The Journal of infection. 2010;61(2):155–63. Epub 2010/05/18. PubMed Central PMCID: PMC Pubmed. doi: 10.1016/j.jinf.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 102.Mosha F, Muchunguzi V, Matee M, Sangeda RZ, Vercauteren J, Nsubuga P, et al. Gender differences in HIV disease progression and treatment outcomes among HIV patients one year after starting antiretroviral treatment (ART) in Dar es Salaam, Tanzania. BMC public health. 2013;13:38 Epub 2013/01/17. PubMed Central PMCID: PMCPMC3623886 Pubmed. doi: 10.1186/1471-2458-13-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Franke MF, Kaigamba F, Socci AR, Hakizamungu M, Patel A, Bagiruwigize E, et al. Improved retention associated with community-based accompaniment for antiretroviral therapy delivery in rural Rwanda. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56(9):1319–26. [DOI] [PubMed] [Google Scholar]
- 104.Karasi JC, Musonera F, Iranyumviye K, Servais JY, Devaux C, Binagwaho A, et al. Assessing the WHO recommended first-line ART regimens for resource-limited settings: Comparing the relative virologic efficacy and resistance patterns of TDF/3TC/NVP to AZT/3TC/NVP in a Rwandan cohort. Journal of the International AIDS Society. 2012;15. [Google Scholar]
- 105.Shipton LK, Wester CW, Stock S, Ndwapi N, Gaolathe T, Thior I, et al. Safety and efficacy of nevirapine- and efavirenz-based antiretroviral treatment in adults treated for TB-HIV co-infection in Botswana. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2009;13(3):360–6. Epub 2009/03/12. PubMed Central PMCID: PMCPMC2696339. [PMC free article] [PubMed] [Google Scholar]
- 106.Brennan AT, Shearer K, Maskew M, Long L, Sanne I, Fox MP. Impact of choice of NRTI in first-line antiretroviral therapy: a cohort analysis of stavudine vs. tenofovir. Tropical medicine & international health: TM & IH. 2014;19(5):490–8. Epub 2014/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kassa D, Gebremichael G, Alemayehu Y, Wolday D, Messele T, van Baarle D. Virologic and immunologic outcome of HAART in Human Immunodeficiency Virus (HIV)-1 infected patients with and without tuberculosis (TB) and latent TB infection (LTBI) in Addis Ababa, Ethiopia. AIDS research and therapy. 2013;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Igumbor JO, Scheepers E, Ebrahim R, Jason A, Grimwood A. An evaluation of the impact of a community-based adherence support programme on ART outcomes in selected government HIV treatment sites in South Africa. AIDS Care—Psychological and Socio-Medical Aspects of AIDS/HIV. 2011;23(2):231–6. [DOI] [PubMed] [Google Scholar]
- 109.WHO. Consolidated Guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommandation for a public Health Approch. June 2013. Available at http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. Accessed September 2014. 2013.
- 110.WHO. Consolidated guidelines on the use of antiretroviral therapy and preventing HIV infection—What's new (http://apps.who.int/iris/bitstream/10665/198064/1/9789241509893_eng.pdf?ua=1 accessed April 2016). 2015.
- 111.Delaugerre C, Gallien S, Flandre P, Mathez D, Amarsy R, Ferret S, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PloS one. 2012;7(5):e36673 PubMed Central PMCID: PMCPMC3349708. doi: 10.1371/journal.pone.0036673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li JZ, Gallien S, Do TD, Martin JN, Deeks S, Kuritzkes DR, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrobial agents and chemotherapy. 2012;56(11):5998–6000. PubMed Central PMCID: PMCPMC3486544. doi: 10.1128/AAC.01217-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Monleau M, Aghokeng AF, Eymard-Duvernay S, Dagnra A, Kania D, Ngo-Giang-Huong N, et al. Field evaluation of dried blood spots for routine HIV-1 viral load and drug resistance monitoring in patients receiving antiretroviral therapy in Africa and Asia. Journal of clinical microbiology. 2014;52(2):578–86. Epub 2014/01/31. PubMed Central PMCID: PMCPMC3911301 Pubmed. doi: 10.1128/JCM.02860-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rottinghaus EK, Ugbena R, Diallo K, Bassey O, Azeez A, Devos J, et al. Dried blood spot specimens are a suitable alternative sample type for HIV-1 viral load measurement and drug resistance genotyping in patients receiving first-line antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54(8):1187–95. Epub 2012/03/14. PubMed Central PMCID: PMC Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johannessen A. Dried blood spots in HIV monitoring: applications in resource-limited settings. Bioanalysis. 2010;2(11):1893–908. Epub 2010/11/19. PubMed Central PMCID: PMC Pubmed. doi: 10.4155/bio.10.120 [DOI] [PubMed] [Google Scholar]
- 116.Smit PW, Sollis KA, Fiscus S, Ford N, Vitoria M, Essajee S, et al. Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis. PloS one. 2014;9(3):e86461 PubMed Central PMCID: PMCPMC3945725. doi: 10.1371/journal.pone.0086461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aoudjane S, Chaponda M, Gonzalez Del Castillo AA, O'Connor J, Noguera M, Beloukas A, et al. Hepatitis B Virus Sub-genotype A1 Infection Is Characterized by High Replication Levels and Rapid Emergence of Drug Resistance in HIV-Positive Adults Receiving First-line Antiretroviral Therapy in Malawi. Clin Infect Dis. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maskew M, MacPhail AP, Whitby D, Egger M, Fox MP. Kaposi sarcoma-associated herpes virus and response to antiretroviral therapy: a prospective study of HIV-infected adults. Journal of acquired immune deficiency syndromes (1999). 2013;63(4):442–8. Epub 2013/04/26. PubMed Central PMCID: PMCPMC3712196 Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.WHO. Surveillance of HIV drug resistance in adults initiating antiretroviral therapy (pre-treatment HIV drug resistance) Concept note. Available at http://apps.who.int/iris/bitstream/10665/112802/1/9789241507196_eng.pdf?ua=1. Accessed on november 2014. 2014.
- 120.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2 [DOI] [PubMed] [Google Scholar]
- 121.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15 Suppl 1:1–15. PubMed Central PMCID: PMC2948795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Estimation of the rate of virological success based on different threshold (in copies/mL) on on-treatment (full box) and intention-to treat (empty box) at: A) at 6 months of ART, B) 12 months of ART, c) 24 months of ART, and D) 36 months of ART.
(DOCX)
A) OT analysis at 12 months; B) ITT analysis at 12 months; C) OT analysis at 24 months; D) ITT analysis at 24 months.
(DOCX)
(DOCX)
Data Availability Statement
Relevant data can be found in Figshare, as follows: https://figshare.com/s/942c5e220b758357b703; DOI: 10.6084/m9.figshare.4811500.