Abstract
In this study, we investigated whether loss of GH receptor (GHR) signaling in postnatal skeletal muscle alters muscle mass and regenerative ability in adult mice and whether this was dependent on IGF-1 receptor (IGF-1R) signaling. To do so, we used mouse models with skeletal muscle-specific loss of GHR signaling (mGHRKO), IGF-1R and insulin receptor signaling (MKR), or both GHR and IGF-1R/insulin receptor signaling (mGHRKO/MKR). We did not find a reduction in muscle cross-sectional area, fiber type composition, or response to pathological muscle injury in male mGHRKO and mGHRKO/MKR mice when compared with control and MKR mice, respectively. This could potentially be explained by unchanged skeletal muscle Igf-1 expression in mGHRKO and mGHRKO/MKR mice relative to control and MKR mice, respectively. Furthermore, MKR and mGHRKO/MKR mice, but not mGHRKO mice, demonstrated reduced fiber fusion after cardiotoxin injection, suggesting that IGF-1, and not GH, promotes fiber fusion in adult mice. In summary, our data suggest that GHR signaling in postnatal skeletal muscle does not play a significant role in regulating muscle mass or muscle regeneration. Additionally, in our model, muscle Igf-1 expression is not dependent on GHR signaling in postnatal skeletal muscle.
The GH/IGF-1 axis is a critical regulator of somatic growth and other developmental processes. This involves GH stimulating the production and secretion of hepatic IGF-1, which then exerts its biological effects on the other tissues (1, 2). One of the major target tissues of IGF-1 is the skeletal muscle, where IGF-1 is an important regulator of muscle mass and function and physiological and pathological hypertrophy in response to exercise and muscle injury, respectively (3–9).
However, endocrine IGF-1 is not the only source of IGF-1 in the body. Targeted deletion of hepatic Igf-1 in mice decreases circulating IGF-1 levels by 75% but does not result in growth defects or other gross abnormalities, whereas whole-body IGF-1 knockout mice have decreased mortality and are severely growth-retarded (10–12). Furthermore, overexpression of IGF-1 specifically in the muscle increases muscle mass and strength and accelerates muscle regeneration in dystrophic mice (13). Additionally, satellite cells, the quiescent self-renewing population of muscle precursor cells that are resident in between the differentiated, senescent muscle fibers, are activated in response to muscle injury and locally produce IGF-1 (3, 4, 14). Local IGF-1 production is also required for the induction of inflammatory responses after muscle injury, and this is critical for the initiation of the muscle regenerative process (15).
However, although hepatic IGF-1 is regulated by GH to a major extent, the data regarding the regulation of autocrine/paracrine IGF-1 production in the muscle by GH is inconclusive (16–20). Administration of recombinant human GH improves muscle strength after injury, and although this is associated with increased circulating IGF-1, the production of autocrine/paracrine IGF-1 was not assessed (21). Furthermore, GH has been shown to regulate muscle fiber fusion and fiber-type composition; its dependence on IGF-1 for these processes has been a matter of debate (16–19, 22, 23).
Thus, in this study, we used mice with loss of GH receptor (GHR) signaling (muscle GHR knockout [mGHRKO] mice) to explore whether GHR signaling in postnatal skeletal muscle is required for regulating muscle mass and response to pathological injury. We also sought to determine whether the effects of GH in the muscle were dependent on IGF-1 signaling. For this purpose, we used the MKR mouse that overexpresses dominant-negative human IGF-1 receptors (hIGF-1Rs) specifically in postnatal skeletal muscle and consequently have significantly decreased muscle insulin/IGF-1 signaling. MKR mice exhibit reduced muscle mass, lower twitch force, and delayed muscle regeneration in response to local injury (24). Additionally, negligible insulin signaling in the muscles of the MKR mice results in the development of overt diabetes primarily due to a marked reduction in insulin-stimulated glucose uptake in the muscle leading to secondary metabolic alterations in other tissues (24, 25).
Our data suggest that muscle GHR signaling is not required for maintaining postnatal skeletal muscle mass and muscle regeneration in response to pathological injury. We also show that Igf-1 expression is not regulated by GH in postnatal skeletal muscle.
Materials and Methods
Generation of mice
All experimental procedures were performed in accordance with the Institutional Animal Care and Use Committee at the Mount Sinai School of Medicine. The MKR mice, overexpressing dominant-negative hIGF-1Rs specifically in postnatal skeletal muscle, have been previously described (25). The mGHRKO mice were generated by the Cre-loxP system using the muscle-specific creatine kinase (MCK) promoter and have been characterized on the C57BL/6 background (20, 26). For this study, GHRflox/flox mice were backcrossed to the FVB/N background for 6 generations, crossed with MCK-Cre+/+ mice on the FVB/N background (The Jackson Laboratory; stock no. 006475). MKR mice were crossed with mGHRKO mice to generate the double transgenic mGHRKO/MKR mice. The following groups of age-matched male mice on the FVB/N background were used in all resulting experiments: control (GHRflox/flox), mGHRKO (MCK-Cre+/−, GHRflox/flox), MKR (GHRflox/flox, MKR+/+), and mGHRKO/MKR (MCK-Cre+/−, GHRflox/flox, MKR+/+). The mice were kept under a 12-hour light, 12-hour dark cycle and had ad libitum access to standard diets and water.
Gene expression
mRNA from quadriceps was extracted with Qiazol using the RNeasy Lipid Tissue Mini kit (QIAGEN) with on-column deoxyribonuclease treatment, and the concentration of RNA was determined using the NanoDrop ND-1000 Spectrophotometer (Thermo Scientific). 1 μg of RNA was subjected to reverse transcription according to the manufacturer's instructions (Invitrogen Corp). Real-time PCR was performed using the QuantiTect SYBR green PCR kit (QIAGEN) in ABI PRISM 7900HT sequence detection systems (Applied Biosystems). For each gene, a single sample was assayed in triplicate, and gene expression was normalized to Gapdh. The following primer sequences were used: Ghr forward GGAGACATCCAAGTGAGTTGG and reverse ATCCATTCTCAATGAGTACAC, higf-1r forward CCAAGGGTGTGGTGAAAGAT and reverse TCCATGATGACCAGTGTTGG, Igf-1 forward GGACCAGAGACCCTTTGCGGGG and reverse GGCTGCTTTTGTAGGCTTCAGTGG, Mhc1 forward CTCCCAAGGAGAGACGACTG and reverse TTAAGCAGGTCGGCTGAGTT, Mhc2a forward GAACCCTCCCAAGTACGACA and reverse TAAGGGTTGACGGTGACACA, Mhc2b forward CCAGAGTCACCTTCCAGCTC and reverse CTTCCCTTTGCTTTTGCTTG, and Gapdh forward TGAAGGTCGGTGTCAACGGATTTGGC and reverse CATGTAGGCCATGAGGTCCACCAC.
Body weight and composition
Mice were weighed every 2 weeks starting at 4 weeks of age. Body composition was assessed at 8 and 16 weeks of age using an Echo MRI 3-in-1 NMR system (Echo Medical Systems).
Blood chemistry and metabolic tests
Blood glucose levels were measured from the tail vein using an automated glucometer (Contour; Bayer). Plasma, collected in the random fed state, was used for measurement of plasma insulin levels by RIA (Millipore) according to the manufacturer's instructions. Glucose tolerance tests (GTTs) were performed in overnight fasted animals at 10 and 16 weeks of age after ip injections of dextrose (Sigma-Aldrich) (2 and 1 g/kg body weight for 10- and 18-week-old mice, respectively). Blood glucose levels were measured immediately before (time 0) and 15, 30, 60, and 120 minutes after glucose injection. Insulin tolerance tests (ITTs) were performed at 8 and 16 weeks of age after a brief 2- to 3-hour fast by ip injection of insulin (HumulinR; Eli Lilly) (0.75 and 1.25 U/kg body weight for 8- and 16-week-old mice, respectively). Blood glucose levels were measured immediately before (0) and 15, 30, and 60 minutes after insulin injection. The data are represented as the percent reduction of blood glucose from basal levels at time 0.
Western immunoblot analysis
Proteins were homogenized and extracted from the quadriceps muscle in lysis buffer (50mM Tris, 150mM NaCl, 1mM EDTA, 1.25% CHAPS, 1mM sodium orthovanadate, 2mM sodium fluoride, 10mM sodium pyrophosphate, 8mM β-glycerophosphate, and Complete EDTA-free Protease Inhibitor Cocktail tablets [Roche Diagnostics] [pH 7.4]) and 25–30 μg of proteins were subjected to SDS-PAGE and Western blot analysis using previously described protocols (26). The primary antibodies used were phospho-AktSer473 (no. 9271; Cell Signaling); Akt (Cell Signaling; no. 2920); β-tubulin (sc-9104; Santa Cruz Biotechnology). The signal was developed using the Odyssey Infrared Imaging System (Licor Biosciences). The blots were quantified using the NIH ImageJ software.
Muscle injury
The 8- and 24-week-old mice were anesthetized using isoflurane (Baxter), and 30 μl of 50μM cardiotoxin (Sigma-Aldrich) was injected into the tibialis anterior of one leg, whereas sterile PBS was injected into the tibialis anterior of the contralateral leg. Ten days after cardiotoxin injection, the mice were euthanized and the tibialis anterior muscles were harvested and fixed in 10% formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and assessed using an Olympus AX70 microscope (Olympus) at ×10 objective magnification. Fiber cross-sectional area (CSA) was determined by measuring the area of 250 fibers per mouse using the NIH ImageJ software. Regenerating fibers with centralized nuclei were counted, and fusion index was calculated as the fraction of regenerating fibers with >1 nucleus.
Statistical analysis
All data are represented as mean ± SEM 2-tailed Student's t test (for comparisons of 2 groups), and 2-way ANOVA with Holm-Sidak post hoc test (for comparisons of 3 or more groups) were performed using SigmaStat for Windows (version 3.5; Systat Software, Inc).
Results
Loss of muscle GHR signaling does not improve insulin sensitivity in diabetic MKR mice
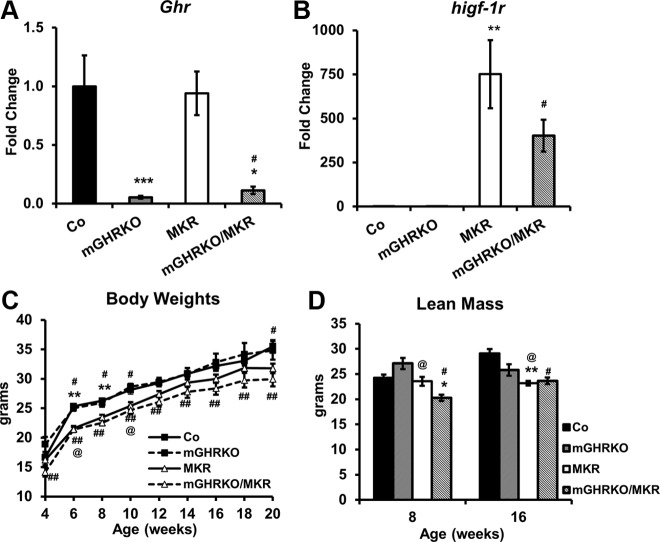
The 8-week-old mGHRKO and mGHRKO/MKR mice demonstrated a significant reduction in muscle Ghr expression compared with the control and MKR mice, respectively (Figure 1A). The MKR and mGHRKO/MKR mice overexpressed dominant-negative hIGF-1R in their muscles, and thus showed a 500- to 1500-fold increase in muscle higf-1r expression, although the expression of higf-1r was negligible in the control and mGHRKO mice (Figure 1B). Body length and serum GH levels were similar between the genotypes at 20 weeks of age, suggesting an intact global GH/IGF-1 axis in the mGHRKO and mGHRKO/MKR mice (data not shown).
Figure 1.
Deletion of GHR in muscle of MKR mice does not alter their body weights. A and B, Gene expression of mouse Ghr and higf-1r in quadriceps muscles of 8-week-old control, mGHRKO, MKR, and mGHRKO/MKR mice as assessed by real-time PCR. Gene expression was normalized to Gapdh and expressed as a fold change compared with controls (n = 2–10 mice per group). C and D, Body weight (C) and absolute lean mass (D) were measured at indicated times (n = 3–9 mice per group). Results are expressed as mean ± SEM. ***, P < .05, control vs mGHRKO; **, P < .05, control vs MKR; *, P < .05, MKR vs mGHRKO/MKR; #, P < .05, control vs mGHRKO/MKR; ##, P < .05, mGHRKO vs mGHRKO/MKR; @, P < .05 mGHRKO vs MKR (2-tailed Student's t test).
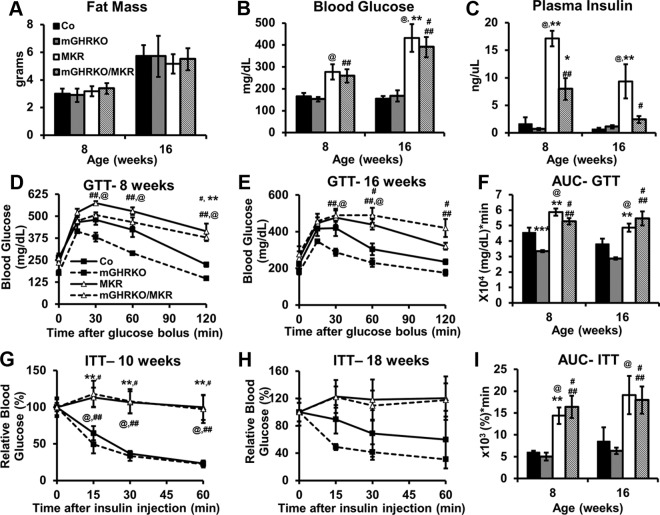
The MKR mice had lower body weights than control and mGHRKO mice. Furthermore, whereas the mGHRKO/MKR mice demonstrated lower body weights than the control and mGHRKO mice, there was no difference in body weight between the MKR and mGHRKO/MKR mice (Figure 1C). The MKR and mGHRKO/MKR mice displayed lower lean mass compared with controls at 16 weeks; however, this parameter was statistically different only in the mGHRKO/MKR mice at 8 weeks of age. The mGHRKO/MKR mice had significantly lower lean mass than the MKR mice at 8 weeks; however, this difference was lost by 16 weeks of age (Figure 1D). There was no difference in absolute fat mass between the genotypes at either 8 or 16 weeks of age (Figure 2A).
Figure 2.
Loss of muscle GHR signaling in MKR mice does not improve their glucose homeostasis or insulin sensitivity. A–C, Absolute fat mass (A), blood glucose levels (B), and plasma insulin levels (C) were measured in the random fed state at the indicated times (n = 5–6 per group). D and E, GTTs in 8-week-old (n = 3–13 per group) (D) and 16-week-old (n = 6–8 per group) (E) mice. F, Area under the curve (AUC) for the GTTs. G and H, ITTs were performed at 10 weeks (n = 3–8 per group) (G) and 18 weeks of age (n = 3–7 per group) (H). I, AUC calculated for the ITTs. Results are expressed as mean ± SEM for each group. ***, P < .05, control vs mGHRKO; **, P < .05, control vs MKR; *, P < .05, MKR vs mGHRKO/MKR; #, P < .05, control vs mGHRKO/MKR; ##, P < .05, mGHRKO vs mGHRKO/MKR; @, P < .05, mGHRKO vs MKR (2-way ANOVA).
The MKR mice had significantly elevated fed-state blood glucose levels, as has previously been shown (25), but glucose was not altered in the mGHRKO and mGHRKO/MKR mice relative to control and MKR mice, respectively (Figure 2B). The MKR and mGHRKO/MKR mice had higher insulin levels than the control and mGHRKO mice, although there was no difference in insulin levels between the control and mGHRKO mice. However, the mGHRKO/MKR mice demonstrated significantly lower insulin levels compared with MKR mice (Figure 2C). In line with our previous findings (20), the mGHRKO mice had improved glucose tolerance in a GTT, but not enhanced insulin sensitivity in an ITT, compared with control mice (Figure 2, D–I). However, the mGHRKO/MKR mice did not demonstrate improved glucose tolerance or insulin sensitivity compared with the MKR mice, despite lower insulin levels (Figure 2, D–I).
Loss of muscle GHR signaling does not have an additionally negative effect on muscle fiber size, fiber type, and IGF-1R/insulin receptor signaling in MKR mice
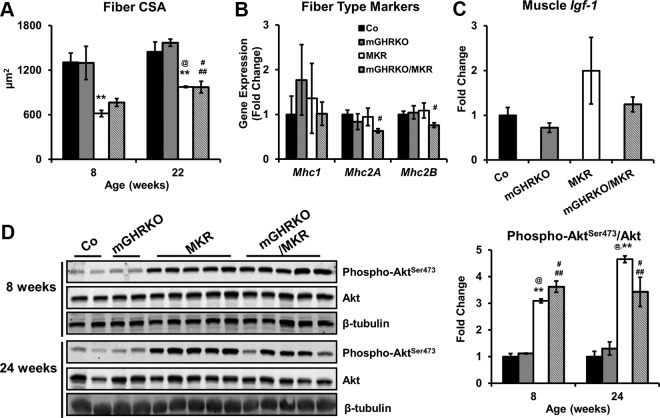
The MKR and mGHRKO/MKR mice demonstrated lower muscle CSA compared with the control and mGHRKO mice (Figure 3A). However, neither the mGHRKO nor the mGHRKO/MKR mice demonstrated significant changes in fiber CSA compared with control and MKR mice, respectively, at either 8 or 22 weeks of age (Figure 3A). To assess muscle fiber type, we measured gene expression of myosin heavy chain isoforms. mRNA expression of Mhc1 and Mhc2A (markers of oxidative fibers) and Mhc2B (marker for glycolytic fibers) was not significantly different in 8-week-old mGHRKO and mGHRKO/MKR mice compared with control and MKR mice, respectively (Figure 3B). Moreover, muscle Igf-1 expression was also similar between the genotypes at 8 weeks of age, suggesting that GHR signaling does not regulate Igf-1 expression in adult skeletal muscle (Figure 3C).
Figure 3.
Loss of muscle GHR signaling does not affect muscle physiology or fiber type composition in MKR mice. A, Fiber CSA was quantified from tibialis anterior muscles at 8 and 22 weeks of age. B and C, mRNA expression of myosin heavy chain (MHC) isoforms (B), indicative of fiber type, and Igf-1 (C) were assessed by real-time PCR in the quadriceps of 8-week-old mice. Gene expression was normalized to Gapdh and expressed as a fold change compared with controls (n = 2–10 per group). D, Left, Phosphorylated and total protein content of Akt was measured in muscle lysates of 8- and 24-week-old mice using immunoblotting (left), with β-tubulin used to evaluate equal loading of proteins; right, levels of phosphorylated Akt were normalized to total Akt content and represented as a fold change compared with the control mice (n = 2–5 per group). Results are expressed as mean ± SEM for each genotype. **, P < .05, control vs MKR; #, P < .05, control vs mGHRKO/MKR; ##, P < .05, mGHRKO vs mGHRKO/MKR; @, P < .05, mGHRKO vs MKR (2-tailed Student's t test).
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway has been implicated in mediating the intracellular effects of IGF-1 on muscle hypertrophy (13, 27), and surprisingly, MKR and mGHRKO/MKR mice display significant hyperphosphorylation of Akt (Figure 3D). This suggests the up-regulation of compensatory mechanisms to maintain muscle mass in the absence of IGF-1R/insulin receptor (IR) signaling in the MKR and mGHRKO/MKR mice.
Loss of muscle IGF-1R/IR, but not GHR, signaling impairs muscle response to injury
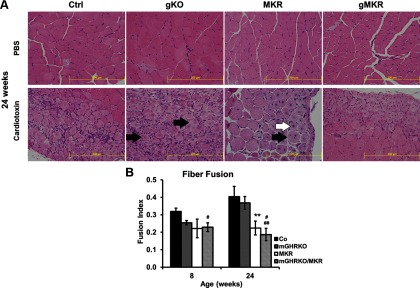
Local muscle injury initiates a program of muscle regeneration that occurs in several stages starting with the recruitment of inflammatory cells, myonecrosis, activation of satellite cells, myogenesis, myoblast fusion, and finally hypertrophy to form the regenerated muscle fiber (28). Histological examination of the tibialis anterior muscles 10 days after cardiotoxin injection showed the initiation of myogenesis (identified by the presence of centralized nuclei) (Figure 4A, white arrow) and fiber fusion (shown by the presence of >1 centralized nucleus per myofiber) (Figure 4A, black arrow) in the different genotypes at 8 and 24 weeks of age (Figure 4A, data not shown). However, the fusion index, defined as the fraction of regenerating fibers with >1 myonucleus, was significantly lower in the MKR and mGHRKO/MKR mice 24 weeks of age compared with the control mice (Figure 4B). The mGHRKO/MKR also displayed significantly lower fusion index than the mGHRKO mice at 8 and 24 weeks of age (Figure 4B). However, there was no difference in fusion index at either 8 or 24 weeks between the MKR and mGHRKO/MKR mice or the mGHRKO and control mice (Figure 4B). These data suggest that although loss of IGF-1R/IR signaling in MKR mice impairs muscle regeneration, additional loss of GHR does not further impair regenerative ability in the mGHRKO/MKR mice.
Figure 4.
Loss of muscle IGF-1R/IR, but not GHR signaling, impairs fiber fusion after cardiotoxin injury. A, Histological examination of tibialis anterior muscles of 24-week-old mice 10 days after PBS or cardiotoxin injections. Muscle regeneration was identified by the presence of fibers with centralized nuclei (black arrow). Fiber fusion was identified by the presence of fibers with >1 centralized nucleus (white arrow). All pictures were taken at ×40 objective magnification (n = 2–5 per group; representative images are shown). B, Fiber fusion at 8 and 24 weeks of age was assessed by counting the number of regenerating cells with >1 centralized nucleus and is represented as a fraction of the total number of regenerating fibers (fusion index) (n = 2–5 mice per group). Results are expressed as mean ± SEM for each genotype. ***, P < .05, control vs mGHRKO; **, P < .05, control vs MKR; *, P < .05, MKR vs mGHRKO/MKR; #, P < .05, control vs mGHRKO/MKR; ##, P < .05, mGHRKO vs mGHRKO/MKR; @, P < .05, mGHRKO vs MKR (2-tailed Student's t test).
Discussion
In this study, we sought to determine whether GHR signaling had an effect on postnatal skeletal muscle mass and regeneration in response to pathological injury and whether its effects were dependent on IGF-1 signaling in the skeletal muscle. Our data suggest that GHR signaling does not independently have a major impact on muscle mass, fiber type composition, or regenerative capacity in the postnatal skeletal muscle. These findings are in contrast to previous reports that suggest that muscle GHR signaling affects muscle mass and function (16–18). Muscle-specific deletion of GHR in a Cre-loxP manner using the Mef2C promoter-driven Cre (Mef2C-GHRKO mice) results in decreased fiber CSA, fiber fusion ability, oxidative fiber content, and muscle Igf-1 expression (18). Similarly, Myf5-STAT5KO mice with loss of STAT5, the predominant downstream target of GHR signaling, in skeletal muscle (using the Myf5-driven Cre) had smaller muscles, decreased muscle Igf-1 expression, and lower-frequency oxidative fibers. Interestingly, the Myf5-STAT5KO mice also demonstrated lower circulating IGF-1 levels as well as reduced body length (18, 19). A major point of contrast between the aforementioned studies and our study is that the Mef2C and Myf5 promoters are activated in early myocytes and mesenchymal stem cells, whereas the MCK promoter is activated only in postnatal skeletal muscle (29–32). Thus, the effects seen in the Mef2C-GHRKO and the Myf5-STAT5KO mice could reflect off-target effects in other tissues. Alternatively, GH may play a role in muscle mass accumulation and myogenesis during development, but not in postnatal, differentiated muscle fibers.
Another potential reason for the discrepancies between the studies could be the expression levels of Igf-1 in the muscle. The mGHRKO and mGHRKO/MKR mice did not demonstrate any changes in muscle Igf-1 expression, in agreement with our previous report (20). In contrast, both the Mef2C-GHRKO and Myf5-STAT5KO mice demonstrate significant reductions in muscle Igf-1 expression. Furthermore, deletion of IGF-1R in muscle using the Mef2C-Cre recapitulated the phenotype of the Mef2C-GHRKO mice (17, 18). Together, this suggests that the local GH/IGF-1 axis in the muscle plays a significant role during prenatal myogenesis at which time GH exerts its effects in an IGF-1-dependent manner, but in postnatal skeletal muscle, IGF-1 is not entirely GH-dependent. This conclusion is further supported by reports showing that, in addition to GH, postnatal muscle IGF-1 expression and production is stimulated by other factors, such as androgen receptor signaling (16, 19, 33–36).
The effects of IGF-1 on muscle mass, function, and regeneration have been attributed to functional PI3K/Akt signaling (13, 27, 37). However, surprisingly, the MKR and mGHRKO/MKR mice displayed hyperphosphorylation of Akt in the muscle at 8 and 22 weeks of age. This correlates with increased fiber CSA and bromodeoxyuridine incorporation in the muscles of the MKR mice between 5 and 8 weeks of age (24). Inducible transgenic activation of Akt in the muscle for 3 weeks also results in hypertrophy, increased muscle strength, and bromodeoxyuridine incorporation in the muscle (38). Furthermore, Akt can be activated in myocytes and cardiomyocytes by factors such as Wnt proteins, testosterone, heat-shock protein 27, and sarcospan in an IGF-1–independent manner (39–44). Together, these suggest that in the absence of functional IGF-1R/IR signaling, compensatory activation of the PI3K/Akt pathway in an IGF-1–independent manner may occur to maintain muscle mass.
Loss of muscle GHR signaling did not alter the recovery process in response to injury. However, MKR and mGHRKO/MKR mice displayed decreased fiber fusion after cardiotoxin injury, suggesting that IGF-1 regulates muscle fiber fusion. Isolated myoblasts from the Mef2C-GHRKO and Mef2C-IGF-1RKO mice demonstrated decreased fiber fusion during differentiation, suggesting that GH regulated fiber fusion in an IGF-1–dependent manner (18). Our in vivo data suggest that GHR signaling in postnatal skeletal muscle does not have an effect on muscle fiber fusion, suggesting that the process is IGF-1–dependent.
GH exerts systemic metabolic effects that are independent of, and in opposition to, the metabolic effects exerted by IGF-1 (45). We recently reported that the mGHRKO mice on the C57BL/6 background demonstrated slight improvements in glucose tolerance on a regular chow diet. Furthermore, the mGHRKO mice are protected from the development of high-fat diet-induced insulin resistance. This was in part mediated by increased insulin-stimulated glucose disposal into the muscle and adipose tissue of the mGHRKO mice, in addition to other systemic effects such as increased energy expenditure and retention of metabolic flexibility (20, 26). Similarly, here we show that on the FVB/N background, the mGHRKO mice also demonstrate slightly improved glucose tolerance. The MKR mice are severely diabetic primarily due to a marked reduction in insulin-stimulated glucose uptake in the muscle leading to secondary metabolic alterations in other tissues (24, 25). Although the mGHRKO/MKR mice displayed lower circulating insulin levels, they were not more insulin-sensitive than the MKR mice. This could potentially be due to the inability of the MKR muscles to up-regulate insulin-stimulated glucose disposal owing to abrogation of insulin/IGF-1 signaling in this tissue.
In summary, our data suggest that postnatal IGF-1R/IR and not GHR signaling is critical for the regulation of muscle mass and regeneration in response to injury. Therefore, this suggests that GH treatment improves muscle regeneration by increasing circulating IGF-1 and muscle IGF-1R signaling, rather than through direct effects of GH on muscle GHR signaling.
Acknowledgments
We thank Hui Sun, Aviva Tobin-Hess, Ashley Polin, Darren Gorman, and Joshua Vazhappilly for their help with experiments and genotyping of the mice.
Current address for A.V.: Division of Endocrinology, Diabetes, and Metabolism, Beth Israel Deaconess Medical Center, and Department of Medicine, Harvard Medical School, Boston Massachusetts.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CSA
- cross-sectional area
- GHR
- GH receptor
- GTT
- glucose tolerance test
- hIGF-1R
- human IGF-1 receptor
- ITT
- insulin tolerance test
- IR
- insulin receptor
- MCK
- muscle-specific creatine kinase
- PI3K
- phosphatidylinositol 3-kinase.
References
- 1. Laron Z, Kowadlo-Silbergeld A, Eshet R, Pertzelan A. Growth hormone resistance. Ann Clin Res. 1980;12:269–277. [PubMed] [Google Scholar]
- 2. List EO, Sackmann-Sala L, Berryman DE, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr Rev. 2011;32:356–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jennische E, Hansson HA. Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol Scand. 1987;130:327–332. [DOI] [PubMed] [Google Scholar]
- 4. Edwall D, Schalling M, Jennische E, Norstedt G. Induction of insulin-like growth factor I messenger ribonucleic acid during regeneration of rat skeletal muscle. Endocrinology. 1989;124:820–825. [DOI] [PubMed] [Google Scholar]
- 5. Lefaucheur JP, Sébille A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor I. J Neuroimmunol. 1995;57:85–91. [DOI] [PubMed] [Google Scholar]
- 6. Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. [DOI] [PubMed] [Google Scholar]
- 7. Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89:1365–1379. [DOI] [PubMed] [Google Scholar]
- 8. Chakravarthy MV, Booth FW, Spangenburg EE. The molecular responses of skeletal muscle satellite cells to continuous expression of IGF-1: implications for the rescue of induced muscular atrophy in aged rats. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S44–S48. [DOI] [PubMed] [Google Scholar]
- 9. Shefer G, Benayahu D. The effect of exercise on IGF-I on muscle fibers and satellite cells. Front Biosci (Elite Ed). 2012;4:230–239. [DOI] [PubMed] [Google Scholar]
- 10. Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 11. Wu Y, Sun H, Yakar S, LeRoith D. Elevated levels of insulin-like growth factor (IGF)-I in serum rescue the severe growth retardation of IGF-I null mice. Endocrinology. 2009;150:4395–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jennische E, Skottner A, Hansson HA. Satellite cells express the trophic factor IGF-I in regenerating skeletal muscle. Acta Physiol Scand. 1987;129:9–15. [DOI] [PubMed] [Google Scholar]
- 15. Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011;25:3344–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klover P, Chen W, Zhu BM, Hennighausen L. Skeletal muscle growth and fiber composition in mice are regulated through the transcription factors STAT5a/b: linking growth hormone to the androgen receptor. FASEB J. 2009;23:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klover P, Hennighausen L. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology. 2007;148:1489–1497. [DOI] [PubMed] [Google Scholar]
- 18. Mavalli MD, DiGirolamo DJ, Fan Y, et al. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120:4007–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H, Barton E, Muja N, Yakar S, Pennisi P, Leroith D. Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology. 2005;146:1772–1779. [DOI] [PubMed] [Google Scholar]
- 20. Vijayakumar A, Wu Y, Sun H, et al. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes. 2012;61:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Przkora R, Herndon DN, Suman OE, et al. Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients. Ann Surg. 2006;243:796–801; discussion 801–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schuenke MD, Kopchick JJ, Hikida RS, Kraemer WJ, Staron RS. Effects of growth hormone overexpression vs growth hormone receptor gene disruption on mouse hindlimb muscle fiber type composition. Growth Horm IGF Res. 2008;18:479–486. [DOI] [PubMed] [Google Scholar]
- 23. Sotiropoulos A, Ohanna M, Kedzia C, et al. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci U S A. 2006;103:7315–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernández AM, Dupont J, Farrar RP, Lee S, Stannard B, Le Roith D. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest. 2002;109:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernández AM, Kim JK, Yakar S, et al. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001;15:1926–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijayakumar A, Wu Y, Buffin NJ, et al. Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS One. 2012;7:e44777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quinn LS, Anderson BG, Plymate SR. Muscle-specific overexpression of the type 1 IGF receptor results in myoblast-independent muscle hypertrophy via PI3K, and not calcineurin, signaling. Am J Physiol Endocrinol Metab. 2007;293:E1538–E1551. [DOI] [PubMed] [Google Scholar]
- 28. Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224:7–16. [DOI] [PubMed] [Google Scholar]
- 29. Caplan AI, Fiszman MY, Eppenberger HM. Molecular and cell isoforms during development. Science. 1983;221:921–927. [DOI] [PubMed] [Google Scholar]
- 30. Vong LH, Ragusa MJ, Schwarz JJ. Generation of conditional Mef2cloxP/loxP mice for temporal- and tissue-specific analyses. Genesis. 2005;43:43–48. [DOI] [PubMed] [Google Scholar]
- 31. Naya FJ, Wu C, Richardson JA, Overbeek P, Olson EN. Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development. 1999;126:2045–2052. [DOI] [PubMed] [Google Scholar]
- 32. Ott MO, Bober E, Lyons G, Arnold H, Buckingham M. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 1991;111:1097–1107. [DOI] [PubMed] [Google Scholar]
- 33. Ostrovsky O, Eletto D, Makarewich C, Barton ER, Argon Y. Glucose regulated protein 94 is required for muscle differentiation through its control of the autocrine production of insulin-like growth factors. Biochim Biophys Acta. 2010;1803:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gentile MA, Nantermet PV, Vogel RL, et al. Androgen-mediated improvement of body composition and muscle function involves a novel early transcriptional program including IGF1, mechano growth factor, and induction of {beta}-catenin. J Mol Endocrinol. 2010;44:55–73. [DOI] [PubMed] [Google Scholar]
- 35. Ahtiainen JP, Lehti M, Hulmi JJ, et al. Recovery after heavy resistance exercise and skeletal muscle androgen receptor and insulin-like growth factor-I isoform expression in strength trained men. J Strength Cond Res. 2011;25:767–777. [DOI] [PubMed] [Google Scholar]
- 36. List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52:1647–1655. [DOI] [PubMed] [Google Scholar]
- 37. Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267–278. [DOI] [PubMed] [Google Scholar]
- 38. Blaauw B, Canato M, Agatea L, et al. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009;23:3896–3905. [DOI] [PubMed] [Google Scholar]
- 39. Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 40. Marshall JL, Holmberg J, Chou E, et al. Sarcospan-dependent Akt activation is required for utrophin expression and muscle regeneration. J Cell Biol. 2012;197:1009–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Maltzahn J, Bentzinger CF, Rudnicki MA. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat Cell Biol. 2012;14:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abiola M, Favier M, Christodoulou-Vafeiadou E, Pichard AL, Martelly I, Guillet-Deniau I. Activation of Wnt/β-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. PLoS One. 2009;4:e8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu L, Zhang XJ, Jiang SR, et al. Heat shock protein 27 regulates oxidative stress-induced apoptosis in cardiomyocytes: mechanisms via reactive oxygen species generation and Akt activation. Chin Med J (Engl). 2007;120:2271–2277. [PubMed] [Google Scholar]
- 45. Vijayakumar A, Novosyadlyy R, Wu Y, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm IGF Res. 2010;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]