ABSTRACT
The role of T-type calcium currents is rarely considered in the extensive literature covering the mechanisms of long-term synaptic plasticity. This situation reflects the lack of suitable T-type channel antagonists that till recently has hampered investigations of the functional roles of these channels. However, with the development of new pharmacological and genetic tools, a clear involvement of T-type channels in synaptic plasticity is starting to emerge. Here, we review a number of studies showing that T-type channels participate to numerous homo- and hetero-synaptic plasticity mechanisms that involve different molecular partners and both pre- and post-synaptic modifications. The existence of T-channel dependent and independent plasticity at the same synapse strongly suggests a subcellular localization of these channels and their partners that allows specific interactions. Moreover, we illustrate the functional importance of T-channel dependent synaptic plasticity in neocortex and thalamus.
KEYWORDS: Cav3.1, Cav3.2, Cav3.3, long-term depression, long-term potentiation, neuron, synapse, thalamus, visual system
The interest in the low-voltage activated T-type calcium currents goes back to the beginning of the 80s. At that time, the group of R. Llinas identified a Ca2+-mediated rebound depolarization, the so-called low-threshold spike (LTS), that follows a transient hyperpolarization of inferior olive and thalamic neurons.1-4 Functionally, the Ca2+ current responsible for this depolarization was rapidly recognized as one of the key conductances underlying the characteristic rhythmic high-frequency burst firing activity of thalamic neurons during various NREM sleep stages.5,6 In parallel, studies mainly performed in primary sensory neurons7-10 demonstrated that this low-threshold Ca2+ current, named T-type current, is activated around −60 mV and fully inactivated after a few tens of milliseconds. Since steady-state inactivation of these channels is nearly complete at membrane potentials more depolarized than −60 mV, a hyperpolarization that allows some channels to recover from inactivation is required before a substantial T-type current can be evoked. Three genes (Cav3.1, 3.2, and 3.3) displaying multiple alternative splicings were later identified,11-13 with the 3 isoforms presenting specific expression in various regions of the nervous system.14 All T-type channels share the same basic biophysical properties with some key differences in the time courses of activation, inactivation, deactivation and recovery from inactivation and in the precise activation and inactivation voltage-dependence. From a functional point of view, these differences in the biophysical properties of the 3 isoforms and their splice variants should not be overlooked since they significantly impact the generation of LTSs.15
While the contribution of T-type currents in neuron excitability was building up, evidence also started to accumulate concerning a putative role of these currents in long-term synaptic plasticity. However, pinpointing the contribution of T-type currents remained for a long time a challenging task since no tools were available to specifically suppress these currents. To circumvent this difficulty, some authors took advantage of the peculiar biophysical properties of these channels. For example, using the same protocol, the induction of a long-term potentiation (LTP) or a long-term depression (LTD) at a given synapse could be prevented by changing the potential of the postsynaptic neurons from an hyperpolarized value at which a large fraction of the T-type channel population was available to a depolarized potential where no LTSs were evoked due to channel inactivation.16-18 Another approach relied on the use of poorly selective T-type channel blockers. Among these pharmacological agents the divalent cation Ni2+ was popular16-19 although, even at low concentrations, it also affects high-voltage activated Ca2+ currents, in particular the Cav2.3 “R” current. Since “R” current activation range partly overlaps with those of the Cav3 channels,20 the biophysical properties could not be used to definitely distinguish between these 2 Ca2+ currents in the early studies. The hypertensive drug mibefradil was also largely used although it inhibits Na+ and high-voltage Ca2+ currents as well as voltage-dependent and ATP-sensitive K+ channels.21-26 Despite these limitations, good indications of a role for T-type channels in long-term synaptic plasticity were nevertheless provided and definite evidence has been recently brought about by the generation of knock-out Cav3 channel mice27 and the synthesis of the first potent and selective T-type channel antagonists, mainly based on a piperidine chemical structure28,29 (Table 1). Not surprisingly most evidence was obtained in CNS areas showing a large expression of T-type channels, i.e. cortex, hippocampus, thalamus, cerebellum and spinal cord.14 In the following, we will mainly present the basic mechanisms identified in T-type channel dependent synaptic plasticity using results obtained in these CNS areas but a more complete list of the relevant studies is provided in Table 1.
Table 1.
Summary of T-type channel dependent synaptic plasticity.
| Brain area | Synapse | Nature of synapse | Type of plasticity | Evidence of T currents | Other partners | Ref. |
|---|---|---|---|---|---|---|
| Thalamus | TC->NRT | Glut | LTP postsynaptic |
Cav3.3 KO Voltage dependence |
NMDAR (GluN2B) | 72 |
| NRT -> TC VB | GABA | LTD postsynaptic | TTA-P2 | mGluR1 GABAAR CamK |
45 | |
| |
NRT -> TC PO |
GABA |
LTP presynaptic |
Voltage dependence |
L type Ca2+ current NO Guanylate cyclase |
46 |
| Cortex | White matter -> layer II/III (cat) | Glut | LTP | Voltage dependence | 16 | |
| Layer IV-> layer II/III (rat) | ? LTP ? |
Ni2+ Voltage dependence Ni2+, Mibefradil, Kurtoxin, Efonidipine |
82 | |||
| Pairs of layer V pyramidal neurons | Glut | LTP postsynaptic |
Ni2+ | NMDA R Backpropagating APs |
36 | |
| layer IV -> layer II/III | Glut | LTD presynaptic |
Voltage dependence Ni2+ |
mGluR5 IP3R-dependent stores L type Ca2+ current Presynaptic NMDAR CB1R |
74 | |
| layer II/III -> layer V pyramidal neurons | Glut | LTD ? |
Ni2+ | Group I/II mGluR Backpropagating APs |
38 | |
| |
layer II/III -> layer II/III pyramidal neurons |
Glut |
LTD presynaptic |
Ni2+ |
Group I mGluR PLC-activation L type Ca2+ current CB1R |
44 |
| Hippocampus | Schaffer collaterals -> CA1 | Glut | LTP ? |
Ni2+ | 55 | |
| Schaffer collaterals -> CA1 | Glut | LTP ? |
Ni2+ | NMDAR P type Ca2+ current |
19 | |
| -> CA1 | Glut | LTP ? |
Ni2+ | NMDAR L type Ca2+ current Backpropagating APs |
39 | |
| Schaffer collaterals -> CA1 | Glut | LTD presynaptic |
Voltage dependence Ni2+ |
mGluR5 PKC |
17 | |
| Medial perforant pathway -> dentate granule cells | Glut | LTP ? |
Voltage dependence Ni2+ |
Group I/II mGluR | 18 | |
| |
Stratum radiatum ->CA1 |
Glut |
LTP ? |
Cav3.2 KO mibefradil |
|
92 |
| Cerebellum | Parallel fibers -> Purkinje cells | Glut | LTP postsynaptic |
Cav3.1 KO TTA-P2 |
77 | |
| Mossy fibers ->cerebellar nuclear neurons | Glut | LTP ? |
Voltage dependence | NMDAR (NR2D) | 93 | |
| |
Purkinje cells -> cerebellar nuclear neurons |
GABA |
LTD LTP ? |
Voltage dependence Voltage dependence |
– Action potentials |
41 |
| Basal ganglia |
Globus pallidus -> subthalamic nucleus |
GABA |
LTP ? |
mibefradil |
L type Ca2+ current |
94 |
| Spinal cord | C fibers -> Lamina I neurons | Glut | LTP ? |
Ni2+ | Neurokinin1R Action potentials NMDAR |
73 |
Activation of T-type channels mediates membrane depolarization and calcium influx
Most forms of LTP and LTD described so far report an initial rise in postsynaptic Ca2 concentration occurring through various sources.30,31 Activation of T-type currents may contribute to such increase in 2 ways. On one hand, due to their low-threshold of activation, they can initiate a first depolarization that further recruit additional Ca2+ sources either through the activation of other voltage activated Ca2+ currents or/and by removing the voltage-dependent magnesium block of NMDA receptor. On the other hand, the Ca2+ ions funneled through T-type channels may constitute per se the required Ca2+ rise that triggers the molecular cascades leading to LTP or LTD.
The contribution of low-threshold Ca2+ channels to the dendritic depolarization associated to action potential back-propagation has been highlighted in a number of studies.32-34 For example in cortical layer V pyramidal neurons, while single action potentials are significantly attenuated when invading the distal regions of basal dendrites, action potential bursts efficiently depolarize these distal dendrites as a result of the activation of dendritic T- (or R) type calcium channels and the generation of calcium spikes. In these dendritic regions which receive the majority of synaptic inputs,35 the T-(R) bursts lead to a supra-linear increase in intracellular calcium compared to the generation of single action potentials or trains of spikes at lower frequencies.36,37 Interestingly, at the synapses between layer V pyramidal cells, pairing unitary excitatory postsynaptic potentials (EPSPs) with high-frequency action potential bursts, but not single action potentials, induced a robust LTP. Both Ni2+ at low concentration and NMDA receptor antagonists precluded this spike-timing dependent plasticity indicating that the large and long-lasting dendritic depolarization evoked by the T-(R) mediated action potential bursts allows the development of synaptic NMDA currents which contribute to LTP induction.36,37 The importance of T- (R) burst evoked dendritic spikes for the antidromic propagation of action potentials and the induction of synaptic plasticity was also demonstrated at the synapses between layer 2/3 neurons and layer V pyramidal neurons. Addition of low Ni2+ concentration or intracellular application of QX-314 that blocked action potentials precluded the induction of the LTD evoked when pairing action potential bursts in layer V pyramidal neurons with extracellular synaptic stimulations of layer 2/3 neurons.38 Similarly in CA1 hippocampal neurons, Magee and Johnston39 demonstrated that the coincidence of synaptic stimulation and action potential generation resulted in a large and widespread increase in dendritic Ca2+ and induced a significant spike-timing dependent potentiation of the EPSPs that was inhibited by both low Ni2+ concentration and nimodepine, suggesting the involvement of T-(R) and high threshold L-type Ca2+ channels.
The role played by the T-type current mediated depolarization in the mechanisms of long-term plasticity is not restricted to excitatory synapses, but it was also demonstrated at inhibitory synapses such as the connection between Purkinje neurons and neurons of the deep cerebellar nucleus. Indeed, following the first reports describing the generation of low-threshold Ca2+ spikes at the end of transient hyperpolarization, it was soon established that inhibitory postsynaptic potentials (IPSPs) are ideally suited to activate rebound LTSs.40 Accordingly, in deep cerebellar neurons Aizenman et al.41 showed that trains of IPSPs reliably evoked an LTS crowned by a burst of action potentials that resulted in large Ca2+ transients and the induction of LTP. Conversely, conditions that limited this rebound firing, and the associated Ca2+ transient, resulted in LTD.41
The contribution of the T-type current induced depolarization to the induction of long-term synaptic plasticity is fairly well established. However, to what extent does the rise in postsynaptic Ca2+ necessary to trigger synaptic plasticity specifically occur through T-type channels? Indeed, not only LTSs mediate large Ca2+ entry but also since T-type channels deactivate slowly, the calcium influx associated to the potential recruitment of T-type currents during brief depolarizing events, such as back-propagating action potentials or fast synaptic events, is especially large42,43 and mediate significant dendritic Ca2+ rises. However, to the best of our knowledge, the requirement of a specific funneling of Ca2+ through T-type channels as a prerequisite to induce synaptic plasticity was seldom investigated44 and only demonstrated at the inhibitory synapse between neurons of the nucleus reticularis thalami (NRT) and thalamocortical neurons of the ventrobasal somatosensory nucleus (Fig. 1A, synapse 1).45 At this synapse, pairing the stimulation of the reticulothalamic input to activation of the post-synaptic T-type current induced an LTD (Fig. 1B) that depends on a rise in intracellular Ca2+ concentration in the dendritic arbor (Fig. 1B). In the presence of a specific T-type channels blocker (TTA-P2), recruitment of high-voltage activated (HVA) Ca2+ currents, which evoked dendritic Ca2+ rise matching in amplitude the increase observed upon T-type channel activation, did not induce any synaptic plasticity (Fig. 2B & D). Furthermore, protocols that allowed significant Ca2+ entry from both T and HVA Ca2+ channels reintroduced an LTD that was resistant to HVA channel antagonist application (Fig. 2F). In contrast, in other studies reporting T-type dependent long-term plasticity, such as in the same CNS area at the synapse between NRT neurons and thalamocortical neurons of the associative posterior medial nucleus (PO; Fig. 1A synapse 2), application of the L-type HVA Ca2+ channel antagonist, nimodipine, precluded the induction of plasticity (Fig. 1Cb,46), suggesting that at these synapses Ca2+ entry through T-type channels was not sufficient to trigger plasticity. The link between the initial T-mediated depolarization and the recruitment of HVA Ca2+ channels can nevertheless be complex. Although at this synapse, the LTP required a LTS mediated postsynaptic depolarization that further activated L-type Ca2+ channels, the magnitude of the LTP was smaller if the LTS was evoked from a membrane potential of −76 mV compared to −62mV. This is surprising since hyperpolarization of the posterior medial neuron increases the fraction of available T-type channels and hence should enhances the amplitude of the evoked T-type currents: it may be that activation of a fraction of the T-type channel population already induces a maximal amplitude LTS in thalamic neurons.29,47 As a consequence, the hyperpolarization from −62 to −76 mV should have had little effect on the amplitude of the LTS and only increased the T-mediated Ca2+ entry. Therefore, one may hypothesize that either the stronger Ca2+ entry specifically funneled through T-type channels impedes the LTP development or the more hyperpolarized value of the membrane potential at the onset of the LTS makes it difficult to recruit the dendritic L-type Ca2+ channels.
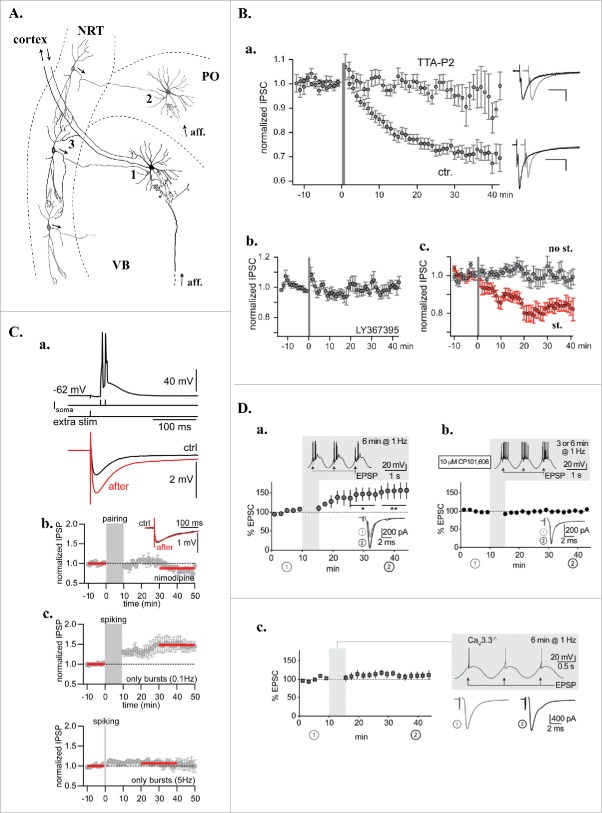
Figure 1.
T-dependent plasticity in the intra-thalamic network. A. Schematic drawing of the thalamic network presenting 3 synapses (1,2,3) where long-term plasticity mechanisms were described. VB: ventrobasal nucleus; PO: posterior medial nucleus; NRT: nucleus reticularis thalami; aff.: extra-thalamic afferences. B. Homo and hetero synaptic LTD of the rat NRT-VB GABAergic synapse (synapse 1 in A). a. Mean normalized inhibitory current amplitude evoked in thalamocortical neurons by NRT fiber stimulation. At time 0 (gray bar) short series of periodic (1.6 Hz) high-frequency stimulations (200 Hz) were applied to the NRT afferences while depolarizing the postsynaptic thalamocortical neurons from −80 to −30 mV to mimic both the incoming bursting activity of the NRT fibers and the concomitant LTS-associated depolarizations of the thalamocortical neurons that occur during sleep slow waves. Following the induction protocol an LTD of the GABAergic synapses developed in control condition (ctr., n = 22) but was not induced in the presence of the T-type current antagonist, TTA-P2 (3µM, n = 10). Examples of inhibitory currents evoked before (black traces) and after (gray traces) the induction protocol in both conditions are presented on the right. b. Demonstrating its heterosynaptic nature, LTD was suppressed in the presence of LY-367385 (100 µM, n = 11), a selective antagonist of the type 1 mGluRs that are expressed post-synaptically at the cortico-thalamic synapses .c. Mean normalized inhibitory current amplitudes evoked by stimulating 2 independent NRT pathways converging on the same thalamocortical neurons. LTD was only observed in the NRT pathway stimulated during the induction protocols (red dots) confirming the homosynaptic nature of the plasticity mechanism (n = 11). C. LTP of GABAergic synapses in rat thalamic posterior median thalamus (synapse 2 in A). a. top - induction protocol: Two current steps (100 Hz) injected in the thalamocortical neurons to evoke action potentials on top of a low-threshold Ca2+ spike were paired with an inhibitory post-synaptic potential (IPSP) triggered by extracellular stimulation. Bottom: Averaged IPSPs during baseline (ctrl, black) and after LTP induction (after, red). b. Normalized and averaged IPSP amplitudes over time demonstrating that LTP did not develop in the continuous presence of the L-type Ca2+ channel antagonist, nimodipine (10µM; n = 5). c. Normalized and averaged IPSP amplitudes over time showing that LTP can be induced with postsynaptic stimulation alone (bursting rhythmicity 0.1Hz, n = 7) in the absence of presynaptic stimulation. The LTP was reduced upon increasing the bursting rhythmicity and disappeared at frequencies higher than 5 Hz (n = 7). In b. and c., red bars indicate IPSPs used to calculate the amplitude ratio between control and post-induction protocol periods. D. Burst discharge via Cav3.3 channels induced LTP at thalamoreticular inputs (synapse 3 in A) when coactivated with GluN2B-containing NMDA receptors. a. Normalized and averaged excitatory post-synaptic currents (EPSCs) evoked in NRT neurons during baseline and after pairing synaptic input with LTS mediated bursting activities for 6 min (see shadowed inset). The pairing protocol induced a significant potentiation of the EPSCs (*p < 0.05, **p < 0.01; n = 7). Traces in the lower insets are average EPSCs evoked during baseline (1, gray) and during the last 10 min of recording (2, black). b. LTP was prevented in the presence of the GluN2B-NMDA receptor antagonist CP101,606 (n = 9). c. In Cav3.3−/− KO mice where low-threshold bursting is largely suppressed, pairing synaptic input with action potential firing elicited by sinusoidal current injection did not allow LTP induction (n = 8). Modified with permission from: (B),45 (C),46 (D).72.
Figure 2.
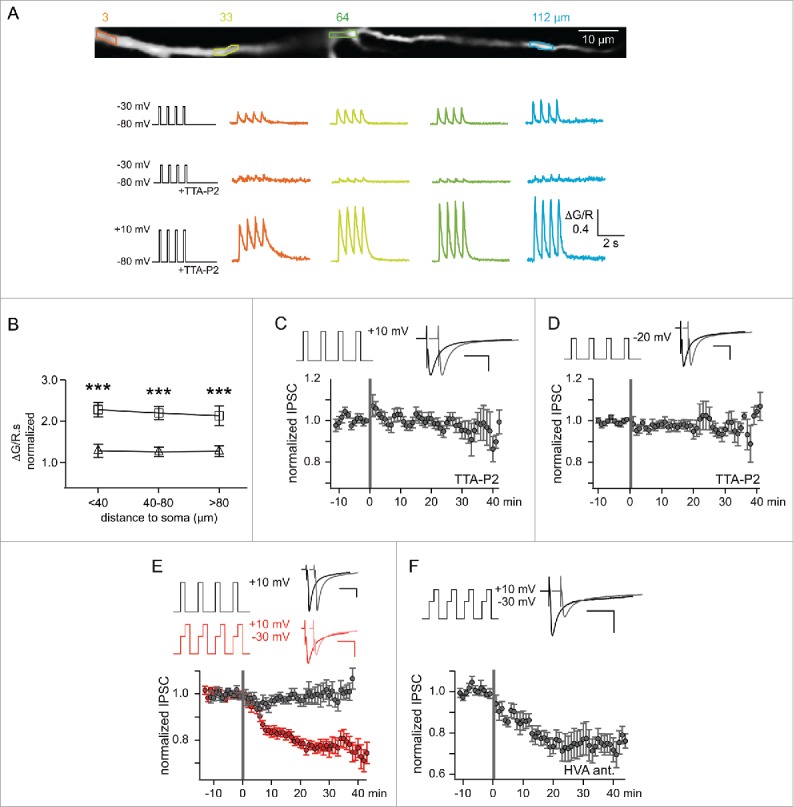
Long-term depression of the rat NRT-VB GABAergic synapse requires a specific funneling of Ca2+ through T-type channels. A. Dendritic Ca2+ responses evoked by somatic depolarizations in a thalamocortical neuron. Top, Stacked 2-photon microscopy image of a thalamocortical neuron dendritic branch filled with Alexa Fluor 594 and the Ca2+-sensitive dye Fluo-5F (4 regions of interest are highlighted). The traces below present variations in the fluorescent dye ratio (ΔG/R) triggered at these locations by successive step depolarizations at 1.6 Hz demonstrating that the T-type channel antagonist, TTA-P2 (3 µM), almost abolished the Ca2+ influx evoked in response to depolarizing pulses from −80 to −30 mV while a large Ca2+ entry is still observed for step depolarizations to +10 mV that strongly recruit HVA Ca2+ channels. B. Average Ca2+ influx (estimated by integrating over time the fluorescent ratio, ΔG/R.s) at different distances from the soma were evoked by specifically recruiting HVA Ca2+ channels in the presence of TTA-P2 using somatic step depolarizations from −80 to −20 (triangles) or +10 mV (squares). Normalizing the values to the ones previously obtained by activating T-type channels in the absence of TTA-P2, showed that the Ca2+ responses evoked at +10 mV through HVA channels were double (***p < 0.001) while HVA-mediated Ca2+ entry at −20 mV were equivalent. C. Mean normalized amplitude of inhibitory currents evoked in thalamocortical neurons by nucleus reticularis thalami (NRT) fiber stimulation. Depolarizing the postsynaptic thalamocortical neurons from −80 mV to +10 mV in the continuous presence of TTA-P2 while applying short series of periodic (1.6 Hz) high-frequency stimulations (200 Hz, gray bar) to the NRT afferents, specifically and strongly recruited HVA Ca2+ channels but failed to induce LTD (n = 10). D. Similar results were obtained when the amplitude of the step depolarization was decreased to −20 mV to reduce HVA-channel activation, therefore matching the Ca2+ entry evoked by T-type channel activation (see B; n = 11). E. In the absence of TTA-P2, recruiting both HVA- and T-type channels during the induction protocol triggered LTD only when a significant Ca2+ influx through the T-type channels was evoked by a depolarization to −30 mV preceding the +10 mV step (n = 12). F. In this condition, LTD induction was not affected by the presence of the HVA-channel antagonists (1 µM ω-conotoxin GVIA, 20 µM nifedipine, 500 nM SNX-482; HVA ant; n = 7). Modified with permission from45.
In conclusion, it is now well established that due to their biophysical properties and dendritic localization, T-type channels are major actors of both excitatory and inhibitory synaptic plasticity in many brain areas although at a number of synapses whether the crucial event is a specific Ca2+ entry through the T-type channels or the resulting depolarization remains to be clarified.
Subcellular localization of T-type channels and synaptic plasticity
The contribution of T-type channels to synaptic plasticity raises the question of the subcellular localization of these channels, in particular their proximity with the postsynaptic receptors within the dendritic arbor. Although few data are already available concerning their precise localization in the various neuronal compartments,48,49 calcium imaging technique, immunocytochemistry and electrophysiological recordings revealed that T-(R) calcium channels are not evenly distributed along dendrites.50-53 For example, in CA1 pyramidal neurons where Ni2+ dependent synaptic plasticity mechanisms have been described at the Schaffer collateral synapses,19,39 Ni2+ sensitive channels are more abundant in the distal area of the apical dendrites.54 Accordingly, by recording field potentials at different locations, “proximal,” “middle” and “distal” along the apical dendrite of CA1 pyramidal neurons, Isomura et al.55 showed that while no significant difference among the magnitudes of the induced LTP was observed at these 3 dendritic locations in control condition, Ni2+ strongly inhibited the LTP induction in distal dendrites, slightly in middle dendrites, and did not significantly influenced LTP at proximal dendrites. Thus, for a given synaptic type, the specific T-(R) channel distribution may underlie different induction mechanisms of LTP along a dendrite. While the LTP of proximal synapses does not require T-type channel activation, recruitment of distal T-type channels is necessary to boost the amplitude of backpropagating action potentials allowing the activation of the NMDA receptors and the induction of LTP.
The relationship between the spatial extend of calcium influx through T-type channels and its ability to evoke synaptic plasticity has not been studied yet although recent electron microscopic studies reported a close proximity of Cav3.3 channels to synaptic contacts in cortical interneurons and NRT neurons49 and of Cav3.2 channels in NRT neurons,56 entorhinal cortical neurons57 and neurons located in lamina II/III of the dorsal horn of the spinal cord (Fig. 3Bab,58). Where are these channels located in respect to the other actors of the signaling mechanisms and how far should the Ca2+ entering through T-type channels spread to trigger synaptic plasticity? At the synapse between GABAergic NRT neurons and somatosensory thalamocortical neurons (Fig. 1A synapse 1), the only synapse where the specific requirement of calcium influx through T-type channels was demonstrated (see above), both T-type channels and GABA-A receptors are present on the whole dendritic shafts of thalamocortical neurons.59,60 Although T-type activation evokes a widespread Ca2+ increase in the dendritic arbor, LTD was only present at the GABA-A synapses that undergo the stimulation protocol (Fig. 1Bc).45 In addition, this LTD was blocked by transient application of a GABA-A receptor antagonist during the induction protocol, demonstrating that not only GABA release but also GABA-A receptor activation was required to trigger the postsynaptic LTD. Since these experiments were performed while voltage-clamping the postsynaptic neuron, they suggest that the intracellular mechanisms involved in this LTD require chloride influx or more likely state-dependent modification(s) of the GABA-A receptors. Finally, the LTD was also blocked by calcineurin, an antagonist of the calcium-sensitive phosphatase. Therefore, one may hypothesize that a spatially restricted interaction occurs between the T-mediated Ca2+ influx and calcineurin that results in a state-dependent dephosphorylation of the activated GABA-A receptors. However, such mechanism remains hypothetical since data on a potential co-localization of GABA-A receptors, calcineurin and T-type channels are lacking. For now, such local functional interactions for T-type channels including direct protein-protein interactions have only been demonstrated for various K+ channels61-64 and pre-synaptic proteins.65
Figure 3.
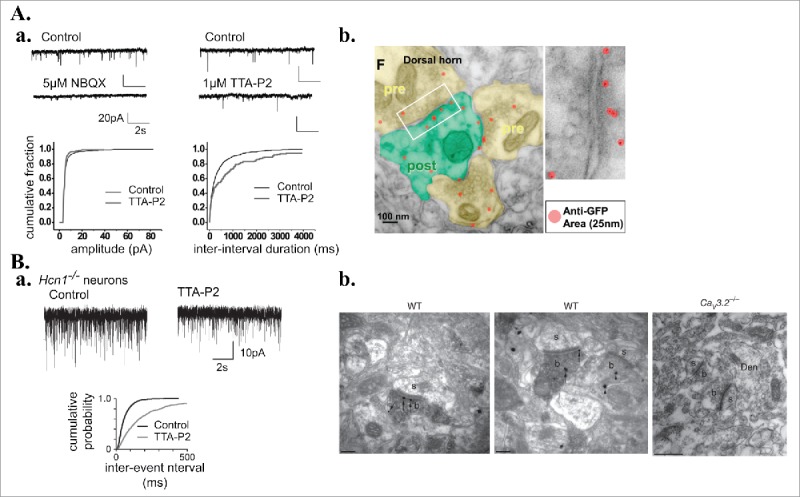
Presynaptic T-type Ca2+ channels regulate neurotransmission. A. Presynaptic T-type channels in the superficial lamina of the dorsal horn of the spinal cord. a. Top: Recordings of spontaneous miniature excitatory post-synaptic currents (mEPSCs) from lamina I neurons (Control) and after application of the AMPA receptor antagonist, NBQX (left) or the T-type channel antagonist, TTA-P2 (right). Bottom: Cumulative distributions of events amplitude (left) and frequency (right) in control and TTA-P2 conditions demonstrating the presynaptic regulation by T-type channels of the spontaneous vesicular release. b. Transmission electron microscopy images from pre-embedded GFP silver staining of spinal cord dorsal horn coronal sections from knocking mice expressing GFP within an extracellular loop of the Cav3.2 channels. Red dots represent GFP antibody complexes around individual silver dot (25 mm). Areas in yellow are presynaptic regions containing dense synaptic vesicles. Green area is a postsynaptic region recognized by the postsynaptic density. Right panel is a magnification of the box in the left image. B. Presynaptic T-type channels at excitatory synapses in the entorhinal cortical layer III. a. Typical recordings of mEPSCs performed in slices from Hcn1−/− KO mice (devoid of the type cationic h current activated upon hyperpolarization) in control condition and upon application of the T-type channel antagonist (TTA-A2, 500nM). As demonstrated by the cumulative probability graph (bottom), T-type channel blocker decreases the mEPSC frequency. b. Electron micrographs using pre-embedding double-labeling methods showing immunoreactivity for Hcn1 (peroxidase reaction product) and Cav3.2 (immunoparticles) channels in wild-type sections (WT) of entorhinal cortex. Immunogold particles for Cav3.2 were absent in Cav3.2−/− sections. b, axon terminals; s, dendritic spines; Den, dendrite. Scale bars: 0.2μm. Modified with permission from: (A.a.)91; (A.b.)58; (B)57.
So far all reported examples of T-type channel dependent synaptic plasticity, resulting in either pre or postsynaptic modification, involve T-type channels located on the postsynaptic neuron. However some electron microscope studies reported the presence of Cav3.2 channels on presynaptic-terminals (Fig. 3Ab,58)57 and Cav3.1 channels are expressed at the terminals of inhibitory parvalbumin-positive interneuron in the CA1 hippocampal region.66 In addition, Cav3.2 channels colocalize with proteins involved in synaptic release as syntaxin 1A in NRT neurons67 while T-type channels are associated with syntaxin-1A and SNAP-25 in chromafin cells.68 Functionally, Cav3.2 channels localized in layer III presynaptic-terminals of the entorhinal cortex were shown to contribute to the spontaneous release of glutamate (Fig. 3B,57) while in the hippocampal CA1 region calcium influx through Cav3.1 channels present in the terminals of parvalbumin positive axons triggers the asynchronous quantal GABA release evoked following nicotinic receptor activation.66 Whether these presynaptically located T-type channels also participate to long-term synaptic changes remains an open question.
T-type channels: Partner of diverse long-term synaptic plasticity mechanisms
As it can be inferred from the various studies presented so far, T-type channels are not associated to a specific type of synapses, excitatory or inhibitory, nor to a peculiar form of plasticity, LTP or LTD. Many reviews have been written on the molecular mechanisms of long-term synaptic changes (see69). Here, we selected a few examples to emphasize that T-type channels act in synergy with a number of the well-known signaling pathways and are implicated in both homo- and heterosynaptic plasticity.
Following the discovery of LTP at excitatory synapses,70 the requirement of NMDA receptor activation was clearly identified as one of its essential initial step.71 As already stated, T-type channels may contribute to this type of plasticity through the backpropagation of action potentials that relieves the magnesium block of synaptic NMDA receptors, resulting in calcium entry through these receptors and a Ca2+ rise in dendritic spines.36 Recently, the use of transgenic mice has allowed to definitely demonstrate the synergistic contribution of NMDA receptors and T-type channels to LTP in the thalamus. At the glutamatergic synapse between thalamocortical neurons and NRT neurons (Fig. 1A synapse 3), pairing synaptic inputs with LTS in the NRT neuron resulted in subsequent LTP of excitatory postsynaptic currents that required GluN2B-NMDA receptors and could not be evoked in Cav3.3 KO mice or in the presence of NMDA receptor antagonist (Fig. 1D). Notably, since the LTP could be induced even in the absence of Na+ action potentials, the low-threshold potential per se was sufficient to enable coincidence detection via NMDA receptors, and did not require backpropagating action potentials.72 Another type of T- and NMDA- dependent mechanism was suggested in the spinal cord. At the synapses between afferent C-fibers and lamina I projecting neurons, high-frequency stimulation induced an LTP that required the activation of neurokin 1 receptors. The transduction pathways produce a rise in calcium, likely by calcium release from intracellular stores, and a substance P-facilitated calcium influx through NMDA receptors.73 Although the high-frequency stimulation during the LTP induction protocol did not evoke an LTS but only action potential discharges, this LTP was also blocked by Ni2+ application. Detailed studies of the action potential waveforms revealed a significant action potential broadening that was attributed to T-(R) type channel activation and significantly participated to the firing induced Ca2+ rise. This study therefore suggests a cooperation of intracellular Ca2+ store, synaptically evoked NMDA current and action potential-triggered T-(R) type current to the increase in intracellular Ca2+ required to induce LTP at synapses from nociceptive fibers.73
T-type channels not only contribute to NMDA receptor- but also to metabotropic glutamatergic (mGlu) receptor-dependent LTP and LTD. For example, at the excitatory cortical layer IV to layer II synapse, T-type channels contribute to the LTD that involves the activation of group I mGlu receptors, the downstream activation of IP3 receptor-gated Ca2+ stores, together with calcium entry through L-type calcium channels. This plasticity is of presynaptic expression, since it is downstream to the postsynaptic calcium increase, and requires a retrograde endocannabinoid signaling together with the activation of apparently presynaptic NMDA receptors.74 Similarly, T-type channels contribute to the presynaptic LTD observed at the synapse between layer II/III pyramidal cortical neurons that also required mGlu1 receptor activation, PLC activation and endocannabinoid retrograde messenger.44
The mechanisms presented so far to illustrate NMDA and mGlu receptor-dependent plasticity were homosynaptic. However, T-type channel dependent plasticity can also be both hetero and homosynaptic. Indeed, as already described (see above and Fig. 1Bc), the LTD at the GABAergic synapses between NRT neurons and somatosensory thalamocortical neurons (Fig. 1A synapse 1) is clearly homosynaptic since it requires activation of the depressed GABAergic synapses. However the full mechanism also requires heterosynaptic activation of mGlu receptors by the corticothalamic afferents (Fig. 1Bb). This activation, coupled to large influxes of calcium through T-type channels, triggers the calcium-sensitive phosphatase calcineurin that will then dephosphorylate GABA-A receptors inducing their long-term desensitization (Fig. 1B).45
Finally, it should be noted that T-type channels may differentially contribute to the multiple forms of synaptic plasticity expressed a given synapse. As already mentioned, activation of the GABAergic synapse between Purkinje cells and deep cerebellar neurons evoked T-mediated rebound depolarizations that can produce either LTD or LTP. Aizenman and collaborators showed that the polarity of these synaptic changes is linked to the number of action potentials evoked by T-mediated rebound depolarizations. Whereas LTP were induced in conditions where pronounced rebound depolarization elicited significant spiking (>5 action potentials), LTD required rebound depolarizations that had little or no spiking.41,75 Although activation of high-voltage Ca2+ channels by action potentials can explain why a strong rebound firing is required to trigger LTP, the exact biochemical mechanisms coupling the rebound depolarization to either LTP or LTD of IPSPs are still unknown. These data highlight the fact that either form of plasticity can be evoked depending on the pattern of LTS activation. While at these synapses T-type channel activation is required to trigger both type of plasticity, it has also been shown at other synapses that these channels may contribute to one but not the other.
At the glutamatergic Schaffer collateral–CA1 cell synapse, either an NMDA or an mGlu receptor-dependent LTD can be induced depending on the recording conditions. While both forms are pathway specific, and require membrane depolarization and a rise in postsynaptic calcium, the activation of T-type channels was needed for the expression of mGlu but not NMDA receptor-LTD. Importantly, the mGlu receptor LTD is favored in conditions of reduced excitability, i.e., when the amplitude of the synaptic input is small and in the presence of GABAergic inhibition that would help deinactivating T-type channels.17 Both glutamate receptor subtypes are present in postsynaptic spines but the metabotropic and the ionotropic receptors are localized perisynaptically and at the post-synaptic density, respectively.76 A remarkable compartmentalization of the signaling transduction pathways could therefore be hypothesized with a localization of the T-type channels in the immediate vicinity of mGlu receptors limiting the activation of the specific pathway to the appropriate trigger.17
Another example of the fine-tuning of T-type channel recruitment according to the type of synaptic plasticity was reported in Purkinje cells that show a high expression of Cav3.1 channels. Using both Cav3.1 KO mice and specific T-type channel antagonist (TTA-P2) application, Ly et al.77 showed that T-type channels are required for LTP induction at the glutamatergic parallel fiber–Purkinje cell synapse. However, the functional inactivation of Cav3.1 channels did not affect the LTD expressed at the same synapse when the induction protocol paired the stimulation of the parallel fibers with a subsequent stimulation of the climbing fibers (Fig. 4). In this case, high postsynaptic Ca2+ levels are required that presumably result from the activation of P/Q calcium channels after the strong depolarization ensuing climbing fiber activity and from release from intracellular Ca2+ stores.78
Figure 4.
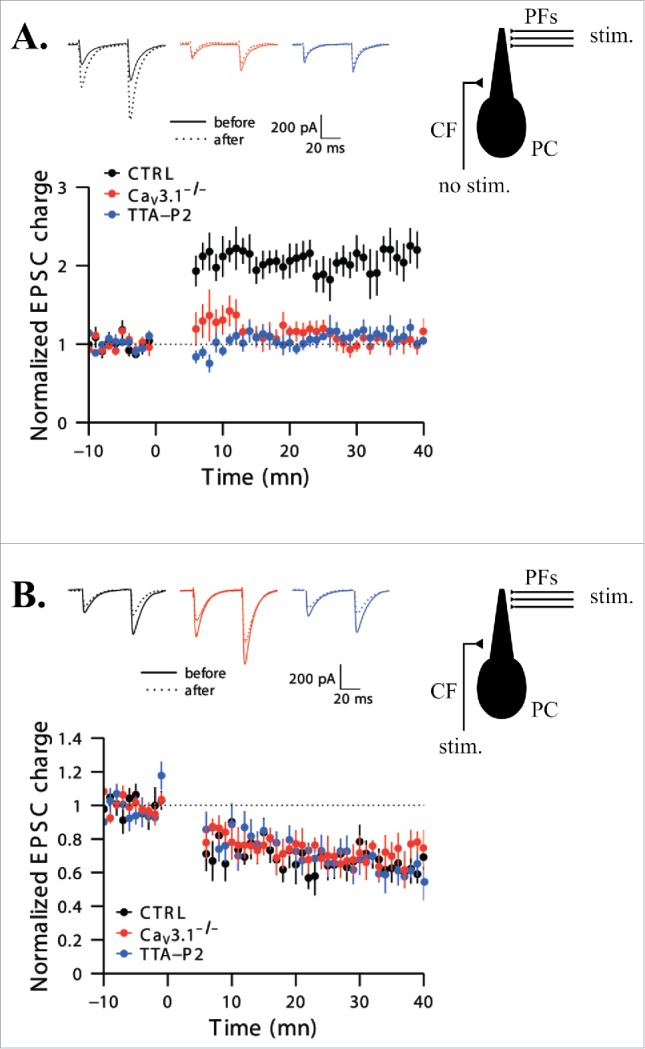
T-type Ca2+ channel activation is necessary for long-term potentiation but not depression induction at the parallel fibers to Purkinje cell synapses. A. Time course of normalized excitatory post-synaptic current (EPSC) charges. The LTP induction protocol (performed at time 0) comprised 5 high-frequency (200Hz) stimulations of the parallel fibers (PFs) repeated each second for 5 min without stimulating the climbing fiber (CF) (see schematic representation on the right). LTP was induced in control condition (black dots, n = 8) but was blocked by the specific T-type channel antagonist, TTA-P2 (blue dots, n = 7), and absent in slices from Cav3.1−/− mice (red dots, n = 7). Representative traces from 10 successive sweeps before and 30 min after the induction protocol are presented at the top in each case. B. Same data as in A for the LTD induction protocol. Doublets of PF stimuli (2 pulses, 200 Hz) followed by a 100 ms burst of CF activation (4 pulses, 400 Hz) every second during 5 min were used to successfully trigger LTD in each cases (n = 7 for the 3 sets of conditions). Modified with permission from77.
Therefore, as briefly presented here and illustrated in the articles listed in Table 1, T-type channels participate to numerous synaptic plasticity mechanisms, both homo and heterosynaptic, involving different partners and both pre- and post-synaptic modifications (the main pathways underlying T-channel dependent plasticity at both excitatory and inhibitory synapses are schematically presented in Fig. 5). Although few data are available, the existence of T-dependent and T-independent plasticity at the same synapse strongly suggests a subcellular localization of these channels and their partners that allows specific interactions.
Figure 5.
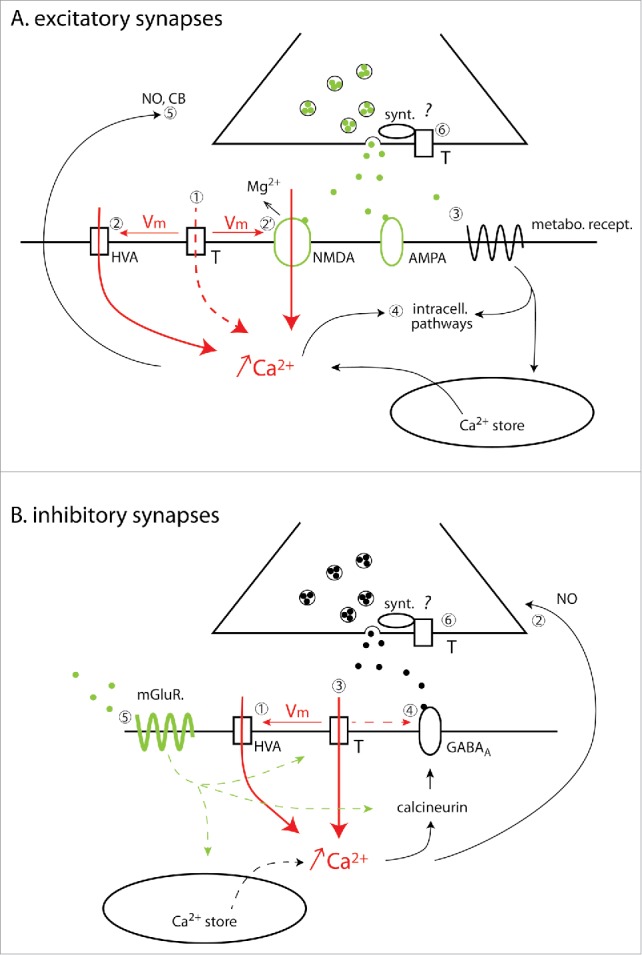
T-type calcium channel-dependent LTP/LTD mechanisms at excitatory and inhibitory synapses. A. Excitatory synapses. Activation of postsynaptic T-type calcium channels induces a post-synaptic Ca2+ increase either directly (1) and/or as a consequence of the membrane potential (Vm) depolarization that can in turn either trigger the opening of the HVA Ca2+ channel (2) and/or relieve the Mg2+ block of NMDA receptors (2′). In addition to T channels activation, various types of metabotropic receptors can be recruited (3) further enhancing intracellular Ca2+ concentration by mobilizing the Ca2+ stores. The increase in Ca2+ concentration activates multiple intracellular pathways (4) mediating post-synaptic long-term plasticity or producing the retrograde messengers, nitric oxide (NO) or cannabinoid (CB) (5) that modify the presynaptic release probability. Finally, presynaptic T-type channels (6) directly interact with proteins involved in the exocytotic machinery like syntaxin 1A (synt). Further studies will clarify whether these presynaptic T-type channels are also involved in synaptic LTP/LTD. Note that a postsynaptic Ca2+ increase specifically supported by the Ca2+ influx through T-type channels has not yet been demonstrated as a required step for LTP/LTD induction at excitatory synapses. B. Inhibitory synapses. T-type channel activation depolarizes the postsynaptic membrane potential (Vm), opening L-type HVA Ca2+ channels (1). The resulting increase in intracellular Ca2+ concentration triggers NO production that diffuses retrogradely to the presynaptic element (2) and induces pre-synaptic LTP through guanylate cyclase activation. A different mechanism, independent of HVA channel activation, requires Ca2+ ions specifically entering in the post-synaptic dendrite through the T-type channels to activate the phosphatase calcineurin and trigger LTD (3). In this case, only activated GABA receptors are down-regulated suggesting a state-dependent interaction with either calcineurin or the T-type channels (4). In addition, LTD also requires the concomitant activation of metabotropic glutamate receptors (5) that may potentiate the T-mediated Ca2+ influx, recruit intracellular Ca2+ stores and/or modulate intracellular pathways. Note that T-type channels are also potentially present on inhibitory pre-synaptiC-terminals (6) where they directly interact with proteins involved in the exocytotic machinery like the syntaxin 1A (synt). In both A and B, dashed lines are used when no definite experimental evidence are available to support the proposed mechanism.
Physiological consequences of T-dependent plasticity
How these various T-dependent synaptic plasticities contribute to brain functions is an emerging issue that can now be tackled with the help of the new pharmacological and genetic tools. Two sets of studies performed in the visual cortex and the thalamus illustrate their functional importance in developmental and sleep related plasticity, respectively.
Plasticity of visual responses
During development, long-term synaptic plasticity contributes to the refinement of the responses of neuronal networks to sensory experiences.79 An attractive model to study this phenomenon is ocular dominance, i.e. the relative effectiveness of the left and right eyes in driving visual cortical neurons that drastically shifts toward the non-deprived eye after monocular deprivation.80 In the rat visual cortex, this susceptibility of ocular dominance preference to monocular deprivation is small around the time of eye opening, peaks at around 4 weeks, and disappears in adults.81 Interestingly, in vitro experiments showed that an LTP could be elicited at the excitatory layer IV and layers II/III synapses during this critical time period. Dark rearing delays similarly both the age-dependent decline of LTP and the period of ocular dominance plasticity illustrating the time correlation between these phenomena. The synaptic plasticity requires the activation of T-type channels since it is precluded if the post-synaptic neuron is maintained at a depolarized potential or in the presence of T-type channel blockers.82 Direct measurement of the T-type current, presumably of the Cav3.2 type, showed a developmental profile in these neurons. The amplitude of the currents was very small before eye opening, peaked during the critical period and returned to a small value by adulthood while dark rearing prevented this developmental decline until adulthood.83 Similarly in the kitten visual cortex, in vitro experiments showed that the LTP evoked in layers II/IV neurons by white matter stimulation, which is maximal at the time of the critical period, requires the activation of LTSs in the post-synaptic neuron.16 The contribution of T-type channels to the visual response plasticity was also investigated in vivo. In rats subjected to monocular deprivation for a time period long enough to allow the full development of both depression of deprived eye responses and potentiation of non-deprived eye responses, infusion of mibefradil into the visual cortex abolished the potentiation of the visual evoked potentials. In contrast, the depression of the deprived eye responses that are likely mediated by NMDA receptor-dependent LTD was unchanged.82 Similarly, infusion of the specific T-type channel blocker TTA-I1 in the cat visual cortex reduced cortical plasticity triggered by monocular deprivation while preserving normal visual response properties.84 Altogether the results from these in vitro and in vivo experiments strongly suggest that T-type channels are essential to the development of experience-dependent enhancement of visual responses. How this synaptic plasticity could be related to changes in the number or properties of cortical Cav3 channels remain to be resolved.
Sleep-associated synaptic plasticity
Among the various theories on the cognitive roles of sleep, it is suggested that at least part of the sleep memory consolidation processes, involving reshaping of synaptic connectivity, occurs during the slow waves of non-REM sleep.85 At the network/cellular level these EEG rhythms are associated to oscillatory activities within the intrathalamic network (Fig. 1A) that are characterized by the rhythmic occurrence of high-frequency bursts of action potentials mediated by LTSs in both thalamocortical and NRT neurons.86 Using induction protocols that mimic the activities occurring in these neurons during slow sleep oscillations, 3 in vitro studies recently tackled the issue of long-term synaptic changes within the intrathalamic network. As already described above, different synapses were investigated, the excitatory thalamocortical to NRT neuron synapse (Fig. 1A synapse 3)72 and 2 inhibitory synapses between NRT neurons and either associative (PO) (Fig. 1A synapse 2)46 or sensory (VB) thalamocortical neurons45 (Fig. 1A synapse 1). Interestingly activation of T-type channels is required for the expression of long-term changes at the 3 synaptic types, albeit the sign of the changes, the signaling pathways and the plasticity loci were different.45,46,72 How these synaptic mechanisms contribute to sleep-associated cognitive functions remains to be established through in vivo investigations. It can however be hypothesized that the 2 long-term changes present at the inhibitory synapses may occur at different periods of the sleep rhythms and target specific thalamic networks. For example, the LTP of inhibitory synapses in associative thalamus is a homeostatic plasticity since it only required postsynaptic repetitive bursting activity (Fig. 1Cc) and therefore should develop in every thalamocortical neurons and affect every GABAergic synapse. Conversely, the LTD between NRT and sensory thalamocortical neurons required not only T-type current activation but also GABA-A and mGlu receptor activation (Fig. 1B). Therefore, this is a more restricted process that specifically affects sleep activated GABAergic synapses in a subset of thalamocortical neurons submitted to strong activation of their corticothalamic inputs, and hence may be involved in the precise functional reshaping of the sensory information pathway during sleep. Moreover from the requirement of the induction protocol frequencies, it can be inferred that the LTP at the NRT to associative thalamocortical neuron synapses should develop at early stages of NREM sleep when LTSs mainly occur at very low frequency during the thalamocortical neuron transitions from DOWN to UP states (Fig. 1Cc). Conversely, LTD at the NRT to sensory thalamocortical neuron synapses should be triggered during a deeper sleep state when the DOWN to UP state transitions are intermixed with delta-activity characterized by the occurrence of LTSs at a relatively higher frequency.87
Conclusion – perspectives
Although the first evidences pointing to a synaptic role of T-type channels were obtained more than 20 y ago, they have so far contributed little to the vast literature on synaptic plasticity (Table 1). Two main reasons may underlie this limited interest. First, for a long time, the current dogma has affirmed that T-type currents could play little role in physiological functions such as behaviorally related memory formation, which take place during the wake state when neurons are supposed to be depolarized and therefore T-type channel inactivated. However, it is now clear that in some brain areas, the density of T-type channels expressed in neurons is high enough to allow a significant number of deinactivated T-type channels at the membrane potentials reported in awake animals.88 For example, in the thalamus, the fraction of T-type channels that is available at depolarized potential participates to EPSP amplification and has a drastic effect on spike probability during wake states.89 Second, the lack of suitable T-type channel antagonists has hampered for many years the discovery of subtle functions for neuronal T-type currents and made impossible to definitely prove their implication in synaptic plasticity. However, the development of specific and potent antagonists29 now sets this issue, opening the way to a growing number of studies demonstrating new roles for T-type currents (see review in.90).
From the studies reported here, it can already be concluded that T-type currents are not restricted to a specific form of long-term synaptic plasticity and play a role in both excitatory and inhibitory synapses with the help of various intracellular partners (Fig. 5). Although, only postsynaptically localized T-type channels have been considered so far, some recent results point to a localization of the channels in presynaptic-terminals. Therefore, one may assume that data will rapidly be obtained demonstrating a role of presynaptic T-type channels in either long-term or short-term synaptic plasticity.
Finally, the specific requirement of Ca2+ entering through T-type channels to trigger long-term plasticity at some synapses45 and the observation that at a given synapse where multiple forms of plasticity occur, only one depends on T-type channel activation77 open new exciting perspectives. Indeed, both results strongly suggest that the different molecular actors of the long-term plasticity are spatially localized and/or directly interact with T-type channels. Molecular approaches based on new genetically engineered tools should help to decipher these complex protein-protein interactions and bring about totally new insights into the physiology of these channels.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. V. Crunelli for critical reading of the manuscript.
References
- [1].Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol 1981; 315:549-67; PMID:6273544; http://dx.doi.org/ 10.1113/jphysiol.1981.sp013763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature 1982; 297:406-8; PMID:7078650; http://dx.doi.org/ 10.1038/297406a0 [DOI] [PubMed] [Google Scholar]
- [3].Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol 1984; 349:205-26; PMID:6737292; http://dx.doi.org/ 10.1113/jphysiol.1984.sp015153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol 1984; 349:227-47; PMID:6737293; http://dx.doi.org/ 10.1113/jphysiol.1984.sp015154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deschenes M, Paradis M, Roy JP, Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol 1984; 51:1196-219; PMID:6737028 [DOI] [PubMed] [Google Scholar]
- [6].Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically projecting and reticularis neurones. J Physiol (Lond) 1986; 379:429-49; PMID:3560000; http://dx.doi.org/ 10.1113/jphysiol.1986.sp016262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bossu JL, Feltz A. Inactivation of the low-threshold transient calcium current in rat sensory neurones: evidence for a dual process. J Physiol 1986; 376:341-57; PMID:2432232; http://dx.doi.org/ 10.1113/jphysiol.1986.sp016157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature 1984; 310:501-2; PMID:6087159; http://dx.doi.org/ 10.1038/310501a0 [DOI] [PubMed] [Google Scholar]
- [9].Fedulova SA, Kostyuk PG, Veselovsky NS. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol 1985; 359:431-46; PMID:2582115; http://dx.doi.org/ 10.1113/jphysiol.1985.sp015594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 1985; 316:440-3; PMID:2410796; http://dx.doi.org/ 10.1038/316440a0 [DOI] [PubMed] [Google Scholar]
- [11].Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 1998; 391:896-900; PMID:9495342; http://dx.doi.org/ 10.1038/36110 [DOI] [PubMed] [Google Scholar]
- [12].Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 2003; 83:117-61; PMID:12506128; http://dx.doi.org/ 10.1152/physrev.00018.2002 [DOI] [PubMed] [Google Scholar]
- [13].Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P. Alternatively spliced α(1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca(2+) channel gating properties. Biophys J 2001; 80:1238-50; PMID:11222288; http://dx.doi.org/ 10.1016/S0006-3495(01)76100-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 1999; 19:1895-911; PMID:10066243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tscherter A, David F, Ivanova T, Deleuze C, Renger JJ, Uebele VN, Shin HS, Bal T, Leresche N, Lambert RC. Minimal alterations in T-type calcium channel gating markedly modify physiological firing dynamics. J Physiol 2011; 589:1707-24; PMID:21320888; http://dx.doi.org/ 10.1113/jphysiol.2010.203836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Komatsu Y, Iwakiri M. Low-threshold Ca2+ channels mediate induction of long-term potentiation in kitten visual cortex. J Neurophysiol 1992; 67:401-10; PMID:1349036 [DOI] [PubMed] [Google Scholar]
- [17].Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron 1997; 18:969-82; PMID:9208864; http://dx.doi.org/ 10.1016/S0896-6273(00)80336-0 [DOI] [PubMed] [Google Scholar]
- [18].Wang Y, Rowan MJ, Anwyl R. LTP induction dependent on activation of Ni2+-sensitive voltage-gated calcium channels, but not NMDA receptors, in the rat dentate gyrus in vitro. J Neurophysiol 1997; 78:2574-81; PMID:9356407 [DOI] [PubMed] [Google Scholar]
- [19].Ito K, Miura M, Furuse H, Zhixiong C, Kato H, Yasutomi D, Inoue T, Mikoshiba K, Kimura T, Sakakibara S, et al.. Voltage-gated Ca2+ channel blockers, omega-AgaIVA and Ni2+, suppress the induction of theta-burst induced long-term potentiation in guinea-pig hippocampal CA1 neurons. Neuroscience Letters 1995; 183:112-5; PMID:7746467; http://dx.doi.org/ 10.1016/0304-3940(94)11127-5 [DOI] [PubMed] [Google Scholar]
- [20].Zamponi GW, Bourinet E, Snutch TP. Nickel block of a family of neuronal calcium channels: subtype- and subunit-dependent action at multiple sites. J Membr Biol 1996; 151:77-90; PMID:8661496; http://dx.doi.org/ 10.1007/s002329900059 [DOI] [PubMed] [Google Scholar]
- [21].Bezprozvanny I, Tsien RW. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967). Mol Pharmacol 1995; 48:540-9; PMID:7565636 [PubMed] [Google Scholar]
- [22].Gomora JC, Enyeart JA, Enyeart JJ. Mibefradil potently blocks ATP-activated K(+) channels in adrenal cells. Mol Pharmacol 1999; 56:1192-7; PMID:10570046 [DOI] [PubMed] [Google Scholar]
- [23].Liu JH, Bijlenga P, Occhiodoro T, Fischer-Lougheed J, Bader CR, Bernheim L. Mibefradil (Ro 40-5967) inhibits several Ca2+ and K+ currents in human fusion-competent myoblasts. Br J Pharmacol 1999; 126:245-50; PMID:10051142; http://dx.doi.org/ 10.1038/sj.bjp.0702321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mishra SK, Hermsmeyer K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circulation Res 1994; 75:144-8; PMID:8013072; http://dx.doi.org/ 10.1161/01.RES.75.1.144 [DOI] [PubMed] [Google Scholar]
- [25].Nilius B, Prenen J, Kamouchi M, Viana F, Voets T, Droogmans G. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca(2+)- and volume-activated Cl− channels in macrovascular endothelial cells. Br J Pharmacol 1997; 121:547-55; PMID:9179399; http://dx.doi.org/ 10.1038/sj.bjp.0701140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Viana F, Van den Bosch L, Missiaen L, Vandenberghe W, Droogmans G, Nilius B, Robberecht W. Mibefradil (Ro 40-5967) blocks multiple types of voltage-gated calcium channels in cultured rat spinal motoneurones. Cell Calcium 1997; 22:299-311; PMID:9481480; http://dx.doi.org/ 10.1016/S0143-4160(97)90068-3 [DOI] [PubMed] [Google Scholar]
- [27].Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α(1G) T-type Ca(2+) channels. Neuron 2001; 31:35-45; PMID:11498049; http://dx.doi.org/ 10.1016/S0896-6273(01)00343-9 [DOI] [PubMed] [Google Scholar]
- [28].Shipe WD, Barrow JC, Yang ZQ, Lindsley CW, Yang FV, Schlegel KA, Shu Y, Rittle KE, Bock MG, Hartman GD, et al.. Design, synthesis, and evaluation of a novel 4-aminomethyl-4-fluoropiperidine as a T-type Ca2+ channel antagonist. J Med Chem 2008; 51:3692-5; PMID:18540666; http://dx.doi.org/ 10.1021/jm800419w [DOI] [PubMed] [Google Scholar]
- [29].Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci 2010; 30:99-109; PMID:20053892; http://dx.doi.org/ 10.1523/JNEUROSCI.4305-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci 1993; 16:480-7; PMID:7507622; http://dx.doi.org/ 10.1016/0166-2236(93)90081-V [DOI] [PubMed] [Google Scholar]
- [31].Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 2004; 44:5-21; PMID:15450156; http://dx.doi.org/ 10.1016/j.neuron.2004.09.012 [DOI] [PubMed] [Google Scholar]
- [32].Magee JC, Christofi G, Miyakawa H, Christie B, Lasser-Ross N, Johnston D. Subthreshold synaptic activation of voltage-gated Ca2+ channels mediates a localized Ca2+ influx into the dendrites of hippocampal pyramidal neurons. J Neurophysiol 1995; 74:1335-42; PMID:7500154 [DOI] [PubMed] [Google Scholar]
- [33].Markram H, Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci U S A 1994; 91:5207-11; PMID:8197208; http://dx.doi.org/ 10.1073/pnas.91.11.5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci 1997; 20:125-31; PMID:9061867; http://dx.doi.org/ 10.1016/S0166-2236(96)10075-8 [DOI] [PubMed] [Google Scholar]
- [35].Larkman AU. Dendritic morphology of pyramidal neurones of the visual cortex of the rat: III. Spine distributions. J Comp Neurol 1991; 306:332-43; PMID:1711059; http://dx.doi.org/ 10.1002/cne.903060209 [DOI] [PubMed] [Google Scholar]
- [36].Kampa BM, Letzkus JJ, Stuart GJ. Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity. J Physiol 2006; 574:283-90; PMID:16675489; http://dx.doi.org/ 10.1113/jphysiol.2006.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kampa BM, Stuart GJ. Calcium spikes in basal dendrites of layer 5 pyramidal neurons during action potential bursts. J Neurosci 2006; 26:7424-32; PMID:16837590; http://dx.doi.org/ 10.1523/JNEUROSCI.3062-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Birtoli B, Ulrich D. Firing mode-dependent synaptic plasticity in rat neocortical pyramidal neurons. J Neurosci 2004; 24:4935-40; PMID:15163685; http://dx.doi.org/ 10.1523/JNEUROSCI.0795-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 1997; 275:209-13; PMID:8985013; http://dx.doi.org/ 10.1126/science.275.5297.209 [DOI] [PubMed] [Google Scholar]
- [40].Crunelli V, Leresche N. A role for GABAB receptors in excitation and inhibition of thalamocortical cells. Trends Neurosci 1991; 14:16-21; PMID:1709527; http://dx.doi.org/ 10.1016/0166-2236(91)90178-W [DOI] [PubMed] [Google Scholar]
- [41].Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron 1998; 21:827-35; PMID:9808468; http://dx.doi.org/ 10.1016/S0896-6273(00)80598-X [DOI] [PubMed] [Google Scholar]
- [42].Kozlov AS, McKenna F, Lee JH, Cribbs LL, Perez-Reyes E, Feltz A, Lambert RC. Distinct kinetics of cloned T-type Ca2 + channels lead to differential Ca2 + entry and frequency-dependence during mock action potentials. Eur J Neurosci 1999; 11:4149-58; PMID:10594640; http://dx.doi.org/ 10.1046/j.1460-9568.1999.00841.x [DOI] [PubMed] [Google Scholar]
- [43].Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P. Specific contribution of human T-type calcium channel isotypes (α(1G), α(1H) and α(1I)) to neuronal excitability. J Physiol 2002; 540:3-14; PMID:11927664; http://dx.doi.org/ 10.1113/jphysiol.2001.013269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci 2006; 26:11001-13; PMID:17065442; http://dx.doi.org/ 10.1523/JNEUROSCI.1749-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pigeat R, Chausson P, Dreyfus FM, Leresche N, Lambert RC. Sleep slow wave-related homo and heterosynaptic LTD of intrathalamic GABAAergic synapses: involvement of T-type Ca2+ channels and metabotropic glutamate receptors. J Neurosci 2015; 35:64-73; PMID:25568103; http://dx.doi.org/ 10.1523/JNEUROSCI.2748-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sieber AR, Min R, Nevian T. Non-Hebbian long-term potentiation of inhibitory synapses in the thalamus. J Neurosci 2013; 33:15675-85; PMID:24089475; http://dx.doi.org/ 10.1523/JNEUROSCI.0247-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bessaih T, Leresche N, Lambert RC. T current potentiation increases the occurrence and temporal fidelity of synaptically evoked burst firing in sensory thalamic neurons. Proc Natl Acad Sci U S A 2008; 105:11376-81; PMID:18685097; http://dx.doi.org/ 10.1073/pnas.0801484105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Parajuli LK, Fukazawa Y, Watanabe M, Shigemoto R. Subcellular distribution of alpha1G subunit of T-type calcium channel in the mouse dorsal lateral geniculate nucleus. J Comp Neurol 2010; 518:4362-74; PMID:20853512; http://dx.doi.org/ 10.1002/cne.22461 [DOI] [PubMed] [Google Scholar]
- [49].Liu XB, Murray KD, Jones EG. Low-threshold calcium channel subunit Ca(v) 3.3 is specifically localized in GABAergic neurons of rodent thalamus and cerebral cortex. J Comp Neurol 2011; 519:1181-95; PMID:21344408; http://dx.doi.org/ 10.1002/cne.22567 [DOI] [PubMed] [Google Scholar]
- [50].Chausson P, Leresche N, Lambert RC. Dynamics of intrinsic dendritic calcium signaling during tonic firing of thalamic reticular neurons. PLoS One 2013; 8:e72275; PMID:23991078; http://dx.doi.org/ 10.1371/journal.pone.0072275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Crandall SR, Cox CL. Local dendrodendritic inhibition regulates fast synaptic transmission in visual thalamus. J Neurosci 2012; 32:2513-22; PMID:22396424; http://dx.doi.org/ 10.1523/JNEUROSCI.4402-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Destexhe A, Contreras D, Steriade M, Sejnowski TJ, Huguenard JR. In vivo, in vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons. J Neurosci 1996; 16:169-85; PMID:8613783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Joksovic PM, Bayliss DA, Todorovic SM. Different kinetic properties of two T-type Ca2+ currents of rat reticular thalamic neurones and their modulation by enflurane. J Physiol 2005; 566:125-42; PMID:15845580; http://dx.doi.org/ 10.1113/jphysiol.2005.086579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Christie BR, Eliot LS, Ito K, Miyakawa H, Johnston D. Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. J Neurophysiol 1995; 73:2553-7; PMID:7666160 [DOI] [PubMed] [Google Scholar]
- [55].Isomura Y, Fujiwara-Tsukamoto Y, Imanishi M, Nambu A, Takada M. Distance-dependent Ni(2+)-sensitivity of synaptic plasticity in apical dendrites of hippocampal CA1 pyramidal cells. J Neurophysiol 2002; 87:1169-74; PMID:11826086 [DOI] [PubMed] [Google Scholar]
- [56].Wang G, Bochorishvili G, Chen Y, Salvati KA, Zhang P, Dubel SJ, Perez-Reyes E, Snutch TP, Stornetta RL, Deisseroth K, et al.. CaV3.2 calcium channels control NMDA receptor-mediated transmission: a new mechanism for absence epilepsy. Genes Dev 2015; 29:1535-51; PMID:26220996; http://dx.doi.org/ 10.1101/gad.260869.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Huang Z, Lujan R, Kadurin I, Uebele VN, Renger JJ, Dolphin AC, Shah MM. Presynaptic HCN1 channels regulate Cav3.2 activity and neurotransmission at select cortical synapses. Nat Neurosci 2011; 14:478-86; PMID:21358644; http://dx.doi.org/ 10.1038/nn.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Francois A, Schuetter N, Laffray S, Sanguesa J, Pizzoccaro A, Dubel S, Mantilleri A, Nargeot J, Noel J, Wood JN, et al.. The low-threshold calcium channel Cav3.2 determines Low-Threshold mechanoreceptor function. Cell Rep 2015; 10: 370-382, http://dx.doi.org/ 10.1016/j.celrep.2014.12.042 [DOI] [PubMed] [Google Scholar]
- [59].Pinault D, Deschenes M. Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: a three-dimensional, graphic, and morphometric analysis. J Comp Neurol 1998; 391:180-203; PMID:9518268; http://dx.doi.org/ 10.1002/(SICI)1096-9861(19980209)391:2%3c180::AID-CNE3%3e3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- [60].Connelly WM, Crunelli V, Errington AC. The Global Spike: Conserved dendritic properties enable unique Ca2+ spike generation in Low-threshold spiking neurons. J Neurosci 2015; 35:15505-22; PMID:26609149; http://dx.doi.org/ 10.1523/JNEUROSCI.2740-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Anderson D, Rehak R, Hameed S, Mehaffey WH, Zamponi GW, Turner RW. Regulation of the KV4.2 complex by CaV3.1 calcium channels. Channels (Austin) 2010; 4:163-7; PMID:20458163; http://dx.doi.org/ 10.4161/chan.4.3.11955 [DOI] [PubMed] [Google Scholar]
- [62].Anderson D, Engbers JD, Heath NC, Bartoletti TM, Mehaffey WH, Zamponi GW, Turner RW. The Cav3-Kv4 complex acts as a calcium sensor to maintain inhibitory charge transfer during extracellular calcium fluctuations. J Neurosci 2013; 33:7811-24; PMID:23637173; http://dx.doi.org/ 10.1523/JNEUROSCI.5384-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Engbers JD, Anderson D, Asmara H, Rehak R, Mehaffey WH, Hameed S, McKay BE, Kruskic M, Zamponi GW, Turner RW. Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proc Natl Acad Sci U S A 2012; 109:2601-6; PMID:22308379; http://dx.doi.org/ 10.1073/pnas.1115024109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rehak R, Bartoletti TM, Engbers JD, Berecki G, Turner RW, Zamponi GW. Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PLoS One 2013; 8:e61844; PMID:23626738; http://dx.doi.org/ 10.1371/journal.pone.0061844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Weiss N, Legrand C, Pouvreau S, Bichraoui H, Allard B, Zamponi GW, De Waard M, Jacquemond V. In vivo expression of G-protein beta1gamma2 dimer in adult mouse skeletal muscle alters L-type calcium current and excitation-contraction coupling. J Physiol 2010; 588:2945-60; PMID:20547679; http://dx.doi.org/ 10.1113/jphysiol.2010.191593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tang AH, Karson MA, Nagode DA, McIntosh JM, Uebele VN, Renger JJ, Klugmann M, Milner TA, Alger BE. Nerve terminal nicotinic acetylcholine receptors initiate quantal GABA release from perisomatic interneurons by activating axonal T-type (Cav3) Ca(2)(+) channels and Ca(2)(+) release from stores. J Neurosci 2011; 31:13546-61; PMID:21940446; http://dx.doi.org/ 10.1523/JNEUROSCI.2781-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Weiss N, Hameed S, Fernandez-Fernandez JM, Fablet K, Karmazinova M, Poillot C, Proft J, Chen L, Bidaud I, Monteil A, et al.. A Ca(v)3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem 2012; 287:2810-8; PMID:22130660; http://dx.doi.org/ 10.1074/jbc.M111.290882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Weiss N, Zamponi GW. Control of low-threshold exocytosis by T-type calcium channels. Biochim Biophys Acta 2013; 1828:1579-86; PMID:22885170; http://dx.doi.org/ 10.1016/j.bbamem.2012.07.031 [DOI] [PubMed] [Google Scholar]
- [69].Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron 2013; 80:704-17; PMID:24183021; http://dx.doi.org/ 10.1016/j.neuron.2013.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 1973; 232:331-56; PMID:4727084; http://dx.doi.org/ 10.1113/jphysiol.1973.sp010273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol 1983; 334:33-46; PMID:6306230; http://dx.doi.org/ 10.1113/jphysiol.1983.sp014478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Astori S, Luthi A. Synaptic plasticity at intrathalamic connections via CaV3.3 T-type Ca2+ channels and GluN2B-containing NMDA receptors. J Neurosci 2013; 33:624-30; PMID:23303941; http://dx.doi.org/ 10.1523/JNEUROSCI.3185-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 2003; 299:1237-40; PMID:12595694; http://dx.doi.org/ 10.1126/science.1080659 [DOI] [PubMed] [Google Scholar]
- [74].Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci 2006; 26:4166-77; PMID:16624937; http://dx.doi.org/ 10.1523/JNEUROSCI.0176-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Aizenman CD, Huang EJ, Manis PB, Linden DJ. Use-dependent changes in synaptic strength at the Purkinje cell to deep nuclear synapse. Prog Brain Res 2000; 124:257-73; PMID:10943131; http://dx.doi.org/ 10.1016/S0079-6123(00)24022-3 [DOI] [PubMed] [Google Scholar]
- [76].Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci 1996; 8:1488-500; PMID:8758956; http://dx.doi.org/ 10.1111/j.1460-9568.1996.tb01611.x [DOI] [PubMed] [Google Scholar]
- [77].Ly R, Bouvier G, Schonewille M, Arabo A, Rondi-Reig L, Lena C, Casado M, De Zeeuw CI, Feltz A. T-type channel blockade impairs long-term potentiation at the parallel fiber-Purkinje cell synapse and cerebellar learning. Proc Natl Acad Sci U S A 2013; 110:20302-7; PMID:24277825; http://dx.doi.org/ 10.1073/pnas.1311686110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 2004; 44:691-700; PMID:15541316; http://dx.doi.org/ 10.1016/j.neuron.2004.10.031 [DOI] [PubMed] [Google Scholar]
- [79].Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res 2008; 169:211-23; PMID:18394476; http://dx.doi.org/ 10.1016/S0079-6123(07)00012-X [DOI] [PubMed] [Google Scholar]
- [80].Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature 1982; 299:583-91; PMID:6811951; http://dx.doi.org/ 10.1038/299583a0 [DOI] [PubMed] [Google Scholar]
- [81].Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res 1994; 34:709-20; PMID:8160387; http://dx.doi.org/ 10.1016/0042-6989(94)90210-0 [DOI] [PubMed] [Google Scholar]
- [82].Yoshimura Y, Inaba M, Yamada K, Kurotani T, Begum T, Reza F, Maruyama T, Komatsu Y. Involvement of T-type Ca2+ channels in the potentiation of synaptic and visual responses during the critical period in rat visual cortex. Eur J Neurosci 2008; 28:730-43; PMID:18657180; http://dx.doi.org/ 10.1111/j.1460-9568.2008.06384.x [DOI] [PubMed] [Google Scholar]
- [83].Horibe S, Tarusawa E, Komatsu Y, Yoshimura Y. Ni(2+)-sensitive T-type Ca(2+) channel currents are regulated in parallel with synaptic and visual response plasticity in visual cortex. Neurosci Res 2014; 87:33-9; PMID:25017998; http://dx.doi.org/ 10.1016/j.neures.2014.07.001 [DOI] [PubMed] [Google Scholar]
- [84].Uebele VN, Nuss CE, Santarelli VP, Garson SL, Kraus RL, Barrow JC, Stauffer SR, Koblan KS, Renger JJ, Aton S, et al.. T-type calcium channels regulate cortical plasticity in-vivo. [corrected]. Neuroreport 2009; 20:257-62; PMID:19212242; http://dx.doi.org/ 10.1097/WNR.0b013e3283200111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci 2010; 11:114-26; PMID:20046194; http://dx.doi.org/ 10.1038/nrn2762-c2 [DOI] [PubMed] [Google Scholar]
- [86].Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993; 262:679-85; PMID:8235588; http://dx.doi.org/ 10.1126/science.8235588 [DOI] [PubMed] [Google Scholar]
- [87].Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium 2006; 40:175-90; PMID:16777223; http://dx.doi.org/ 10.1016/j.ceca.2006.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Urbain N, Salin PA, Libourel PA, Comte JC, Gentet LJ, Petersen CC. Whisking-Related Changes in Neuronal Firing and Membrane Potential Dynamics in the Somatosensory Thalamus of Awake Mice. Cell Rep 2015; 13:647-56; PMID:26489463; http://dx.doi.org/ 10.1016/j.celrep.2015.09.029 [DOI] [PubMed] [Google Scholar]
- [89].Deleuze C, David F, Behuret S, Sadoc G, Shin HS, Uebele VN, Renger JJ, Lambert RC, Leresche N, Bal T. T-type calcium channels consolidate tonic action potential output of thalamic neurons to neocortex. J Neurosci 2012; 32:12228-36; PMID:22933804; http://dx.doi.org/ 10.1523/JNEUROSCI.1362-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lambert RC, Bessaih T, Crunelli V, Leresche N. The many faces of T-type calcium channels. Pflugers Arch 2014; 466:415-23; PMID:24043572; http://dx.doi.org/ 10.1007/s00424-013-1353-6 [DOI] [PubMed] [Google Scholar]
- [91].Jacus MO, Uebele VN, Renger JJ, Todorovic SM. Presynaptic Cav3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J Neurosci 2012; 32:9374-82; PMID:22764245; http://dx.doi.org/ 10.1523/JNEUROSCI.0068-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chen CC, Shen JW, Chung NC, Min MY, Cheng SJ, Liu IY. Retrieval of context-associated memory is dependent on the Ca(v)3.2 T-type calcium channel. PLoS One 2012; 7:e29384; PMID:22235292; http://dx.doi.org/ 10.1371/journal.pone.0029384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 2006; 51:113-23; PMID:16815336; http://dx.doi.org/ 10.1016/j.neuron.2006.05.021 [DOI] [PubMed] [Google Scholar]
- [94].Wang L, Kitai ST, Xiang Z. Activity-dependent bidirectional modification of inhibitory synaptic transmission in rat subthalamic neurons. J Neurosci 2006; 26:7321-7; PMID:16837578; http://dx.doi.org/ 10.1523/JNEUROSCI.4656-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]