Abstract
Cardiac hypertrophy in humans can progress to cardiac failure if the underlying impetus is poorly controlled. An important direct stimulator of hypertrophy and its progression is the angiotensin II (AngII) peptide. AngII also causes hypertension that indirectly contributes to cardiac hypertrophy. Others and we have shown that estrogens acting through the estrogen receptor (ER)-β can inhibit AngII-induced or other forms of cardiac hypertrophy in mice. However, the proliferative effects of estrogen in breast and uterus that promote the development of malignancy preclude using the steroid to prevent cardiac disease progression. We therefore tested whether an ERβ selective agonist, β-LGND2, can prevent hypertension and cardiac pathology in female mice. AngII infusion over 3 weeks significantly stimulated systolic and diastolic hypertension, cardiac hypertrophy, and cardiac fibrosis, all significantly prevented by β-LGND2 in wild-type but not in ERβ genetically deleted mice. AngII stimulated the Akt kinase to phosphorylate and inhibit the glycogen synthase kinase-3β kinase, leading to GATA4 transcription factor activation and hypertrophic mRNA expression. As a novel mechanism, all these actions were opposed by estradiol and β-LGND2. Our findings provide additional understanding of the antihypertrophic effects of ERβ and serve as an impetus to test specific receptor agonists in humans to prevent the worsening of cardiovascular disease.
Estrogens have been shown to reduce arterial resistance, stimulate arterial dilation and blood flow, lower blood pressure (BP), mitigate the damage of arteries and the heart to various forms of injury, decrease vascular inflammation and atherosclerosis, and prevent cardiac hypertrophy in animals and humans (reviewed in Reference 1).
An especially important role for estrogen is to mitigate the development of cardiac hypertrophy. Mice deficient for the FKBP12.6 gene/protein have abnormal sarcoplasmic reticulum calcium regulation in the heart due to abnormal control of the cardiomyocyte ryanodine receptor. This leads to profound cardiac hypertrophy and failure in the male mice (2). Interestingly, postnatal female mice do not develop cardiac hypertrophy, unless administered tamoxifen, implicating estrogen receptor (ER) protection.
Numerous publications implicate a high activity of the calcium-sensitive, protein phosphatase 2B (calcineurin) in cardiac hypertrophy (3). Calcineurin activity is increased by a variety of hypertrophic stimuli, including angiotensin, pressure overload, overexpression of the cardiac calcium channel Kv4.2N, or isoproterenol exposure (3). Calcineurin activation dephosphorylates serine residues of nuclear factor of activated T-lymphocyte (NFAT) transcription factors, leading to their nuclear translocation and subsequent up-regulation of genes that promote cardiac hypertrophy (4). We previously published that 17β-estradiol (E2) inhibits calcineurin activity through up-regulating the transcription of the modulatory calcineurin-interacting protein (MCIP1) gene. This occurred from membrane ERβ signaling through the Akt kinase, preventing cardiomyocyte hypertrophy in vitro (5) and in vivo (6). Inhibiting dephosphorylation of NFATs by calcineurin led to the retention of the NFAT transcription factors in cytoplasm and could account for the ability of E2 to dampen the downstream effects of calcium dysregulation in female mice (2).
In addition, the progression of cardiac hypertrophy to dilation and heart failure is portended by and partially results from interstitial myocardial fibrosis. Fibrosis interferes with coordinated excitation-contraction coupling of cardiomyocytes in systole and diastole and induces diastolic stiffness, impairing cardiac output (7). We recently showed that E2 signaling through membrane ERβ in cardiac fibroblasts prevents angiotensin II (AngII) or endothelin-1-induced fibrosis in vitro and in vivo (8). This results from E2 preventing TGF-β production and subsequent stimulation of the conversion of fibroblasts to myofibroblasts, severely limiting collagen production that is crucial to cardiac fibrosis (8).
Regarding human hypertrophy, studies indicate that heart failure and death postmyocardial infarction occur less frequently in women who are taking hormone replacement therapy (9) and improved survival during heart failure is associated with estrogen use (10, 11). With aging, the incidence of hypertrophy in postmenopausal women exceeds that of age-matched men (10) but can be reversed by hormone replacement therapy (11). Hormone replacement also lowers vascular resistance and decreases left ventricular (LV) hypertrophy in hypertensive, postmenopausal women (12). However, E2 engaging ERα promotes breast and uterine proliferation that contributes to sex-steroid responsive cancers (13, 14). Thus, selective, agonistic engagement of ERβ might prove useful to prevent multiple events leading to cardiac hypertrophy and progression and avoid undesirable actions that stem from ERα activation.
In these studies, we investigated whether a novel and selective ERβ agonist (15) could prevent AngII-induced hypertension, cardiac hypertrophy, and fibrosis as a preclinical proof of concept for testing such compounds in humans.
Materials and Methods
Animal models
All studies were approved by the Animal Care and Research and Development Committees at the Department of Veterans Affairs Medical Center (Long Beach, California). Ten-week-old, ovariectomized female C57/BJ6 mice were obtained from Harlan/Sprague Dawley and were housed in 12-hour on/off lighting and fed rodent chow devoid of soy or most plant products. Osmotic minipumps (Alzet; DURECT Corp) were filled with either AngII (0.7 mg/kg·d) in saline, saline alone (control), AngII plus 100 μL of an ERβ agonist, β-LGND2 (0.5 mg) or β-LGND2 alone. The minipumps were inserted sc under inhaled chlorofluorane anesthesia to provide a 21-day infusion. In some mice, an E2 pellet (0.1 mg, 21 d release pellets; Innovative Research of America) was inserted under the skin, but these mice did not receive the ERβ agonist. This pellet is well documented to produce physiological levels of E2 in the serum of mice (6). In all studies, five to six mice were used per experimental condition.
At inception and after 21 d, the mice were weighed. Blood pressure was measured four times during the 21-day period, using the CODA noninvasive BP measuring system (Kent Scientific). At the experimental conclusion, the hearts were removed and weighed, the LV dissected free, and the ratio of heart or LV to total body weight was determined. The heart was then processed for a variety of studies. Estrogen loss or administration did not significantly affect body weight over the 3-week period of the study, but heart weight was normalized to initial body weight. For comparison, ovariectomized female wild-type, and ERβ gene-deleted mice were obtained from Ken Korach (National Institute of Environmental Health Sciences) and Harlan and were originally created by Smithies and colleagues (16). These mice were subjected to the same conditions described for wild-type, ovariectomized female mice, involving administration of AngII ± E2 or ERβ agonist, and all mice were identically housed and fed the same chow at the Veterans Affairs animal research facility.
mRNA and protein expression
Total RNA was extracted using the QIAGEN RNeasy minikit following the manufacturers' protocol. All the samples were treated with deoxyribonuclease-free (Ambion). RNA purity and concentrations were measured by UV spectrophotometry (A260 and A280). cDNA was synthesized using approximately 500 ng RNA and Oligodeoxythymidine primers with the Improm-II reverse transcription system (Promega). Quantitative PCR (qPCR) was used to examine the relative expression of β-myosin heavy chain (MHC), inositol polyphosphate-5-phosphatase (Inpp5f), MCIP1, and GATA4, and expression was normalized using expression of the housekeeping mRNA glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers were designed using Primer3 (http://frodo.wi.mit.edu/) and were blasted to check specificity (primers: GATA4 forward, 5′-ggctcccagagattcttcct-3′, GATA4 reverse, 5′-ctctgctacggccagtaagg-3′; β-MHC forward, 5′-atagcaacagcgaggctctttctg-3′, β-MHC reverse, 5′-ggagctcacctaccagacaga-3′; Inpp5f forward, 5′-aacttgggaaaggcctgg-3′, Inpp5f reverse, 5′-catggagctgcggatctt-3′; MCIP11 forward, 5′-gactggagcttcattgactgcgaga-3′, MCIP11 reverse, 5′-aaggaacctacagcctcttggaaag-3′; GAPDH forward, 5′-ccacagtccatgccatca-3′; GAPDH reverse, 5′-ggatgaccttgcccacag-3′).
Primers were designed to have an annealing temperature of 55°C and to amplify regions of approximately 150 bp. PCR amplicons sizes were confirmed by agarose gel electrophoresis prior to quantitative RT-PCR analysis. For quantitative RT-PCR analysis, 500 ng of cDNA was used in a 50-μL reaction consisting of 25 μL of SYBR Green ER qPCR Supermix (Invitrogen), 1 μL of 10 μM forward/reverse primer stocks, and nuclease-free water. Thermocycling was carried out using the iCycler (Bio-Rad Laboratories) with a melting curve temperature of 60°C. Relative mRNA levels were calculated using the cycle threshold method.
For relative protein detection, immunoblots were carried out on protein extracted from the left ventricles of mice from all conditions, after separation by SDS-PAGE and transfer to nitrocellulose, as we described (6, 8). Antibodies to cardiac myosin heavy chain, collagen I, MCIP1, phospho-ERK1(tyr 202) and ERK2(tyr204), and total, phospho-Ser473 and total Akt, and serine 9 phospho-glycogen synthase kinase (GSK)-3β and GATA4 antibodies were from Abcam and Santa Cruz Biotechnology Inc. Brain natriuretic peptide (BNP) antibodies (Penninsula Laboratories) were also used. GATA4, Inpp5F, and control small interfering RNAs (siRNAs) and a GATA4-luciferase reporter plasmid was from QIAGEN.
Fibrosis
Left ventricular tissues were fixed in 4% paraformaldehyde solution. Paraffin-embedded tissue sections (5 μm) were stained with Masson's trichrome for the presence of interstitial collagen fiber accumulation that is indicative of cardiac fibrosis. The ratio of interstitial fibrosis to the total LV area was calculated from 10 randomly selected microscopic fields from each of five sections per heart using National Institutes of Health ImageJ analysis software (n = 5–6 mice per condition).
Further quantification of collagen deposition was made by ventricular content of hydroxyproline, a breakdown product of collagen, determined by a modified method of Bergman and Loxley (17). The ventricular tissues (0.5 g from each mouse) were homogenized and hydrolyzed in 6 M HCl at 110°C for 24 hours in a sealed reaction vial. The sample was dried and the residue resuspended in sterile water; 0.5 mL of chloramine T was added for 5 minutes, and then Ehrlich's reagent (3 mL) was added and the mixture left for 18 hours at room temperature. The intensity of the red coloration that developed was measured by a spectrophotometer at 558 nm.
Ligand binding assay
Ligand binding assay was performed as previously described (15). Briefly, recombinant ER-α or ER-β ligand binding domain (LBD) protein was expressed, isolated, and purified for in vitro binding studies. Each LBD was combined with [3H]estradiol (PerkinElmer) in buffer A (10 mM Tris, pH 7.4; 1.5 mM disodium EDTA; 0.25 M sucrose; 10 mM sodium molybdate; 1 mM phenylmethylsulfonyl fluoride) to determine the equilibrium dissociation constant of [3H]E2 binding. Increasing concentrations of β-LGND2 (range 10−11 to 10−6 M) were incubated with [3H]E2 (1–3 nM) and ER LBD (α or β) using the conditions described above. Results are represented as relative binding affinity (RBA) to ERα or ERβ with an RBA of E2 considered as 1. Nine binding curves were generated.
Transient transfection and reporter gene assay
Transactivation assays were performed as described earlier (15, 18). Human ERs (ERα and ERβ) were cloned from prostate cDNA into a pCR3.1 vector backbone. Human embryonic kidney-293 cells were plated at 125 000 cells/well of a 24-well plate in DMEM +5% charcoal-stripped fetal bovine serum. The cells were transfected using Lipofectamine (Invitrogen) with 0.25 μg estrogen response element-luciferase (a gift from Dr Carolyn L. Smith, Baylor College of Medicine, Houston, Texas), 0.02 μg cytomegalovirus-luciferase (renilla luciferase for correction purposes), and 12.5 ng of plasmid for expressing full-length human ERα or 25 ng of plasmid for human ERβ. The cells were treated 24 hours after transfection with various concentrations of β-LGND2 (1 pM to 10 μM), and luciferase assays were performed 48 hours after transfection.
Isolation and experiments in cardiomyocytes
Neonatal rat cardiomyocytes were isolated from the hearts of 1- to 3-day-old rats or from term pregnant female rats (Charles River) using a cardiomyocyte isolation kit (Worthington), according to the manufacturer's instructions, as previously described (5). The myocytes were incubated in DMEM/F12 supplemented with 10% fetal bovine serum, 1× insulin-transferrin-selenium (Sigma) antibiotic and antimycotic, and 10 μg/mL fibronectin (to aid adherence). In some studies, a GATA4 luciferase DNA construct (QIAGEN) with a Renilla luciferase control was expressed in the myocytes using lipofectamine for a subsequent luciferase determination that represents transactivation activity (luciferase assay kit from Promega). Transfected cells were recovered for 24 hours and synchronized overnight in medium containing very low serum (0.1%) and then exposed to AngII with or without estrogenic compounds for 24 hours. Myocytes were then lysed with a reporter lysis buffer (Promega) and a dual luciferase assay was performed, and the promoter activity was measured using a luminometer. Values are expressed as arbitrary units using a Renilla luciferase reporter for internal normalization. Experiments were done in triplicates per condition, and the study was repeated three times.
Rat uterotrophic assay
All animal studies were performed in accordance with the current guidelines for animal welfare. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center (Memphis, Tennessee). Female Sprague Dawley rats, 200–250 g of body weight, were randomized into groups of five animals. The animals were either sham operated or ovariectomized and were then injected with saline vehicle, 50 μg/kg·d estradiol sc, or 10 mg/kg·d β-LGND2 (sc) for 14 days. At the end of the treatment, the animals were killed, and the uteri were weighed.
Statistics
The results are shown as bar graphs based on the calculation of mean ± SEM data from five to six mice per condition except for mice treated with β-LGND2 or E2 alone (four mice). Significance was determined by ANOVA plus Scheffe's post hoc test and was taken at P < .05.
Results
Angiotensin-induced cardiac hypertrophy is inhibited by E2 and an ERβ agonist
Numerous noncardiac properties of β-LGND2 were described in a previous publication that demonstrated the in vivo ERβ selectivity of this compound (15). β-LGND2 is a nonsteroidal selective ligand that binds to ERβ with a relative binding affinity compared with estradiol of 1 but to ERα with an estradiol-compared relative affinity of 112 (15). In our previous work, β-LGND2 did not affect ER-α selective pathways involving the hypothalamic-pituitary-gonadal axis and testes weights in male mice and uterine weight in ovariectomized female mice, indicating the absence of any cross-reactivity with ER-α at the administered dose, 30 mg/kg·d daily by sc injection (15). Here we present ligand binding assay results, indicating that although β-LGND2 bound to ERβ with an affinity equipotent to that of estradiol (1.2 RBA), it bound to ERα 153-fold less strongly than the same concentration of estradiol used for the ERα binding assay (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Similarly, transactivation studies indicate that β-LGND2 activated the estrogen response element-luciferase reporter via expressed ERβ with an EC50 of 5.1 nM and through expressed ERα with an EC50 of 119 nM, a selectivity of approximately 24-fold.
Because the effect of ER ligands on the uterus is mediated by ERα, a utertrophic assay was used to demonstrate the selectivity of β-LGND2 in vivo. Administration of 10 mg/kg·d β-LGND2 for 14 days did not result in noticeable increase in uterine weight compared with ovariectomized vehicle-treated animals (Supplemental Figure 2). As expected, estradiol increased the uterine weight significantly, corroborating previous publications and validating the assay. These results confirm that administration of approximately 50-fold more β-LGND2 in the uterotrophic studies, compared with the total amount used in our cardiac hypertrophy and fibrosis studies (see below), did not result in ERα activation.
To determine the effects of β-LGND2 in the cardiovascular system, we used a model that is relevant for human cardiac disease by infusing AngII or saline (control) for 3 weeks into ovariectomized, female mice by implanted osmotic minipump. AngII infusion resulted in significant cardiac hypertrophy, particularly of the LV (Figure 1, A and B). Coadministration of E2 (by sc pellet) or the ERβ agonist, β-LGND2 (infusion of 0.5 mg over 21 d), comparably and significantly inhibited approximately 65% of the AngII-induced hypertrophy in wild type (WT) mice. In contrast, neither E2 nor β-LGND2 inhibited AngII-induced hypertrophy in ovariectomized, ERβ knockout (KO) mice (Figure 1, A and B). In ERβ KO ventricles, ERα protein expression was comparable with WT mouse ventricles (Supplemental Figure 3). Neither β-LGND2 nor E2 administration alone to four mice each was different from saline, consistent with our previous studies for E2 (6). Previously we determined that E2 had no protective effects against induced cardiac hypertrophy in ERβ KO mice (6). The current results affirm that it is ERβ that mediates the antihypertrophic effects of E2 and establish here that β-LGND2 is effective in this regard as a specific ERβ agonist (15).
Figure 1.
E2 and an ERβ agonist prevent cardiac hypertrophy induced by AngII. A, Single representative mouse hearts from each group are shown. Whole hearts were removed at 3 weeks and weighed, and the ratio of heart weight (HW) to body weight (BW) was calculated. The left ventricles were then dissected and weighed (LVW). The mean ± SEM data presented on the bar graph are from individual values in six mice per condition. *, P < .05 for ovariectomized (Ovx) WT or ERβ KO mice receiving saline infusion compared with same but receiving AngII, and for saline control compared with AngII + β-LGND2 or E2 in ERβ KO mice; +, P < .05 for Ovx WT mice receiving AngII vs AngII + E2 or β-LGND2. E2 or β-LGND2 administration by themselves in WT mice produced results similar to saline (data not shown). Bar, 2 mm. B, Transverse sections of the ventricles stained with hematoxylin and eosin. All mice were ovariectomized. The bar graph represents the mean ± SEM from individual values in six mice per condition. *, P < .05 for WT (saline control) vs other condition; +, P < .05 for AngII vs the same + β-LGND2 or E2 in WT mice. Bar, 2 mm. RV, right ventricle.
ERβ inhibits markers of cardiac hypertrophy
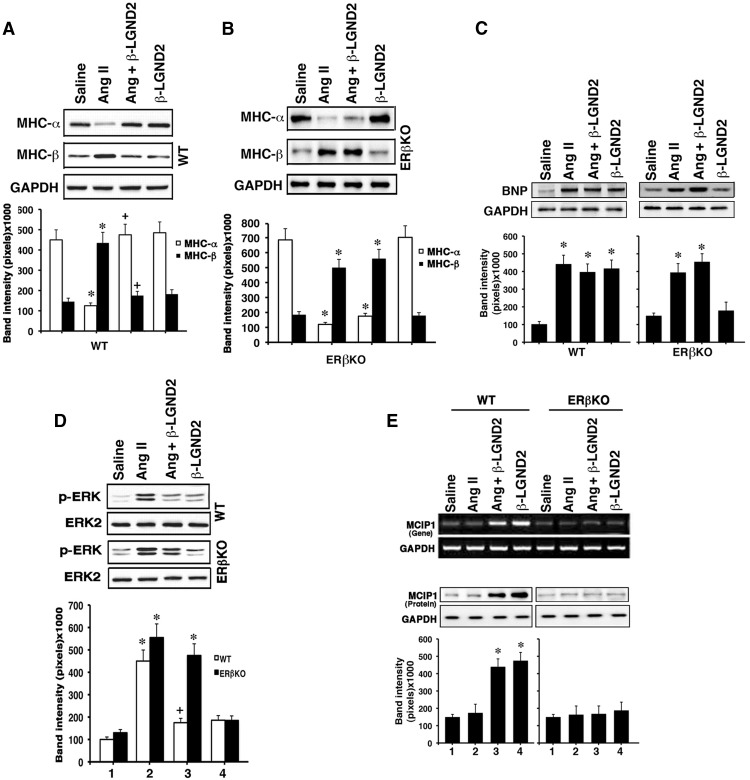
Proteins and signaling mechanisms that are important to the hypertrophic process in the heart were also investigated. In the normal ventricle, MHC α is dominantly expressed compared with a small amount of MHCβ that is produced; however, this is reversed in the hypertrophic heart (19). We found strong expression of MHCα and weaker expression of MHCβ proteins in the ventricles of saline-infused, ovariectomized WT or ERβ KO mice (n = 6) (Figure 2A). The relative expression of the two MHC protein isoforms in both mice was reversed from 3 weeks of AngII treatment, and the AngII effect was prevented significantly by β-LGND2 coinfusion in WT mice. In contrast, coinfusion of β-LGND2 and AngII had no effect on AngII-induced expression of MHC isoforms determined from the ventricular protein of ERβ KO mice (Figure 2B). Administration of β-LGND2 had no effects on expression of either MHCα or -β isoforms in the absence of AngII (absence of effects for E2 alone was previously shown in reference 6).
Figure 2.
E2 and an ERβ agonist modulates important markers of and signals for cardiac hypertrophy. A, MHC-α and -β proteins were determined by immune blot from the ventricles of each of six WT mice per condition. GAPDH is shown as a loading control, and blots are from a single representative mouse from each group. B, Same studies in ERβ KO mice. *, P < .05 comparing saline vs AngII infusion in WT mice and saline vs AngII or AngII + β-LGND2 in ERβ KO mice; +, P < .05 for AngII vs AngII + β-LGND2 in WT mice. C, Endogenous BNP protein in the left ventricles. Individual mice ventricles were processed with protein extraction for BNP expression by immune blot (n = 6 per condition). Single representative results from each condition are shown. *, P < .05 for saline compared with AngII or β-LGND2 infusion or both together in both mouse types. D, Activation of ERK by AngII is inhibited by β-LGND2 in WT mice. Kinase activity in the LV was determined at 3 weeks of treatment from equal amounts of ERK1 and ERK2 proteins immunoprecipitated from the mouse hearts and used for activating phosphorylation-immunoblots of ERK1 and ERK2. Total ERK2 protein is shown as loading control. Bar graph is the mean ± SEM densitometries from the samples (n = 6). *, P < .05 for control vs AngII in both models or AngII + β-LGND2 in ERβ KO mice; +, P < .05 for AngII vs AngII+ β-LGND2 in WT mice. E, MCIP1 mRNA (top panel) and protein (bottom panel) are stimulated by β-LGND2 in WT mice ventricles. mRNA expression was determined by RT-PCR, and protein was demonstrated by immunoblots with representative single samples per each condition shown. Bar graph of the mean ± SEM densitometry protein data are from individual mouse results combined. *, P < .05 for saline control vs β-LGND2 or β-LGND2 + AngII in WT mice.
We also determined the protein expression of BNP in the ventricles of the various mice. BNP is reexpressed as a fetal gene/protein in the ventricles of the hypertrophied heart, mainly to antagonize hypertrophic stimuli, thereby preventing decompensation and progression to heart failure (20, 21). We found that AngII and β-LGND2 each strongly induced BNP protein expression in WT mouse ventricles, compared with saline infusion, but only AngII accomplished this in ERβ KO mouse ventricles (Figure 2C). We previously showed that E2 stimulated BNP expression in both WT and ERα KO mouse heart but not strongly in ERβ KO mice (6).
Peptides such as AngII or endothelin-1 and catecholamines signal through their respective G protein-coupled receptors to cardiac hypertrophy. In part this occurs through activating ERK (22). AngII administration strongly induced ERK activity in the ventricles of both mouse models (Figure 2D), and the ERβ agonist significantly inhibited the ERK activation by AngII but only in WT mice.
Numerous stimuli for the induction of cardiac hypertrophy activate protein phosphatase 2B (calcineurin) that is highly implicated in the pathogenesis of this heart disorder (3, 4). An important protein that binds to and inhibits the activity of calcineurin is MCIP1 (23, 24). Here we assessed the ability of β-LGND2 to regulate MCIP1 gene and protein expression in the LV, correlating to regulating in vivo hypertrophy. As seen in Figure 2E, MCIP mRNA (Figure 2E, top panel) and protein (Figure 2E, bottom panel) expressions were strongly stimulated by the ERβ agonist in WT mice ventricles in the presence of AngII but insignificantly in ERβKO mice hearts. Importantly, AngII had no effect itself or on MCIP1-modifing actions of β-LGND2 in WT mice. We previously reported that E2 by itself up-regulates MCIP1 mRNA expression in cultured cardiomyocytes, and knockdown of MCIP1 with siRNA in these cells reversed the ability of E2 to inhibit both calcineurin activity and cell hypertrophy in vitro (5). E2 also stimulated MCIP expression in the hearts of WT but not ERβ KO mice, unaffected by coadministration of AngII to the former (6). Our data here and in conjunction with previous results (5) indicate an ERβ agonist up-regulates MCIP1 expression in vivo as a likely mechanism to inhibit calcineurin activity and AngII-induced cardiac hypertrophy.
Novel targets of ERβ signaling to suppress cardiomyocyte hypertrophy
Active glycogen synthase kinase, GSK3β, suppresses cardiac hypertrophy. GSK3β activity is negatively modulated from phosphorylation at serine 9 by Akt, the latter activated by prohypertrophic stimuli (25). An important target for GSK3β in opposing cardiac hypertrophy is the prohypertrophic transcription factor, GATA4 (26, 27). GSK3β directly phosphorylates GATA4, inhibiting the ability of this transcription factor to transactivate genes associated with the hypertrophic phenotype (27). We therefore investigated whether AngII and E2/ERβ interact to regulate this important pathway. In cultured cardiomyocytes, AngII stimulated GSK3βSer9 phosphorylation that was significantly blocked by E2 or β-LGND2 (Figure 3A, left panel). In vivo, AngII infusion caused a similar increase in this inactivating phosphorylation of GSK3β in WT and ERβ KO mouse ventricles that was prevented by coadministration of either estrogenic compound, the latter only in WT mice (Figure 3B, right panel). AngII also stimulated the activating phosphorylation of Akt (ser473) both in vitro and in vivo (Figure 3B), and E2 or β-LGND2 prevented Akt activation in cardiomyocytes and the WT mice only. Linking these actions, the AngII-induced inactivating phosphorylation of GSK3β in cardiomyocytes was prevented by LY294002, a selective AKT inhibitor (Figure 3C).
Figure 3.
GSK3β and its targets are regulated by ERβ ligands to prevent cardiac hypertrophy. A, Left panels, AngII causes the inhibitory phosphorylation of GSK3β at serine 9 in cultured cardiomyocytes, inhibited by E2 or β-LGND2. Total GSK3β serves as loading control. Data are from three experiments combined. *, P < .05 for control vs AngII; +, P < .05 for AngII vs AngII + E2 or β-LGND2. Right panels, In vivo stimulation of GSK3β phosphorylation at serine 9 by AngII and inhibition by E2 or β-LGND2. The latter was seen only in WT mouse ventricles, and bar graph is from four to six mice per condition. *, P < .05 for control vs AngII in both mouse models or AngII + β-LGND2 in ERβ KO mice; +, P < .05 for AngII vs AngII + β-LGND2 in WT mice. B, Left panels, Serine 473 Akt phosphorylation in response to AngII ± E2 or β-LGND2 in vitro. Right panels, In vivo regulation of Akt phosphorylation. All data are analyzed as in panel A. C, Ser9 phosphorylation of GSK3β induced by AngII in cardiomyocytes is prevented by an Akt inhibitor (LY294002). Data are from three experiments combined, and GAPDH serves as loading control. *, P < .05 for control vs AngII; +, P < .05 for AngII vs AngII + LY294002. D, GATA4 mRNA (upper panel) and protein (lower panel) expression in cardiomyocytes are stimulated by AngII but inhibited by E2 or β-LGND2. *, P < .05 for control vs AngII; +, P < .05 for AngII vs AngII plus E2 or β-LGND2. E, AngII activates GATA4 transcriptional activity, inhibited by estrogenic compounds. Cardiomyocytes were transfected with a GATA4-luciferase reporter construct and recovered, and luciferase activity was determined after 24 hours of incubation. The study was done three times and data were combined. *, P < .05 for control vs AngII; +, P < .05 for AngII vs AngII + E2 or β-LGND2. F, AngII-stimulated β-MHC mRNA in cardiomyocytes is dependent on GATA4. GAPDH is the loading control. siRNA validation is shown. Data are from three experiments; +, P = .05 for control vs AngII + control siRNA; +, P < .05 for AngII + control siRNA vs AngII + GATA4 siRNA. G, Inpp5f protein (left panel) and mRNA (right panel) expression are suppressed by AngII but prevented by E2 or β-LGND2 in cardiomyocytes. Data are from three experiments. *, P < .05 for control vs AngII; +, P < .05 for AngII vs AngII + E2 or β-LGND2. H, E2 or β-LGND2 derepresses the effects of AngII on Inpp5f protein (left panels) and mRNA (right panels) expression only in WT mouse ventricles. AngII also stimulates β-MHC mRNA expression in vivo, prevented by E2 or β-LGND2. *, P < .05 for control vs AngII or AngII + E2 or β-LGND2 in ERβ KO mice; +, P < .05 for AngII vs AngII plus E2 or β-LGND2 in WT mice. I, Inpp5f knockdown prevents β-LGND2 inhibition of AngII activation of Akt and the resulting inhibitory phosphorylation of GSK3β. Data are from three experiments. *, P < .05 for control vs AngII under either siRNA condition or AngII + β-LGND2 + control siRNA vs AngII + β-LGND2 + Inpp5f siRNA; +, P < .05 for AngII vs AngII + E2 or β-LGND2.
As mentioned, an important downstream target of active GSK3β is the GATA4 transcription factor (27). We found here that AngII stimulated both the mRNA and protein expression of this transcription factor in cardiomyocytes, prevented by coincubation with E2 or β-LGND2 (Figure 3D). Importantly, AngII also stimulated GATA4 transcriptional activity, determined from a GATA4-luciferase reporter and likely resulting from GSK3β inactivation in cardiomyocytes (Figure 3E). AngII-stimulated GATA4 activity was also inhibited by E2 and β-LGND2. The β-MHC gene is up-regulated in cardiomyocytes as an important marker of the transition to hypertrophy (5) and is a known target for GATA4 in these cells (28). To further link this pathway, cardiomyocytes were transfected with control or GATA4 siRNA and β-MHC expression was determined by qPCR. The ability of AngII to stimulate β-MHC mRNA expression was prevented by GATA4 knockdown (Figure 3F). We also determined β-MHC expression in vivo and found that AngII stimulated this mRNA in both WT and ERβ KO mouse ventricles, whereas E2 or β-LGND2 prevented this action of AngII only in WT mice (Figure 3H, right panel).
How do E2 and β-LGND2 potentially inhibit Akt, resulting in increased GSK3β activity and the downstream effects through GATA4 that represent a novel antihypertrophic pathway for E2/ERβ action? It is recognized that Akt activity is negatively regulated by several phosphatases, including the Inpp5F in cardiomyocytes (29, 30). Inpp5F degrades phosphoinositide (3–5) P3, thus inactivating Akt (30). We found here that AngII inhibited the mRNA expression and protein abundance of this phosphatase in vitro (Figure 3G) and in vivo (Figure 3H). Interestingly, E2 and β-LGND2 each prevented these AngII actions but did not influence phosphatase expression themselves (Figure 3, G and H). Thus, E2/ERβ derepresses Inpp5f in the setting of AngII. To further implicate Inpp5f, we knocked down endogenous phosphatase expression with siRNA (Figure 3I) and determined Akt activating phosphorylation. AngII caused the stimulation of Akt and resulting GSK3β Ser9 phosphorylation comparably in control siRNA and Inpp5f siRNA conditions, although some enhancement was seen with the latter. In contrast, the ability of β-LGND2 to inhibit both Akt and GSK3β phosphorylations was significantly reversed only by Inpp5f siRNA (Figure 3I). Thus, by derepressing Inpp5f, β-LGND2 restores GSK3β activity. As a result, GATA4 is not activated by AngII, resulting in decreased expression of the hypertrophic marker βMHC mRNA that we show. These findings overall indicate a new mechanism by which ERβ inhibits cardiac hypertrophy.
ERβ activation inhibits AngII-induced hypertension
Angiotensin has direct effects on the myocardium (31) but also has actions on the vasculature that promote cardiac hypertrophy through increased systemic vascular resistance (32). Angiotensin is a potent vasoconstrictor and is considered to be a key peptide in the development of arterial hypertension in humans, often serving as a primary target for antihypertensive therapies (33). ERβ has been implicated to prevent increased BP because ERβ KO male and female mice develop hypertension with aging (34). Therefore, we determined whether β-LGND2 modulates AngII effects on BP. In the ovariectomized, WT female mice, AngII infusion significantly stimulated increases in both systolic and diastolic arterial blood pressures, and this was substantially and comparably prevented by equimolar β-LGND2 or E2 (Table 1). AngII-induced hypertension in ERβ KO mice was comparable with BP increases in WT mice because endogenous ERβ in WT mice was not engaged by E2 due to ovariectomy. Importantly, attenuation of hypertension by either β-LGND2 or E2 in ERβ KO mice was insignificant. Therefore, opposition to the AngII hypertensive response was significantly different in WT and ERβ KO mice. Our results suggest that ERβ importantly opposes both the direct and indirect effects of AngII that lead to hypertension and cardiac hypertrophy.
Table 1.
Systolic/Diastolic BP of C57BL/6J (WT) and ERβ KO Mice During Drug Treatments
| Time, d | Saline | Ang II | Ang + β-LGND2 | Ang + E2 |
|---|---|---|---|---|
| WT | ||||
| 0 | 122/83 | 121/81 | 120/85 | 123/85 |
| 7 | 119/82 | 143/95* | 120/88+ | 121/82+ |
| 14 | 123/85 | 145/96* | 125/87+ | 124/86+ |
| 21 | 121/83 | 153/94* | 130/86+ | 131/88+ |
| ERβ KO | ||||
| 0 | 123/84 | 121/85 | 123/87 | 126/86 |
| 7 | 125/85 | 149/98* | 143/98* | 149/95* |
| 14 | 123/85 | 155/96* | 147/98* | 143/98* |
| 21 | 121/83 | 156/94* | 146/96* | 145/96* |
, P < .05 vs saline; + P < .05 vs Ang II.
ERβ agonist inhibits cardiac fibrosis
Interstitial fibrosis of the myocardium develops during the progression of cardiac hypertrophy to myocardial dilation and contributes to the resulting heart failure (35). As a result of fibrosis, the heart stiffens and is progressively incapable of maintaining cardiac output. Impaired cardiac output can lead to a paucity of oxygenation of vital organs including the heart itself, contributing to the apoptotic death of cardiac myocytes that has been observed from this oxidative stress (36).
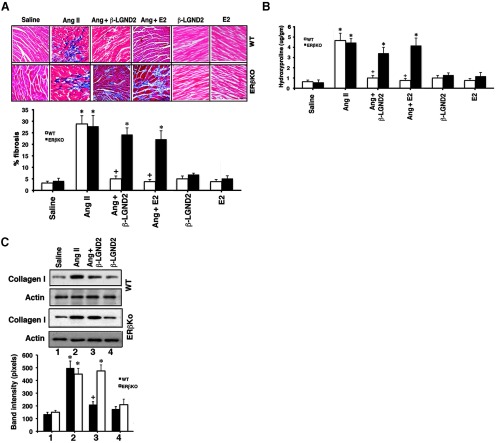
We previously showed that E2, acting through membrane ERβ in the cardiac fibroblast, substantially prevents the events that lead to AngII-induced fibrosis (8). Here we compared the effects of β-LGND2 and E2 to prevent this important development in myocardial disease progression. We found that each estrogenic compound caused a 90%–95% reduction in AngII-induced fibrosis in ovariectomized, WT female mice heart ventricles (Figure 4A). This was also reflected as β-LGND2 or E2 inhibition of hydroxyproline content in the ventricles of the AngII-infused WT mice (Figure 4B). Hydroxyproline is a major breakdown product from metabolism of collagen that is a critical protein and feature of fibrosis. In addition, the estrogenic compounds inhibited collagen I protein content in the ventricles of these mice (Figure 4C), an important form of collagen found in human myocardium undergoing fibrosis (37). In contrast, there were insignificant effects of β-LGND2 or E2 on AngII-induced fibrosis or hydroxyproline or β-LGND2 on collagen I in the ERβ KO mice. Ventricles from WT or ERβ KO mice infused with saline (control), administered E2 (6), or infused β-LGND2 in the absence of AngII (n = 3 mice) showed no effects on the small amount of fibrosis seen. These results indicate that an ERβ agonist is extremely effective in suppressing cardiac fibrosis that results from AngII action in vivo.
Figure 4.
β-LGND2 prevents AngII-induced cardiac fibrosis. A, Representative Masson trichrome staining of collagen deposition in the LV of ovariectomized female mice exposed to the indicated conditions is shown (n = 5 mice per condition). Arrows indicate fibrosis. Bar, 0.1 mm. The percent area of fibrosis is quantified as described in Materials and Methods, and the bar graph data are the mean ± SEM (n = 5 mice per condition). *, P < .05 for saline control vs AngII in either mouse type or versus β-LGND2 or E2 + AngII in ERβKO mice. +, P < .05 for AngII vs AngII + β-LGND2 or E2 in WT mice. B, Hydroxyproline content of the ventricle was measured by spectrophotometry and mean ± SEM was calculated from individual data from each ventricle. *, P < .05 for saline control vs AngII in either mouse type or vs β-LGND2 or E2 + AngII in ERβ KO mice; +, P < .05 for AngII vs AngII + β-LGND2 or E2 in WT mice. C, Collagen I protein expression is inhibited by estrogenic compounds in WT mice. Actin serves as the protein loading control, and bar graphs are mean ± SEM. *, P < .05 for saline vs AngII; +, P < .05 for AngII vs the same + β-LGND2 or E2 in WT mice.
Discussion
Preventing the worsening of cardiac hypertrophy and fibrosis that can ultimately progress to heart failure would have great value to intervene in this common cause of death in humans. Previous studies by multiple groups, including our own, have shown that estrogen can prevent the development of cardiac hypertrophy and fibrosis in various female mouse models and in humans. In ovariectomized mice, E2 administration prevents ventricular remodeling after myocardial infarction (38) or genetic models of cardiac hypertrophy (39). Jazbutyte et al (40) showed that administration of an ERβ-selective agonist, 8b-VE2 (Schering Pharmaceutical), to ovariectomized spontaneously hypertensive rats lowered systolic BP and attenuated hypertrophy of the left cardiac ventricle. In addition, remodeling of the right ventricle is induced by pulmonary hypertension and is prevented by estrogen acting through ERβ (41). Whether E2 attenuates pulmonary hypertension per se is controversial (42). In humans, postmenopausal women taking sex steroids showed a significant decrease in LV mass, compared with such women not taking hormone replacement (43). Hormone replacement also significantly decreases the LV mass index in hypertensive women (12, 44). In these latter studies, the mechanisms and ER isoforms that mediate these desirable effects of estrogen were not determined.
We report that a novel ERβ agonist, β-LGND2, strongly inhibits the cardiac hypertrophic response to AngII infusion in WT but not ERβ KO mice. In these studies, we found that β-LGND2 was comparable with E2 in preventing cardiac hypertrophy. The ERβ agonist also impressively prevented the increased interstitial fibrosis/collagen deposition in the myocardium that is induced by AngII and eventually impairs cardiac function (7, 35). Several laboratories have reported that AngII acting through its type I receptor induces TGF-β1 production that results in phosphorylated mothers against decapentaplegic-2/3 phosphorylation and trafficking to the nucleus, in which these transcription factors induce the genes responsible for cardiac fibrosis (45, 46). This may be in part related to perivascular fibrosis, resulting from altered extracellular matrix composition and from the transition of cardiac fibroblasts to myofibroblasts (45). We previously showed E2/membrane ERβ inhibits these events, and here we report comparable inhibition of fibrosis by β-LGND2.
AngII activates several pathways to produce hypertrophy, but a prominent signal for many hypertrophic stimuli is calcium signaling to the up-regulation of calcineurin (protein phosphatase 2B) activity (3, 4). When calcineurin is induced, it dephosphorylates and promotes the translocation of the transcription factor NFATc3 to the nucleus. In the nucleus, NFATc3 cooperates with GATA4 and myocyte enhancing factor transcription factors to stimulate hypertrophic gene expression (47, 48). An important protein that prevents calcineurin activity is the antihypertrophic protein MCIP1. Here we report that β-LGND2 stimulates MCIP1 mRNA and protein expression in the ventricle. Previously we found that siRNA directed to MCIP1 substantially reversed the inhibitory effects of E2 on calcineurin activity, new protein synthesis, and hypertrophy of the cardiomyocyte. This mechanism accounted for the modulation of NFAT protein translocation to the nucleus and related transcriptional activity, stimulated by AngII and inhibited by E2 (5).
Another well-established signal by which G protein-coupled receptors including the AngII type I receptor signal to cardiomyocyte hypertrophy is through activating ERK (22). Here we find that AngII administration over 3 weeks induces ERK activation in the ventricles of WT and ERβ KO mouse models (Figure 2D). β-LGND2 significantly inhibited ERK activation by AngII in WT mice but failed to do so in ERβ genetically deleted mice.
A key feature of cardiac hypertrophy is the reversal of the normal pattern of MHC isoform expression in ventricular muscle that results from the actions of hypertrophic agents. Here we found that the ERβ agonist maintains the ratio of MHCα/MHCβ expression in the normal heart by reversing the effects of AngII. Furthermore, BNP expression was stimulated by β-LGND2 in the WT mouse hearts. Interestingly, the hypertrophic peptide, AngII, also stimulated the production of BNP expression that maintains fluid and salt homeostasis and thereby limits cardiac decompensation (21). Genetic deletion of the guanylate cyclase A protein, the functional receptor for BNP (and atrial natriuretic peptide), results in pronounced hypertrophy in response to numerous stimuli (49). Previously we showed that E2-induced ANP was important for the inhibition of AngII-induced ERK in cultured myocytes (5). Based on those results, we propose that the ability of β-LGND2 by itself to stimulate BNP in vivo, as we show here, could limit some hypertrophic signaling by AngII.
We also investigated an important mechanism of cardiac hypertrophy involving the action of the antihypertrophic kinase, GSK3β, and show for the first time that estrogen/ERβ inhibits a pathway involving inactivation of this kinase, both in cardiomyocytes and in the in vivo heart. AngII caused an inactivating phosphorylation of GSK3β at serine 9 through Akt activation, resulting in enhanced GATA4 transcriptional activity. GATA4 is a transcription factor that has been highly implicated to contribute to the remodeling of the ventricle, underlying cardiac hypertrophy (26, 27). Here we show that E2 and β-LGND2 prevent AngII-induced Akt activation and the resulting inactivating phosphorylation of GSK3β. It is established that active GSK3β physically associates with, phosphorylates, and thereby down-regulates GATA 4 transcriptional activity in cardiomyocytes (27). Therefore, estrogenic compound(s) prevention of GSK3β inactivation provides a mechanism by which AngII-induced GATA4 transcriptional activity is inhibited. We report that GATA4 transcriptional activity that is stimulated by AngII is inhibited by the estrogenic compounds and reduces the expression of the β-MHC mRNA, a target of GATA4 (28) and a marker of cardiac hypertrophy/ventricular remodeling. We further showed that the mRNA and protein for the Inpp5F phosphatase that inactivates Akt in cardiomyocytes (29) was down-regulated by AngII, but this was reversed by E2 and β-LGND2. Derepression of Inpp5F by β-LGND2 was then shown to be required for the prevention of AngII-stimulated Akt and the resulting GSK3β inactivating phosphorylation. Overall, ERβ inhibits multiple signals that underlie AngII-induced cardiac hypertrophy.
AngII administration in vivo has been reported to cause hypertension (50), potentially inducing cardiac hypertrophy indirectly. ERβ stimulates nitric oxide synthase activity and nitric oxide production, preventing hypertension as deduced from a mouse model of ERβ deletion (34). Nitric oxide is generated in the heart and in endothelial and vascular smooth muscle cells and is a well-recognized antihypertrophic factor (51). We previously showed that E2 stimulates nitric oxide production in cardiomyocytes (5) and in several arteries in vivo, the latter involving ERα and ERβ (52). We report here that AngII infusion causes significant increases in both systolic and diastolic blood pressures that persist for the duration of the experiment in both WT and ERβ KO mice. Importantly, E2 and the ERβ agonist have comparably significant effects to lower BP and restore normotension only in WT but not ERβKO mice. Thus, β-LGND2 has the potential to lower BP seen from AngII excess, a situation found in human hypertension (53). This also is likely to be important for the antihypertrophic effects of the ERβ agonist, as mentioned. Interestingly, E2-induced prevention of the hypertensive response to AngII also required ERβ presence. This is consistent with the in vivo development of hypertension that results from the aging of ERβ genetically deleted male and female mice (34).
In summary, engaging ERβ with an agonist significantly inhibits the ability of AngII to stimulate hypertension, cardiac hypertrophy, and cardiac fibrosis in vivo. In our experimental model, the relatively short duration of AngII infusion does not result in significant cardiac functional compromise (6), thus allowing us to determine whether starting estrogenic compounds early diminishes disease induction. It will be important to test in the future whether more advanced disease could be reversed by ERβ agonists to prevent progressive cardiac pathology. Selective ERβ agonists are particularly attractive because they lack the breast and uterine proliferative effects of E2 or selective ER modulators that act through ERα to promote the malignant transformation of these tissues (13, 14). We also speculate that males might also benefit from ERβ agonists, and we intend to test this hypothesis in additional rodent models. Positive additional data would support the testing of select ERβ agonists in humans.
Acknowledgments
This work was supported by grants from the Research Service of the Department of Veterans Affairs and National Institutes of Health Grant CA-10036 (to E.R.L.).
Disclosure Summary: R.N. and J.T.D. are employees of GTx Inc Pharmaceutical Company and have equity interests in the company. A.P., M.R., K.S.K., and E.R.L. have nothing to declare.
Funding Statement
This work was supported by grants from the Research Service of the Department of Veterans Affairs and National Institutes of Health Grant CA-10036 (to E.R.L.).
Footnotes
- AngII
- angiotensin II
- BNP
- brain natriuretic peptide
- BP
- blood pressure
- E2
- 17β-estradiol
- ER
- estrogen receptor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GSK
- glycogen synthase kinase
- Inpp5f
- inositol polyphosphate-5-phosphatase
- KO
- knockout
- LBD
- ligand binding domain
- LV
- left ventricle
- MCIP1
- modulatory calcineurin-interacting protein
- MHC
- myosin heavy chain
- NFAT
- nuclear factor of activated T-lymphocyte
- qPCR
- quantitative PCR
- RBA
- relative binding affinity
- siRNA
- small interfering RNA
- WT
- wild type.
References
- 1. Kim J, Levin ER. Estrogen signaling in the cardiovascular system. Nucl Recept Signaling. 2006;4:e013–e017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xin HB, Senbonmatsu T, Cheng DS, et al. . Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416:334–338. [DOI] [PubMed] [Google Scholar]
- 3. Molkentin JD, Lu JR, Antos CL, et al. . A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vega RB, Bassel-Duby R, Olson EN. Control of cardiac growth and function by calcineurin signaling. J Biol Chem. 2003;278:36981–36984. [DOI] [PubMed] [Google Scholar]
- 5. Pedram A, Razandi R, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro: antagonism of calcineurin-related hypertrophy through Induction of MCIP1. J Biol Chem. 2005;280:26339–26348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor β to inhibit calcineurin. Endocrinology. 2008;149:3361–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor β prevents cardiac fibrosis. Mol Endocrinol. 2010;24:2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shlipak MG, Angeja BG, Go AS, Frederick PD, Canto JG, Grady D. Hormone therapy and in-hospital survival after myocardial infarction in postmenopausal women. Circulation. 2001;104:2300–2304. [DOI] [PubMed] [Google Scholar]
- 10. Lindenfeld J, Ghali JK, Krause-Steinrauf HJ, et al. . Hormone replacement therapy is associated with improved survival in women with advanced heart failure. J Am Coll Cardiol. 2003;42:1238–1245. [DOI] [PubMed] [Google Scholar]
- 11. Reis SE, Holubkov R, Young JB, Whie BG, Cohn JN, Feldman AM. Estrogen is associated with improved survival in aging women with congestive heart failure: analysis of the Vesnarinone studies. J Am Coll Cardiol. 2000;36:529–533. [DOI] [PubMed] [Google Scholar]
- 12. Light KC, Hinderliter AL, West SG, et al. . Hormone replacement improves hemodynamic profile and left ventricular geometry in hypertensive and normotensive postmenopausal women. J Hypertens. 2001;19:269–278. [DOI] [PubMed] [Google Scholar]
- 13. Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer. 2006;6:360–368. [DOI] [PubMed] [Google Scholar]
- 14. Pearce ST, Jordan VC. The biological role of estrogen receptors α and β in cancer. Crit Rev Oncol Hematol. 2004;50:3–22. [DOI] [PubMed] [Google Scholar]
- 15. Yepuru M, Eswaraka J, Kearby JD, et al. . Estrogen receptor β selective ligands alleviate high-fat diet and ovariectomy-induced obesity in mice. J Biol Chem. 2010;285:31292–31303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krege JH, Hodgin JB, Couse JF, et al. . Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA. 1998;95:15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergman I, Loxley R. New spectrophotometric method for the determination of proline in tissue hydrolyzates. Anal Chem. 1970;42:702–706. [DOI] [PubMed] [Google Scholar]
- 18. Narayanan R, Coss CC, Yepuru M, Kearbey JD, Miller DD, Dalton JT. Steroidal androgens and nonsteroidal, tissue-selective androgen receptor modulator, S-22, regulate androgen receptor function through distinct genomic and nongenomic signaling pathways. Mol Endocrinol. 2008;22:2448–2465. [DOI] [PubMed] [Google Scholar]
- 19. Lompre AM, Schwartz K, d'Albis A, Lacombe G, Thiem NV, Swynghedauw B. Myosin isozymes redistribution in chronic heart overloading. Nature. 1979;282:105–107. [DOI] [PubMed] [Google Scholar]
- 20. Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. [DOI] [PubMed] [Google Scholar]
- 21. Woods RL. Cardioprotective functions of atrial natriuretic peptide and B-type natriuretic peptide: a brief review. Clin Exp Pharmacol Physiol. 2004;31:791–794. [DOI] [PubMed] [Google Scholar]
- 22. Wang L, Proud CG. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor antagonists in adult cardiomyocytes. Circ Res. 2002;91:821–829. [DOI] [PubMed] [Google Scholar]
- 23. Rothermel BA, McKinsey TA, Vega RB, et al. . Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2001;98:3328–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vega RB, Yang J, Rothermel BA, Bassel-Duby R, Williams RS. Multiple domains of MCIP1 contribute to inhibition of calcineurin activity. J Biol Chem. 2002;277:30401–30407. [DOI] [PubMed] [Google Scholar]
- 25. Antos CL, McKinsey TA, Frey N, et al. . Activated glycogen synthase-3β suppresses cardiac hypertrophy in vivo. Proc Nat Acad Sci USA. 2002;99:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res. 2003;92:1079–1088. [DOI] [PubMed] [Google Scholar]
- 27. Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3β regulates GATA4 in cardiac myocytes. J Biol Chem. 2001;276:28586–28597. [DOI] [PubMed] [Google Scholar]
- 28. Charron F, Paradis P, Bronchain O, Nemer G, Nemer M. Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol Cell Biol. 1999;19:4355–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trivedi CM, Luo Y, Yin Z, et al. . HDAC2 regulates the cardiac hypertrophic response by modulating GSK3β activity. Nat Med. 2007;13:234–331. [DOI] [PubMed] [Google Scholar]
- 30. Minagawa T, Ijuin T, Mochizuki Y, Takenawa T. Identification and characterization of a sac domain-containing phosphoinositide 5-phosphatase. J Biol Chem. 2001;276:22011–22015. [DOI] [PubMed] [Google Scholar]
- 31. Higaki J, Aoki M, Morishita R, et al. . In vivo evidence of the importance of cardiac angiotensin-converting enzyme in the pathogenesis of cardiac hypertrophy. Arterioscler Thromb Vasc Biol. 2000;20:428–434. [DOI] [PubMed] [Google Scholar]
- 32. Jacobi J, Schlaich MP, Delles C, Schobel HP, Schmieder RE. Angiotensin II stimulates left ventricular hypertrophy in hypertensive patients independently of blood pressure. Am J Hypertens. 1999;12:418–422. [PubMed] [Google Scholar]
- 33. Paulis L, Steckelings UM, Unger T. Key advances in antihypertensive treatment. Nat Rev Cardiol. 2012;9:276–285. [DOI] [PubMed] [Google Scholar]
- 34. Zhu Y, Bian Z, Lu P, et al. . Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science. 2002;295:505–508. [DOI] [PubMed] [Google Scholar]
- 35. Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. [DOI] [PubMed] [Google Scholar]
- 36. Izumiya Y, Araki S, Usuku H, Rokutanda T, Hanatani S, Ogawa H. Chronic C-type natriuretic peptide infusion attenuates angiotensin II-induced myocardial superoxide production and cardiac remodeling. Int J Vasc Med. 2012;2012:246058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. González A, López B, Díez J. Fibrosis in hypertensive heart disease: role of the renin-angiotensin-aldosterone system. Med Clin North Am. 2004;88:83–97. [DOI] [PubMed] [Google Scholar]
- 38. Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1560–H1569. [DOI] [PubMed] [Google Scholar]
- 39. Satoh M, Matter CM, Ogita H, et al. . Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007;115:3197–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jazbutyte V, Arias-Loza PA, Hu K, et al. . Ligand-dependent activation of ER lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized SHR. Cardiovasc Res. 2008;77:774–781. [DOI] [PubMed] [Google Scholar]
- 41. Nadadur RD, Umar S, Wong G, et al. . Reverse right ventricular structural and extracellular matrix remodeling by estrogen in severe pulmonary hypertension. J Appl Physiol. 2012;113:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paulin R, Michelakis ED. The estrogen puzzle in pulmonary arterial hypertension. Circulation. 2012;126:1016–1019. [DOI] [PubMed] [Google Scholar]
- 43. Lim WK, Wren B, Jepson N, Roy S, Caplan G. Effect of hormone replacement therapy on left ventricular hypertrophy. Am J Cardiol. 1999;83:1132–1134. [DOI] [PubMed] [Google Scholar]
- 44. Miya Y, Sumino H, Ichikawa S, et al. . Effects of hormone replacement therapy on left ventricular hypertrophy and growth-promoting factors in hypertensive postmenopausal women. Hypertens Res. 2002;25:153–159. [DOI] [PubMed] [Google Scholar]
- 45. Weber KT, Swamynathan SK, Guntaka RV, Sun Y. Angiotensin II and extracellular matrix homeostasis. Int J Biochem Cell Biol. 1999;31:395–403. [DOI] [PubMed] [Google Scholar]
- 46. Sorescu D. Smad3 mediates angiotensin II and TGF-β1-induced vascular fibrosis: Smad3 thickens the plot. Circ Res. 2006;98:988–989. [DOI] [PubMed] [Google Scholar]
- 47. Bueno OF, Wilkins BJ, Tymitz KM, et al. . Impaired cardiac hypertrophic response in calcineurin Aβ-deficient mice. Proc Natl Acad Sci USA. 2002;99:4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilkins BJ, De Windt LJ, Bueno OF, et al. . Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol. 2002;22:7603–7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klinger JR, Warburton RR, Pietras L, et al. . Targeted disruption of the gene for natriuretic peptide receptor-A worsens hypoxia-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2002;282:H58–H65. [DOI] [PubMed] [Google Scholar]
- 50. Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. [DOI] [PubMed] [Google Scholar]
- 51. Brede M, Roell W, Ritter O, et al. . Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension. 2003;42:1177–1182. [DOI] [PubMed] [Google Scholar]
- 52. Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors α and β. J Biol Chem. 2005;280:19704–19710. [DOI] [PubMed] [Google Scholar]
- 53. Raizada V, Skipper B, Luo W, Griffith J. Intracardiac and intrarenal rennin-angiotensin systems: mechanism of cardiovascular and renal effects. J Investig Med. 2007;55:341–359. [DOI] [PubMed] [Google Scholar]