Abstract
Current methodology to monitor pulsatile LH release in mice is limited by inadequate assay sensitivity, resulting in the need for collection of large blood volumes. Thus, assessment of pulsatile LH secretion in mice remains highly challenging, and observations are limited to adult mice. To address this, we developed a highly sensitive ELISA for assessment of mouse LH concentrations in small fractions of whole blood. We demonstrate that this assay is capable of reliably detecting LH down to a theoretical limit of 0.117 ng/mL in a 2-μL fraction of whole blood. Using an established frequent blood collection procedure, we validated the accuracy of this method by determining the pulsatile LH secretion in early-adult (10 weeks old) C57BL6/J male mice. Data demonstrate regular pulsatile release of LH, with peaks in LH secretion rarely exceeding 3 ng/mL. Moreover, assessment of LH release in Gpr54 knockout mice demonstrates the lack of pulsatile LH release after the loss of kisspeptin-mediated pubertal maturation. We next determined age-associated changes in pulsatile LH secretion by assessment of LH secretion in prepubertal (28 days old) C57BL6/J male mice and repeated assessment in the same mice in adulthood (120 days old). Data demonstrate that the rise in total LH secretion in mice after pubertal maturation occurs along with an overall rise in the pulsatile LH secretion rate. This was coupled with a significant increase in the number of LH secretory events (number of pulses). In addition, we observed a decrease in the clearance (increased half-life) and a decrease in the regularity (approximate entropy) of LH release. This method will be of wide general utility within the field of reproductive biology.
The transition from childhood into adulthood is accompanied by the activation and maturation of the reproductive axis. This process of puberty is driven by the emergence or reemergence of pulsatile gonadotropin (LH and FSH) secretion in response to increased secretion of GnRH (1–6). Although LH secretion profiles are well characterized across puberty in primates and in larger animal models (1, 2, 7), this is generally not the case for rodents. In particular, the size of the mouse has not enabled the evaluation of plasma gonadotropin concentrations across puberty or, indeed, with any great ease in adults. Seminal studies by Coquelin and coworkers (8, 9) and Kokoris et al (10) were the first to define the pulsatile patterns of LH secretion in the adult male mouse, and subsequent studies have provided similar information for adult female mice (11, 12). However, it has not been technically possible to determine the profile of LH secretion in prepubertal mice.
The accurate assessment of pulsatile LH secretion requires frequent collection of blood samples and measurement of circulating levels of LH over extended periods of time. In mice, repeat measurements of LH secretion remain highly challenging, and current approaches are severely impaired by limitations in assay sensitivity, resulting in the need for collection of large blood volumes. To address these issues, we report here the development of a new highly sensitive ELISA for assessment of LH concentration in mouse blood samples. Incorporation of this assay with a previously validated frequent blood collection procedure (13) enabled repeat blood sampling from mice as young as 4 weeks old. Using this methodology, we determine pulsatile mouse LH (mLH) secretion in pubertal and adult mice for periods of up to 360 minutes, without the need for volume replacement. Using this approach, that is likely to be of wide general utility within the field of reproductive biology, we provide here the profiles of pulsatile LH secretion from the same mice sampled before and after puberty. In addition, we used the ability to undertake high-resolution serial blood sampling to determine the level of pulsatile LH secretion in kisspeptin receptor (Gpr54) knockout mice.
Materials and Methods
Animals
Wild-type C57BL/6 mice (3–16 weeks of age, weighing 9.3–34.2 g at the time of assessment) were obtained from the University of Queensland Biological Resources. Gpr54 knockout mice (14) and aged-matched wild-type littermates (32 weeks of age) were obtained from the Hercus-Taieri Resource Unit, University of Otago (Dunedin, New Zealand). Mice were pair-housed in a 12-hour light/12-hour dark cycle (lights on at 6:00 am and off at 6:00 pm). Room temperature was maintained at 24 ± 2°C. Animals had free to access food (Specialty Feeds) and water for the duration of all experiments. The University of Queensland Animal Ethics Committee approved all experimental procedures. For collection of terminal blood samples, mice were anesthetized by ip administration of sodium pentobarbitone (32.5 mg/mL; Virbac Animal Health).
LH assay
We developed and validated a sensitive sandwich ELISA for the assessment of whole blood LH concentrations. A 96-well high-affinity binding microplate (9018; Corning) was coated with 50 μL of capture antibody (monoclonal antibody, anti-bovine LH beta subunit, 518B7; University of California) at a final dilution of 1:1 000 (in 1× PBS, 1.09 g of Na2HPO4 [anhydrous], 0.32 g of NaH2PO4 [anhydrous], and 9 g of NaCl in 1000 mL of distilled water) and incubated overnight at 4°C. To minimize unspecific binding of the capture antibody, wells were incubated with 200 μL of blocking buffer (5% [w/v] skim milk powder in 1× PBS-T (1× PBS with 0.05% Tween 20) for 2 hours at room temperature (RT). A standard curve was generated using a 2-fold serial dilution of mLH (reference preparation, AFP-5306A; National Institute of Diabetes and Digestive and Kidney Diseases−National Hormone and Pituitary Program [NIDDK-NHPP]) in 0.2% (w/v) BSA−1× PBS-T. The LH standards and blood samples were incubated with 50 μL of detection antibody (polyclonal antibody, rabbit LH antiserum, AFP240580Rb; NIDDK-NHPP) at a final dilution of 1:10 000 for 1.5 hours (at RT). Each well containing bound substrate was incubated with 50 μL of horseradish peroxidase–conjugated antibody (polyclonal goat anti-rabbit, D048701–2; DakoCytomation) at a final dilution of 1:2000. After a 1.5-hour incubation, 100 μL of o-phenylenediamine (002003; Invitrogen), substrate containing 0.1% H2O2 was added to each well and left at RT for 30 minutes. The reaction was stopped by addition of 50 μL of 3 M HCl to each well, and absorbance of each well was read at a wavelength of 490 nm (Sunrise; Tecan Group). The concentration of LH in whole blood samples was determined by interpolating the OD values of unknowns against a nonlinear regression of the LH standard curve.
The capture antibody (monoclonal antibodies raised against bovine LH β subunit [no. 581B7]) was purified as described previously. Cross-reactivity with FSH, TSH, prolactin, and GH from a number of species was negligible, with minimal cross-reactivity attributed to trace levels of LH contamination (15). The immunogen for development of the detecting antibody (rat LH anti-rabbit antiserum, AFP240580Rb; NIDDK-NHPP) was a highly purified rat pituitary LH titer of an antirabbit LH antibody. The cross-reactivity of rat anti-rabbit LH antibody was described previously (NIDDK-NHPP) and is <0.13% for rat TSH and <0.01% for rat FSH, prolactin, and GH.
Validation of the LH assay
The sensitive mLH ELISA was validated following guidelines established by the International Union of Pure and Applied Chemistry, using terminal mouse plasma and whole blood samples. The linear detection range was determined by assessment of 9 serially diluted mLH standard replicates ranging from 0.0039 to 0.5 ng/mL. To interpolate the LH concentration in whole blood samples, a nonlinear regression analysis was performed using serially diluted mLH standard curve replicates (repeated over 32 individual curves), ranging from 0.0039 to 1 ng/mL. For assessment of the accuracy of detection, diluted terminal whole blood samples were supplemented with known amounts of mLH standards (0.031, 0.125, and 0.500 ng/mL, spike recovery test). This test was repeated (n = 3/assay) and validated over 4 assays. Repeatability precision was determined by assessing the intra- and interassay variability of control samples. Quality control samples were prepared using mLH at a concentration of 0.25 ng/mL. These were aliquoted, stored at −80°C, and included as between-assay control samples for all subsequent assays. Intra- and interassay variability was assessed by determination of mLH concentrations in 36 sample replicates containing 0.25 ng/mL mLH, repeated within one assay and across 6 separate assays, respectively. As final validation, plasma concentrations of mLH (as assessed by ELISA) were compared with those obtained from a commercial RIA (NIDDK-NHPP).
Deconvolution and approximate entropy analysis
Mouse LH concentration time series were analyzed using an automated deconvolution method, which was empirically validated using hypothalamo-pituitary sampling and simulated pulsatile time series (16, 17). Sensitivity and specificity were ∼93%. The MATLAB-based algorithm first detrends the data and normalizes concentrations to the unit interval [0, 1]. Second, the program creates multiple successively decremental potential pulse-time sets, each containing one fewer burst by a smoothing process (a nonlinear adaptation of the heat-diffusion equation). Third, a maximum-likelihood expectation estimation method calculates all secretion and elimination parameters simultaneously, conditional on each of the candidate pulse-time sets. Deconvolution parameters comprised basal secretion (β0), secretory-burst mass (η0, η1), random effects on burst mass (σA), procedural/measurement error (σϵ), and a 3-parameter flexible gamma secretory-burst waveform (β1, β2, β3). The fast half-life of LH in mice was represented as 1 minute, constituting 63% of the decay amplitude, and the slow half-life was estimated from individual data series. Based on a false-positive rate of ≤5%, minimal LH concentration thresholds were 16.4% (twice the coefficient of variation [CV] of 120 assay replicates) and 0.126 ng/L (3 times the median SD determined from 10-minute LH profiles sampled in Gpr54 knockout mice). Statistical model selection was performed to distinguish among the independently framed fits of the individual candidate pulse-time sets using the Akaike information criterion. The parameters (and units) were basal and pulsatile secretion rates (concentration units per session), mass secreted per burst (concentration units), and waveform mode (time delay to maximal secretion after objectively estimated burst onset, minutes). The orderliness of regularity of serial LH serum concentrations was calculated by approximate entropy analysis, following established methodology (18).
Experiment 1: Validation of methodology by assessment of pulsatile LH secretion in adult male mice
We completed assessment of pulsatile LH secretion in 10-week-old C57BL6/J mice (n = 8) as an initial validation of the effectiveness of the proposed method for the detection of pulsatile LH secretion in mice. Mice were relocated to the procedure room 10 days before blood collection and handled daily to allow acclimation to sampling conditions. Pulsatile measures of LH secretion were assessed by repeat blood collection following an established method (13). Starting at 7:00 am, we collected 36 sequential 4-μL tail tip blood samples over a 6-hour sampling period. Whole blood samples were immediately suspended in PBS-T (at a final dilution of 1:30), mixed by vortex, immediately placed on dry ice, and stored at −80°C for future batch analysis.
Experiment 2: Assessment of pulsatile LH release in Gpr54 knockout mice
Mutations in the Gpr54 gene in humans result in impaired or absent LH secretion (14, 19, 20). Accordingly, to further validate the LH pulse detection method, we assessed pulsatile LH secretion in 32-week-old male Gpr54 knockout mice. Observations were matched to those for age-matched wild-type littermates. Acclimation, handling, and sampling procedures were performed as detailed in experiment 1.
Experiment 3: Repeat assessment of pulsatile LH secretion from prepubertal to early adult mice
Attainment of reproductive maturation correlates with altered pulsatile LH release. To further validate the method for pulsatile LH secretion, we determined pulsatile LH release from the same mouse before and after sexual maturation. C57BL6/J mice achieve reproductive competency at approximately 6 weeks of age. Accordingly, we determined LH secretion from the same male C57BL6/J mice at 4 and 16 weeks of age (n = 8). All procedures were performed as detailed in experiment 1. For 4-week-old mice, collection volume per sample was restricted to 2 μL (diluted into 58 μL of PBS-T). Accordingly, blood loss at all ages was restricted to less than 7% of total blood volume per animal.
Statistical analysis
Data are presented as means ± SEM. Differences between groups were identified by a Wilcoxon matched-pairs signed rank test. All measures (excluding deconvolution analysis) were performed using GraphPad Prism (version 6.0c; GraphPad Software Inc.). The threshold level for statistical significance was set at P < .05.
Results
Validation of the LH ELISA
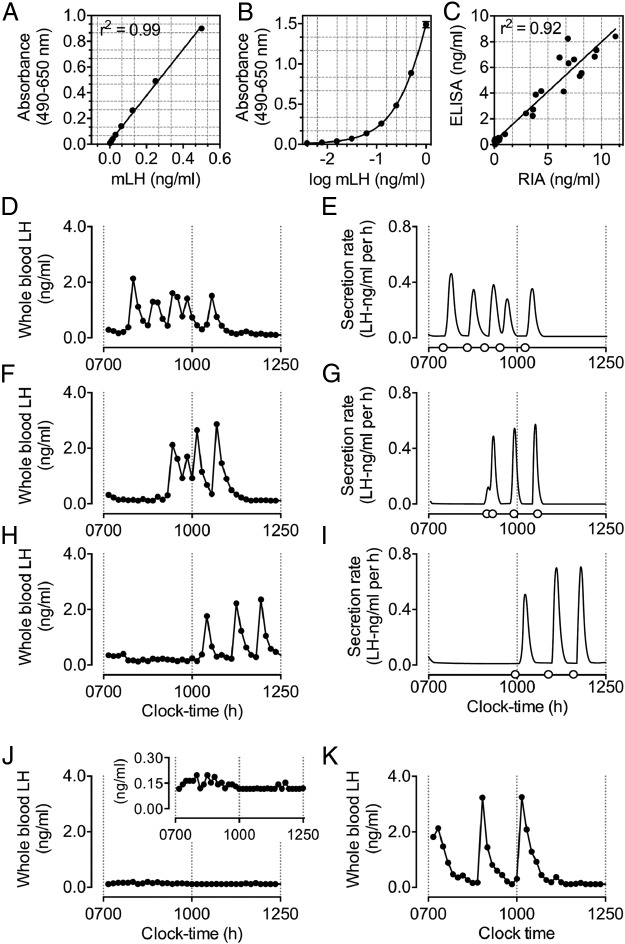
We observed reliable detection of mLH at a final concentration of 0.0039 ng/mL. A linear regression across a standard curve ranging from 0.0039 to 0.5 ng/mL generated an R2 value of 0.987 (Figure 1A). Extension of the standard curve by inclusion of a top standard of 1.0 ng/mL and extrapolation of unknowns after nonlinear regression analysis (Figure 1B) allowed a theoretical detection range of whole blood mLH (in a 1:30 dilution) of 0.117 to 30 ng/mL. Mean recovery of mLH in whole blood and plasma samples was >85%. The intra- and interassay coefficients of variation were 6.05% and 4.29%, respectively. Comparison of plasma mLH with a commercial RIA yielded a near linear relationship (Figure 1C). Assessment outcomes after validation are summarized in Table 1.
Figure 1.
Validation of a sensitive method for the detection of pulsatile LH release in mice. A and B, Linear (A) and nonlinear (B) regression curves generated from mLH standards ranging from 0.0039 to 0.5 and from 0.0039 to 1 ng/mL, respectively (n = 32/curve). C, Direct comparison of the ELISA with a commercial RIA. D–I, Representative examples of measures of pulsatile LH secretion in male C57BL/6 mice at 10 weeks of age. Panels D, F, and H illustrate circulating levels of whole blood LH over a 6-hour sampling period. Starting at 7:00 am, whole blood samples were collected at 10-minute intervals. Panels on the right illustrate the secretion rate of LH after deconvolution analysis, with open circles on the x-axis indicating the onset of a pulse in LH secretion, as determined by deconvolution analysis (E, G, and I). Output parameters are summarized in Table 2. Representative example of measures of pulsatile LH secretion in a 32-week-old Gpr54 knockout (J) and an age-matched wild-type littermate control mouse (K). For all measures of pulsatile LH secretion, whole blood samples were collected at 10-minute intervals, starting at 7:00 am.
Table 1.
Validation of the Sensitive LH ELISA: Spike Recovery, Repeatability Precision, Direct Comparison with RIA, and Deconvolution and ApEN Analysis Parameters of Pulsatile LH Secretion from 10-Week-Old Male C57BL/6J Mice
| Whole Blood | Plasma | |
|---|---|---|
| Spike recovery, ng/mL mLH | ||
| 0.500 ng/mL | 87.87 ± 2.450 | 86.62 ± 1.220 |
| 0.125 ng/mL | 88.30 ± 2.580 | 88.53 ± 2.600 |
| 0.031 ng/mL | 84.93 ± 4.600 | 86.10 ± 7.290 |
| Repeatability precision, ng/mL mLH or % | ||
| Intra-assay CV | 0.250 ± 0.002 | 6.05 |
| Interassay CV | 0.220 ± 0.002 | 4.29 |
| Direct comparison with an established RIA | ||
| Slope (R2) | 1.192 ± 0.071 (0.921) | |
| Deconvolution and ApEn parameters, mean ± SEM (n = 8) | ||
| Total LH secretion, ng/mL/6 h | 13.9 ± 2.26 | |
| Pulsatile LH secretion rate, ng/mL/6 h | 5.86 ± 1.51 | |
| Mass of LH secreted/burst (MPP), ng/mL | 1.76 ± 0.14 | |
| Basal LH secretion rate, ng/mL/6 h | 2.64 ± 0.52 | |
| No. of pulses/6 h | 3.25 ± 0.59 | |
| Half-life, min | 10.4 ± 0.56 | |
| ApEn, 1, 0.35 | 0.46 ± 0.04 |
Abbreviation: CV, coefficient of variation. Recovery of LH in whole blood samples was quantified by spiking samples with 0.5, 0.125, and 0.031 ng/mL mLH. The intra- and inter-assay CVs were determined using the mLH standard. For pulsatile measures of LH secretion, samples were collected at 10-minute intervals between 7:00 am and 1:00 pm.
Experiment 1: Detection of pulsatile LH secretion in adult male mice
Episodic release of LH among 10-week-old male C57BL/6 mice (n = 8) was highly variable and characterized by distinct periods of peak LH secretion. The peak LH pulse amplitude rarely exceeded 3 ng/mL. As is characteristic of pulsatile hormone release, peak periods of LH secretion were defined by an initial rapid rise, followed by a gradual decline in circulating levels of LH (Figure 1, D–I). Measures for total, pulsatile, and basal LH secretion, approximate entropy (ApEn), and mass per pulse (MPP) in these animals are summarized in Table 1.
Experiment 2: Assessment of pulsatile LH release in Gpr54 knockout mice
Assessment of LH secretion in Gpr54 knockout mice confirmed a lack of pulsatile LH release relative to that of age-matched wild-type littermates (Figure 1, J and K). Pulsatile LH release in wild-type littermates was reminiscent of that observed in C57BL/6 mice.
Experiment 3: Repeat assessment of pulsatile LH secretion from prepuberty to early adulthood
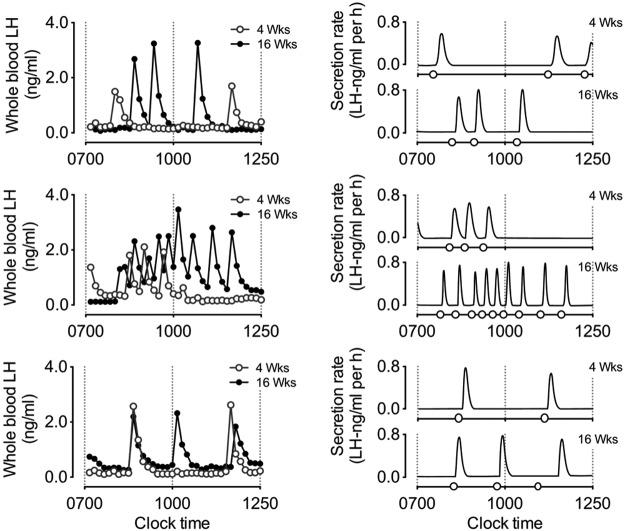
Representative examples of pulsatile LH secretion (and resulting output figures after deconvolution analysis) from the same mice at 4 (○) and 16 (●) weeks of age are illustrated in Figure 2. Output values after deconvolution and approximate entropy analysis are detailed in Table 2. Compared with that in 4-week-old mice, we observed an increase in the rate of total LH secretion by 16 weeks of age. Although not significant, this increase occurred along with an overall rise in pulsatile LH secretion rate and was coupled with a significant increase in the number of secretory events (number of pulses). Accordingly, we observed a decrease in the clearance (increased half-life) and a decrease in the regularity (ApEn) of LH release. Observations suggest the establishment of more frequent, irregular periods of LH release in male mice throughout the period of pubertal maturation. Collectively, this release contributed to an overall increase in total LH secretion.
Figure 2.
Three representative examples of pulsatile LH secretion in prepubertal (○, 4 week old) male C57BL/6 mice and in the same mice in adulthood (●, 16 weeks old). Panels on the left illustrate circulating levels of whole blood LH over a 6-hour sampling period. Starting at 7:00 am, whole blood samples were collected at 10-minute intervals. Panels on the right illustrate the secretion rate of LH after deconvolution analysis, with open circles on the x-axis indicating the onset of a pulse in LH secretion, as determined by deconvolution analysis. Output parameters and comparisons between LH secretion at 4 and 16 weeks of age are summarized in Table 2.
Table 2.
Deconvolution and ApEn Analysis Parameters of Pulsatile LH Secretion from the Same Male C57BL/6 Mice at 4 and 16 Weeks of Age
| 4 Weeks Old (n = 8) | 16 Weeks Old (n = 8) | P | |
|---|---|---|---|
| Total LH secretion, ng/mL/6 h | 12.51 ± 2.207 | 22.35 ± 3.330 | .023a |
| Pulsatile LH secretion rate, ng/mL/6 h | 4.709 ± 0.657 | 8.374 ± 1.694 | .078 |
| Mass of LH secreted/burst (MPP), ng/mL | 1.841 ± 0.289 | 2.010 ± 0.233 | .461 |
| Basal LH secretion rate, ng/mL/6 h | 3.130 ± 0.660 | 4.145 ± 0.650 | .313 |
| No. of pulses/6 h | 2.250 ± 0.313 | 4.250 ± 0.861 | .047a |
| Half-life, min | 9.245 ± 0.749 | 11.88 ± 0.668 | .016a |
| ApEN, 1,0.35 | 0.347 ± 0.032 | 0.481 ± 0.047 | .039a |
Samples were collected at 10-minute intervals between 7:00 am and 1:00 pm. Data are presented as means ± SEM.
P < .05 was considered significant.
Discussion
Pubertal changes in GnRH activity result in sustained changes in pulsatile LH secretion and the consequential maturation of the gonads. Thus, a detailed assessment of LH secretory dynamics in male mice between prepuberty and early adulthood should reflect changes in the pattern of LH secretion that account for the progressive rise in mean LH secretion with age. Here, we demonstrate a method for the assessment of pulsatile LH secretion in mice. To substantiate this method, we demonstrate pulsatile LH secretion in adult male C57BL/6 mice, confirm the absence of pulsatile LH release in Gpr54 knockout mice, and characterize altered LH secretory pulse dynamics that account for the progressive rise in LH secretion throughout pubertal maturation in male mice.
Coquelin and Desjardins (9) first documented measures of pulsatile LH secretion in mice and demonstrated a secretory pattern of LH secretion in mice similar to that seen in other mammals. These laudable experiments established key parameters of LH secretion in intact male mice, and the consequential reporting of peak levels of LH secretion in gonadectomized male or female mice ranging between 3 and 9 ng/mL (10–12, 21, 22). Using a sensitive LH ELISA, we report peak measures of LH secretion within a similar range. Moreover, documented assessments of LH secretion in adult naive and sexually experienced male mice demonstrate striking similarities with measures collected in this study (21).
Previous observations of pulsatile LH secretion in mice required surgical cannulation of mice and collection of large quantities (20–120 μl) of blood for detection of LH by RIA (8–12, 21, 22). Cannulation of pubertal mice is technically impractical, and thus reliable measures demonstrating the LH secretory pattern between puberty and early adulthood in mice do not exist. Moreover, given the difficulties in maintaining the integrity of cannulae, reports of repeat assessment of LH secretion in mice over extended periods of time (ie, pubertal vs postpubertal ages) do not exist. A number of observations assessing LH secretion in one-off measures, however, provide some insights relating to pubertal changes in LH secretion. One of the earliest and most thorough studies to report pubertal-associated changes in LH secretion in male mice demonstrate a dramatic rise in the release of LH at 3 weeks postpartum (23). This result contrasted with the steady rise in LH secretion observed in the rat during a similar developmental stage (24–26). Given large variations in peak and basal levels of LH secretion and the unpredicted nature of patterned LH release, these discrepancies can easily be reasoned through the unreliable nature of one-off measures of LH. To address this, we compared measures of pulsatile LH secretion in prepubertal (4 weeks old) mice with parameters of pulsatile LH secretion in the same mice in adulthood (16 weeks old). As seen in rats (25–27), data demonstrate a progressive rise in LH secretion leading into adulthood. Given the observed parity between LH secretion and GnRH activity during pubertal maturation in the rat (27), we anticipate that this progressive rise in LH secretion in the mouse occurs in response to a similar elevation in synchronous GnRH activity. The observed net rise in LH secretion was attributed to subtle, yet significant, changes in pulse dynamics. These were characterized by a rise in pulse frequency and the half-life of circulating LH and the accompanying decrease in regularity (entropy) of LH secretory events. Although not directly assessed, these observations are reminiscent of matured GnRH pulse dynamics suggestive of synchronous and enhanced GnRH-induced LH secretory events. Presumably, altered LH pulsatility will reflect pubertal changes in testosterone production. However, this finding was not addressed, and thus confirmation of pubertal changes in pulsatile LH release relative to circulating measures of testosterone requires further assessment. Of interest, the maturation of LH secretory dynamics reflects the progressive rise in the GnRH neuronal response to kisspeptin (28).
Mutations in the Gpr54 genes in humans result in an absence of puberty and impaired or absent pulsatile LH secretion (14, 19, 20). The exact impact of these changes on LH secretion in mice has not been well defined. Indeed, these mutant mouse lines usually display relatively normal “single-point” levels of LH secretion in association with markedly reduced FSH concentrations (29). We show here that Gpr54 knockout mice exhibit fluctuations in basal levels of LH secretion but with a complete absence of LH pulses. Overall, observations further demonstrate the capacity for pulsatile measures of LH secretion in mice to negate the potential misconceptions associated with less frequent sampling practices.
In summary, we conclude that the current methodology provides a renewed opportunity for the repeat assessment of pulsatile LH release in mice, while offering superior detection sensitivity to allow more reliable assessment of LH pulse dynamics. Extrapolation of pulsatile measures of LH secretion in the mouse will aid in the assessment of key parameters that contribute to optimal LH pulse generation essential for pubertal maturation and optimal reproductive capacity.
Acknowledgments
This work was supported by the School of Biomedical Sciences, the University of Queensland, the University of Otago, and the Australian National Health and Medical Research Council. Y.W. is a recipient of a postgraduate scholarship from the University of Queensland.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the School of Biomedical Sciences, the University of Queensland, the University of Otago, and the Australian National Health and Medical Research Council. Y.W. is a recipient of a postgraduate scholarship from the University of Queensland.
Footnotes
- ApEn
- approximate entropy
- MPP
- mass per pulse
- NIDDK-NHPP
- National Institute of Diabetes and Digestive and Kidney Diseases−National Hormone and Pituitary Program
- mLH
- mouse LH
- RT
- room temperature.
References
- 1. Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. [DOI] [PubMed] [Google Scholar]
- 2. Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neil JD, ed. Knobil and Neill's Physiology of Reproduction. San Diego: Academic Press; 2006:2177–2230. [Google Scholar]
- 3. Ojeda SR, Skinner MK. Puberty in the rat. In: Neil JD. ed. Knobil and Neill's Physiology of Reproduction. San Diego: Academic Press; 2006:2061–2126. [Google Scholar]
- 4. Bronson FH, Rissman EF. The biology of puberty. Biol Rev Cambridge Philos Soc. 1986;61:157–195. [DOI] [PubMed] [Google Scholar]
- 5. Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. [DOI] [PubMed] [Google Scholar]
- 6. Bouligand J, Ghervan C, Tello JA, et al. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med. 2009;360:2742–2748. [DOI] [PubMed] [Google Scholar]
- 7. Foster DL, Jackson LM, Padmanabhan V. Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol Cell Endocrinol. 2006;254–255:109–119. [DOI] [PubMed] [Google Scholar]
- 8. Coquelin A, Clancy AN, Macrides F, Noble EP, Gorski RA. Pheromonally induced release of luteinizing hormone in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2230–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol. 1982;243:E257–E263. [DOI] [PubMed] [Google Scholar]
- 10. Kokoris GJ, Lam NY, Ferin M, Silverman AJ, Gibson MJ. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology. 1988;48:45–52. [DOI] [PubMed] [Google Scholar]
- 11. Suter KJ, O'Farrell L. Impaired episodic LH secretion in female mice with GFP in GnRH neurons. Am J Physiol Endocrinol Metab. 2008;295:E130–E136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gibson MJ, Miller GM, Silverman AJ. Pulsatile luteinizing hormone secretion in normal female mice and in hypogonadal female mice with preoptic area implants. Endocrinology. 1991;128:965–971. [DOI] [PubMed] [Google Scholar]
- 13. Steyn FJ, Huang L, Ngo ST, et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152:3165–3171. [DOI] [PubMed] [Google Scholar]
- 14. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 15. Matteri RL, Roser JF, Baldwin DM, Lipovetsky V, Papkoff H. Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domest Anim Endocrinol. 1987;4:157–165. [DOI] [PubMed] [Google Scholar]
- 16. Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency, and waveform: a statistical formulation and analysis. Am J Physiol Regul Integr Comp Physiol. 2003;285:R664–R673. [DOI] [PubMed] [Google Scholar]
- 17. Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab. 2009;297:E538–E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus SM. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1975–R1985. [DOI] [PubMed] [Google Scholar]
- 19. Brioude F, Bouligand J, Francou B, et al. A Two families with normosmic congenital hypogonadotropic hypogonadism and biallelic mutations in KISS1R (KISS1 receptor): clinical evaluation and molecular characterization of a novel mutation. PLoS One. 2013;8:e53896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92:1137–1144. [DOI] [PubMed] [Google Scholar]
- 21. Coquelin A. Absence of female-induced luteinizing hormone release in orchidectomized, sexually active mice. Biol Reprod. 1991;45:424–431. [DOI] [PubMed] [Google Scholar]
- 22. Minabe S, Uenoyama Y, Tsukamura H, Maeda K. Analysis of pulsatile and surge-like luteinizing hormone secretion with frequent blood sampling in female mice. J Reprod Dev. 2011;57:660–664. [DOI] [PubMed] [Google Scholar]
- 23. Selmanoff MK, Goldman BD, Ginsburg BE. Developmental changes in serum luteinizing hormone, follicle stimulating hormone and androgen levels in males of two inbred mouse strains. Endocrinology. 1977;100:122–127. [DOI] [PubMed] [Google Scholar]
- 24. Goldman BD, Gorski RA. Effects of gonadal steroids on the secretion of LH and FSH in neonatal rats. Endocrinology. 1971;89:112–115. [DOI] [PubMed] [Google Scholar]
- 25. Negro-Vilar A, Krulich L, McCann SM. Changes in serum prolactin and gonadotropins during sexual development of the male rat. Endocrinology. 1973;93:660–664. [DOI] [PubMed] [Google Scholar]
- 26. Swerdloff RS, Walsh PC, Jacobs HS, Odell WD. Serum LH and FSH during sexual maturation in the male rat: effect of castration and cryptorchidism. Endocrinology. 1971;88:120–128. [DOI] [PubMed] [Google Scholar]
- 27. Harris GC, Levine JE. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology. 2003;144:163–171. [DOI] [PubMed] [Google Scholar]
- 28. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colledge WH. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides. 2009;30:34–41. [DOI] [PubMed] [Google Scholar]