Abstract
Endometriosis is a chronic inflammatory disease of reproductive age women leading to chronic pelvic pain and infertility. Current antiestrogen therapies are temporizing measures, and endometriosis often recurs. Potential nonestrogenic or nonsteroidal targets are needed for treating endometriosis. Peroxisome proliferator-activated receptor (PPAR)γ, a nuclear receptor, is activated by thiazolidinediones (TZDs). In experimental endometriosis, TZDs inhibit growth of endometriosis. Clinical data suggest potential use of TZDs for treating pain and fertility concurrently in endometriosis patients. Study objectives were to 1) determine the effects of PPARγ action on growth and survival of human endometriotic epithelial and stromal cells and 2) identify the underlying molecular links between PPARγ activation and cell cycle regulation, apoptosis, estrogen biosynthesis, and prostaglandin E2 biosynthesis and signaling in human endometriotic epithelial and stromal cells. Results indicate that activation of PPARγ by TZD ciglitazone 1) inhibits growth of endometriotic epithelial cells 12Z up to 35% and growth of endometriotic stromal cells 22B up to 70% through altered cell cycle regulation and intrinsic apoptosis, 2) decreases expression of PGE2 receptors (EP)2 and EP4 mRNAs in 12Z and 22B cells, and 3) inhibits expression and function of P450 aromatase mRNA and protein and estrone production in 12Z and 22B cells through EP2 and EP4 in a stromal-epithelial cell-specific manner. Collectively, these results indicate that PGE2 receptors EP2 and EP4 mediate actions of PPARγ by incorporating multiple cell signaling pathways. Activation of PPARγ combined with inhibition of EP2 and EP4 may emerge as novel nonsteroidal therapeutic targets for endometriosis-associated pain and infertility, if clinically proven safe and efficacious.
Peroxisome proliferator-activated receptor (PPAR)γ is a type II nuclear receptor, encoded by the PPARG gene in human (1–4). The endogenous ligands for PPARγ are free fatty acids and eicosanoids. Upon activation, PPARγ forms heterodimers with retinoid X receptor, another nuclear receptor (1–4). The activated PPAR/retinoid X receptor dimer binds to peroxisome proliferator hormone-response elements in complex with a number of coactivators, such as nuclear receptor coactivator 1 and cAMP response element-binding protein binding protein, and thus causes activation or repression of specific genes (1–4). PPARγ is expressed in many tissues, including colon, skeletal muscle, liver, heart, activated macrophages, and adipocytes (2–4). In addition, PPARγ is expressed in endometrial epithelial (5) and stromal (6) cells.
The thiazolidinediones (TZDs) are known activators of PPARγ. The TZDs derivatives include rosiglitazone, pioglitazone, troglitazone, netoglitazone, rivoglitazone, and ciglitazone (CTZ) (1–4, 7). TZDs have several biological actions. TZDs decrease insulin resistance and thus emerged as a potential treatment option for insulin-resistant type 2 diabetes mellitus (1–4). TZDs regulate differentiation of adipocytes and fat redistributions by decreasing leptin and increasing adiponectin secretions (1–4, 7). In addition, TZD-PPARγ-dependent transrepression mediates antiinflammatory effects (1–4). Activation of PPARγ decreases the coactivators available for binding to proinflammatory transcription factors, such as nuclear factor-κB, and thus inhibits transcription of number of proinflammatory genes, including various ILs and TNFs (1, 7, 8). TZDs have been shown to inhibit migration of monocytes and peritoneal inflammatory cells in a mouse model (9) and to modulate angiogenesis (10).
Endometriosis is a chronic inflammatory disease of reproductive age women characterized by the presence of functional endometrial tissues outside the uterine cavity (11, 12). The prevalence of the disease is approximately 10% in childbearing age women, and it increases to 20%–30% in women with subfertility and to 40%–60% in women with dysmenorrhoea (11–13). The two major symptoms are infertility and pelvic pain. Current medical treatments are aimed at inhibiting the action of estrogen on ectopic implants through suppression of ovarian estrogen production via oral contraceptives, aromatase inhibitors, androgenic agents, and gonadotropin-releasing hormone analogues (11, 12, 14, 15). Antiestrogen hormonal therapies can be prescribed for a short time (∼6–9 mo) because of undesirable side effects, such as bone density loss, pseudomenopause, hot flashes, and mood swings, increased risk for uterine and ovarian cancers, and compromised pregnancy, which profoundly affect the quality of life and emotional and physical wellbeing of endometriosis patients (11, 12, 14, 15). Unexpectedly, the disease reestablishes at the rate of approximately 50%–60% within a year after cessation of antiestrogen therapy (14, 15). The remarkable redundancy of signaling pathways that control growth and survival of endometriosis indicates a crucial need to identify potential cell signaling pathways for nonestrogen or nonsteroidal therapeutic targets for treatment of endometriosis.
Rosiglitazone treatment inhibits endometriotic implant growth, cell proliferation, and vascularization and augments apoptosis in the mouse model of endometriosis (16). Rosiglitazone, CTZ, or pioglitazone reduces growth of established implants in rat model of endometriosis (17–19), decreases postsurgical adhesions with endometriosis lesions in chimeric mouse model (20), and provokes implant regression in baboons with endometriosis (21, 22). TZDs inhibit endothelial cell proliferation and reduce the pathologic vascularization of the lesions, which is associated with repression of the vascular endothelial growth factor gene expression via PPARγ-responsive elements in the vascular endothelial growth factor gene promoter (16, 23–25). CTZ and pioglitazone reduce IL-6 secretion from endometrial stromal cells (8). TZDs inhibit the expression of various inflammatory mediators in chronic inflammatory diseases and may be used to treat pain associated with endometriosis (8, 24, 26). Data from clinical studies involving endometriosis patients treated with rosiglitazone for 6 months suggest the possibility of using TZDs for endometriosis pain relief in conjunction with attempts to conceive (26). It indicates a possible use of some TZDs as a new treatment in endometriosis.
The role of estrogen in growth of endometriosis is well known. P450 aromatase catalyzes the last steps of estrogen biosynthesis from androgens in 2 ways: 1) from androstenedione into estrone and 2) from testosterone into estradiol (11, 27–29). P450 aromatase protein is abundantly expressed in endometriosis (11, 27–29). In endometriotic stromal cells, prostaglandin E2 increases P450 aromatase through PGE2 receptors (EP)2 (11, 27–29). TZDs decrease expression of P450 aromatase expression in human breast adipose tissue and breast cancer (30–32) as well as in ovarian granulosa cells (33, 34). Role for PGE2 is well accepted in the pathogenesis of endometriosis (35–38). Recent studies show that PGE2 via EP2 and EP4 activates the cAMP-protein kinase A-cAMP response element-binding protein pathway, leading to enhanced P450 aromatase transcription and increased aromatase activity (30, 31). Activation of PPARγ by piglitazone decreases P450 aromatase levels by inducing BRCA1 and suppressing PGE2→cAMP→protein kinase A axis in breast cancer (30, 31). These studies, together, indicate interactions between TZDs and PGE2-aromatase P450.
The objectives of the present study were 1) to determine the effects of PPARγ action on growth and survival of human endometriotic epithelial and stromal cells, 2) to unravel the underlined molecular link between PPARγ activation and cell cycle and apoptosis machinery in human endometriotic epithelial and stromal cells, and 3) to determine molecular interactions among PPARγ and P450 aromatase and PGE2 in endometriotic epithelial and stromal cells. Our results indicate that activation of PPARγ by agonist CTZ inhibits proliferation and induces apoptosis of endometriotic epithelial and stromal cells, decreases estrogen biosynthesis, and inhibits EP2- and EP4-mediated PGE2 signaling. These results suggest that these growth inhibitory, apoptotic, and antiestrogenic actions are potentially mediated through PGE2-mediated EP2 and EP4 signaling in endometriosis.
Materials and Methods
Materials
The reagents used in this study were purchased from the following suppliers: prestained protein markers and Bio-Rad assay reagents and standards from Bio-Rad Laboratories; Protran BA83 Nitrocellulose membrane from Whatman, Inc; Pierce ECL from Pierce Biotechnology; protease inhibitor cocktail tablets complete EDTA free and PhosStop from Roche Applied Biosciences; antibiotic-antimycotic and trypsin-EDTA from Invitrogen Life Technologies, Inc; Blue X-Ray film from Phenix Research Products; fetal bovine serum (FBS) from HyClone; and tissue culture dishes and plates from Corning, Inc. EP2 inhibitor (AH6809), EP4 inhibitor (AH23848), PPARγ agonist (CTZ), and androstenidione were purchased from Sigma-Aldrich. PGE2 ELISA kit (Cayman Chemicals) and Estrone ELISA kit (DRG International, Inc) were purchased. Antibodies for cyclin-dependent kinase (CDK)1, CDK2, and CDK4 (Cell Signaling Technology), P450 aromatase (Santa Cruz Biotechnology, Inc), β-actin antibody (Sigma-Aldrich), and goat antirabbit or antimouse IgG conjugated with horseradish peroxidase (Kirkegaard & Perry Laboratories) were purchased. SMART-pool small interfering RNA (siRNA) duplex, siGLO RISC-free siRNA, and DharmaFect-1 were purchased from Dharmacon, Inc. QuantiTect reverse transcription kit and QuantiTect SYBR RT-PCR kit were purchased from QIAGEN, Inc. The chemicals used were molecular biological grade from Fisher Scientific or Sigma-Aldrich.
Human endometriotic cells and culture
Immortalized endometriotic epithelial cells 12Z and stromal cells 22B used in this study were derived from active red peritoneal endometriosis lesions during the proliferative phase of the menstrual cycle from women (39). These 12Z and 22B cells share several phenotypic and molecular characteristics of primary cultured endometriotic cells (39). Although these 12Z and 22B cells are immortalized with simian vacuolating virus 40 antigen, accumulating information from our and other laboratories indicates that 12Z and 22B cells mimic the active/progressive phase of endometriosis (35–37, 39–41). Importantly, xenograft of a mixed population of these 12Z and 22B cells into the peritoneal cavity of nude mice is able to proliferate, attach, invade, reorganize, and establish peritoneal endometriosis-like lesions, and histomorphology are similar to that of spontaneous peritoneal endometriosis in women (42). These well-characterized 12Z and 22B cells were cultured in DMEM/F12 without special steroid treatment containing 10% FBS and penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin-B (2.5 μg/mL) in a humidified 5% CO2 and 95% air at 37°C as we described previously (36, 37, 43, 44).
CTZ concentration-response and growth inhibitory experiment
The 12Z and 22B cells (100 000 cells/well) were cultured in 6-well plates. At 70%–80% confluency, the cells were serum starved in DMEM/F12 with 2% dextran charcoal-treated FBS (DC-FBS) for 12 hours. Then, the cells were treated with CTZ at 0μM, 10μM, 20μM, 25μM, 30μM, 35μM, 40μM, 50μM, 100μM, and 200μM in plain DMEM/F12 for 24 hours. Afterwards, the adherent cells 12Z and 22B cells were washed with PBS and harvested using 0.1% Trypsin-EDTA (Invitrogen, Inc). The cell pellets were resuspended in isotonic buffer (Val Tech Diagnostics, Inc), and cell numbers were counted using a Coulter counter. Three experiments were conducted, and data were expressed as the mean ± SEM. The optimal concentration (25μM) was selected based on its effects on proliferation of 12Z and 22B cells.
Effects of CTZ on cell cycle and apoptosis machinery proteins
The 12Z and 22B cells were cultured in T75-flask. At 70%–80% confluency, the cells were serum starved in DMEM/F12 with 2% DC-FBS for 12 hours. Then, the 12Z and 22B cells were treated with CTZ at 25μM in plain DMEM/F12 for 24 hours. Afterwards, the cells were harvested using 0.1% Trypsin-EDTA, and protein was isolated. Effects of CTZ on expression of CDK1, CDK2, and CDK4 and cleavage of caspase-3 protein were determined by Western blot analysis. Three experiments were conducted, and data were expressed as the mean ± SEM.
Effects of CTZ on P450 aromatase expression and activity
The 12Z and 22B cells were cultured in T75-flask, serum starved, and treated CTZ at 25μM as described above. The culture media were harvested. The cells were harvested using 0.1% Trypsin-EDTA, and total protein was isolated. Expression of P450 aromatase was determined by Western blot analysis. In order to determine the activity of P450 aromatase, the 12Z and 22B cells (50 000 cells/well) were cultured in 24-well plates. At 70%–80% confluency, the 12Z and 22B cells were treated with CTZ at 25μM for 24 hours in phenol red-free plain DMEM/F12. After 24 hours, the culture media were harvested, and the 12Z and 22B cells were further incubated with androstenidione (40μM) in 1 mL of fresh phenol red-free plain DMEM/F12 for 60 minutes, and the culture media were harvested. The concentration of estrone in culture media were measured using ELISA. Three experiments were conducted, and data were expressed as the mean ± SEM.
Effects of CTZ on PGE2 biosynthesis and signaling
The 12Z and 22B cells were cultured in T75-flask, serum starved, and treated with CTZ at 25μM as described above. The culture media were harvested. The cells were harvested using 0.1% Trypsin-EDTA, and total RNA was isolated. The concentration of PGE2 was measured in the culture media by ELISA. Expression of cyclooxygenase-2 (COX-2) and EP2 and EP4 mRNAs was determined by quantitative PCR (qPCR). Three experiments were conducted, and data were expressed as the mean ± SEM.
Effects of EP2 and EP4 inhibition on P450 aromatase expression and activity
The 12Z and 22B cells were cultured in T75-flask. At 70%–80% confluency, the cells were serum starved in DMEM/F12 with 2% DC-FBS for 12 hours. Then, the cells were treated with EP inhibitors (EP-I) for EP2 (AH6809, 75μM) and EP4 (AH23848, 50μM) for 24 hours. Afterwards, culture media were collected, and cells were harvested using 0.1% Trypsin-EDTA, and total was protein isolated. The concentration of estrone in culture media were measured using ELISA. Expression of P450 aromatase protein was determined by Western blot analysis. Three experiments were conducted, and data were expressed as the mean ± SEM. EP2 inhibitor AH6809 and EP4 inhibitor AH23848 competitively bind with the respective EP2 or EP4 receptors and inhibit their activations (45–47) but not their expressions (36). The doses for these inhibitors were selected based on the dose-response experiments as we published previously (37, 41, 42).
Effects of knock down of EP2 and EP4 genes on P450 aromatase expression and activity
The 12Z and 22B cells were cultured in 6-well plates. EP2 and EP4 knock down was as described below. At 72 hours after transfection, the culture media were collected, and concentration of estrone was determined by ELISA. Total RNA was collected, and P450 aromatase mRNA was determined using qPCR as described below.
EP2/EP4 siRNA
Knock down of EP2 and EP4 genes were performed as we described previously (36). The 12Z and 22B cells (3.0 × 105/well) were cultured in antibiotic-free DMEM/F12 with 10% FBS in 6-well tissue culture plates. At 70%–80% confluency, cells were used for EP2 and EP4 knock down experiments using SMART-pool siRNA duplex delivered by DharmaFect-1 as we described previously (36, 41, 43) and per manufacturer's instructions (Dharmacon, Inc). As an internal control, MOCK siRNA was performed using the transfection procedure in the absence of siRNA. Briefly, siRNA duplexes (100nM/well) and DharmaFect-1 (3 μL/well) were diluted in 50-μL antibiotic and serum-free DMEM/F12 medium separately and mixed gently and incubated for 5 minutes at room temperature. Afterwards, EP2 and EP4 siRNA and DharmaFect-1 were mixed (total volume, 100 μL) and incubated at room temperature for 20 minutes. Then, 100-μL siRNA:DharmaFect-1 complex was diluted with 2-mL antibiotic-free media with 10% FBS and added to the well. After 24 hours, the medium was replaced with fresh DMEM/F12 with 10% FBS and incubated for 24 hours. Fluorescence-labeled siGLO RISC-free siRNA was transfected separately, and transfection efficiency was estimated using a fluorescence microscope. Transfection efficiency more than 80% was considered as optimal conditions for further experiments. Efficiency of siRNA on silencing of EP2 and EP4 proteins was assessed by Western blot analysis at 72 hours after transfection. Knock down efficiency was 70%–80% in both 12Z and 22B cells as we reported previously (36).
Protein extraction
Total protein was isolated from endometriotic cells as we described previously (36, 43). Briefly, the cells were harvested using 1% Trypsin-EDTA and pelleted. The cell lysates were sonicated in sonication buffer, which consisted of 20mM Tris-Hcl, 0.5mM EDTA, 100 μM diethyldithiocarbamate, 1% Tween, 1mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail tablets: complete EDTA free (1 tablet/50 mL) and PhosStop (1 tablet/10 mL). Sonication was performed using a Microson ultrasonic cell disruptor (Microsonix, Inc). Protein concentration was determined using the Bradford method (48) and a Bio-Rad protein assay kit.
Western blot analysis
Protein samples (75 μg) were resolved using 7.5%, 10%, or 12.5% SDS-PAGE. Antihuman polyclonal CDK1, CDK2, CDK4, caspase-3, P450 aromatase (1:1000), or β-actin (1:5000) was used as the primary antibody. Goat antirabbit IgG conjugated with horseradish peroxidase was used as the secondary antibody (1:10 000). Chemiluminescent substrate was applied according to the manufacturer's instructions (Pierce Biotechnology). The blots were exposed to Blue X-Ray film, and densitometry of autoradiograms was performed using an Alpha Imager (Alpha Innotech Corp).
RNA isolation and qPCR
Total RNA was isolated using TRIzol reagent according to the manufacturer's (Invitrogen, Inc) instructions. PPARγ, COX-2, EP2, and EP4 mRNAs were measured by qPCR. Reverse transcription was performed using QuantiTect reverse transcription kit (QIAGEN, Inc), and PCR was performed using QuantiTect SYBR RT-PCR kit (QIAGEN, Inc) StepOnePlus Real-Time PCR system (Life Technologies) following manufacturer's instructions. As an internal control, β-actin mRNA was measured. We used the following primers for PPARγ: sense, 5-ATCTCTCCGTAATGGAAGACCACTC-3 and antisense, 5-CCCAAACCTGATGGCATTATGAGAC-3; COX-2: sense, 5-GTGATGAGCAGTTGTTCCAGACAAGCAG-3 and antisense, 5-ACCATAGAG TGCTTCCAACTCTGCAGAC-3; EP2: sense, 5-CTCGATCGGGCACCCCTACTTCTACC-3 and antisense, 5-CAGGAGGCCTAAGGATGGCAAAGACC-3; EP4: sense, 5- TCTTTGCAGTCTATGCGTCCAACGTG-3 and antisense, 5-GGTCTAGGATGGG GTTCACAGAAGC-3; and β-actin: sense, 5-AGAGAGGCATCCTCACCCTGAAGTACC-3 and antisense, 5-GACGTAGCACAGCTT CTCC TTAATGTC-3. PCR cycling conditions were 1 cycle of 42°C for 45 minutes and 94°C for 2 minutes, followed by 35 cycles at 94°C for 30 seconds, 60°C for 1 minute, and 72°C for 1 minute. Data were analyzed by delta comparative cycle threshold (dCT) quantitative method as recommended by the manufacturer.
PGE2 assay
Commercially available PGE2 standards, antiserum, and acetylcholinesterase tracers (Cayman Chemicals) were used. ELISA was performed according to the manufacturers' instructions and as described previously (49). Briefly, 50 μL of culture medium collected from different experiments was used. The reaction mixture was incubated overnight at room temperature. Concentrations of PGE2 were measured at 405 nm using plate reader. A standard curve was developed with standards ranging from 50- to 5000-pg PGE2/mL.
Estrone assay
Concentration of estrone was measured using commercially available ELISA kit (DRG International, Inc) following manufacturer's protocols. Briefly, 50 μL of culture medium collected from different experiments were used. The reaction mixture was incubated 60 minutes at room temperature. Concentrations of estrone were measured at 450 nm using plate reader. A standard curve was developed with standards ranging from 0- to 2000-pg estrone/mL.
Statistical analyses
Statistical analyses were performed using general linear models of Statistical Analysis System (SAS). Effects of PPARγ activation or EP inhibition on cell proliferation, expression levels of different proteins or mRNA, and PGE2 and estrone production in 12Z and 22B cells were analyzed by one-way ANOVA followed by Tukey-Kramer honestly significant difference test. The numerical data are expressed as mean ± SEM. Statistical significance was considered at P < .05.
Results
Expression of PPARγ receptor in 12Z and 22B cells
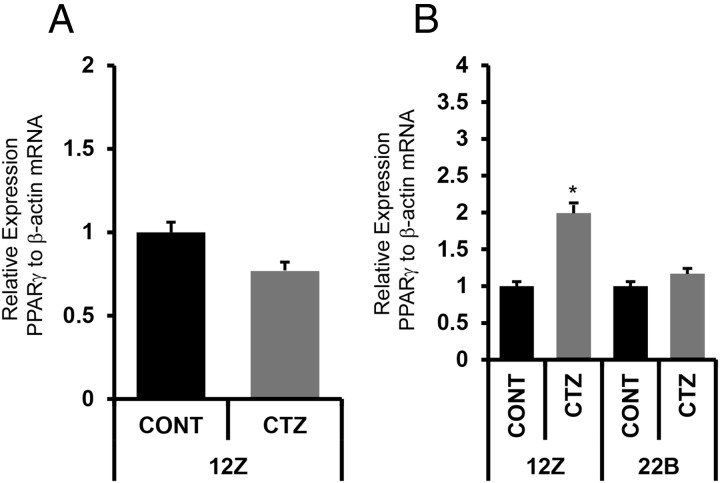
We determined the expression of PPARγ mRNA in endometriotic epithelial cells 12Z and stromal cells 22B using qPCR. Results indicated that PPARγ mRNA was expressed in both 12Z and 22B cells. Interestingly, the expression levels were 2-fold higher (P < .05) in 12Z cells compared with 22B cells (Figure 1A). CTZ increased (P < .01) the expression of PPARγ mRNA specifically in 12Z cells but not in 22B cells (Figure 1B).
Figure 1.
(A) Expression of PPARγ receptor mRNA in human endometriotic epithelial cells 12Z and stromal cells 22B. β-Actin mRNA was measured as an internal control. (B) Effects of PPARγ agonist CTZ on expression of PPARγ mRNA in 12Z and 22B cells. The 12Z and 22B cells were treated with PPARγ agonist CTZ 25 μm for 24 hours. Expression of PPARγ mRNA was performed using qPCR, and data were analyzed using dCT method. Numerical data are expressed in ratio between PPARγ mRNA and β-actin mRNA as mean ± SEM of 3 (n = 3) independent experiments. CONT, control; *, expression levels of PPARγ mRNA in 12Z vs 22B cells, P < .05. More details are provided in Materials and Methods and Result sections.
Activation of PPARγ inhibits growth of endometriotic epithelial and stromal cells
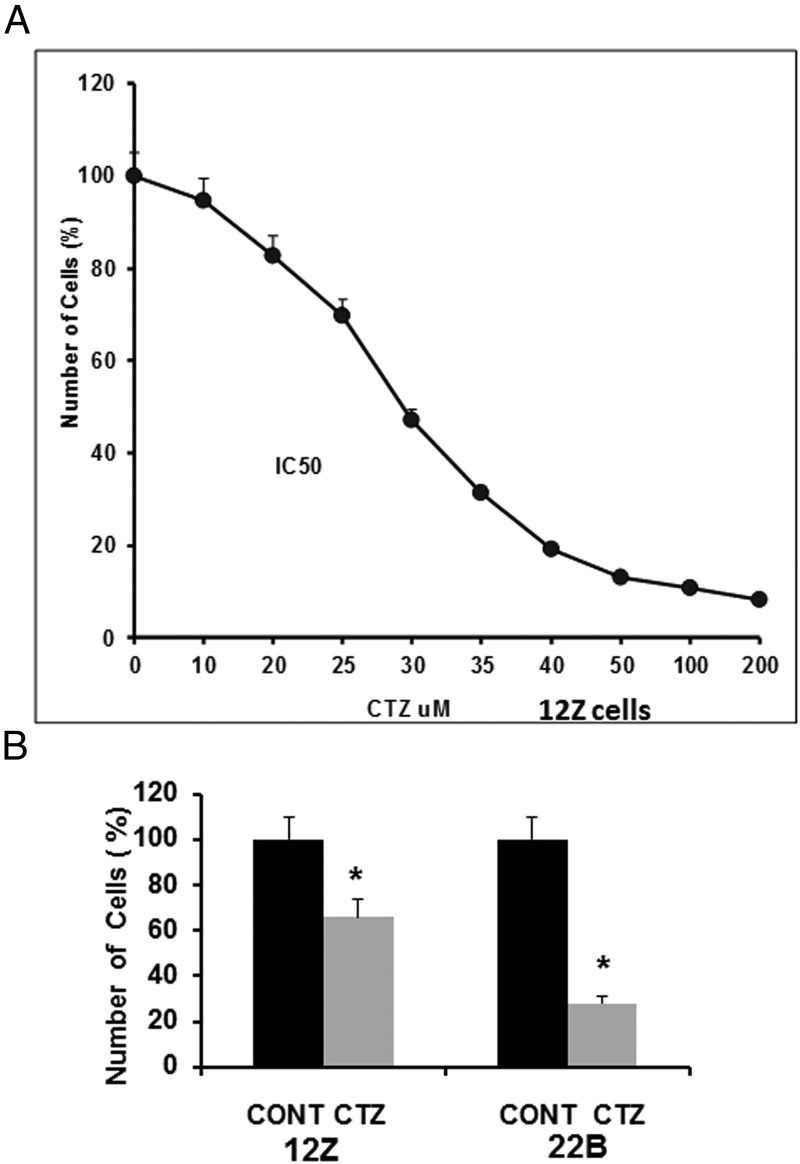
We determined the optimal concentration of PPARγ receptor agonist CTZ by a concentration-response experiment. We used endometriotic epithelial cells 12Z cells as a model system. CTZ concentrations from 0μM to 200μM were tested. Results indicated that CTZ decreased (P < .05) proliferation of 12Z cells in a concentration-dependent manner. Based on these results, the CTZ concentration 25μM was chosen for other experiments (Figure 2A). Next, we determined the effects of CTZ at 25μM on proliferation endometriotic 12Z and 22B cells at the 24-hour time point. Results indicated that CTZ inhibited (P < .05) growth of endometriotic epithelial cells 12Z up to 35% and growth of endometriotic stromal cells 22B up to 70% in a stromal-epithelial cell-specific manner (Figure 2B).
Figure 2.
Effects of activation of PPARγ on growth of human endometriotic epithelial cells 12Z and stromal cells 22B. (A) PPARγ agonist CTZ concentration-response experiment in 12Z cells. (B) Effects of PPARγ agonist CTZ on growth of 12Z and 22B cells. The 12Z and 22B cells were treated with PPARγ agonist CTZ 25 μm for 24 hours. The adherent cells 12Z and 22B cells were harvested, and the cell numbers were counted using a Coulter counter. Numerical data are expressed as mean ± SEM of 3 (n = 3) independent experiments. CONT, control; *, CONT vs CTZ on growth of 12Z and 22B cells, P < .05. More details are provided in Materials and Methods and Result sections.
Activation of PPARγ decreased expression of CDK proteins and cleaved caspase-3 protein in endometriotic epithelial and stromal cells
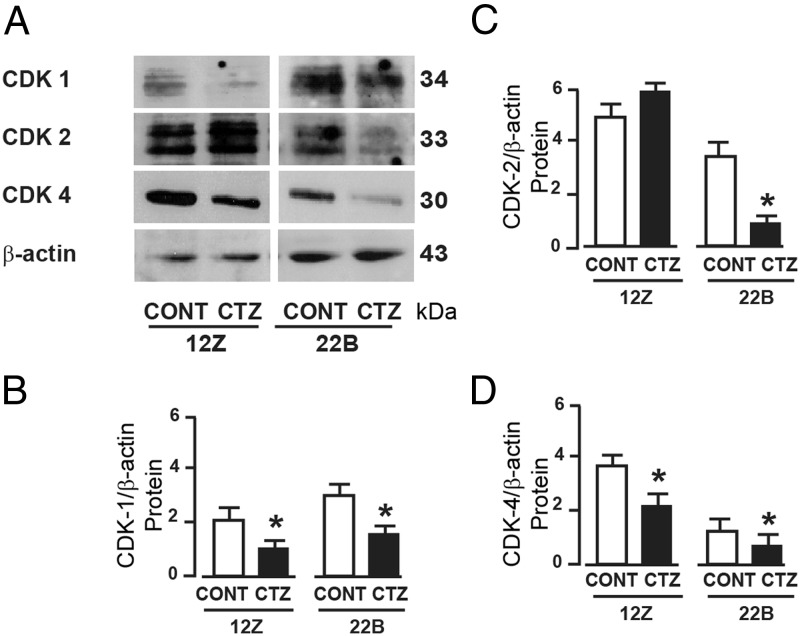
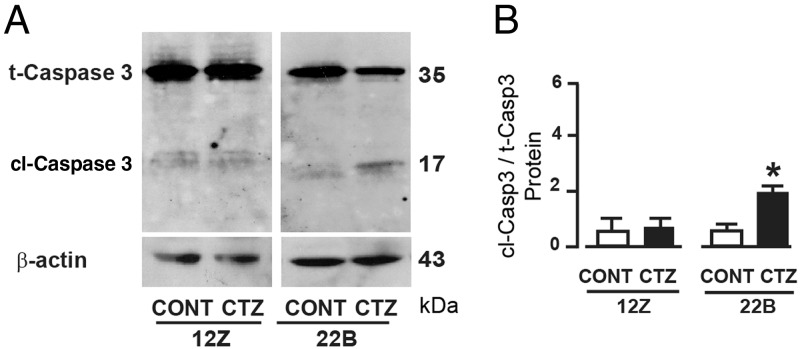
We sought to determine whether activation of PPARγ induced growth inhibition through cell cycle regulatory and/or apoptotic mechanisms. Results indicated that CTZ decreased (P < .05) expression of CDK1 and CDK4 proteins in endometriotic epithelial cells 12Z and stromal cells 22B (Figure 3). Importantly, CTZ decreased (P < .05) expression of CDK2 only in 22B cells but not in 12Z cells. In addition, CTZ cleaved (P < .05) caspase-3 protein in 22B cells but not in 12Z cells (Figure 4).
Figure 3.
Effects of activation of PPARγ on cell cycle machinery proteins in human endometriotic epithelial cells 12Z and stromal cells 22B. (A) Western blot analysis of cell cycle-dependent kinases CDK-1, CDK-2, and CDK-4 proteins. β-Actin protein was measured as an internal control. Densitometry for (B) CDK-1, (C) CDK-2, and (D) CDK-4 proteins was performed by Alpha Imager and expressed in integrated density value (IDV). The 12Z and 22B cells were treated with PPARγ agonist CTZ 25 μm for 24 hours. Numerical data are expressed in ratio between CDK1, CDK2, or CDK4 protein and β-actin protein as mean ± SEM of 3 (n = 3) independent experiments. CONT, control; *, CONT vs CTZ on expression of CDK1, CDK2, or CDK4 protein in 12Z and 22B cells, P < .05. More details are provided in Materials and Methods and Result sections.
Figure 4.
Effects of activation of PPARγ on apoptosis machinery protein caspase-3 in human endometriotic epithelial cells 12Z and stromal cells 22B. (A) Western blot analysis of caspase-3 protein, immunoreactive protein band at 35 kDa is total (inactive) caspase-3 and at 17 kDa was cleaved (active) caspase-3 protein. (B) Densitometry for caspase-3 was performed by Alpha Imager and expressed in integrated density value (IDV). The 12Z and 22B cells were treated with PPARγ agonist CTZ 25 μm for 24 hours. Numerical data are expressed in ratio between cleaved and total caspase-3 proteins as mean ± SEM of 3 (n = 3) independent experiments. CONT, control; *, CONT vs CTZ on cleavage of caspase-3 protein in 12Z and 22B cells, P < .05. More details are provided in Materials and Methods and Result sections. t-Casp3, total caspase3; cl-Casp3, cleaved caspase3.
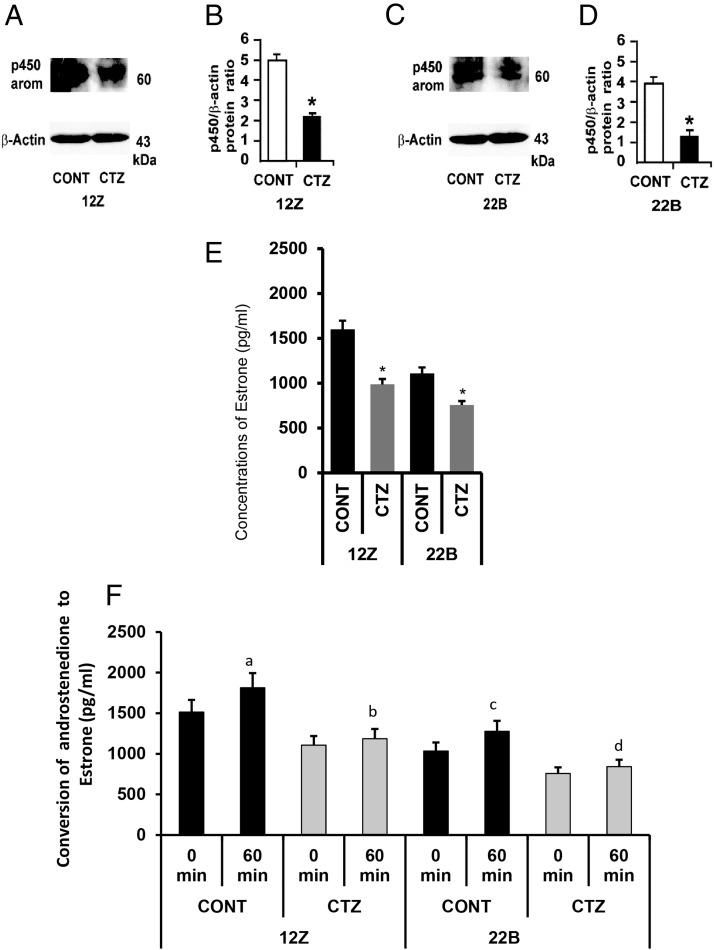
Activation of PPARγ decreased expression and activity of P450 aromatase in endometriotic epithelial and stromal cells
We examined whether activation of PPARγ decreased expression and activity of P450 aromatase in endometriotic epithelial cells 12Z and stromal cells 22B. Results indicated that P450 protein was expressed in both 12Z and 22B cells. CTZ decreased (P < .05) expression of P450 protein in both in 12Z and 22B cells (Figure 5, A–D). CTZ decreased (P < .05) basal conversion of androstenedione to estrone in 12Z and 22B cells in 24 hours (Figure 5E). In addition, exogenous androstenedione was converted into estrone in control 12Z and 22B cells at 60 minutes (P < .05), and this enzymatic conversion by P450 aromatase was inhibited (P < .05) by CTZ-treated 12Z and 22B cells at 60 minutes (Figure 5F).
Figure 5.
Effects of activation of PPARγ on expression and activity of P450 aromatase in human endometriotic epithelial cells 12Z and stromal cells 22B. (A and B) Western blot analysis of P450 aromatase (P450 arom). β-Actin protein was measured as an internal control. (C and D) Densitometry for P450 arom was performed by Alpha Imager and expressed in integrated density value (IDV). The 12Z and 22B cells were treated with PPARγ agonist CTZ 25 μm for 24 hours. Numerical data are expressed in ratio between P450 protein and β-actin protein as mean ± SEM of 3 (n = 3) independent experiments. CONT, control; *, CONT vs CTZ on expression of P450 arom protein in 12Z and 22B cells, P < .05. (E) The 12Z and 22B cells were treated with PPARγ agonist CTZ 25 μm for 24 hours. The culture media were collected, and concentration of estrone was measured using ELISA. Numerical data are expressed as mean ± SEM of 3 (n = 3) independent experiments. *, CONT vs CTZ on estrone biosynthesis by 12Z and 22B cells, P < .05. (F) The 12Z and 22B cells were pretreated or not treated with PPARγ agonist CTZ 25 μm for 24 hours. Afterwards, 12Z and 22B cells were treated with androstenedione (40 μm) for 0 and 60 minutes, and conversion of exogenous androstenedione into estrone was measured in the culture media using ELISA. Numerical data are expressed as mean ± SEM of 3 (n = 3) independent experiments. (a–d) CONT vs CTZ on conversion of exogenous androstenedione into estrone by 12Z and 22B cells at 60 minutes, P < .05. More details are provided in Materials and Methods and Result sections.
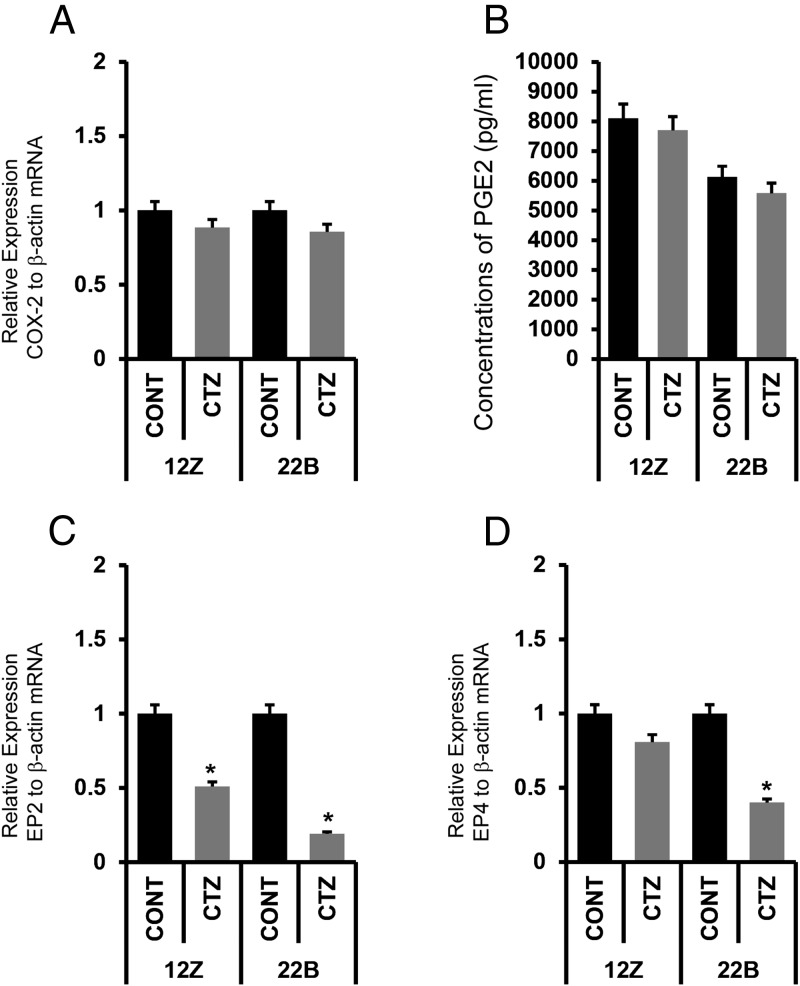
Activation of PPARγ did not decrease PGE2 biosynthesis but inhibits expression of its receptors EP2 and EP4 in endometriotic epithelial and stromal cells
We determined whether activation of PPARγ inhibited PGE2 biosynthesis and signaling in endometriotic epithelial cells 12Z and stromal cells 22B. Results indicated that CTZ did not inhibit expression of COX-2 mRNA (Figure 6, A–D) and total PGE2 production by in 12Z and 22B cells (Figure 6E). By contrast, CTZ inhibits expression of both receptors EP2 and EP4 mRNAs in 22B cells, whereas it inhibits expression of only EP2 mRNA in 12Z cells (Figure 6, E and F).
Figure 6.
Effects of activation of PPARγ on expression of COX-2 mRNA and PGE2 biosynthesis in human endometriotic epithelial cells 12Z and stromal cells 22B. (A) Expression of COX-2 mRNA in 12Z and 22B cells. (B) PGE2 production in 12Z and 22B cells measured by ELISA in the culture media, n = 3. (C) Effects of activation of PPARγ on expression of EP2 receptor mRNA and in 12Z and 22B cells. (D) Effects of activation of PPARγ on expression of EP4 receptor mRNA and in 12Z and 22B cells. Expression of COX-2, EP2, or EP4 mRNA was performed using qPCR, and data were analyzed using dCT method. Numerical data are expressed in ratio between COX-2, EP2, or EP4 mRNA and β-actin mRNA as mean ± SEM of 3 (n = 3) independent experiments. CONT, control. The 12Z and 22B cells were treated with PPARγ agonist CTZ 25 μm for 24 hours. More details are provided in Materials and Methods and Result sections.
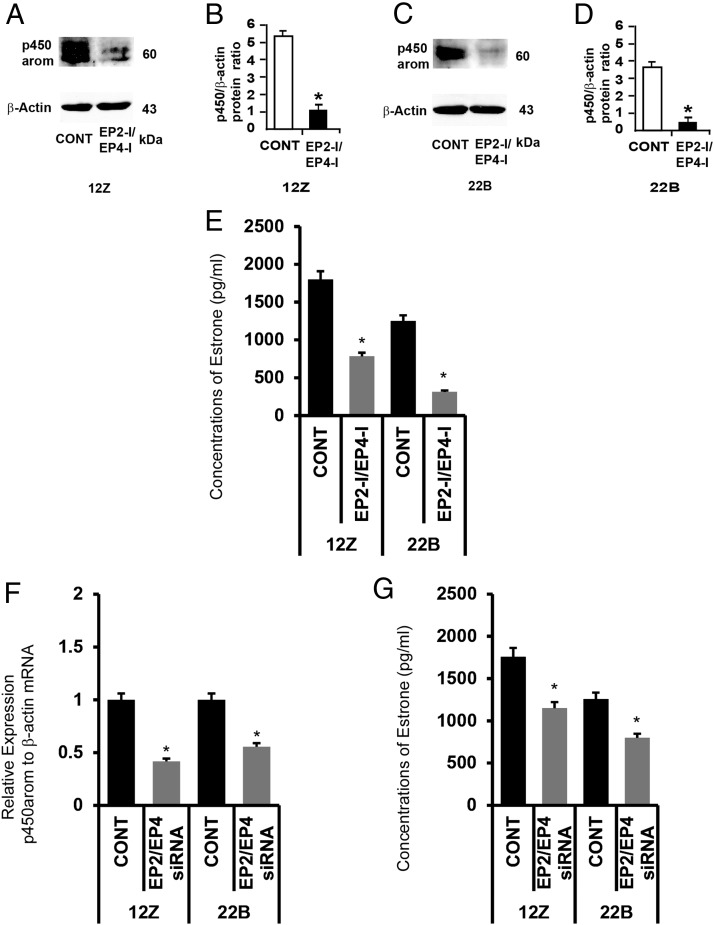
Inhibition of EP2 and EP4 receptor decreased expression of P450 protein in endometriotic epithelial and stromal cells
We determined the effects of inhibition of EP2 and EP4 on expression of P450 aromatase protein in endometriotic epithelial cells 12Z and stromal cells 22B. Results (Figure 7, A–D) indicated that inhibition of EP2 and EP4 decreased expression of P450 aromatase protein in 12Z and 22B cells. In addition, inhibition of EP2 and EP4 decreased (P < .05) basal endogenous conversion of androstenedione to estrone in 12Z and 22B cells at 24 hours (Figure 7E). Further, we confirmed this observed pharmacologic effects by knock down of EP2 and EP4 genes using siRNA. Results indicated that silencing of EP2 and EP4 genes decreased (P < .05) expression of P450 aromatase mRNA (Figure 7F) and concomitantly decreased (P < .05) basal estrone production (Figure 7G) in 12Z and 22B cells.
Figure 7.
Effects of inhibition of EP2 and EP4 receptors on activity of P450 aromatase (P450 arom) in human endometriotic epithelial cells 12Z and stromal cells 22B. (A and B) Western blot analysis of P450 arom in 12Z and 22B cells. β-Actin protein was measured as an internal control. (C and D) Densitometry for P450 arom performed by Alpha Imager and expressed in integrated density value (IDV). The 12Z and 22B cells were treated with inhibitors for EP2 (AH6809, 75μM) and EP4 (AH23848, 50μM) for 24 hours. Numerical data are expressed in ratio between P450 arom protein and β-actin protein as mean ± SEM of 3 (n = 3) independent experiments. CONT, control; *, CONT vs CTZ on expression of P450 arom protein in 12Z and 22B cells, P < .05. (E) Concentrations of estrone. The 12Z and 22B cells were treated with inhibitors (EP-I) for EP2 (AH6809, 75μM) and EP4 (AH23848, 50μM) for 24 hours. The culture media were collected, and concentration of estrone was measured using ELISA. Numerical data are expressed as mean ± SEM of 3 (n = 3) independent experiments. *, CONT vs EP2-I + EP4-I on estrone biosynthesis by 12Z and 22B cells, P < .05. (F and G) Effects of knock down of EP2 and EP4 genes P450 arom gene expression and estrone production. (F) Expression of P450 arom mRNA was performed using qPCR, and data were analyzed using dCT method. (G) Concentration of estrone in the culture media were measured using ELISA. Numerical data are expressed as mean ± SEM of 3 (n = 3) independent experiments. More details are provided in Materials and Methods and Result sections.
Discussion
PPARs are ligand-activated nuclear receptors, which play an important role in inflammation in addition to their well-established role in glucose and lipid metabolisms (1–4). Activation of PPARγ inhibits growth of endometriosis in murine and baboon models (8, 16–22, 24–26). However, the underlying mechanisms are largely unknown. The objective of the present study is to understand growth inhibitory, apoptotic, antiestrogenic, and antiinflammatory actions of PPARγ ligands in human endometriotic epithelial and stromal cells.
Results of the present study indicate that PPARγ mRNA is abundantly expressed in human endometriotic epithelial cells 12Z and stromal cells 22B. Activation of PPARγ by its agonist CTZ regulates expression of PPARγ mRNA through positive feedback mechanisms in epithelial cells but not in stromal cells. At present, the precise underlying molecular mechanism is not known.
Activation of PPARγ by its agonist CTZ inhibits growth of 12Z cells up to 35% and 22B cells up to 70% in an epithelial and stromal cell-specific manner. Therefore, we unraveled the underlying growth inhibitory molecular mechanisms. Selective CDK/cyclin complexes are activated at different phases/check points of the cell cycle. CDK4/6 and cyclin D1/D2/D3 complexes are activated in early to mid G1-phase, CDK2/cyclin E complexes are required for the G1/S transition, CDK2/cyclin A complex is essential for the progression of S-phase/DNA synthesis, and CDK1/cyclin A-B is necessary for G2-M transition (50–53). Our results indicate that activation of PPARγ inhibits CDK1, CDK2, and CDK4 proteins in stromal cells 22B and that in turn may inhibit progression of stromal cells through G1-S and G2-M phases of cell cycle. By contrast, in epithelial cells 12Z, activation of PPARγ inhibits CDK1 and CDK4 proteins that in turn may inhibit progression of epithelial cells through G2-M phase of the cell cycle.
We next examined whether activation of PPARγ induces apoptosis of 12Z and 22B cells. In response to intrinsic apoptotic stimuli, cytochrome C is released form the mitochondria to the cytosol and cleaves caspase-3 enzymes, which in turn cleaves nuclear PARP enzyme (54, 55). Cleavage of caspase-3 protein is the rate-limiting step in intrinsic apoptotic pathways (55). Our results indicate that activation of PPARγ by its agonist CTZ cleaves caspase-3 protein only in stromal cells 22B but not in epithelial cells 12Z. Our results are in agreement with previous reports that the PPARγ agonist rosiglitazone inhibits endometriotic implant growth, by inhibiting cell proliferation (measured by cell nuclear antigen) and inducing apoptosis (measured by terminal deoxynucleotidyl transferase dUTP nick end labeling assay), and inhibits angiogenesis in the mice model of endometriosis (16).
The role of estrogen in growth of endometriosis is well known (11, 27–29). P450 aromatase catalyzes the last steps of estrogen biosynthesis from androgens in 2 ways: 1) from androstenedione into estrone and 2) from testosterone into estradiol. P450 aromatase protein is abundantly expressed in endometriosis (11, 27–29). Studies indicated that activation of PPARγ decreased expression of P450 aromatase expression in human breast adipose tissue (31, 32) as well as in ovarian granulosa cells (33, 34). Therefore, we examined the effects of activation of PPARγ by its agonist CTZ on expression and/or activity of P450 aromatase in human endometriotic epithelial cells 12Z and stromal cells 22B. Our results indicate that activation of PPARγ by its agonist CTZ decreases expression of P450 aromatase protein in both 12Z and 22B cells, which is accompanied by decreased basal production of estrone by these cells. In addition, the 12Z and 22B cells were incubated with exogenous androstenedione for 24 hours, and activity of P450 aromatase was determined by measuring estrone production. Importantly, CTZ decreases enzymatic conversion of androstenedione into estrone by the 12Z and 22B cells. These results suggest that activation of PPARγ inhibits P450 aromatase and estrone production and in turn may lead to decreased estradiol production by endometriotic epithelial and stromal cells.
The role for PGE2 is well accepted in the pathogenesis of endometriosis (35–38). Therefore, we examined the effects of activation of PPARγ on PGE2 biosynthesis in endometriotic epithelial cells 12Z and stromal cells 22B. Results indicate that activation of PPARγ by its agonist CTZ did not decrease COX-2 mRNA expression and PGE2 production by 12Z and 22B cells. These results captivated us to conclude that growth inhibitory, apoptotic, and antiestrogenic effects of PPARγ agonist are independent of PGE2. However, recent studies from our laboratory indicate that inhibition of EP2- and EP4-mediated PGE2 signaling deregulates cell cycle and induces apoptosis in 12Z and 22B cells by integrating multiple cell signaling pathways, such as ERK1/2, protein kinase B, NF-κB, and β-catenin (36). Therefore, we determined the effects of activation of PPARγ on EP2 and EP4 expression and signaling. Importantly, we found that PPARγ agonist CTZ decrease expression of EP2 and EP4 mRNA in endometriotic stromal cells 22B but decrease only EP2 mRNA in endometriotic epithelial cells 12Z. Inhibition of both EP2 and EP4 may be one of the underlying mechanisms by which activation of PPARγ induces 70% growth inhibition in 22B cells compared with 35% growth inhibition in 12Z cells by dysegulating cell cycle and apoptotic machinery in epithelial-stromal cell-specific modes. At present, the underlying mechanisms through which activation of PPARγ decreases EP2 and EP4 receptors genes in endometriosis are not known.
It is well established that PGE2 increases estrogen biosynthesis through P450 aromatase through positive feedback mechanisms and vice versa (11, 28, 29). We hypothesize that activation of PPARγ inhibits P450 aromatase expression through EP2- and EP4-mediated PGE2 signaling. Our results indicate that selective pharmacologic and genomic inhibition of EP2 and EP4 inhibits expression of P450 aromatase protein in both 12Z and 22B cells. These results together suggest that activation of PPARγ inhibits P450 aromatase mRNA expression through EP2- and EP4-mediated PGE2 signaling. At present, the underlying mechanism through which selective inhibition of EP2 and EP4 inhibits expression of P450 protein is not known.
In summary, results of the present study indicate that activation of PPARγ by agonist CTZ 1) inhibits proliferation and induces apoptosis of endometriotic epithelial and stromal cells, 2) decreases estrogen biosynthesis, and 3) does not decrease PGE2 biosynthesis but inhibits EP2- and EP4-mediated PGE2 signaling. In addition, these results suggest that these growth inhibitory, apoptotic, and antiestrogenic actions are potentially mediated through PGE2-mediated EP2 and EP4 signaling in endometriosis. We also strongly believe that impaired PGE2 signaling is one of the important downstream signaling pathways that mediates antiinflammatory actions of PPARγ agonists. Collectively, the results of the present study indicate that combination of PPARγ activation and inhibition of EP2 and EP4 may emerge as novel nonsteroidal therapy for endometriosis-associated pain and infertility, if clinically proven safe and efficacious.
Acknowledgments
We thank Dr Anna Starzinski-Powitz (Department of Biology, University of Frankfurt, Frankfurt am Main, Germany) for providing human endometriotic epithelial cells 12Z and stromal cells 22B as generous gift.
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and Office of Research on Women's Health and NICHD Grants 1R21HD065138–01A1 and 1R21HD066248–01A1 (to J.A.A.) and 5K23HD043952–02 to (D.I.L.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and Office of Research on Women's Health and NICHD Grants 1R21HD065138–01A1 and 1R21HD066248–01A1 (to J.A.A.) and 5K23HD043952–02 to (D.I.L.).
Footnotes
- CDK
- cyclin-dependent kinase
- COX-2
- cyclooxygenase-2
- CTZ
- ciglitazone
- DC-FBS
- dextran charcoal-treated FBS
- dCT
- delta comparative cycle threshold
- EP
- PGE2 receptors
- FBS
- fetal bovine serum
- PPAR
- peroxisome proliferator-activated receptor
- qPCR
- quantitative PCR
- siRNA
- small interfering RNA
- TZD
- thiazolidinedione.
References
- 1. Kahn CR, Chen L, Cohen SE. Unraveling the mechanism of action of thiazolidinediones. J Clin Invest. 2000;106:1305–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Savage DB. PPAR γ as a metabolic regulator: insights from genomics and pharmacology. Expert Rev Mol Med. 2005;7:1–16. [DOI] [PubMed] [Google Scholar]
- 3. Semple RK, Chatterjee VK, O'Rahilly S. PPAR γ and human metabolic disease. J Clin Invest. 2006;116:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem. 2008;77:289–312. [DOI] [PubMed] [Google Scholar]
- 5. Wanichkul T, Han S, Huang RP, Sidell N. Cytokine regulation by peroxisome proliferator-activated receptor γ in human endometrial cells. Fertil Steril. 2003;79(suppl 1):763–769. [DOI] [PubMed] [Google Scholar]
- 6. Pritts EA, Zhao D, Ricke E, Waite L, Taylor RN. PPAR-γ decreases endometrial stromal cell transcription and translation of RANTES in vitro. J Clin Endocrinol Metab. 2002;87:1841–1844. [DOI] [PubMed] [Google Scholar]
- 7. Galli A, Mello T, Ceni E, Surrenti E, Surrenti C. The potential of antidiabetic thiazolidinediones for anticancer therapy. Expert Opin Investig Drugs. 2006;15:1039–1049. [DOI] [PubMed] [Google Scholar]
- 8. Rocha AL, Reis FM, Petraglia F. New trends for the medical treatment of endometriosis. Expert Opin Investig Drugs. 2012;21:905–919. [DOI] [PubMed] [Google Scholar]
- 9. Hornung D, Chao VA, Vigne JL, Wallwiener D, Taylor RN. Thiazolidinedione inhibition of peritoneal inflammation. Gynecol Obstet Invest. 2003;55:20–24. [DOI] [PubMed] [Google Scholar]
- 10. Giaginis C, Margeli A, Theocharis S. Peroxisome proliferator-activated receptor-γ ligands as investigational modulators of angiogenesis. Expert Opin Investig Drugs. 2007;16:1561–1572. [DOI] [PubMed] [Google Scholar]
- 11. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- 12. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- 13. Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118:691–705. [DOI] [PubMed] [Google Scholar]
- 14. Guo SW, Olive DL. Two unsuccessful clinical trials on endometriosis and a few lessons learned. Gynecol Obstet Invest. 2007;64:24–35. [DOI] [PubMed] [Google Scholar]
- 15. Kyama CM, Mihalyi A, Simsa P, et al. Non-steroidal targets in the diagnosis and treatment of endometriosis. Curr Med Chem. 2008;15:1006–1017. [DOI] [PubMed] [Google Scholar]
- 16. Olivares C, Ricci A, Bilotas M, Barañao RI, Meresman G. The inhibitory effect of celecoxib and rosiglitazone on experimental endometriosis. Fertil Steril. 2011;96:428–433. [DOI] [PubMed] [Google Scholar]
- 17. Aytan H, Caliskan AC, Demirturk F, Aytan P, Koseoglu DR. Peroxisome proliferator-activated receptor-γ agonist rosiglitazone reduces the size of experimental endometriosis in the rat model. Aust N Z J Obstet Gynaecol. 2007;47:321–325. [DOI] [PubMed] [Google Scholar]
- 18. Demirturk F, Aytan H, Caliskan AC, Aytan P, Koseoglu DR. Effect of peroxisome proliferator-activated receptor-γ agonist rosiglitazone on the induction of endometriosis in an experimental rat model. J Soc Gynecol Investig. 2006;13:58–62. [DOI] [PubMed] [Google Scholar]
- 19. Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-γ induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004;82(suppl 3):1008–1013. [DOI] [PubMed] [Google Scholar]
- 20. Herington JL, Crispens MA, Carvalho-Macedo AC, et al. Development and prevention of postsurgical adhesions in a chimeric mouse model of experimental endometriosis. Fertil Steril. 2011;95:1295–1301.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lebovic DI, Mwenda JM, Chai DC, et al. PPAR-γ receptor ligand induces regression of endometrial explants in baboons: a prospective, randomized, placebo- and drug-controlled study. Fertil Steril. 2007;88:1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lebovic DI, Mwenda JM, Chai DC, Santi A, Xu X, D'Hooghe T. Peroxisome proliferator-activated receptor-(γ) receptor ligand partially prevents the development of endometrial explants in baboons: a prospective, randomized, placebo-controlled study. Endocrinology. 2010;151:1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peeters LL, Vigne JL, Tee MK, Zhao D, Waite LL, Taylor RN. PPAR γ represses VEGF expression in human endometrial cells: implications for uterine angiogenesis. Angiogenesis. 2005;8:373–379. [DOI] [PubMed] [Google Scholar]
- 24. Sharma I, Dhawan V, Saha SC, Dhaliwal LK. In vitro effects of peroxisome proliferator-activated receptor-γ ligands on gene expression in lipopolysaccharide-induced endometrial and endometriotic stromal cells. Fertil Steril. 2011;95:829–831.e1–e5. [DOI] [PubMed] [Google Scholar]
- 25. Streuli I, de Ziegler D, Borghese B, Santulli P, Batteux F, Chapron C. New treatment strategies and emerging drugs in endometriosis. Expert Opin Emerg Drugs. 2012;17:83–104. [DOI] [PubMed] [Google Scholar]
- 26. Moravek MB, Ward EA, Lebovic DI. Thiazolidinediones as therapy for endometriosis: a case series. Gynecol Obstet Invest. 2009;68:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Attar E, Tokunaga H, Imir G, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bulun SE, Fang Z, Imir G, et al. Aromatase and endometriosis. Semin Reprod Med. 2004;22:45–50. [DOI] [PubMed] [Google Scholar]
- 29. Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Sebastian S. Estrogen production and metabolism in endometriosis. Ann NY Acad Sci. 2002;955:75–85; discussion 86–88, 396–406. [DOI] [PubMed] [Google Scholar]
- 30. Subbaramaiah K, Hudis C, Chang SH, Hla T, Dannenberg AJ. EP2 and EP4 receptors regulate aromatase expression in human adipocytes and breast cancer cells. Evidence of a BRCA1 and p300 exchange. J Biol Chem. 2008;283:3433–3444. [DOI] [PubMed] [Google Scholar]
- 31. Subbaramaiah K, Howe LR, Zhou XK, et al. Pioglitazone, a PPARγ agonist, suppresses CYP19 transcription: evidence for involvement of 15-hydroxyprostaglandin dehydrogenase and BRCA1. Cancer Prev Res (Phila). 2012;5:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Rubin GL, Zhao Y, Kalus AM, Simpson ER. Peroxisome proliferator-activated receptor γ ligands inhibit estrogen biosynthesis in human breast adipose tissue: possible implications for breast cancer therapy. Cancer Res. 2000;60:1604–1608. [PubMed] [Google Scholar]
- 33. Mu YM, Yanase T, Nishi Y, et al. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun. 2000;271:710–713. [DOI] [PubMed] [Google Scholar]
- 34. Fan W, Yanase T, Morinaga H, et al. Activation of peroxisome proliferator-activated receptor-γ and retinoid X receptor inhibits aromatase transcription via nuclear factor-κB. Endocrinology. 2005;146:85–92. [DOI] [PubMed] [Google Scholar]
- 35. Banu SK, Lee J, Satterfield MC, Spencer TE, Bazer FW, Arosh JA. Molecular cloning and characterization of prostaglandin transporter in ovine endometrium: role of mitogen activated protein kinase pathways in release of prostaglandin F2 α. Enodcrinology. 2008;149:219–231. [DOI] [PubMed] [Google Scholar]
- 36. Banu SK, Lee J, Speights VO Jr, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFκB and b-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009;23:1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee J, Banu SK, Rodriguez R, Starzinski-Powitz A, Arosh JA. Selective blockade of prostaglandin E2 receptors EP2 and EP4 signaling inhibits proliferation of human endometriotic epithelial cells and stromal cells through distinct cell cycle arrest. Fertil Steril. 2010;93:2498–2506. [DOI] [PubMed] [Google Scholar]
- 38. Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med (Maywood). 2010;235:668–677. [DOI] [PubMed] [Google Scholar]
- 39. Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159:1839–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu Y, Starzinski-Powitz A, Guo SW. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod Sci. 2007;14:374–382. [DOI] [PubMed] [Google Scholar]
- 41. Lee J, Banu SK, Subbarao T, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol Cell Endocrinol. 2011;332:306–313. [DOI] [PubMed] [Google Scholar]
- 42. Banu SK, Starzinski-Powitz A, Speights VO, Burghardt RC, Arosh JA. Induction of peritoneal endometriosis in nude mice with use of human immortalized endometriosis epithelial and stromal cells: a potential experimental tool to study molecular pathogenesis of endometriosis in humans. Fertil Steril. 2009;91:2199–2209. [DOI] [PubMed] [Google Scholar]
- 43. Banu SK, Lee J, Speights VO Jr, Starzinski-Powitz A, Arosh JA. Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology. 2008;149:1180–1189. [DOI] [PubMed] [Google Scholar]
- 44. Banu SK, Lee J, Starzinski-Powitz A, Arosh JA. Gene expression profiles and functional characterization of human immortalized endometriotic epithelial and stromal cells. Fertil Steril. 2008;90:972–987. [DOI] [PubMed] [Google Scholar]
- 45. Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 46. Woodward DF, Pepperl DJ, Burkey TH, Regan JW. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem Pharmacol. 1995;50:1731–1733. [DOI] [PubMed] [Google Scholar]
- 47. Crider JY, Griffin BW, Sharif NA. Endogenous EP4 prostaglandin receptors coupled positively to adenylyl cyclase in Chinese hamster ovary cells: pharmacological characterization. Prostaglandins Leukot Essent Fatty Acids. 2000;62:21–26. [DOI] [PubMed] [Google Scholar]
- 48. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 49. Asselin E, Lacroix D, Fortier MA. IFN-tau increases PGE2 production and COX-2 gene expression in the bovine endometrium in vitro. Mol Cell Endocrinol. 1997;132:117–126. [DOI] [PubMed] [Google Scholar]
- 50. Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999;39:295–312. [DOI] [PubMed] [Google Scholar]
- 51. Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. [DOI] [PubMed] [Google Scholar]
- 52. Sánchez I, Dynlacht BD. New insights into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol. 2005;16:311–321. [DOI] [PubMed] [Google Scholar]
- 53. Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. [DOI] [PubMed] [Google Scholar]
- 54. Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. [DOI] [PubMed] [Google Scholar]