ABSTRACT
The broadly expressed volume-sensitive outwardly rectifying anion channel (VSOR, also called VRAC) plays essential roles in cell survival and death. Recent findings have suggested that LRRC8A is a core component of VSOR in human cells. In the present study, VSOR currents were found to be largely reduced by siRNA against LRRC8A in mouse C127 cells as well. In contrast, LRRC8A knockdown never affected activities of 4 other types of anion channel activated by acid, Ca2+, patch excision or cAMP. While cisplatin-resistant KCP-4 cells poorly expressed endogenous VSOR activity, molecular expression levels of LRRC8A, LRRC8D and LRRC8E were indistinguishable between VSOR-deficient KCP-4 cells and the parental VSOR-rich KB cells. Furthermore, overexpression of LRRC8A alone or together with LRRC8D or LRRC8E in KCP-4 cells failed to restore VSOR activity. These results show that deficiency of VSOR currents in KCP-4 cells is not due to insufficient expression of the LRRC8A/D/E gene, suggesting an essential involvement of some other factor(s), and indicate that further study is required to better understand the complexities of the molecular determinants of VSOR, including the precise role of LRRC8 proteins.
KEYWORDS: anion channel, cisplatin resistance, LRRC8, volume-sensitive, VSOR
Introduction
Volume-sensitive outwardly rectifying anion channel (VSOR) is one of the volume-regulated anion channels (VRACs) and functionally expressed in almost all cell types.1 The roles of VSOR in cell volume regulation, proliferation, migration and cell death are well established, but its molecular identity has not fully been clarified until recently.1,2
In the 1990s, several proteins, including P-glycoprotein, pIcln, and ClC-3, were proposed as molecular identities of VSOR,3-5 but none of them has survived scrutiny.6-12 In 2014, 2 research groups independently reported that the leucine-rich repeat containing 8A (LRRC8A), which has 4 transmembrane domains and a leucine-rich repeat (LRR) domain at the C-terminus, is a core factor of VSOR in human cells.13,14 They reported in common that knockdown of LRRC8A diminished endogenous VSOR currents in human cells, and such reduced currents could be rescued by introduction of exogenous LRRC8A, but overexpression of LRRC8A alone in normal cells paradoxically reduced endogenous VSOR currents.13,14 Also, VSOR was shown to be a heteromeric channel containing not only LRRC8A but also other LRRC8 family members.13 Indeed, a multimeric complex mainly of these members with a molecular mass of ∼800 kDa was found to be sufficient to form the osmo- and ionic strength-sensitive anion channels in lipid bilayers.15 More recently, Jentsch's group has discovered the roles of LRRC8D in cisplatin and taurine transport and in cisplatin resistance acquirement in human KBM7 cells.16
LRRC8s may exhibit diverse functions through LRR domain-mediated protein-protein interactions. It has been reported that truncation of LRRD in LRRC8A causes deficiency of B cell development.17,18 Such truncated LRRC8A mutants may not exhibit normal functions due to lack of normal protein-protein interactions or may exert a dominant-negative action. LRRC8A has also been suspected to have a ‘ligand’, and LRRC8A ligation activates the PI3K-AKT pathway in T cell development and function.19 Therefore, it is feasible that LRRC8A is involved in regulation of a variety of functional proteins, presumably including anion channel molecules, via protein-protein interactions and/or via the PI3K-AKT pathway. However, there has not been any study about a possible interaction between LRRC8A and distinct anion channel types other than VSOR: acid-sensitive outwardly rectifying anion channel (ASOR), Ca2+-activated Cl− channel (CaCC), maxi-conductance Cl− channel (Maxi-Cl) and cAMP-activated anion channel (CFTR).
In our most recent study, we have confirmed an involvement of LRRC8A in VSOR activity in human HeLa cells and suggested independence of LRRC8A in ASOR activity therein.20,21 In the present study, we focused on the following 3 questions: whether LRRC8A is essentially involved in VSOR activity in mouse cells as well, whether LRRC8A is involved in activities of other distinct types of anion channel, and whether LRRC8A, LRRC8D or LRRC8E represents a missing factor in human cisplatin-resistant, VSOR-deficient KCP-4 cells that are derived from the parental cisplatin-sensitive, VSOR-rich KB cells.
Results
LRRC8A is involved in VSOR current generation in a murine cell line
To examine whether species specificity exists in the role of LRRC8A in VSOR current generation, we performed siRNA-mediated knockdown experiments in a mouse cell line of mammary gland origin, C127. Transfection of siRNA against mouse LRRC8A gene (Lrrc8a) successfully reduced expression level of mRNA in C127 cells (by ∼77%: Fig. 1A). Whole-cell patch-clamp analysis showed that VSOR currents were robustly evoked by hypotonic stimulation in control C127 cells (Fig. 1B), and knockdown of Lrrc8a significantly reduced VSOR currents when compared to control siRNA transfection (Fig. 1C, D). These results indicate that LRRC8A is a key component for VSOR in murine cells, as is the case in human cells.13,14,20
Figure 1.
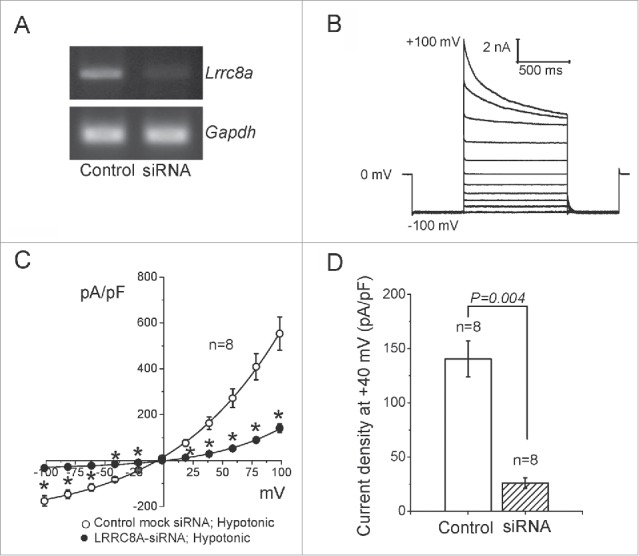
Suppressive effects of siRNA for LRRC8A on VSOR currents in murine C127 cells. (A) RT-PCR data confirming a knockdown effect of siRNA for LRRC8A. Data represent duplicate experiments. GAPDH was used as an internal control. (B) Whole-cell VSOR current responses to voltage steps in mock-transfected control cells after maximal activation by hypoosmotic stimulation (244 mosmol/kg-H2O). The holding potential was 0 mV. After a pre-pulse to −100 mV (500 ms), currents were elicited by application of step pulses (1000 ms) from −100 to +100 mV in 20-mV increments followed by 500 ms at −100 mV. (C) Instantaneous current-to-voltage relationships of VSOR in cells treated with non-targeting siRNA (Mock control; open circles) and in cells treated with siRNA against LRRC8A (filled circles). The current density (normalized by cell capacitance) was measured at the beginning of test pulses from current recordings similar to those shown in (B). *Significantly different from the mock control at P < 0.05. (D) Mean values of current density recorded at +40 mV in mock-transfected and LRRC8A-siRNA-transfected cells.
LRRC8A is not involved in current generation of other distinct types of anion channels
Since it is not known whether LRRC8A contributes to generation only of swelling-activated VSOR currents, we examined the knockdown effect of Lrrc8a on 4 other different types of Cl− channel currents functionally expressed in murine C127/CFTR cells: ASOR, CaCC, Maxi-Cl and CFTR currents activated by acid, Ca2+, patch excision and cAMP, respectively. All four types of anion channel currents recorded in the cells exhibited their phenotypical current profiles (Fig. 2) similar to those reported previously.22-25 siRNA-mediated knockdown of LRRC8A failed to suppress any of these Cl− currents (Fig. 2). Thus, it is concluded that the LRRC8A is specific to VSOR and is not involved in the generation and regulation of activities of 4 other Cl− channels.
Figure 2.
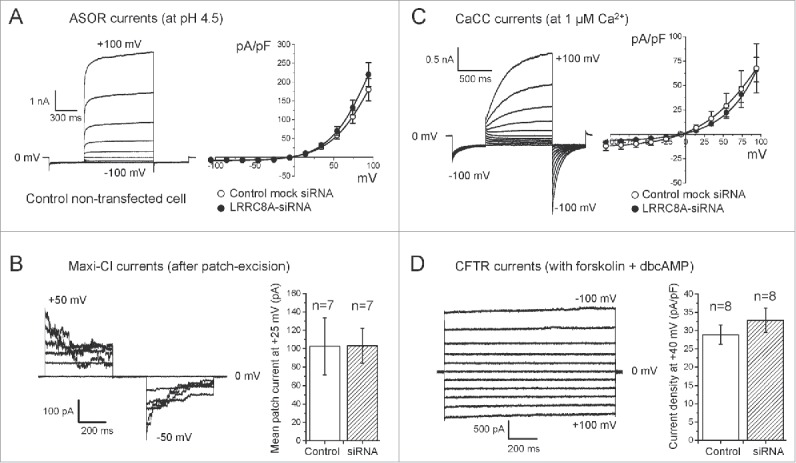
No significant effects of siRNA for LRRC8A on 4 other Cl− channel currents in C127/CFTR cells. (A) Effects of siRNA for LRRC8A on the acid-sensitive outwardly rectifying (ASOR) anion channel currents. Left panel: Representative whole-cell ASOR current responses to voltage steps. ASOR currents were evoked by a low-pH stimulation (pH 4.5). Whole–cell currents were elicited by a pulse-protocol same as in Figure 1B. Right panel: Current-to-voltage relationships in cells treated with non-targeting siRNA (Mock control; open circles) and in cells treated with siRNA against LRRC8A (filled circles). Currents were measured at the end of test pulses from current recordings similar to those shown on the left panel. (B) Effects of siRNA for LRRC8A on the maxi-conductance Cl− channel (Maxi-Cl) currents. Left panel: Representative Maxi-Cl current responses to voltage steps recorded after full activation upon patch excision (inside-out mode) from the cells transfected with non-targeting siRNA (Mock control). The holding potential was 0 mV. Currents were elicited by application of step pulses (500 ms) from −50 to +50 mV in 10-mV increments. Right panel: Mean values of macropatch Maxi-Cl current measured at +25 mV after full activation upon patch excision from mock-transfected (open column) and LRRC8A-siRNA transfected cells (hatched column). (C) Effects of siRNA for LRRC8A on the Ca2+-activated Cl− channel (CaCC) currents. Left panel: Representative whole-cell CaCC current responses to voltage steps (a pulse-protocol same as in Figure 1B) in non-transfected control cells. Right panel: Current-to-voltage relationships in cells treated with non-targeting siRNA (Mock control: open circles) and with siRNA against LRRC8A (filled circles). Currents were measured at the end of test pulses from current recordings similar to those shown on left panel. (D) Effects of siRNA for LRRC8A on the cAMP-activated anion channel (CFTR) currents. Left panel: Representative whole-cell CFTR current responses to voltage steps in the cells transfected with non-targeting (Mock control) siRNA. The holding potential was 0 mV. Currents were elicited by application of step pulses (1000 ms) from −100 to +100 mV in 20-mV increments. CFTR currents were activated by bath-application of a cocktail containing forskolin (5 µM) + dibutyryl-cAMP (dbcAMP: 1 mM) in standard isotonic Ringer solution. Right panel: Mean values of CFTR current density recorded at +40 mV in mock-transfected (open column) and LRRC8A-siRNA-transfected cells (hatched column). No statistically significant difference was observed between anion channel currents elicited from mock-transfected and LRRC8A-siRNA-transfected cells.
LRRC8A/D/E is abundantly expressed in a VSOR-deficient cell line
A human cisplatin-resistant cell line, KCP-4, is known to poorly express VSOR activity when compared to its parental cisplatin-sensitive cell line, KB.26,27 Recently, cisplatin resistance in other cell lines has been shown to be associated with loss of LRRC8D16 and with reduced expression of LRRC8A.28,29 Here, we examined whether deficiency of VSOR current generation in KCP-4 cells is related to a lack of or insufficient expression of LRRC8A or LRRC8D. When expression of human LRRC8A mRNA (LRRC8A) was compared between KCP-4 and KB cells by RT-PCR, it was nearly equal (KCP-4/KB = 94.2 ±0 .9%: Fig. 3A). The RT-PCR results were supported by microarray analysis with using isolated mRNAs from VSOR-deficient KCP-4 cells and 4 VSOR-rich human cell lines, including the parental KB cells as well as HeLa, HEK293T and Intestine 407 cells. Expression levels of LRRC8A were not significantly different among 5 human cell lines (Table 1). When expression of LRRC8A protein was monitored by western blotting, its expression level was nearly equal in KCP-4 and KB cells and was not significantly different among 4 human cell lines tested (Fig. 3B). Microarray data indicated that the expression levels of mRNAs of LRRC8D and LRRC8E in KCP-4 cells were not distinguishable from those of parental KB cells as well as of HEK293, HeLa and Intestine 407 cells (Table 1). Taken together, it is concluded that insufficiency of the LRRC8A/D/E gene is not a causal factor for deficiency of VSOR currents in cisplatin-resistant KCP-4 cells.
Figure 3.
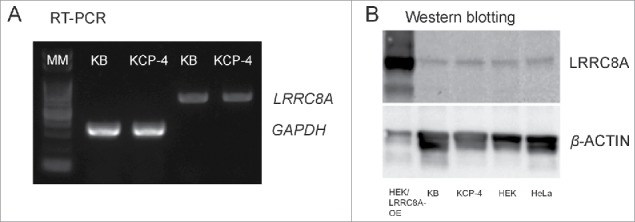
Expression profiles of LRRC8A in KB, KCP-4, HEK293T and HeLa cells. (A) RT-PCR data. The expression level of mRNA of LRRC8A was almost identical between the VSOR-deficient KCP-4 cell line and its parental VSOR-rich KB cell line. Data represent triplicate experiments. GAPDH was used as an internal control. MM: molecular marker. (B) Western blotting data. Expression of LRRC8A protein in KCP-4 was almost identical when compared with 3 other human cell lines, KB, HEK293T (HEK) and HeLa. We used protein samples isolated from LRRC8A-transfected HEK293T cells as a positive control where LRRC8A was overexpressed (HEK/LRRC8A-OE). Data represent duplicate experiments.
Table 1.
Expression levels of mRNA of LRRC8A, LRRC8D and LRRC8E monitored by microarray assays in KCP-4 cells and 4 other human cell lines.
| Gene_Symbol | Gene_Description | KCP-4_signal | KB_signal | HEK293T_signal | HeLa_signal | Intestine 407_signal |
|---|---|---|---|---|---|---|
| LRRC8A | leucine rich repeat containing 8 family, member A | 554.73 | 443.51 | 304.35 | 523.4 | 517.19 |
| (Ratio) | (1) | (0. 80) | (0.55) | (0.94) | (0.93) | |
| LRRC8D | leucine rich repeat containing 8 family, member D | 1157.35 | 1241.54 | 1294.62 | 1325.61 | 1595.98 |
| (Ratio) | (1) | (1.07) | (1.12) | (1.15) | (1.38) | |
| LRRC8E | leucine rich repeat containing 8 family, member E | 99.046 | 111.85 | 77.82 | 97.86 | 151.35 |
| (Ratio) | (1) | (1.13) | (0.79) | (0.99) | (1.53) |
Exogenous expression of LRRC8 proteins failed to restore VSOR activity in a VSOR-deficient cell line
Next, we examined the subcellular localization of LRRC8A by transfecting GFP-tagged LRRC8A. Confocal microscopic observations revealed that a substantial part of LRRC8A-GFP protein located in the cell periphery in both KB and KCP-4 cells (Fig. 4A: green). When the plasma membrane was visualized by counterstaining with the Cell Mask™ Orange Plasma Membrane Stain (Fig. 4A: red), LRRC8A-GFP proteins look largely localized at the plasma membrane (Fig. 4A: merged yellowish).
Figure 4.
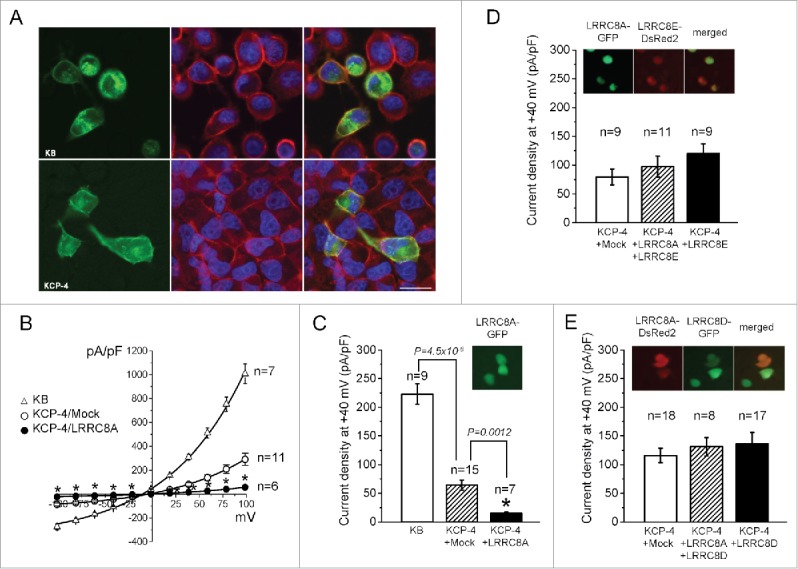
Expression of exogenous LRRC8A and its suppressing, but not augmenting, effect on VSOR currents in KCP-4 cells. (A) GFP-tagged LRRC8A overexpressed in KB cells (upper row) and KCP-4 cells (lower row) was observed under a confocal microscope at 1 day after transfection. The plasma membrane was stained by Cell Mask™ Orange Plasma Membrane Stain (red) and nuclei by Hoechst stain (blue). (B) Current-to-voltage relationships of VSOR in KB cells (triangles) and KCP-4 cells (circles) transfected with mock (open circles) and with LRRC8A-IRES-EGFP vector (filled circles). Current recordings were performed as described in Figure 1B. (C) Mean values of VSOR current density recorded at +40 mV in KB cells (open column), mock-transfected KCP-4 cells (hatched column) and LRRC8A-IRES-EGFP vector-transfected KCP-4 cells (filled column). Effective transfection of LRRC8A was confirmed by GFP fluorescence (Inset). (D) Mean values of VSOR current density recorded at +40 mV in mock-transfected KCP-4 cells (open column) and in KCP-4 cells transfected with a combination of LRRC8A-IRES-EGFP and LRRC8E-IRES-DsRed2 vectors (hatched column) or in KCP-4 cells with LRRC8E-IRES-DsRed2 vector alone (filled column). No significant difference was observed compared to Mock control, while effective transfections of LRRC8A and LRRC8E were confirmed by GFP and DsRed2 fluorescence (Inset). (E) Mean values of VSOR current density recorded at +40 mV in KCP-4 cells transfected with mock (open column) and with a combination of LRRC8A-IRES-DsRed2 and LRRC8D-IRES-EGFP vectors (hatched column) or with LRRC8D-IRES-EGFP vector alone (filled column). No significant difference was observed as compared with mock control, while effective transfections of LRRC8A and LRRC8D were confirmed by DsRed2 and GFP fluorescence (Inset).
Consistent with our previous observations,26,27 endogenous VSOR currents in mock transfected KCP-4 cells were markedly smaller compared to those in parental KB cells (Fig. 4B, C). We then transfected pCMV-LRRC8A-ires-EGFP vector to KCP-4 cells and recorded their VSOR currents by whole-cell patch-clamp recordings. Overexpression of LRRC8A failed to rescue, but instead significantly reduced the swelling-activated Cl− currents in VSOR-deficient KCP-4 cells (Fig. 4B, C). The latter result appears paradoxical but is consistent with the previous observations that overexpression of LRRC8A rather suppressed endogenous VSOR currents in VSOR-rich human cells.13,14,20,30
LRRC8 family consists of 5 members denoted as A, B, C, D and E. LRRC8E was found to be one of the essential VSOR components necessary for its rapid inactivation at high depolarizing positive potentials in human colonic tumor HCT116 cells.13,31 In our experiments, coexpression of LRRC8A with LRRC8E or overexpression of LRRC8E alone did not affect significantly the amplitude of VSOR currents in KCP-4 cells (Fig. 4D). Similar results were obtained also with LRRC8D (Fig. 4E), which has recently been identified as a necessary component of the VSOR-related transport system for blasticidin32 and Pt-derived anti-cancer drugs, cisplatin and carboplatin.16 Thus, it is concluded that deficiency of VSOR activity in cisplatin-resistant KCP-4 cells is caused by an as-yet-unidentified factor other than LRRC8A/D/E genes.
Discussion
The molecular identity of VSOR has been debated over the past 25 y. Although several candidates, including P-glycoprotein, pICln and ClC-3, have been suggested, all of them were eventually dismissed. LRRC8A is a recently proposed molecule for the core component of VSOR in human cell lines (see for reviews refs.2,35-37). In a mouse mammary cell line C127, in the present study, we observed that swelling-activated VSOR currents were significantly reduced by siRNA-mediated knockdown of LRRC8A (Fig. 1). This result shows that LRRC8A is actually important for VSOR currents in mouse cells, as was previously reported for human cells.13,14 VSOR activity is known to be involved in swelling-induced release of several organic compounds.33,37-40 Recently, siRNA-mediated knockdown of LRRC8A was found to reduce the swelling-induced release of glutamate and taurine from rat astrocytes presumably mediated via VSORs,41 suggesting an involvement of LRRC8A in VSOR activity in rodent cells. In fact, here, we have provided direct evidence for the LRRC8A role in VSOR activity in murine cells.
Because the molecular entities corresponding to ASOR and Maxi-Cl are still unidentified,22,42 we were especially interested in a possible molecular relationship between LRRC8A and ASOR or Maxi-Cl. However, here, no significant effect of its knockdown on the currents of ASOR or Maxi-Cl was observed in mouse C127/CFTR cells (Fig. 2A, B). Studies hitherto performed have excluded a number of molecules including ClC-3a,22 ClC-3d,12 ClC-7,43 and TMEM16A/D/F/H/K44 from the ASOR candidates. Also, from the Maxi-Cl candidates, we have ruled out VDAC,45 the human TTYH1 gene homolog to a Drosophila gene called tweety,1 and pannexin 1/2.46 The present study provided clear evidence that LRRC8A is not related to the candidate molecules for ASOR and Maxi-Cl. The currents of CFTR and CaCC, the molecular entities of which are well established,47-49 were also unaffected by knockdown of Lrrc8a in C127/CFTR cells (Fig. 2C, D). Therefore, it is likely that LRRC8A is not involved in regulation of CFTR and CaCC activities.
mRNAs of LRRC8A are expressed in the VSOR-deficient, cisplatin-resistant human cell line, KCP-4, at a level nearly equivalent with that in a parental VSOR-rich, cisplatin-sensitive cell line, KB (Fig. 3A and Table 1). The LRRC8A protein expression level in KCP-4 was also similar to those in other VSOR-rich cell lines (Fig. 3B). Thus, clear correlation does not exist between expression levels of LRRC8A and VSOR current in KCP-4 cells. In addition, observations of subcellular localization suggested that transfer of LRRC8A protein to plasma membrane may be normal in KCP-4 cells (Fig. 4A). Furthermore, mRNA levels of LRRC8D and LRRC8E in KCP-4 cells were also nearly the same as that in KB cells and comparable to those in VSOR-rich HEK293T, HeLa and Intestine 407 cells (Table 1). Thus, it appears that VSOR current deficiency in KCP-4 cells is due to some differences in posttranscriptional modifications such as glycosylation and phosphorylation of LRRC8A/D/E proteins or due to aberrations in some essential factor(s) other than LRRC8A/D/E.
Exogenous LRRC8A did not rescue VSOR currents in a cell line with disruption of all 5 LRRC8 genes (LRRC8−/−),13 suggesting that LRRC8A alone cannot compose the functional channel. Previous reports also showed the possibility that LRRC8A forms a heteromer with other LRRC8 family member(s).13,15 Actually, co-transfection of LRRC8A together with other LRRC8 family members rescued VSOR currents in LRRC8−/− cells,13 indicating that generation of VSOR currents requires combinatorial expression of LRRC8A and its paralogs. Also, a multimeric complex of LRRC8A with its paralogs was found to form swelling-activated anion channels, when reconstituted in lipid bilayers.15 However, it must be noted that several fundamental properties of this channel, including lack of intracellular ATP dependence and of inactivation kinetics at large positive potentials, are distinct from those of VSOR. Moreover, combinatory overexpression of LRRC8A with any other isoform was reported to fail to increase VSOR activity above wild-type values in HEK293 cells.13 Furthermore, in the present study, combinatory overexpression of LRRC8A with LRRC8D or LRRC8E could not restore VSOR activity in cisplatin-resistant KCP-4 cells (Fig. 4D, E). These facts may indicate that, in addition to LRRC8A and its paralogs, some as-yet-unidentified component, X, is required for VSOR activity. Figure 5A schematically depicts a hypothetical model of the VSOR pore domain, which is formed by LRRC8A (A), LRRC8B/C/D/E (non-A) and an as-yet-unidentified essential component (X), by assuming a hexameric structure of LRRC8A-containing pore.50
Figure 5.
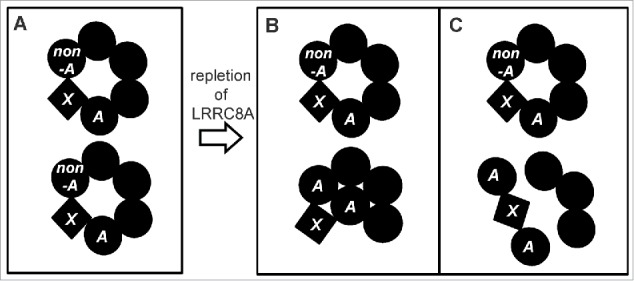
Hypothetical model of the VSOR pore domain depicted by presuming a heteromeric hexamer structure before (A) and after (B, C) repletion of LRRC8A. A and non-A represent LRRC8A and LRRC8B/C/D/E, respectively, and X represents an as-yet-unidentified essential component. Here, we assume that incorporation of the A-X-A complex into the VSOR pore domain results in closure (B) or disruption of the VSOR pore (C).
Whereas LRRC8A is irrefutably linked to VSOR current generation, overexpression of LRRC8A has been found to surprisingly diminish native VSOR currents in VSOR-rich human cells,13,14,20,30 apparently in an LRR domain-independent manner.30 In the present study, overexpression of LRRC8A similarly reduced natively small VSOR currents in KCP-4 cells (Fig. 4B, C). This paradoxical effect may be explained by a U-shaped concentration dependence of transfected LRRC8A. It is, however, noted that LRRC8D or LRRC8E coexpressed with LRRC8A abolished the suppressive effect of transfection of LRRC8A alone (Fig. 4D, E: cf. Fig. 4C). A possible explanation could be that the interaction of LRRC8A with LRRC8E or LRRC8D prevents redundant LRRC8A proteins from suppressing the endogenous VSOR activity by some mechanisms mediated by protein-protein interactions between LRRC8A and LRRC8D/E and presumably X as well. When repletion of LRRC8A was induced by overexpression, protein-protein interactions between LRRC8A, LRRC8A and X would predominate over those between LRRC8A, LRRC8D/E and X, thereby somehow inducing destruction of the VSOR pore which is normally maintained by protein-protein interactions between LRRC8A, LRRC8B/C/D/E and X (Fig. 5A). Figures 5B and 5C depict 2 possible examples of such mechanisms by assuming that the LRRC8A-X-LRRC8A complex exerts a pore-closing or -disrupting action.
The molecular mechanisms by which LRRC8 family members contribute to VSOR currents must be studied in further details. In any case, the present study clearly shows that VSOR activity essentially depends not only on LRRC8 family members but also on some other additional component(s), and that deficiency of VSOR activity in cisplatin-resistant KCP-4 cells is independent of expression of the LRRC8A/D/E genes.
Materials and methods
Cell culture
For patch clamp experiments, cells were grown on glass coverslips and were transferred to the chamber immediately before experiments. Murine mammary C127 cells and the cells stably transfected with the cDNA for human cystic fibrosis transmembrane conductance regulator (CFTR) protein (C127/CFTR cells)25 as well as human adenocarcinoma cell lines, KB and KCP-4, were used. Those were cultured in DMEM (Nissui, Tokyo, Japan) supplemented with 100 U/ml penicillin plus 100 μg/ml streptomycin as well as with 10% fetal calf serum or fetal bovine serum at 37°C with 5% CO2. In the case of KCP-4 cells, cisplatin (23 µM) was added in the culture medium. For RT-PCR, western blot, microarray analysis and histochemical observations, KB and KCP-4 cells were provided. Human HeLa, HEK293T and Intestine 407 cells were also cultured in the same medium and provided for microarray analysis. In double/single LRRC8 protein expression experiments, only GFP- and/or DsRed2-posotive cells (green and/or red fluorescence) were selected under a microscope for patch-clamp. In these experiments, we employed a bi-cistronic vector expression system where both LRRC8 gene products and fluorescent reporter gene products are translated from a single mRNA ensuring identification of target protein-expressing cells with 100% efficiency.51
siRNA transfection
C127 and C127/CFTR cells sparsely seeded on 12-well plates were transfected with siRNA against mouse Lrrc8A (#MSS280623, Invitrogen/Thermo Fisher Scientific, Carlsbad, CA) at a concentration of 50 nM using HiPerFect transfection reagent (Qiagen, Hilden, Germany). One day after transfection, the cells were washed with culture medium and grown in the same medium for 2 d at 37°C before use for RNA isolation or patch-clamp experiments. Stealth RNAi™ siRNA Negative Control (Invitrogen/Thermo Fisher Scientific) was also transfected into C127 cells as a mock control.
Validation of knockdown efficiency
The effect of siRNA-mediated knockdown on Lrrc8a expression was assessed by RT-PCR at 2 d after transfection. Total RNA was isolated from control or gene-specific siRNA-transfected cells using Sepasol RNA I reagent (Nacalai Tesque, Kyoto, Japan). Contamination of genomic DNA in total RNA was removed by DNase1 treatment (RT-grade: Nippongene, Tokyo, Japan). The cDNA was synthesized using Primscript™II 1st strand cDNA synthesis kit (Takara Bio, Shiga, Japan). Semi-quantitative RT-PCR was carried out using KOD+ DNA polymerase (TOYOBO, Tokyo, Japan) with a following primer set for Lrrc8a: 5′-gagcgcctctacctgaacc-3′ (forward primer) and 5′-ggagttctgggagaagctaggc-3′ (reverse primer). Gapdh was also amplified as an internal control using a following primer set: 5′-gcacagtcaaggccgagaat-3′ (forward) and 5′-ttcaccaccatggagaaggc-3′ (reverse).
Cloning and vector construction
To clone the full-coding sequence of LRRC8A gene, we performed nested PCR using the following PCR primer set with the sequences of 5′-atagcagagccatccttgg-3′ (1st, forward), 5′-agttctgggagaagctagg-3′ (1st, reverse) and 5′-cacaaccatgattccggtgacagagc-3′ (2nd, forward), and 5′-tcaggcctgctccttgtcagc-3′ (2nd, reverse). The cDNA was prepared from KB cells as mentioned above. PCR products were cloned into the pGEM®-T Easy vector (Promega, Madison, WI) and sequenced. To generate expression vector for electrophysiological studies in several cell lines, the cDNA of LRRC8A was subcloned into the Sal1-Sac2 site of CMV-pIRES2-EGFP vector or CMV-pIRES-DsRed2 vector (Clontech/Takara, Mountain View, CA). To observe subcellular distribution of LRRC8A, we tagged GFP to the C terminus of LRRC8A. The Sac1 site was added to 5′ end of coding sequence of LRRC8A by PCR using a primer set: 5′-cacaaccatgattccggtgacagagc-3′ (forward) and 5′-ccgcggggcctgctccttgtcagc-3′ (reverse). Then it was subcloned into the Sal1-Sac2 site of pEGFP-N1 vector (Clontech/Takara). We also cloned LRRC8E and LRRC8D using following primer sets: for LRRC8E, 5′-cacaaccatgatcccagtggccgagttc-3′ (forward) and 5′-tcattcctcctccatcttgtcc-3′ (reverse); and for LRRC8D, 5′-ttggtccaggaatgtttacc-3′ (1st, forward), 5′-gttcctgcacatcactgtgc-3′ (1st, reverse), 5′-cacaaccatgtttacccttgcggaag-3′ (2nd, forward), and 5′-tgcatatattatcttagtttaaatcc-3′ (2nd, reverse). Then, LRRC8D and LRRC8E were subcloned into CMV-pIRES2-EGFP vector or CMV-pIRES-DsRed2 vector, respectively.
Comparison of LRRC8 expression
Expression levels of mRNA of LRRC8 family members in human cell lines were analyzed by semi-quantitative RT-PCR and gene microarray. For RT-PCR analysis, cDNAs of KB and KCP-4 cells were prepared as described in Validation of knockdown efficiency. Semi-quantitative RT-PCR was carried out using a following primer set for LRRC8A; 5′-ttgtgtaccgcctctacatgc-3′ (forward primer) and 5′-agtgtcttcaggctatagatcc-3′ (reverse primer). GAPDH was also amplified as an internal control using a following primer set: 5′-gtcatccctgagctgaacgg-3′ (forward) and 5′-gggtcttactccttggaggc-3′ (reverse).
For microarray analysis, the total RNA samples were isolated from 5 human cell lines (HeLa, KB, KCP-4, HEK293T, and Intestine 407) by Sepasol RNA I reagent (Nacalai Tesque) and purified by RNeasyR Minikit (Qiagen), and the gene expression analysis was performed using Affymetrix GeneChipR Mouse Gene 1.0 ST Array (Affymetrix, Santa Clara, CA). Microarray analysis was supported by Cell Innovator, Ltd (Fukuoka, Japan).
The expression level of LRRC8A protein in human cell lines was analyzed by western blotting. Cells were lysed using RIPA buffer (Chromo Tec, Hauppauge, NY) and incubated on ice for 30 min. The cell lysates were passed several times through a 23-gauge needle with syringe and further fractured by brief sonication. Then lysates were incubated on ice for 30 min and centrifuged at 10,000g for 10 min. The supernatants were boiled in Tris-glycine SDS sample buffer (2x) (Invitrogen/Thermo Fisher Scientific) at 95°C for 5 min. 5-15 µl of samples were loaded on 12% precast gel (Mini-protean® TGX™: BIO-RAD, Hercules, CA) and transferred to blotting membrane using iBLOT Blotting system (Invitrogen/Thermo Fisher Scientific). LRRC8A proteins were detected using anti-LRRC8A monoclonal antibody (1/1,000, Sigma-Aldlich, St. Louis, MO) with ECL™ western Blotting Analysis System (Amersham™/GE Healthcare, Buckinghamshire, UK).
Observations of LRRC8A subcellular localization
Subcellular localization of GFP-tagged LRRC8A was observed in living KCP-4 and KB cells using a confocal laser microscope (A1Rs: Nikon, Tokyo, Japan). pEGFP-N1-LRRC8A vector was transfected to the cells using Lipofectamine 3000 (Invitrogen/Thermo Fisher Scientific) according to manufacturer's instructions. To visualize plasma membrane, cells were counterstained with Cell Mask™ Orange Plasma Membrane Stain (1:1000 in culture medium; Molecular Probes/Thermo Fisher Scientific, Eugene, OR) for 30 min and washed twice with culture medium before observations.
Electrophysiology
Standard Ringer solution contained (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 Na-HEPES, 6 HEPES, and 5 glucose (pH 7.4, 290 mosmol/kg-H2O). For measurements of VSOR currents, hypotonic solution was prepared by reducing the NaCl concentration to 110 mM (244 mosmol/kg-H2O, 84% hypotonic). For measurements of whole-cell ASOR currents, standard isotonic Ringer solution was supplemented with 6 mM MES, and the pH value was adjusted at 7.4 with NaOH or at 4.5 with HCl. For measurements of whole-cell CFTR currents, the standard Ringer solution was supplemented with 5 µM forskolin and 1 mM dibutyryl-cAMP. The standard pipette solution for whole-cell experiments contained (in mM): 125 CsCl, 2 CaCl2, 1 MgCl2, 3 Na2ATP, 5 HEPES (pH 7.4 adjusted with CsOH), and 10 EGTA (pCa 7.65; 275 mosmol/kg-H2O). For measurements of whole-cell CaCC currents, the free Ca2+ concentration of the pipette solution was adjusted at 1 μM by adding 4.62 mM CaCl2. For measurements of Maxi-Cl currents in excised inside-out patches, patch pipettes were filled with standard Ringer solution. Osmolality of experimental solutions was measured with a vapor pressure osmometer VAPOR 5600 (WESCOR, South Logan, UT).
Patch electrodes were fabricated from borosilicate glass capillaries (outer diameter 1.4 mm, inner diameter 1.0 mm) with a micropipette puller (model P-97; Sutter Instruments, Novato, CA) and had a tip resistance of 2–4 MΩ when filled with pipette solution. Fast and slow capacitative transients were routinely compensated for. For whole-cell recordings, the access resistance did not exceed 10 MΩ and was always compensated for by 70–80%. Membrane currents were measured with an EPC-9 patch-clamp system (Heka-Electronics, Lambrecht/Pfalz, Germany) or with an Axopatch 200A patch-clamp amplifier coupled to a Digidata 1320 interface (Axon Instruments, Union City, CA). Data acquisition and analysis were done using Pulse+PulseFit (Heka-Electronics) or with pClamp software (version 9.0.2; Axon Instruments) and WinASCD software (kindly provided by Dr. G. Droogmans, KU Leuven, Belgium). The membrane potential was controlled by shifting the pipette potential (Vp) and is reported as Vp for whole-cell recordings. Currents were filtered at 1 kHz and sampled at 5–10 kHz. When appropriate, off-line correction was made for a shift in bath electrode potential upon applying hypotonic solution (calculated from Nernst equation) as well as for changes in liquid junction potentials calculated using pCLAMP 8.1 (Axon Instruments, Foster, CA) algorithms. All experiments were performed at room temperature (23−25°C).
Data analysis
Data were analyzed by OriginPro 7-8 (MicroCal Software, Northampton, MA). Pooled data are given as means ± SEM of n observations. Statistical differences of the data were evaluated by ANOVA and the paired or unpaired Student's t test where appropriate, and considered significant at P < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank K. Sato-Numata for discussion and K. Shigemoto for technical assistance.
Funding
This work was supported by the JSPS KAKENHI grants-in-aid (Numbers 15K15028 and 25670112 to Y.O. and Number 16K08510 to T.O.). M.R.I. was supported by JSPS Postdoctoral Fellowship.
References
- [1].Okada Y, Sato K, Toychiev AH, Suzuki M, Dutta AK, Inoue H, Sabirov RZ. The puzzles of volume-activated anion channels In Physiology and Pathology of Chloride Transporters and Channels in the Nervous System. From Molecules to Diseases, Alvarez-Leefmans FJ, Delpire E, eds. (San Diego: Elsevier; ) 2009; pp. 283-306 [Google Scholar]
- [2].Pedersen SF, Okada Y, Nilius B. Biophysics and physiology of the volume-regulated anion channel (VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR). Pflugers Arch Eur J Physiol 2016; 468:371-83; http://dx.doi.org/ 10.1007/s00424-015-1781-6 [DOI] [PubMed] [Google Scholar]
- [3].Valverde MA, Diaz M, Sepúlveda FV, Gill DR, Hyde SC, Higgins CF. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature 1992; 355:830-3; PMID:1371598; http://dx.doi.org/ 10.1038/355830a0 [DOI] [PubMed] [Google Scholar]
- [4].Paulmichl M, Li Y, Wickman K, Ackerman M, Peralta E, Clapham D. New mammalian chloride channel identified by expression cloning. Nature 1992; 356:238-41; PMID:1313151; http://dx.doi.org/ 10.1038/356238a0 [DOI] [PubMed] [Google Scholar]
- [5].Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature 1997; 390:417-21; PMID:9389484; http://dx.doi.org/ 10.1038/37151 [DOI] [PubMed] [Google Scholar]
- [6].Voets T, Buyse G, Tytgat J, Droogmans G, Eggermont J, Nilius B. The chloride current induced by expression of the protein pICln in Xenopus oocytes differs from the endogenous volume-sensitive chloride current. J Physiol 1996; 495:441-7; PMID:8887755; http://dx.doi.org/ 10.1113/jphysiol.1996.sp021605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buyse G, Voets T, Tytgat J, De Greef C, Droogmans G, Nilius B, Eggermont J. Expression of human pICln and ClC-6 in Xenopus oocytes induces an identical endogenous chloride conductance. J Biol Chem 1997; 272:3615-21; PMID:9013613; http://dx.doi.org/ 10.1074/jbc.272.6.3615 [DOI] [PubMed] [Google Scholar]
- [8].Tominaga M, Tominaga T, Miwa A, Okada Y. Volume-sensitive chloride channel activity does not depend on endogenous P-glycoprotein. J Biol Chem 1995; 270:27887-93; PMID:7499263; http://dx.doi.org/ 10.1074/jbc.270.46.27887 [DOI] [PubMed] [Google Scholar]
- [9].Miwa A, Ueda K, Okada Y. Protein kinase C-independent correlation between P-glycoprotein expression and volume sensitivity of Cl− channel. J Membrane Biol 1997; 157:63-9; http://dx.doi.org/ 10.1007/s002329900216 [DOI] [PubMed] [Google Scholar]
- [10].Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bösl MR, Ruether K, Jahn H, Draguhn A, et al.. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 2001; 29:185-96; PMID:11182090; http://dx.doi.org/ 10.1016/S0896-6273(01)00189-1 [DOI] [PubMed] [Google Scholar]
- [11].Weylandt KH, Valverde MA, Nobles M, Raguz S, Amey JS, Diaz M, Nastrucci C, Higgins CF, Sardini A. Human ClC-3 is not the swelling-activated chloride channel involved in cell volume regulation. J Biol Chem 2001; 276:17461-7; PMID:11278960; http://dx.doi.org/ 10.1074/jbc.M011667200 [DOI] [PubMed] [Google Scholar]
- [12].Okada T, Akita T, Sato-Numata K, Islam Md. R, Okada Y. A newly cloned ClC-3 isoform, ClC-3d, as well as ClC-3a mediates Cd2+-sensitive outwardly rectifying anion currents. Cell Physiol Biochem 2014; 33:539-56; PMID:24603049; http://dx.doi.org/ 10.1159/000358633 [DOI] [PubMed] [Google Scholar]
- [13].Voss FK, Ullrich F, Münch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume regulated anion channel VRAC. Science 2014; 344:634-8; PMID:24790029; http://dx.doi.org/ 10.1126/science.1252826 [DOI] [PubMed] [Google Scholar]
- [14].Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 2014; 157:447-58; PMID:24725410; http://dx.doi.org/ 10.1016/j.cell.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Syeda R, Qiu Z, Dubin AE, Murthy SE, Florendo MN, Mason DE, Mathur J, Cahalan SM, Peters EC, Montal M, et al.. LRRC8 proteins form volume-regulated anion channels that sense ionic strength. Cell 2016; 164:499-511; PMID:26824658; http://dx.doi.org/ 10.1016/j.cell.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, et al.. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J 2015; 34:2993-3008; PMID:26530471; http://dx.doi.org/ 10.15252/embj.201592409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sawada A, Takihara Y, Kim JY, Matsuda-Hashii Y, Tokimasa S, Fujisaki H, Kubota K, Endo H, Onodera T, Ohta H, et al.. A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J Clin Invest 2003; 112:1707-13; PMID:14660746; http://dx.doi.org/ 10.1172/JCI18937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kubota K, Kim JY, Sawada A, Tokimasa S, Fujisaki H, Matsuda-Hashii Y, Ozono K, Hara J. LRRC8 involved in B cell development belongs to a novel family of leucine-rich repeat proteins. FEBS Lett 2004; 564:147-52; PMID:15094057; http://dx.doi.org/ 10.1016/S0014-5793(04)00332-1 [DOI] [PubMed] [Google Scholar]
- [19].Kumar L, Chou J, Yee CS, Borzutzky A, Vollmann EH, von Andrian UH, Park SY, Hollander G, Manis JP, Poliani PL, et al.. Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J Exp Med 2014; 211:929-42; PMID:24752297; http://dx.doi.org/ 10.1084/jem.20131379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sato-Numata K, Numata T, Inoue R, Okada Y. Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel. Pflugers Arch Eur J Physiol 2016; 468:795-803; http://dx.doi.org/ 10.1007/s00424-015-1786-1 [DOI] [PubMed] [Google Scholar]
- [21].Sato-Numata K, Numata T, Inoue R, Sabirov RZ, Okada Y. Distinct contributions of LRRC8A and its paralogs to the VSOR anion channel from those of the ASOR anion channel. Channels 2017; 11(2): TK; PMID:27579940; http://dx.doi.org/ 10.1080/19336950.2016.1230574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sato-Numata K, Numata T, Okada T, Okada Y. Acid-sensitive outwardly rectifying (ASOR) anion channels in human epithelial cells are highly sensitive to temperature and independent of ClC-3. Pflugers Arch Eur J Physiol 2013; 465:1535-43; http://dx.doi.org/ 10.1007/s00424-013-1296-y [DOI] [PubMed] [Google Scholar]
- [23].Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008; 322:590-4; PMID:18772398; http://dx.doi.org/ 10.1126/science.1163518 [DOI] [PubMed] [Google Scholar]
- [24].Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol 2001; 118:251-66; PMID:11524456; http://dx.doi.org/ 10.1085/jgp.118.3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl− conductances in murine C127 cells. J Physiol 2000; 523:1-11; PMID:10673540; http://dx.doi.org/ 10.1111/j.1469-7793.2000.t01-6-00001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee EL, Shimizu T, Ise T, Numata T, Kohno K, Okada Y. Impaired activity of volume-sensitive Cl− channel is involved in cisplatin resistance of cancer cells. J Cell Physiol 2007; 211:513-21; PMID:17186499; http://dx.doi.org/ 10.1002/jcp.20961 [DOI] [PubMed] [Google Scholar]
- [27].Shimizu T, Lee EL, Okada Y. Volume-sensitive Cl− channel as a regulator of acquired cisplatin resistance. Anticancer Res 2008; 28:75-84; PMID:18383827 [PubMed] [Google Scholar]
- [28].Sørensen BH, Nielsen D, Thorsteinsdottir UA, Hoffmann EK, Lambert IH. Downregulation of LRRC8A protects human ovarian and alveolar carcinoma cells against cisplatin-induced expression of p53, MDM2, p21Waf1/Cip1, and caspase-9/-3 activation. Am J Physiol Cell Physiol 2016; 310:C857-73; PMID:26984736; http://dx.doi.org/ 10.1152/ajpcell.00256.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sørensen BH, Dam CS, Stürup S, Lambert IH. Dual role of LRRC8A-containing transporters on cisplatin resistance in human ovarian cancer cells. J Inorg Biochem 2016; 160:287-95; PMID:27112899; http://dx.doi.org/ 10.1016/j.jinorgbio.2016.04.004 [DOI] [PubMed] [Google Scholar]
- [30].Sirianant L, Wanitchakool P, Ousingsawat J, Benedetto R, Zormpa A, Cabrita I, Schreiber R, Kunzelmann K. Non-essential contribution of LRRC8A to volume regulation. Pflugers Arch Eur J Physiol 2016; 468:805-16; http://dx.doi.org/ 10.1007/s00424-016-1789-6 [DOI] [PubMed] [Google Scholar]
- [31].Ullrich F, Reincke SM, Voss FK, Stauber T, Jentch TJ. Inactivation and anion selectivity of volume-regulated VRAC channels depend on carboxy-terminal residues of the first extracellular loop. J Biol Chem 2016; 291:17040-8; PMID:27325695; http://dx.doi.org/ 10.1074/jbc.M116.739342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee CC, Freinkman E, Sabatini DM, Ploegh HL. The protein synthesis inhibitor blasticidin S enters mammalian cells via leucine-rich repeat-containing protein 8D. J Biol Chem 2014; 289:17124-31; PMID:24782309; http://dx.doi.org/ 10.1074/jbc.M114.571257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol 1997; 273:C755-89 [DOI] [PubMed] [Google Scholar]
- [34].Pedersen SF, Klausen TK, Nilius B. The identification of a volume-regulated anion channel: an amazing Odyssey. Acta Physiol (Oxf) 2015; 213:868-81; PMID:25565132; http://dx.doi.org/ 10.1111/apha.12450 [DOI] [PubMed] [Google Scholar]
- [35].Jentsch TJ. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 2016; 17:293-307; PMID:27033257; http://dx.doi.org/ 10.1038/nrm.2016.29 [DOI] [PubMed] [Google Scholar]
- [36].Jentsch TJ, Lutter D, Planells-Cases R, Ullrich F, Voss FK. VRAC: molecular identification as LRRC8 heteromers with differential functions. Pflugers Arch Eur J Physiol 2016; 468:385-93; http://dx.doi.org/ 10.1007/s00424-015-1766-5 [DOI] [PubMed] [Google Scholar]
- [37].Mongin AA. Volume-regulated anion channel - a frenemy within the brain. Pflugers Arch Eur J Physiol 2016; 468:421-41; http://dx.doi.org/ 10.1007/s00424-015-1765-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol 1996; 270:711-30 [DOI] [PubMed] [Google Scholar]
- [39].Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 2009; 89:193-277; PMID:19126758; http://dx.doi.org/ 10.1152/physrev.00037.2007 [DOI] [PubMed] [Google Scholar]
- [40].Akita T, Okada Y. Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience 2014; 275:211-31; PMID:24937753; http://dx.doi.org/ 10.1016/j.neuroscience.2014.06.015 [DOI] [PubMed] [Google Scholar]
- [41].Hyzinski-García MC, Rudkouskaya A, Mongin AA. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J Physiol 2014; 592:4855-62; http://dx.doi.org/ 10.1113/jphysiol.2014.278887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sabirov RZ, Merzlyak PG, Islam Md.R, Okada T, Okada Y. The properties, functions and pathophysiology of maxi-anion channels. Pflugers Arch Eur J Physiol 2016; 468:405-20; http://dx.doi.org/ 10.1007/s00424-015-1774-5 [DOI] [PubMed] [Google Scholar]
- [43].Sato K, Numata T, Lee E, Okada Y. Exploration of the molecular identity of acid-sensitive outwardly rectifying Cl− channel (ASOR). J Physiol Sci 2012; 62 (Supplement 1):S50 [Google Scholar]
- [44].Capurro V, Gianotti A, Caci E, Ravazzolo R, Galietta LJV, Zegarra-Moran O. Functional analysis of acid-activated Cl− channels: Properties and mechanisms of regulation. Biochim Biophys Acta - Biomembranes 2015; 1848:105-14; http://dx.doi.org/ 10.1016/j.bbamem.2014.10.008 [DOI] [PubMed] [Google Scholar]
- [45].Sabirov RZ, Sheiko T, Liu H-T, Deng D, Okada Y, Craigen WJ. Genetic demonstration that the plasma membrane maxi-anion channel and voltage-dependent anion channels are unrelated proteins. J Biol Chem 2006; 281:1897-904; PMID:16291750; http://dx.doi.org/ 10.1074/jbc.M509482200 [DOI] [PubMed] [Google Scholar]
- [46].Islam MR, Uramoto H, Okada T, Sabirov RZ, Okada Y. Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells. Am J Physiol Cell Physiol 2012; 303:C924-35; PMID:22785119; http://dx.doi.org/ 10.1152/ajpcell.00459.2011 [DOI] [PubMed] [Google Scholar]
- [47].Hwang TC, Sheppard DN. Gating of the CFTR Cl− channel by ATP-driven nucleotide-binding domain dimerisation. J Physiol 2009; 587:2151-61; PMID:19332488; http://dx.doi.org/ 10.1113/jphysiol.2009.171595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 2008; 134:1019-29; PMID:18805094; http://dx.doi.org/ 10.1016/j.cell.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, et al.. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008; 455:1210-5; PMID:18724360; http://dx.doi.org/ 10.1038/nature07313 [DOI] [PubMed] [Google Scholar]
- [50].Abascal F, Zardoya R. LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. Bioessays 2012; 34:551-60; PMID:22532330; http://dx.doi.org/ 10.1002/bies.201100173 [DOI] [PubMed] [Google Scholar]
- [51].Trouet D, Nilius B, Voets T, Droogmans G, Eggermont J. Use of a bicistronic GFP-expression vector to characterise ion channels after transfection in mammalian cells. Pflugers Arch 1997; 434:632-8; PMID:9242728; http://dx.doi.org/ 10.1007/s004240050445 [DOI] [PubMed] [Google Scholar]


