ABSTRACT
Plant hormones are a class of organic substances which are synthesized during the plant metabolism. They have obvious physiological effect on plant growth at very low concentrations. Generally, plant hormones are mainly divided into 5 categories: auxins, cytokinins, ethylene, gibberellins (GAs) and abscisic acid (ABA). With the deepening of research, some novel plant hormones such as brassinosteroid and salicylates have been found and identified. The plant hormone products are mainly obtained through plant extraction, chemical synthesis as well as microbial fermentation. However, the extremely low yield in plants and relatively complex chemical structure limit the development of the former 2 approaches. Therefore, more attention has been paid into the microbial fermentative production. In this commentary, the developments and technological achievements of the 2 important plant hormones (GAs and ABA) have been discussed. The discovery, producing strains, fermentation technologies, and their accumulation mechanisms are first introduced. Furthermore, progresses in the industrial mass scale production are discussed. Finally, guidelines for future studies for GAs and ABA production are proposed in light of the current progress, challenges and trends in the field. With the widespread use of plant hormones in agriculture, we believe that the microbial production of plant hormones will have a bright future.
KEYWORDS: abscisic acid, fermentation, filamentous fungi, gibberellins, plant hormones, secondary metabolites
Introduction
The term “hormone” was first proposed by animal physiologist Bayliss and Starling in 1904.1 At that time, the classical definition of a hormone was of a chemical messenger which left one area of the body by the blood stream and arriving at another part of the body, caused a change in behavior there.2 Several years later, by 1910, Fitting formally introduced the word “hormone” into the field of plant physiology. In 1948, through a long time discussion, the term was eventually coined “plant hormones” or “phytohormones” by Thimann referring to trace organic substances which were synthesized in certain parts of the plant and can be transported to other parts to realize the special physiological function.3 With the further research, more and more plant hormones have been identified which greatly enriches our understanding of hormone biosynthesis, metabolism, transport, and signal transduction and directly made the important contribution to the chemical control of crop.4-6 At the same time, with the rapid development of society and increase of population, plant hormones are playing an indispensable role in agriculture, such as promoting fruit ripening and leaf drop, accelerating seed germination and budding, resisting agricultural natural disaster and increasing crop yields, etc.7-10
Plant hormones are found not only in higher plants, but also in algae, and in plant-associated bacteria and fungi.11-13 However, in this case, plant hormones are not the essential small molecular necessary to the growth and reproduction of the microorganisms. Thus they are regarded as secondary metabolites.14 The molecular structures of common plant hormones are shown in Fig. 1. In the early stage, plant hormones are obtained mainly by plant extraction. What is unacceptable is that the hormones concentration in plants is extremely low. For example, only 9 mg abscisic acid (ABA) which is essential to plant growth, can be extracted from 200 kg cotton boll, which contributes to the enormous waste of resources.15 In view of this situation, chemical synthesis gradually begin to be utilized. Nevertheless, due to the relatively complex chemical structure of plant hormones, chemical synthesis suffers from a number of problems such as the complicated operation, high cost and lower purity, etc.16 Compared with the former 2 methods, microbial production of plant hormones overcomes the above shortcomings. The final products obtained through this method not only possess higher bio-activity and purity, but also have a much lower cost. Therefore, microbial fermentative production of these products has broad application prospects.
Figure 1.
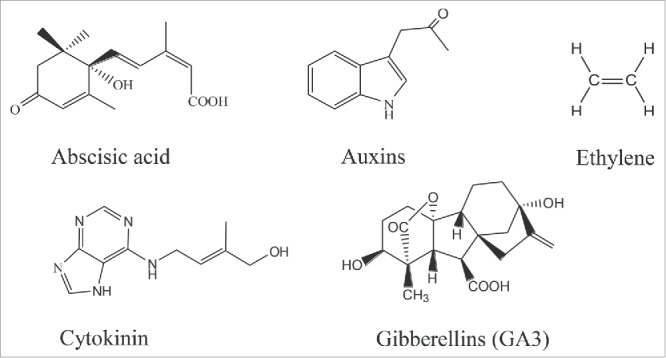
Molecular structures of different plant hormones.
Gibberellins and abscisic acid accumulated as secondary metabolites in filamentous fungi
Gibberellins (GAs) are a family of typically tetracyclic diterpene compounds which can stimulate growth and regulate various developmental processes during the plant's whole life cycle, such as seed germination, stem elongation, sex expression and fruit formation.17 GAs were firstly discovered in 1926 when Kurosawa noticed that a chemical synthesized in Gibberella fujikuroi can contribute to the disease of rice plants.18 In 1934, Yabuta et al. isolated and identified 2 active substance from the filtrate of the culture medium and then named them as “gibberellins A and B.”19 In 1955, Takahashi et al. succeeded in separating GAs A into 3 components, GA1, GA2 and GA3.20 Since then, new GAs components were continuously being discovered. Up till now, more than 136 different GAs have been identified in plants and microorganisms, but only a few of them are biologically active including GA1, GA3, GA4 and GA7.21 Although GAs are widely found in plants, algae, fungi and bacteria, but at present, filamentous fungus G. fujikuroi is mainly utilized as the strain for industrial production due to its relatively high titers. The GAs titer in filamentous fungi is summarized in Table 1. In order to further increase the GAs titer, a large number of work has revolved around the mutagenesis and screening of G. fujikuroi. For example, Lale et al. obtained mutant strains with the shorter mycelium length which greatly improved the level of dissolved oxygen in the fermentation process, the yield of GA3 was nearly 2 times higher than the original strain.22 In addition, the novel fermentation methods, such as cell immobilization and extractive fermentation techniques, were also successfully applied in the microbial production of GAs. Lu and Xie immobilized G. fujikuroi on the surface of the cellulose carrier which could continuously and efficiently produce GA3 in 84 days, the ultimate GA3 titer reached 0.21 g/L.23 Eleazar et al. chose Ca-polygalacturonate as the immobilized carrier in a fluidized bioreactor, the maximum titer reached 3.9 g/L, more than 3-times greater than previously reported.24 The utilization of the extractive fermentation method to produce GA3 can also increase over 2-fold compared with the shake-flask fermentation.44-45 These research greatly laid the foundation for the large-scale production.
Table 1.
Titers of gibberellins and abscisic acid produced by different filamentous fungi.
| Gibberellins | ||
|---|---|---|
| Filamentous fungi | Titer | References |
| G. fujikuroi | 0.21 g/L | 23 |
| G. fujikuroi NRRL2278 | 0.16 g/L | 33 |
| G. fujikuroi CDBB H-984 | 0.29 g/L | 34 |
| G. fujikuroi NCIM 1019 | 0.72 g/L | 35 |
| G. fujikuroi NRRL2284 | 1.68 g/L | 36 |
| G.fujikuroi 978# | 2.1 g/L | 37 |
| F. moniliforme | 2.4 g/L | 38 |
| G. fujikuroi NRRL2284 | 2.8 g/L | 39 |
| G. fujikuroi CDBB H-984 | 3.9 g/L | 24 |
| Abscisic acid | ||
| C. rosicola CBS 138.35 | 0.060 g/L | 28 |
| C. rosicola CBS 138.35 | 0.027 g/L | 29 |
| C. coerulescens RWD 390 | 3.50 ug/L | 40 |
| A. niger NX-53 | 0.276 g/L | 30 |
| R. solani Rs 4. IU To | 16.9 ng/g | 31 |
| C. coerulescens RWD 390 | 31.0 ng/g | 31 |
| F. oxysporum CBS 758.68 | 31.9 ng/g | 31 |
| B. cinerea Bc 1.Sa | 23.7 ng/g | 31 |
| B. cinerea L-13 | 0.622 g/L | 41 |
| B. cinerea TB-3 | 1.4 g/L | 42 |
ABA is another kind of most important plant growth hormone, which plays a significant role in regulating plant growth, resisting agricultural natural disaster and increasing crop yields. Before its chemical properties were fully known, it was discovered and researched under 2 different names. The first name given was “abscisin II” because it was thought to control the abscission of cotton bolls. At almost the same time, another group named it “dormin” for a purported role in bud dormancy.25 In 1965, Wareing et al. compared the chemical properties of dormin and abscisin II and eventually proved that they were the same substance. Subsequently, the name “abscisic acid” was officially accepted as a replacement for dormin and abscisin II.26 The earliest discovery of ABA in microorganisms occurred in 1969, when Rudnicki et al. isolated a substrate from Penicillium italicum. However, they could not demonstrate whether the ABA was from the filamentous fungus itself.27 In 1977, Assante et al. firstly discovered microbial production of ABA in Cercospora rosicola.28 However, over 30-days fermentation time and extremely low titer limited the development of industrial mass scale production using this fungus. After that, other filamentous fungi, including the genuses of Botrytis, Aspergillus and Rhizopus, were also found to be able to accumulate ABA.29-31 In 1982, Marumo et al. began to utilize Botrytis cinerea as the strain to produce ABA.32 Different from the former, the fermentation time was significantly shortened and the titer was improved. Therefore, more research work has been carried out to mutagenize and screen high yielding strains, explore the complete metabolic pathways of Botrytis cinerea as well as optimize the liquid submerged fermentation technology, which greatly promoted the industrialization of ABA.46
Progress in industrialization
For the industrial production of plant hormones such as GAs and ABA, several processes using fungal fermentation were successfully set up in China. As early as 1950s–1960s, the solid state fermentation of GAs has been applied. However, due to the low yield, tedious operation and other shortcomings, the solid state fermentation was gradually replaced by liquid submerged fermentation, the titer of the process is generally at about 2.0 g/L. At present, in China, some enterprises like Jiangxi New Reyphon Biochemical Co. Ltd, Zhejiang Qianjiang Biochemical Co. Ltd, and Jiangsu Fenyuan Bioengineering Co. Ltd are continuing to explore new fermentation technologies and have made some achievements, which greatly promotes the further applications of GAs in the agricultural field. Compared with GAs, the industrial microbial fermentation of ABA started relatively late. Until the beginning of this century, the scientist in Chengdu Institute of Biology, Chinese Academy of Sciences, firstly solved the key technical problems and successfully obtained ABA overproducing strain. Through the optimization of liquid submerged fermentation process, the ABA maximum production titer was eventually up to 6.0 g/L. Subsequently, the world's first industrial production line of ABA was formally established through the cooperation with the company Sichuan Lomon Bio Co. Ltd, which greatly reduced the cost and price of ABA and further promoted the application of ABA in agriculture.43
Conclusions and perspectives
With the rapid development of society and increase of population, the plant hormones, including both GAs and ABA, are playing a more and more important role in the agricultural field. However, the high price of the microbial derived plant hormones still limited their extensive application. Such as the final low product titer and high consuming energy of the fungal fermentation process, and the low efficiency product extraction method still limited the reduction of the cost and price of the final product and thus restrict the development of the industry. Therefore, in future, much effort is still needed to make the existed fungal process more efficient and economical. With the help of numerous novel research tools and more integrative information based upon studies in genomics, proteomics, metabolomics and systems biology, fungal strains would not only achieve higher titers of GAs and ABA but would also obtain higher GAs and ABA productivities. Furthermore, one of the major challenges to the microbial derived GAs and ABA process will be the development of an efficient and economical downstream process with fewer steps and high efficiency. In summary, the microbial production is a green and sustainable process for the plant hormones (GAs and ABA) production. With the continuous deepening of research, we believe that the microbial production of plant hormones will have a bright future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by the National Science Foundation for Distinguished Young Scholars of China (No. 21225626), the National Natural Science Foundation of China (Nos. 21376002, 21476111), the Jiangsu Provincial Natural Science Foundation of China (No. BK20131405), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- [1].Hertig AT. Allen and Doisy's ‘an ovarian hormone'. J Am Med Asso 1983;250(19):2684-2688; http://dx.doi.org/ 10.1001/jama.1983.03340190086042 [DOI] [PubMed] [Google Scholar]
- [2].Atkinson AB. From then to now: lessons from developments in our understanding of the pituitary gland. Ulster Med J 2010;79(2):89-94; PMID:21116427 [PMC free article] [PubMed] [Google Scholar]
- [3].Thimann KV. Fifty years of plant hormone research. Plant Physiol 1974;54(4):450-453; PMID:16658908; http://dx.doi.org/ 10.1104/pp.54.4.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McSteen P, Zhao Y. Plant hormones and signaling. common themes and new developments. Dev Cell 2008;14:467-473; PMID:18410724; http://dx.doi.org/ 10.1016/j.devcel.2008.03.013 [DOI] [PubMed] [Google Scholar]
- [5].Arshad M, Frankenberger WT Jr. Microbial production of plant hormones. Plant Soil 1991;133(1):1-8; http://dx.doi.org/ 10.1007/BF00011893 [DOI] [Google Scholar]
- [6].Ballingall AM. The use of hormone herbicides for resistance management and control of difficult weeds in cereal crops in the UK (with special reference to Scotland). Julius-Kühn-Archiv 2014;443:268-272. [Google Scholar]
- [7].Lu Q, Zhang W, Gao J, Lu M, Zhang L, Li J. Simultaneous determination of plant hormones in peach based on dispersive liquid-liquid microextraction coupled with liquid chromatography-ion trap mass spectrometry. J Chromatogr B 2015;992:8-13; http://dx.doi.org/ 10.1016/j.jchromb.2015.04.014 [DOI] [PubMed] [Google Scholar]
- [8].Kang J, Yim S, Choi H, Kim A, Lee KP, Lopez-Molina L, Martinoia E, Lee Y. Abscisic acid transporters cooperate to control seed germination. Nat Commun 2015;6(8): 113; PMID:26334616; http://dx.doi.org/ 10.1038/ncomms9113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mer LN. Twenty years of gibberellin research. Nat Prod Rep 2003;20(1):49-69; PMID:12636083; http://dx.doi.org/ 10.1039/b007744p [DOI] [PubMed] [Google Scholar]
- [10].Adato I, Gazit S. Role of ethylene in avocado fruit development and ripening: I. fruit drop. J Exp Bot 2014;28(3):636-643; http://dx.doi.org/ 10.1093/jxb/28.3.636 [DOI] [Google Scholar]
- [11].Lu Y, Xu J. Phytohormones in microalgae: a new opportunity for microalgal biotechnology?. Trends Plant Sci 2015;20(5):273-282; PMID:25697753; http://dx.doi.org/ 10.1016/j.tplants.2015.01.006 [DOI] [PubMed] [Google Scholar]
- [12].Wang C, Liu Y, Li SS, Han GZ Insights into the origin and evolution of the plant hormone signaling machinery.[J]. Plant Physiology, 2015, 167(3):872–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tarakhovskaya ER, Maslov YI, Shishova MF. Phytohormones in algae. Russ J Plant Physl 2007;54(2):163-170; http://dx.doi.org/ 10.1134/S1021443707020021 [DOI] [Google Scholar]
- [14].Rademacher W. Gibberellin formation in microorganisms. Plant Growth Regul 1994;15(15):303-314; http://dx.doi.org/ 10.1007/BF00029903 [DOI] [Google Scholar]
- [15].Lu LZ, Lao XZ, Zheng Y. Abscisic Acid production by fermentation and its application. Amino Acids Biot Resour 2011;33(2):23-26. [Google Scholar]
- [16].Zhang H, Liu J, He W. Screening of abscisic acid producing fungi and optimization of its fermentation conditions. Industrial Microbiol 2008;38(1):49-52. [Google Scholar]
- [17].Bömke C, Tudzynski B. Diversity, regulation, and evolution of the gibberellin biosynthetic pathway in fungi compared to plants and bacteria. Phytochem 2009;70(15-16):1876-93; http://dx.doi.org/ 10.1016/j.phytochem.2009.05.020 [DOI] [PubMed] [Google Scholar]
- [18].Kurosawa E. Experimental studies on the nature of the substance secreted by the “bakanae” fungus. Lancet Infect Dis 2003;3(3):125-126; PMID:12614725; http://dx.doi.org/ 10.1016/S1473-3099(03)00539-5 [DOI] [PubMed] [Google Scholar]
- [19].Yabuta T, Kobe K, Hayashi T. Biochemical studies of the ‘bakanae’ fungus of rice. I. fusarinic acid, a new product of the ‘Bakanae’ fungus. Zeitschrift Für Pflanzenkrankheiten 1934;1059-1068 [Google Scholar]
- [20].Takahashi N, Kitamura H, Kawarada A, Seta Y, Takai M, Tamura S, Sumiki Y. Isolation of gibberellins and their properties. Bull Agric Chem Soc 1955;19:267-277. [Google Scholar]
- [21].Csukasi F, Merchante C, Valpuesta V. Modification of plant hormone levels and signaling as a tool in plant biotechnology. Biotechnol J 2009;4(9):1293-1304; PMID:19585532; http://dx.doi.org/ 10.1002/biot.200800286 [DOI] [PubMed] [Google Scholar]
- [22].Lale G, Jogdand VV, Gadre RV. Morphological mutants of Gibberella fujikuroi for enhanced production of gibberellic acid. J Appl Microbiol 2006;100:65-72; PMID:16405686; http://dx.doi.org/ 10.1111/j.1365-2672.2005.02754.x [DOI] [PubMed] [Google Scholar]
- [23].Lu ZX, Xie ZC, Kumakura M. Production of gibberellic acid in Gibberella fujikuroi adhered onto polymeric fibrous carriers. Process Biochem 1995;30(7):661-665; http://dx.doi.org/ 10.1016/0032-9592(94)00042-5 [DOI] [Google Scholar]
- [24].Eleazar ME, Dendooven L, Magaña IP, Parra R, De la Torre M. Optimization of gibberellic acid production by immobilized Gibberella fujikuroi, mycelium in fluidized bioreactors. J Biotechnol 2000;76:147-155; PMID:10656329; http://dx.doi.org/ 10.1016/S0168-1656(99)00182-0 [DOI] [PubMed] [Google Scholar]
- [25].Davies PJ. The plant hormones: their nature, occurrence, and functions. Plant Hormones 2009;1-11. [Google Scholar]
- [26].Cornforth JW, Milborrow BV, Ryback G, Wareing PF. Isolation of sycamore dormin and its identity with abscisin II. Tetrahedron 1966;22(01):603-610; http://dx.doi.org/ 10.1016/S0040-4020(01)90962-7 [DOI] [Google Scholar]
- [27].Rudnicki R, Borecka H, Pieniaek J. Abscisic acid in Penicillium italicum. Planta 1969;86(2):195-196; PMID:24515793; http://dx.doi.org/ 10.1007/BF00379827 [DOI] [PubMed] [Google Scholar]
- [28].Assante G, Merlini L, Nasini G. (+)-Abscisic acid, a metabolite of the fungus Cercospora rosicola. Experientia 1977;33(12):1556-1557; http://dx.doi.org/ 10.1007/BF01933993 [DOI] [Google Scholar]
- [29].Griffin DH, Walton DC. Regulation of abscisic acid formation in Mycosphaerella (Cercospora) rosicola by Phosphate. Mycologia 1982;74(4):614-618; http://dx.doi.org/ 10.2307/3792749 [DOI] [Google Scholar]
- [30].Hui Z, Shang SH, Liu J, He W, Liu XD, Xie XZ. Screening of abscisic acid producing fungi and studies of its fermentation conditions. J Shenyang Institute Chem Technol 2007;21(3):170-173. [Google Scholar]
- [31].Dörffling K, Petersen W, Sprecher E, Urbasch I, Hanssen HP. Abscisic acid in phytopathogenic fungi of the genera Botrytis, Ceratocystis, Fusarium, and Rhizoctonia. Zeitschrift Für Naturforschung C 2014;39(6):683-684. [Google Scholar]
- [32].Marumo S, Katayama M, Komori E, Ozaki Y, Natsume M, Kondo S. Microbial production of abscisic acid by Botrytis Cinerea. Agricultural Biol Chem 1982;46(7):1967-1968. [Google Scholar]
- [33].Gkdere M, Ate S. Extractive fermentation of gibberellic acid with free and immobilized Gibberella fujikuroi. Prep Biochem Biotechnol 2014;44(1):80-89; PMID:24117154; http://dx.doi.org/ 10.1080/10826068.2013.792275 [DOI] [PubMed] [Google Scholar]
- [34].Rios-Iribe EY, Flores-Cotera LB, Chávira MM, Gonzalez-Alatorre G, Escamilla-Silva EM. Inductive effect produced by a mixture of carbon source in the production of gibberellic acid by Gibberella fujikuroi. World J Microb Biotechnol 2011;27(6):1499-1505; http://dx.doi.org/ 10.1007/s11274-010-0603-4 [DOI] [PubMed] [Google Scholar]
- [35].Lale G, Jogdand VV, Gadre RV. Morphological mutants of Gibberella fujikuroi, for enhanced production of gibberellic acid. J Appl Microbiol 2006;100(1):65-72; PMID:16405686; http://dx.doi.org/ 10.1111/j.1365-2672.2005.02754.x [DOI] [PubMed] [Google Scholar]
- [36].Ruchi S, Chand S, Srivastava AK. Improvement of gibberellic acid production using a model based fed-batch cultivation of Gibberella fujikuroi. Process Biochem 2005;40(6):2045-2050; http://dx.doi.org/ 10.1016/j.procbio.2004.07.017 [DOI] [Google Scholar]
- [37].Wang W. Study on gibberellin production by immobilized Gibberella fujikuroi in calcium alginate. Chin Biotechnol 2012;32(1):36-41. [Google Scholar]
- [38].Meleigy SA, Khalaf MA. Biosynthesis of gibberellic acid from milk permeate in repeated batch operation by a mutant Fusarium moniliforme, cells immobilized on loofa sponge. Bioresour Technol 2009;100(1):374-379; PMID:18684618; http://dx.doi.org/ 10.1016/j.biortech.2008.06.024 [DOI] [PubMed] [Google Scholar]
- [39].Pastrana LM, Gonzalez MP, Murado MA. Production of gibberellic acid from mussel processing wastes in submerged batch culture. Bioresource Technol 1993;45(3):213-221; http://dx.doi.org/ 10.1016/0960-8524(93)90114-Q [DOI] [Google Scholar]
- [40].Jens K, Karl D. Abscisic acid metabolism in Ceratocystis coerulescens. Physiol Plantarum 1987;69(69):278-282. [Google Scholar]
- [41].Liang Y, Zheng H, Wu L, Wang M. Effect of Carotenoids on Abscisic Acid Production of Botrytis Cinerea. Pharma Biotechnol 2004;11(2):96-98. [Google Scholar]
- [42].Tan H, Gong G, Li ZD, Peng SL, Lei BL, Liu DJ, Ding LS High-yield ABA producing strain obtained by UV irradiQ6 ation of protoplasts. Chin J Appl Environ Biol 1998;4(3):281-285. [Google Scholar]
- [43].Tan H, Lei BL. A new process for preparing natural abscisic acid, WO/2008/092297. 2008. [Google Scholar]
- [44].Muge G, Selma AT. Extractive fermentation of gibberellic acid with free and immobilized Gibberella fujikuroi. Prep Biochem Biotechnol 2014;44(1):80-89; PMID:24117154; http://dx.doi.org/ 10.1080/10826068.2013.792275 [DOI] [PubMed] [Google Scholar]
- [45]. Hollmann D, Switalski J, Geipel S, Onken U. Extractive fermentation of gibberellic acid by Gibberella fujikuroi. J Ferm Bioeng 1995;79(6):594-600; http://dx.doi.org/ 10.1016/0922-338X(95)94754-F [DOI] [Google Scholar]
- [46].Siewers V, Smedsgaard J, Tudzynski P. The P450 monooxygenase bcaba1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Appl Environ Microbiol 2004;70(7):3868-3876; PMID:15240257; http://dx.doi.org/ 10.1128/AEM.70.7.3868-3876.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]


