Abstract
Objective
The aim of this study was used meta-analysis to investigate changes of serum tumor necrosis factor-alpha (TNF-α) in patients with type 1 diabetes mellitus (T1DM).
Methods
Relevant literatures were identified from PubMed, Cochrane Library, CNKI, WanFang and Chinese-Cqvip databases (published from January 1, 1999 to September 30, 2016). Eligible reports were included for pooled analysis of serum TNF-α level and subgroup analysis was performed in relation with age, disease duration and ethnicity.
Results
A total of 23 articles (1631 T1DM cases, 1429 healthy controls) were included for this meta-analysis. Compared with the controls, the patients had significantly increased serum TNF-α level (P < 0.001). Similar results were also found among all subgroup analysis of different age, disease duration and ethnicity (with the exception of Asian) (all P < 0.05). Regression analysis indicated that age (P = 0.680), disease duration (P = 0.957), and ethnicity (P = 0.526) of patients were not significant impact factors for the high heterogeneity. The results were stable according to the sensitivity analysis and no publication bias existed in this meta-analysis.
Conclusions
Serum TNF-α level in T1DM patients has significantly elevated among all age, disease duration and ethnicity groups.
Introduction
Type 1 diabetes mellitus (T1DM) is a systemic disease leading to abnormal fat, carbohydrate, and protein metabolism due to insulin deficiency [1]. Metabolic proinflammatory disorder, such as chronic hyperglycemia and increased levels of circulating cytokines, suggests immunological disturbances [2–5], which seriously affects the quality of life of the patients and imposes a large economic burden on the national health care system [6]. The reasons responsible for this disease are almost summarized as genetic and environmental factors.
The role of inflammation in diabetes mellitus (DM) has recently been implicated [7] and that inflammatory reaction, mediated by acute phase proteins and cytokines, could lead to the prevention or promotion of diabetes [8, 9]. Inflammatory cytokines such as interleukine-6 (IL-6) [10, 11], IL-17 [12, 13], transforming growth factor-beta (TGF-β) [14] and C-reactive protein (CRP) [15] have been shown to be elevated in DM patients, and the elevated cytokines play an important role in the development and progression of cardiovascular complications.
Tumor necrosis factor-alpha (TNF-α) produced by activated macrophages, CD4+ lymphocytes, natural killer cells, neutrophils, mast cells, eosinophils and neurons, is a cytokine involved in systemic inflammation and always results in acute phase reaction [16]. TNF-α may induce insulin resistance through direct effects on the insulin signaling pathway, and thus participates in the pathogenesis of type 2 DM and obesity [17–20]. As an endogenous factor, TNF-α not only influences energy balance, but also is associated with weight loss, hypermetabolism and resting energy expenditure in malignant diseases [21]. Many researchers focus on the change of serum TNF-α level [21–26] in DM patients, yet findings are inconsistent. In the present study, we performed a pooled analysis of data to define the change of serum TNF-α in T1DM patients.
Methods
Search strategies
This study was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria [27]. The work described here was performed in accordance with the Declaration of Helsinki. This study was approved by the Ethical Committee of Guilin Medical University. We systematically searched five databases (PubMed, Cochrane Library, CNKI, WanFang and Chinese-Cqvip) about the studies published from January 1, 1999 to September 30, 2016. The search strategy using medical terms as following: (“tumor necrosis factor alpha” or “TNF-α”) and (“type 1 diabetes” or “diabetic patients” or “diabetes mellitus” or “DM” or “T1DM”). Otherwise, we identified the additional reports through references cited in recruited articles.
Inclusion criteria and exclusion criteria
All related articles were reviewed using the criteria as follows: (1) Studies focusing on the change of serum TNF-α level in T1DM patients; (2) Case-control research; (3) Patients used insulin alone; (4) Data expressed as Mean ± SD; and (5) Definitions of T1DM met the criteria recommended by the World Health Organization [28].
Exclusion criteria: (1) For duplicated studies and reports, we only included the latest paper into our final analysis; (2) Animal studies, reviews, editorials, case reports, and personal experience summaries; (3) No healthy controls in the study; (4) Original data displayed as figures or no original data reported; and (5) Inconsistent with the inclusion criteria as described above.
Quality assessment and data extraction
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of all eligible studies [29] and the following information from each eligible study was extracted independently by two reviewers: (1) first author’s surname; (2) date of publication; (3) country of the studied population; (4) mean age of patients; (5) sample size of case-control; (6) mean disease duration of patients; (7) Mean ± SD displayed the level of serum TNF-α. Considering disagreement, we invited the third investigator to assess such articles through discussion.
Statistical analysis
We extracted the data (sample size, Mean ± SD) to clarify the change of TNF-α in T1DM patients versus controls, followed by Chi-squared Q test and I2 statistics to estimate the heterogeneity [30, 31]. When P < 0.1 or I2 > 50%, we selected a random-effect model to account for possible heterogeneity between studies; otherwise a fixed-effect model was used in the absence of heterogeneity [32, 33]. In order to evaluate the age and disease duration impact on serum TNF-α, all the patients in the included studies were stratified into three groups by age (<12, 12–24, >24 years old) and by disease duration (<5, 5–10, >10 years) respectively. And the impact of different ethnicity of patients was explored by subgroup analysis of five ethnicity groups (Asia, Europe, America, Africa and Oceania). Regression analysis was used to investigate sources of heterogeneity. In addition, we conducted sensitivity analysis by excluding individual studies or selecting articles with high NOS score (≥) or changing the Cochran’s Q statistic methods to check the stability of the results. Publication bias was judged by an Egger’s test (P < 0.05 was considered representative of statistically significant publication bias) [34]. Stata 12.0 software was used in this pooled analysis.
Results
The process and results of selection
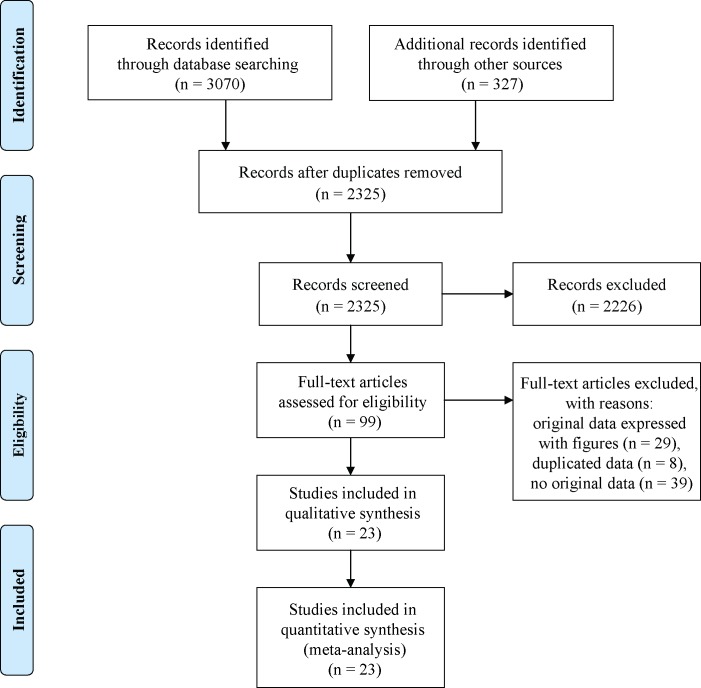
The study selection process was displayed in Fig 1. With our search criteria, we collected 3397 potential studies and excluded 1074 due to duplication. After reading the titles and abstracts, 2226 articles were excluded for no controls, not DM relevant, review and editorial articles or animal studies. Then, we excluded 76 articles due to original data expressed with figures, duplicated data, or no original data. Finally, 20 articles (23 case-control studies) met the criteria and were included in this meta-analysis [1, 7, 21–25, 35–47] (S1 Appendix), involving 1631 T1DM patients and 1429 healthy controls. The specific characteristics of the 23 studies are shown in Table 1.
Fig 1. The flow chart of the articles search and inclusion process.
Table 1. Characteristics of studies about serum TNF-α (ng/L) included in this meta-analysis.
| Author | Year | Country | Quantification Methods |
Duration (years) |
Case | Control | NOS score |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SZ | M/F | Age | Mean | SD | SZ | M/F | Age | Mean | SD | ||||||
| Hegazy[35] | 2013 | Egypt | ELISA | 4.3±2.1 | 15 | 7/8 | 11.1±2.3 | 9 | 0.9 | 15 | 7/8 | 11.5±1.4 | 5.4 | 1.7 | 8 |
| Hegazy[35] | 2013 | Egypt | ELISA | 4.4±3.0 | 15 | 7/8 | 11.9±1.4 | 9.1 | 0.9 | 15 | 7/8 | 11.5±1.4 | 5.4 | 1.7 | 8 |
| Yuan[36] | 2010 | China | ELISA | NR | 15 | NR | 18.53±8.70 | 36.47 | 16.33 | 90 | NR | 28.33±6.12 | 35.37 | 14.97 | 6 |
| Poplawska[1] | 2014 | Poland | ELISA | NR | 62 | NR | 42.6±12.7 | 1.6 | 1.2 | 6 | NR | 48.3±2.1 | 0.8 | 0.5 | 6 |
| Talaat[37] | 2016 | Saudi Arabia |
FCM | 3.50±0.39 | 250 | NR | 8.50±0.5 | 19.81 | 8.75 | 250 | NR | 8.50±0.5 | 3.22 | 0.45 | 7 |
| Balic a [38] | 2009 | Chile | ELISA | 0.21±0.12 | 300 | 158/142 | 10.8±4.1 | 2.31 | 36.8 | 310 | 146/164 | 11.0±2.2 | 1.39 | 0.88 | 8 |
| Balic a [38] | 2009 | Chile | ELISA | 0.21±0.12 | 300 | 158/142 | 10.8±4.1 | 5.22 | 31.7 | 310 | 146/164 | 11.0±2.2 | 1.23 | 1.08 | 8 |
| Machnica[39] | 2014 | Poland | ELISA | 5.09±1.97 | 52 | 19/33 | 14.07±3.03 | 16.63 | 8.32 | 20 | 8/12 | 13.09±3.05 | 9.41 | 4.23 | 8 |
| Alexandrakib [7] | 2008 | Athens | ELISA | 11.87±2.36 | 20 | 13/7 | NR | 0.67 | 0.27 | 34 | 17/17 | NR | 0.53 | 0.29 | 7 |
| Romano a [40] | 2001 | Italy | ELISA | 0.35±0.32 | 20 | 11/9 | 10.6±3.4 | 21.9 | 9.48 | 10 | 6/4 | 9.6±2.6 | 4.4 | 1.68 | 7 |
| Araya[25] | 2003 | Chile | ELISA | 12.0±5.9 | 15 | 8/7 | 22.2±3.1 | 5.7 | 1.5 | 14 | 5/9 | 25.1±3.7 | 1.3 | 0.2 | 8 |
| Mitrovic c [41] | 2011 | Serbia | ELISA | 20.01±8.78 | 76 | 32/44 | 35.24±11.09 | 0.65 | 0.37 | 30 | 12/18 | 38.10±12.97 | 0.2 | 0.11 | 8 |
| Pham c [42] | 2011 | Germany | ELISA | 0.1±0.40 | 90 | 62/28 | 43.2±4.45 | 2.4 | 0.5 | 41 | 16/25 | 47.7±3.38 | 1.8 | 0.35 | 7 |
| Pertynska[22] | 2010 | Poland | ELISA | 9.5±5.41 | 14 | 0/14 | 27.06±5.35 | 5.19 | 8.81 | 16 | 0/16 | 25.21±4.21 | 9.69 | 21.25 | 8 |
| El-Samahy[24] | 2015 | Scoland | ELISA | 6.1±1.6 | 32 | 12/20 | 12.4±2.6 | 17.6 | 1.9 | 30 | 11/19 | 13.4±1.9 | 6.5 | 1.22 | 8 |
| Martos[43] | 2006 | Spain | ELISA | NR | 20 | 10/10 | 7.34±0.88 | 3 | 3.1 | 40 | 20/20 | 8.16±0.46 | 1.4 | 1.2 | 7 |
| Aguilera[44] | 2015 | Spain | ECMA | 20.4±8.1 | 150 | NR | 38.6±8.1 | 7.5 | 2.4 | 50 | NR | 38.1±7.2 | 7.8 | 1.8 | 7 |
| Gabbay[45] | 2012 | Brazil | FCM | NR | 35 | NR | 13.0±5.0 | 13.8 | 26.9 | 25 | NR | 13.6±5.4 | 0.11 | 0.38 | 6 |
| Lo[23] | 2004 | Taiwan | ELISA | NR | 58 | 22/36 | 10.98±4.61 | 11.15 | 14.34 | 33 | 16/17 | 10.06±4.90 | 14.22 | 17.92 | 8 |
| Abdel[46] | 2001 | Egypt | ECMA | 5.48±3.22 | 15 | 8/7 | 13.71±4.26 | 14 | 10.67 | 15 | 8/7 | 13.21±3.20 | 6.61 | 1.97 | 8 |
| Lechleitner[21] | 2000 | Austria | ELISA | 12.8± 8.1 | 29 | 29/0 | 31.7±6.0 | 19.9 | 8.4 | 24 | NR | 30.5± 6.8 | 11.1 | 5.8 | 7 |
| Lechleitner[21] | 2000 | Austria | ELISA | 8.1±7.7 | 15 | 0/15 | 29.3±6.4 | 18.3 | 5.2 | 24 | NR | 30.5± 6.8 | 11.1 | 5.8 | 7 |
| Lv[47] | 2013 | China | ELISA | 0.16±0.08 | 33 | 18/15 | 12±4 | 27 | 14 | 27 | 15/12 | 11±4 | 26 | 6 | 8 |
FCM, flow cytometry method; ELISA, enzyme-linked immunosorbent assay; ECMA, enzyme chemiluminescence immunometric assay; SZ, sample size; M/F, male/female; SD, standard deviation; NR, not report.
aData converted from median (range).
bSD data converted from SE.
cData converted from median (interquartile range).
Results of meta-analysis and subgroup analysis
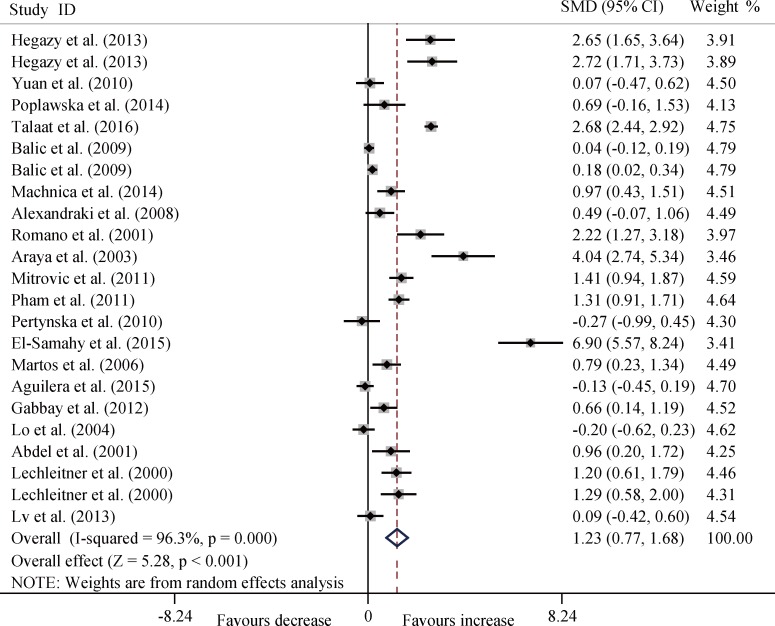
The T1DM patients had significantly increased serum TNF-α level compared with the controls (SMD, 1.23; 95% CI, 0.77 to 1.68; P < 0.001) (Fig 2) and significant heterogeneity (P < 0.001) existed in this meta-analysis.
Fig 2. Forest plots for the level of serum TNF-α between T1DM patients and healthy controls with random effects model (SMD, 1.23, 95% CI, 0.77 to 1.68, P < 0.001).
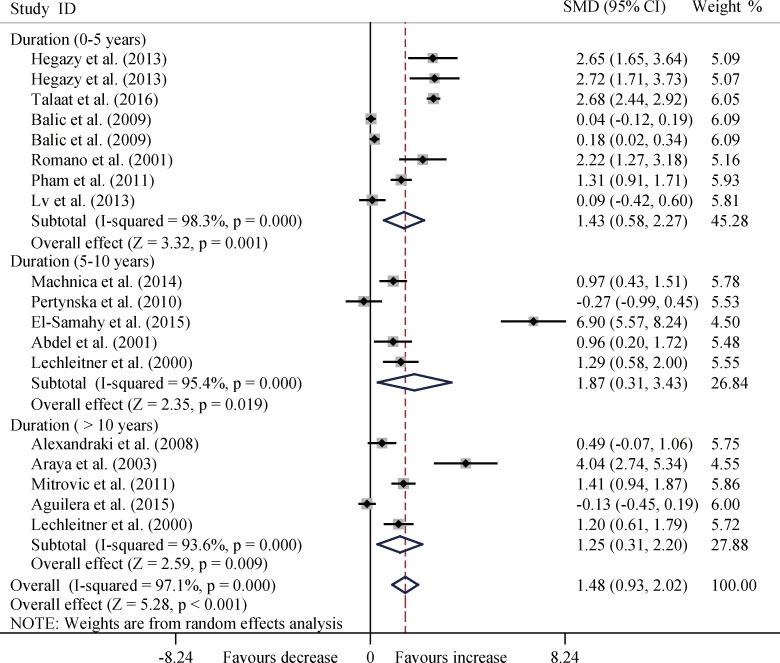
In the subgroup analysis by age, disease duration, and ethnicity, T1DM patients consistently had significantly increased levels of TNF-α among the three age groups (all P < 0.01) (Fig 3), and three disease duration groups (all P < 0.05) (Fig 4), and four ethnicity subgroups (all P < 0.05) except Asia group (SMD, 0.67; 95%CI, -1.03 to 2.36; P = 0.439) (Fig 5).
Fig 3. Forest plots about subgroup analysis for the level of TNF-α between T1DM patients and controls with random effects model among different age groups.
Age, 0–12 years old, SMD, 1.18, 95% CI, 0.40 to 1.96, P = 0.003; Age, 12–24 years old, SMD, 2.14, 95% CI, 0.79 to 3.49, P = 0.002; Age, > 24 years old: SMD, 0.79, 95% CI, 0.19 to 1.39, P = 0.010; Overall, SMD, 1.26, 95% CI, 0.79 to 1.73, P < 0.001.
Fig 4. Forest plots about subgroup analysis for the level of TNF-α between T1DM patients and controls with random effects model among different disease duration groups.
Duration, 0–5 years, SMD, 1.43, 95% CI, 0.58 to 2.27, P = 0.001; Duration, 5–10 years, SMD, 1.87, 95% CI, 0.31 to 3.43, P = 0.019; Duration, > 10 years, SMD, 1.25, 95% CI, 0.31 to 2.20, P = 0.009; Overall, SMD, 1.48, 95% CI, 0.93 to 2.02, P < 0.001.
Fig 5. Forest plots about subgroup analysis for the level of TNF-α between T1DM patients and controls with random effects model among different ethnicity groups.
Asia, SMD, 0.67, 95% CI, -1.03 to 2.36, P = 0.439; Europe, SMD, 1.30, 95% CI, 0.59 to 2.01, P < 0.001; America, SMD, 0.67, 95% CI, 0.15 to 1.19, P = 0.012; Africa, SMD, 2.07, 95% CI, 0.86 to 3.29, P = 0.001; Oceania, SMD, 1.24, 95% CI, 0.78 to 1.69, P < 0.001; Overall, SMD, 1.23, 95% CI, 0.77 to 1.68, P < 0.001.
Regression analysis
Regression analysis was used to explore the source of high heterogeneity of age, disease duration, and ethnicity for the serum level of TNF-α, and the results displayed as follows: age (t = -0.42; 95% CI, -1.035 to 0.689; P = 0.680), disease duration (t = -0.06; 95% CI, -1.091 to 1.036; P = 0.957), and ethnicity of patients (t = 0.64; 95% CI, -0.298 to 0.567; P = 0.526), which indicated that age, disease duration, and ethnicity of patients were not significant impact factors for the high heterogeneity in this meta-analysis.
Sensitivity analysis
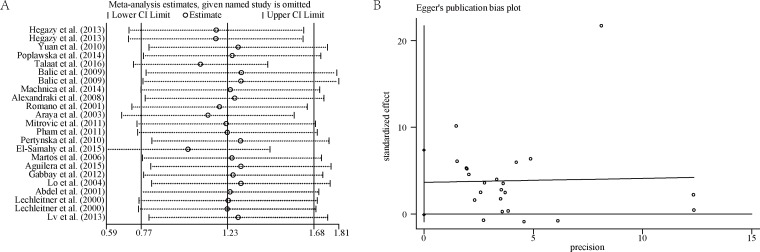
We first performed sensitivity analysis by excluding individual studies and found the results remaining consistent (Fig 6A). Next, sensitivity analysis was conducted by selecting studies with high NOS score (≥) or excluding studies with high risk of bias and we found that all the outcomes still had no significant changes. Otherwise, fixed effects model was chosen to pool the data, all the results were similar to those generated by random effects model.
Fig 6. Sensitivity analysis and publication bias about the serum TNF-α level in T1DM.
A: sensitivity analysis; B: publication bias, t = 2.04; P = 0.054; 95% CI, -0.074 to 7.370.
Publication bias
Egger’s test showed that no publication bias existed in this meta-analysis (t = 2.04; P = 0.054; 95% CI, -0.074 to 7.370) (Fig 6B).
Discussion
TNF-α is highly involved with macrophage activation and increased serum TNF-α level have been observed in insulin resistance stages and diabetes mellitus development [48], however, inconsistency still existed and no meta-analysis was conducted about the change of serum TNF-α level in T1DM patients. The results of this study clearly demonstrate that T1DM patients had significantly elevated serum level of TNF-α and significant correlation existed between TNF-α level and patients’ age, disease duration and ethnicity.
TNF-α level may play an important role and many factors may contribute to the serum TNF-α level in diabetes. TNF-α, as the major physiological and pathophysiological regulators of vascular adhesion molecules, is a key proinflammatory cytokine with widespread metabolic effects, and directly regulate the production of several cardiovascular risk factors [49, 50]. TNF-α via effects on soluble intercellular adhesion molecule-1, may promote vascular adhesion, otherwise plasma levels of TNF-α are associated with dyslipidaemia and increase blood pressure, adding to vascular disease risk, besides, the actions of TNF-α is probably modified by altered production of soluble receptors in type 1 diabetic patients [51]. Increased TNF-α and interleukine-6 (IL-6) levels through metabolic control exist in types 1 and 2 diabetic patients, which suggest that the control of diabetes improves the capacity of activation and maintenance of these pro-inflammatory cytokines [52–54]. Other study [21] demonstrated TNF-α levels were elevated in T1DM which was correlated positively with HbA1c and inversely with HDL cholesterol levels. In addition, a significant relationship between TNF-α levels and both BMI and WHR also was observed in analysis of the combined groups [51].
Owning the various biological effects, TNF-α has been proved to have certain catabolic effects on fat cells, and neutralization of TNF-α in obese rats causes a significant increase in the peripheral uptake of glucose in response to insulin, which indicates an important role in the insulin resistance and diabetes that often accompany obesity [55]. TNF-α, through increasing the activities of the NF-κB transcriptional factor [56, 57], protein kinase C [58], amino terminal kinase and inhibitor kinase, could cause serine/threonine phosphorylation of the insulin receptor substrate, interfere with normal phosphorylation of tyrosine, and weaken signal transduction of insulin, resulting in insulin resistance [36], otherwise, TNF-α may be result in the destruction of pancreatic beta cells and lead to the development of T1DM [59].
Age, disease duration and ethnicity of T1DM patients were focused on in this study. In a previous study [60], serum TNF-α level was not associated with the presence and severity of microalbuminuria, otherwise, the level of urinary TNF-α was only significantly influenced by albumin-creatinine ratio (ACR), although other factors had been included in the multivariate analysis: age, duration of diabetes, BMI, history of cardiovascular disease, presence of retinopathy, hypertension, and HbA1c levels. Lo, H. C., et al. [23] found that serum concentrations of TNF-α had no significant change in type 1 diabetic children compared with healthy siblings between different age groups (1–6 years old group, 6–12 years old group and 12–18 years old group), which was inconsistent with our findings. Small sample size and different statistical approach maybe illuminate the phenomenon. Ethnicity maybe another impact factor for the level of TNF-α and no significant change existed in Asia populations. In this multifactorial disease, Asia populations with varied geographic distribution, linked to climate, diet, lifestyle and economic status may contribute to the discrepancy.
Significant heterogeneity still existed after subgroups analysis and regression analysis indicated that these factors were not potential sources for the high heterogeneity, and we assume that sources of heterogeneity may be attributed to the diversity in design, sample sizes, measurement errors and so on. Otherwise, sensitivity analysis indicated the results were stable and no publication bias existed in this meta-analysis.
Several limitations should be considered when cautiously interpreting the results. Firstly, we could not conduct further subgroup analyses such as by gender, body weight and other factors because of insufficient original data. Secondly, only reports in English and reports in Chinese were chosen and eligible studies might have not been unpublished or published in other languages. Small sample size, limited statistical power and high heterogeneity of the included studies could also influence the results. Furthermore, some reports included in this meta-analysis failed to disclose the status of diabetic complications, which may have impact on the results. All these limitations should be kept in mind when interpreting the findings.
In summary, the present meta-analysis indicates that compared with the healthy controls, the T1DM patients have significantly increased serum level of TNF-α. The role of TNF-α in the development of diabetes and diabetic complications warrant future investigation.
Supporting information
(DOC)
(DOC)
Data Availability
Article DOIs can be found in the Supporting Information file.
Funding Statement
This study was supported by the National Natural Science Foundation of China (81471054) and the Innovation Project of Guangxi Graduate Education (JGY2015128). (http://www.medsci.cn/sci/nsfc.do?action=q).
References
- 1.Poplawska-Kita A, Siewko K, Szpak P, Krol B, Telejko B, Klimiuk PA, et al. Association between type 1 diabetes and periodontal health. Advances in medical sciences. 2014;59(1):126–31. Epub 2014/05/07. doi: 10.1016/j.advms.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 2.Saxena M, Srivastava N, Banerjee M. Association of IL-6, TNF-alpha and IL-10 gene polymorphisms with type 2 diabetes mellitus. Molecular biology reports. 2013;40(11):6271–9. Epub 2013/09/24. doi: 10.1007/s11033-013-2739-4 [DOI] [PubMed] [Google Scholar]
- 3.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40(11):1286–92. doi: 10.1007/s001250050822 [DOI] [PubMed] [Google Scholar]
- 4.Festa A, D'Agostino R Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102(1):42–7. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocrine reviews. 2003;24(3):278–301. Epub 2003/06/06. doi: 10.1210/er.2002-0010 [DOI] [PubMed] [Google Scholar]
- 6.Borges AP, Guidoni CM, Freitas O, Pereira LR. Economic evaluation of outpatients with type 2 diabetes mellitus assisted by a pharmaceutical care service. Arquivos brasileiros de endocrinologia e metabologia. 2011;55(9):686–91. [DOI] [PubMed] [Google Scholar]
- 7.Alexandraki KI, Piperi C, Ziakas PD, Apostolopoulos NV, Makrilakis K, Syriou V, et al. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. Journal of clinical immunology. 2008;28(4):314–21. Epub 2008/01/29. doi: 10.1007/s10875-007-9164-1 [DOI] [PubMed] [Google Scholar]
- 8.Rabinovitch A, Suarez-Pinzon WL. Role of cytokines in the pathogenesis of autoimmune diabetes mellitus. Reviews in endocrine & metabolic disorders. 2003;4(3):291–9. [DOI] [PubMed] [Google Scholar]
- 9.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. The Journal of clinical investigation. 1998;101(2):311–20. doi: 10.1172/JCI1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Targher G, Zenari L, Bertolini L, Muggeo M, Zoppini G. Elevated levels of interleukin-6 in young adults with type 1 diabetes without clinical evidence of microvascular and macrovascular complications. Diabetes care. 2001;24(5):956–7. [DOI] [PubMed] [Google Scholar]
- 11.Oghagbon EK, Jimoh KA, Olaosebikan O, Harbige L. Increased central adiposity may not underlie the marked elevation of IL-6 in diabetes mellitus patients in South-West, Nigeria. West African journal of medicine. 2014;33(2):130–5. Epub 2014/09/23. [PubMed] [Google Scholar]
- 12.Duarte PM, Miranda TS, Lima JA, Dias Goncalves TE, Santos VR, Bastos MF, et al. Expression of immune-inflammatory markers in sites of chronic periodontitis in patients with type 2 diabetes. Journal of periodontology. 2012;83(4):426–34. doi: 10.1902/jop.2011.110324 [DOI] [PubMed] [Google Scholar]
- 13.Hara Cde C, Franca EL, Fagundes DL, de Queiroz AA, Rudge MV, Honorio-Franca AC, et al. Characterization of Natural Killer Cells and Cytokines in Maternal Placenta and Fetus of Diabetic Mothers. Journal of immunology research. 2016;2016:7154524 Epub 2016/06/14. PubMed Central PMCID: PMCPMC4884836. doi: 10.1155/2016/7154524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores L, Naf S, Hernaez R, Conget I, Gomis R, Esmatjes E. Transforming growth factor beta at clinical onset of Type 1 diabetes mellitus. A pilot study. Diabetic medicine: a journal of the British Diabetic Association. 2004;21(8):818–22. [DOI] [PubMed] [Google Scholar]
- 15.Karavanaki K, Kakleas K, Georga S, Bartzeliotou A, Mavropoulos G, Tsouvalas M, et al. Plasma high sensitivity C-reactive protein and its relationship with cytokine levels in children with newly diagnosed type 1 diabetes and ketoacidosis. Clinical biochemistry. 2012;45(16–17):1383–8. doi: 10.1016/j.clinbiochem.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 16.Gahring LC, Carlson NG, Kulmar RA, Rogers SW. Neuronal expression of tumor necrosis factor alpha in the murine brain. Neuroimmunomodulation. 1996;3(5):289–303. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. The Journal of clinical investigation. 1994;94(4):1543–9. Epub 1994/10/01. PubMed Central PMCID: PMCPMC295304. doi: 10.1172/JCI117495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43(11):1271–8. Epub 1994/11/01. [DOI] [PubMed] [Google Scholar]
- 19.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. The Journal of biological chemistry. 1993;268(35):26055–8. [PubMed] [Google Scholar]
- 20.Van Lint J, Agostinis P, Vandevoorde V, Haegeman G, Fiers W, Merlevede W, et al. Tumor necrosis factor stimulates multiple serine/threonine protein kinases in Swiss 3T3 and L929 cells. Implication of casein kinase-2 and extracellular signal-regulated kinases in the tumor necrosis factor signal transduction pathway. The Journal of biological chemistry. 1992;267(36):25916–21. [PubMed] [Google Scholar]
- 21.Lechleitner M, Koch T, Herold M, Dzien A, Hoppichler F. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. Journal of internal medicine. 2000;248(1):67–76. Epub 2000/08/18. [DOI] [PubMed] [Google Scholar]
- 22.Pertynska-Marczewska M, Glowacka E, Grodzicka A, Sobczak M, Cypryk K, Wilczynski JR, et al. Profile of peripheral blood neutrophil cytokines in diabetes type 1 pregnant women and its correlation with selected parameters in the newborns. American journal of reproductive immunology (New York, NY: 1989). 2010;63(2):150–60. Epub 2009/12/31. [DOI] [PubMed] [Google Scholar]
- 23.Lo HC, Lin SC, Wang YM. The relationship among serum cytokines, chemokine, nitric oxide, and leptin in children with type 1 diabetes mellitus. Clinical biochemistry. 2004;37(8):666–72. Epub 2004/08/11. doi: 10.1016/j.clinbiochem.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 24.El-Samahy MH, Adly AA, Ismail EA, Salah NY. Regulatory T cells with CD62L or TNFR2 expression in young type 1 diabetic patients: relation to inflammation, glycemic control and micro-vascular complications. Journal of diabetes and its complications. 2015;29(1):120–6. Epub 2014/08/13. doi: 10.1016/j.jdiacomp.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 25.Araya AV, Pavez V, Perez C, Gonzalez F, Columbo A, Aguirre A, et al. Ex vivo lipopolysaccharide (LPS)-induced TNF-alpha, IL-1beta, IL-6 and PGE2 secretion in whole blood from Type 1 diabetes mellitus patients with or without aggressive periodontitis. European cytokine network. 2003;14(3):128–33. Epub 2003/12/06. [PubMed] [Google Scholar]
- 26.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54(5):1407–14. Epub 2005/04/28. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery. 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 28.Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation (PDF). Geneva: World Health Organization; 2006:21. [Google Scholar]
- 29.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2011. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. PubMed Central PMCID: PMC192859. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Statistics in medicine. 1995;14(4):395–411. [DOI] [PubMed] [Google Scholar]
- 32.Su PF, Chen X, Chen H, Shyr Y. Statistical aspects of omics data analysis using the random compound covariate. BMC systems biology. 2012;6 Suppl 3:S11. PubMed Central PMCID: PMC3524312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. PubMed Central PMCID: PMC2127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegazy SK, Tolba OA, Mostafa TM, Eid MA, El-Afify DR. Alpha-lipoic acid improves subclinical left ventricular dysfunction in asymptomatic patients with type 1 diabetes. The review of diabetic studies: RDS. 2013;10(1):58–67. Epub 2013/11/01. PubMed Central PMCID: PMCPMC3932072. doi: 10.1900/RDS.2013.10.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan T, Zhao WG, Sun Q, Fu Y, Dong YY, Dong YX, et al. Association between four adipokines and insulin sensitivity in patients with obesity, type 1 or type 2 diabetes mellitus, and in the general Chinese population. Chinese medical journal. 2010;123(15):2018–22. Epub 2010/09/08. [PubMed] [Google Scholar]
- 37.Talaat IM, Nasr A, Alsulaimani AA, Alghamdi H, Alswat KA, Almalki DM, et al. Association between type 1, type 2 cytokines, diabetic autoantibodies and 25-hydroxyvitamin D in children with type 1 diabetes. Journal of endocrinological investigation. 2016. Epub 2016/08/20. [DOI] [PubMed] [Google Scholar]
- 38.Balic I, Angel B, Codner E, Carrasco E, Perez-Bravo F. Association of CTLA-4 polymorphisms and clinical-immunologic characteristics at onset of type 1 diabetes mellitus in children. Human immunology. 2009;70(2):116–20. Epub 2009/01/13. doi: 10.1016/j.humimm.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 39.Machnica L, Deja G, Polanska J, Czupryniak L, Szymanska-Garbacz E, Loba J, et al. Blood pressure disturbances and endothelial dysfunction markers in children and adolescents with type 1 diabetes. Atherosclerosis. 2014;237(1):129–34. Epub 2014/09/23. doi: 10.1016/j.atherosclerosis.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 40.Romano M, Pomilio M, Vigneri S, Falco A, Chiesa PL, Chiarelli F, et al. Endothelial perturbation in children and adolescents with type 1 diabetes: association with markers of the inflammatory reaction. Diabetes care. 2001;24(9):1674–8. Epub 2001/08/28. [DOI] [PubMed] [Google Scholar]
- 41.Mitrovic M, Ilic T, Stokic E, Paro JN, Naglic DT, Bajkin I, et al. Influence of glucoregulation quality on C-reactive protein, interleukin-6 and tumor necrosis factor-alpha level in patients with diabetes type 1. Vojnosanitetski pregled. 2011;68(9):756–61. Epub 2011/11/04. [DOI] [PubMed] [Google Scholar]
- 42.Pham MN, Hawa MI, Pfleger C, Roden M, Schernthaner G, Pozzilli P, et al. Pro- and anti-inflammatory cytokines in latent autoimmune diabetes in adults, type 1 and type 2 diabetes patients: Action LADA 4. Diabetologia. 2011;54(7):1630–8. Epub 2011/02/25. doi: 10.1007/s00125-011-2088-6 [DOI] [PubMed] [Google Scholar]
- 43.Martos-Moreno GA, Barrios V, Soriano-Guillen L, Argente J. Relationship between adiponectin levels, acylated ghrelin levels, and short-term body mass index changes in children with diabetes mellitus type 1 at diagnosis and after insulin therapy. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155(5):757–61. Epub 2006/10/26. [DOI] [PubMed] [Google Scholar]
- 44.Aguilera E, Serra-Planas E, Granada ML, Pellitero S, Reverter JL, Alonso N, et al. Relationship of YKL-40 and adiponectin and subclinical atherosclerosis in asymptomatic patients with type 1 diabetes mellitus from a European Mediterranean population. Cardiovascular diabetology. 2015;14:121 Epub 2015/09/19. PubMed Central PMCID: PMCPMC4574547. doi: 10.1186/s12933-015-0287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabbay MA, Sato MN, Duarte AJ, Dib SA. Serum titres of anti-glutamic acid decarboxylase-65 and anti-IA-2 autoantibodies are associated with different immunoregulatory milieu in newly diagnosed type 1 diabetes patients. Clinical and experimental immunology. 2012;168(1):60–7. Epub 2012/03/06. PubMed Central PMCID: PMCPMC3390495. doi: 10.1111/j.1365-2249.2011.04538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel Aziz MT, Fouad HH, Mohsen GA, Mansour M, Abdel Ghaffar S. TNF-alpha and homocysteine levels in type 1 diabetes mellitus. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2001;7(4–5):679–88. Epub 2004/08/31. [PubMed] [Google Scholar]
- 47.Lv L, Liu JY, Ma J, Lin SX, Huang L. [Factors associated with Th1 cytokine disorders in children with newly diagnosed type 1 diabetes]. Zhongguo dang dai er ke za zhi = Chinese journal of contemporary pediatrics. 2013;15(1):50–2. Epub 2013/01/23. [PubMed] [Google Scholar]
- 48.Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. Effect of the glycemic control on intracellular cytokine production from peripheral blood mononuclear cells of type 1 and type 2 diabetic patients. Diabetes research and clinical practice. 2008;82(3):329–34. Epub 2008/10/14. doi: 10.1016/j.diabres.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 49.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annual review of immunology. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003 [DOI] [PubMed] [Google Scholar]
- 50.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1998;22(12):1145–58. Epub 1999/01/07. [DOI] [PubMed] [Google Scholar]
- 51.Mohamed-Ali V, Armstrong L, Clarke D, Bolton CH, Pinkney JH. Evidence for the regulation of levels of plasma adhesion molecules by proinflammatory cytokines and their soluble receptors in type 1 diabetes. Journal of internal medicine. 2001;250(5):415–21. Epub 2002/03/13. [DOI] [PubMed] [Google Scholar]
- 52.Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. In vitro TNF-alpha and IL-6 production by adherent peripheral blood mononuclear cells obtained from type 1 and type 2 diabetic patients evaluated according to the metabolic control. Annals of the New York Academy of Sciences. 2006;1079:177–80. doi: 10.1196/annals.1375.027 [DOI] [PubMed] [Google Scholar]
- 53.Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. Effect of metabolic control on interferon-gamma and interleukin-10 production by peripheral blood mononuclear cells from type 1 and type 2 diabetic patients. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al]. 2007;40(5):671–7. PubMed [DOI] [PubMed] [Google Scholar]
- 54.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS immunology and medical microbiology. 1999;26(3–4):259–65. [DOI] [PubMed] [Google Scholar]
- 55.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (New York, NY). 1993;259(5091):87–91. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 56.Neish AS, Read MA, Thanos D, Pine R, Maniatis T, Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Molecular and cellular biology. 1995;15(5):2558–69. PubMed Central PMCID: PMC230486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Mattia G, Bravi MC, Costanzo A, Laurenti O, Cassone Faldetta M, Armiento A, et al. Effects of insulin on in vitro vascular cell adhesion molecule-1 expression and in vivo soluble VCAM-1 release. Diabetologia. 1999;42(10):1235–9. [DOI] [PubMed] [Google Scholar]
- 58.Deisher TA, Haddix TL, Montgomery KF, Pohlman TH, Kaushansky K, Harlan JM. The role of protein kinase C in the induction of VCAM-1 expression on human umbilical vein endothelial cells. FEBS letters. 1993;331(3):285–90. [DOI] [PubMed] [Google Scholar]
- 59.Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Annals of periodontology / the American Academy of Periodontology. 2001;6(1):125–37. Epub 2002/03/13. [DOI] [PubMed] [Google Scholar]
- 60.Lampropoulou IT, Stangou M, Papagianni A, Didangelos T, Iliadis F, Efstratiadis G. TNF-alpha and microalbuminuria in patients with type 2 diabetes mellitus. Journal of diabetes research. 2014;2014:394206 Epub 2015/01/15. PubMed Central PMCID: PMCPMC4284977. doi: 10.1155/2014/394206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
Article DOIs can be found in the Supporting Information file.