Abstract
Pheochromocytomas (PHEOs) and paragangliomas (PGLs; extra-adrenal tumors) are rare neuroendocrine chromaffin cell tumors with a hereditary background in about 30%–35%. Those caused by succinate dehydrogenase subunit B (SDHB) germline mutations are associated with a high metastatic potential and ultimately higher patient mortality. Succinate dehydrogenase converts succinate to fumarate, uniquely linking the Krebs cycle and oxidative phosphorylation. SDH mutations result in the accumulation of succinate associated with various metabolic disturbances and the shift to aerobic glycolysis in tumor tissue. In the present study, we measured succinate and fumarate levels in mouse pheochromocytoma (MPC) and mouse tumor tissue (MTT) cells and in 10 apparently sporadic, 10 SDHB-, 5 SDHD-, and 2 neurofibromatosis 1–related PHEOs/PGLs and plasma samples using mass spectrometry. We found that the succinate-to-fumarate ratio was significantly higher in the SDHB- and SDHD-related PGLs than in apparently sporadic and neurofibromatosis 1–related PHEOs/PGLs (P = .0376). To further support our data, we silenced SDHB expression in MPC and MTT cells and evaluated the succinate and fumarate levels. Compared with control samples, SDHB-silenced MTT cells also showed an increase in the succinate-to-fumarate ratio (MTT cells: 2.45 vs 7.53), similar to the findings in SDHB-related PGLs. The present findings for the first time demonstrate a significantly increased succinate-to-fumarate ratio in SDHB/D-related PGLs and thus suggest this ratio may be used as a new metabolic marker for the detection of SDHB/D-related PHEOs/PGLs.
Pheochromocytomas (PHEOs) and their extra-adrenal counterpart paragangliomas (PGLs) are rare catecholamine-producing tumors arising from neural crest-derived chromaffin cells. Although most these tumors are apparently sporadic, approximately 30%–35% have a hereditary basis (1).
More than a decade ago, mutations in the genes that encode subunits D, C, and B of the succinate dehydrogenase (SDH) enzyme were discovered, causing familial PGL syndrome type PGL1, -3, and -4, respectively (2–4). Recently, mutations in SDHAF2 (SDH5, PGL2) and SDHA were identified to cause PGL as well (5, 6).
Succinate dehydrogenase subunit B (SDHB)-related PHEOs/PGLs are mostly abdominal, and they are associated with a high risk of developing metastases (7). About 20%–30% of patients already have metastatic disease at the time of the initial diagnosis (8). The diagnosis of SDHB-related PHEOs/PGLs is often delayed, most likely because of the less typical catecholamine excess-related clinical presentations compared with other apparently sporadic or hereditary PHEOs/PGLs. This is partially due to the fact that these tumors can have either a biochemically silent phenotype, a low intratumoral catecholamine content, or a purely dopaminergic phenotype (8, 9). In contrast, SDHD-related PHEOs/PGLs, especially those derived from the parasympathetic nervous system of the head and neck, are much less aggressive. The presence of SDH mutations impairs oxidative phosphorylation and the Krebs cycle, resulting in metabolic abnormalities, including succinate accumulation (10).
Presently, the ultimate diagnosis of these tumors is based on immunohistochemistry to detect the presence or absence of the SDHB protein or genetic testing for an SDH mutation or deletion (11, 12). Although next-generation sequencing methods will significantly reduce the costs of such testing, currently this genetic testing is still costly and therefore limited or even unavailable in many countries. Neither method can be used (eg, to predict therapeutic responses of these tumors, their resistance to various therapies, for follow-up after a therapy is completed, or to assess their progression over time).
Based on previous pioneering reports about the metabolic consequences of SDHx mutations/deletions in these tumors (13–15), the aim of the present study was to measure the level of two Krebs cycle metabolites to introduce a unique metabolic signature of these tumors and to propose its implementation in their clinical diagnosis.
Materials and Methods
Human PHEOs/PGLs
PHEO/PGL tissue samples were collected at the National Institutes of Health (NIH) under clinical protocol 00-CH-0093, approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Tissue samples were frozen in liquid nitrogen shortly after surgical removal of a tumor. All patients underwent genetic testing for known PHEO/PGL susceptibility genes except SDHAF2 and neurofibromatosis 1 (NF1); the diagnosis of the latter was based on clinical grounds.
In the present study, we included four groups of tumors: SDHB (10 PGLs), SDHD (5 PGLs), apparently sporadic (6 PHEOs, 4 PGLs), and NF1 (2 PHEOs). NF1-related PHEOs were included because of the genetic background of the mouse PHEO (MPC) and mouse tumor tissue (MTT) cells used in the in vitro experiments. A detailed summary of clinical and patient characteristics is described in Table 1.
Table 1.
PHEO/PGL Sample Information
| Sample No. | Genetic Background | Localization | Gender |
|---|---|---|---|
| 1 | Sporadic | Right adrenal | F |
| 2 | Sporadic | Right adrenal | F |
| 3 | Sporadic | Right adrenal | M |
| 4 | Sporadic | Right adrenal | M |
| 5 | Sporadic | Para-aortic | F |
| 6 | Sporadic | Left suprarenal hilum | F |
| 7 | Sporadic | Pleural | F |
| 8 | Sporadic | Right adrenal | F |
| 9 | Sporadic | Left adrenal | F |
| 10 | Sporadic | Lumbar spine | F |
| 11 | SDHB | Right ventricle | F |
| 12 | SDHB | Para-aortic | M |
| 13 | SDHB | Retroperitoneum | M |
| 14 | SDHB | Right pericaval | M |
| 15 | SDHB | Retroperitoneum | F |
| 16 | SDHB | Subdural/epidural | F |
| 17 | SDHB | Thoracic, T1 | M |
| 18 | SDHB | Para-aortic | M |
| 19 | SDHB | Paraspinal | M |
| 20 | SDHB | Lung | F |
| 21 | NF1 | Right adrenal | M |
| 22 | NF1 | Right adrenal | M |
| 23 | SDHD | Right carotid body | F |
| 24 | SDHD | Right carotid body | F |
| 25 | SDHD | Right carotid body | F |
| 26 | SDHD | Left carotid body | M |
| 27 | SDHD | Right carotid body | M |
F, female; M, male.
Plasma samples
Patient blood samples were collected at the NIH under clinical protocol (00-CH-0093), approved by the Institutional Review Board of the NICHD. Blood samples were centrifuged at 3500 rpm at 4°C for 20 minutes, and the plasma was stored at −80°C until further processing.
In the present study we selected three samples for plasma measurements from each group (SDHB, SDHD, and apparently sporadic PHEOs/PGLs).
MPC and MTT cells
The MPC and MTT cell lines were used as described previously (16, 17). MTT cells are known to be more aggressive than MPC cells and show aggressiveness similar to human disease (17). MPC and MTT cells were maintained at 21% O2, 5% CO2, 37°C in DMEM (4.5g/L d-glucose, l-glutamine, 110 mg/L sodium pyruvate; Life Technologies Corporation) supplemented with 10% fetal bovine serum (Gibco), 5% heat-inactivated horse serum (Gibco), and Anti-Anti 100× (Penicillin/Streptomycin, Amphotericin B; Gibco). The medium was changed every 2 to 3 days and cells were passaged when 80%–90% confluence was reached (16).
Silencing of SDHB in MPC and MTT cells
Early passages of MPC and MTT cells were transduced with lentiviral particles carrying either shRNA targeted against mSDHB or control shRNA (Thermo Fisher Scientific Inc). The cells were transduced at multiplicity of infection = 1 and maintained according to the manufacturer's instructions. Medium containing 1 μg/mL puromycin was used to select positive cells.
For the metabolic analysis we seeded 1.5 × 10−6 cells on a 6-cm dish. After 24 hours, cells were harvested in 1.5 mL PBS and snap-frozen in liquid nitrogen.
Western blotting
To evaluate the degree of SDHB silencing in MPC and MTT cells, Western blot analysis was performed. On 35-mm dishes, 1.0 × 10−6 cells were plated. The following day, they were lysed and the protein concentration was determined using the Micro BCA Protein Assay Kit (Thermo Fisher Scientific). Thirty micrograms of total protein per well was loaded into a Criterion TGX Precast Gel, 4%–12% (Bio-Rad Laboratories) and transferred to an Immobilon-P membrane (EMD Millipore Corporation). The membrane was blocked in 5% nonfat dry milk in 0.1% Tween in PBS for 1 hour. It was incubated with anti-SDHB antibody (Sigma-Aldrich Co) for 1 hour. β-Actin (Cell Signaling Technology Inc) was used as a loading control. Proteins were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific), and blots were exposed to High Performance Chemiluminescence film (GE Healthcare) and analyzed with Image J 1.42q software (NIH).
Metabolic measurements
Procedures for the determination of succinate and fumarate have been described elsewhere (18) and are briefly related here. The organic acids were analyzed as their tertiary butyl dimethylsilyl ether derivatives using gas chromatography-mass spectrometry (GC-MS) in the electron impact mode and quantified using the 13C-labeled internal standards for each analyte. The N-methyl-N-(tert-butylmethylsilyl) trifluoroacetamide with 1% tert-butyldimethylchlorosilane reagent was purchased from Pierce Chemical Co, and the 13C-labeled organic acids were procured from Sigma Chemical Co.
Samples for GC-MS analysis were prepared by perchloric acid extraction as previously described (18). The 13C-labeled internal standards were added in two-fold excess of the concentrations of the individual analytes in the tumor tissue to the neutralized PCA extracts. Extracts (5.0 μL) were evaporated under a stream of nitrogen to dryness and were immediately reacted with 5 μL of the sylilating reagent in 15 μL of acetonitrile in 1.5 mL screw-capped glass vials and heated to 60°C for 5 minutes. Samples were analyzed on an Agilent 5973 quadrupole GC-MS (Agilent). One microliter of the sample solution was injected onto a 250-μm × 30 m capillary DB-1 (Agilent) column in the splitless injection mode. The mass spectrometer was operated in the electron impact mode (70 eV) and the quadrupole mass analyzer scanned for ions, which corresponded to a loss of 15 mass units (–CH3) from the molecular ion and the base peak of each analyte and its corresponding 13C-labeled internal standard using selected ion monitoring.
Statistical analysis
Data are expressed as means ± SD with coefficient of variation (COV). Student's t test was applied to determine the significance between the groups, with a P value of less than .05 considered significant. Grubbs' test was performed using GraphPad to determine whether there were any outliers among the values.
The NF1 PHEOs were not statistically analyzed due to the small sample size.
Results
The present study included 27 tumor samples and SDHB-silenced and control MPC and MTT cells. The samples included 10 SDHB PGLs, 5 SDHD PGLs, 2 NF1 PHEOs, and 10 apparently sporadic PHEOs/PGLs (Table 1).
Succinate concentration
In SDHB-related PGLs, the results showed a mean succinate concentration of 2.692 ± 1.979 mmol/L with the COV of 0.71, in comparison with the apparently sporadic PHEO/PGL group (0.219 ± 0.066 mmol/L; COV of 0.3; P = .0009). One SDHB-related PGL showed a much higher succinate level than the other SDHB tumors (7.916 mmol/L) (Table 2). This sample is a significant outlier (P < .05 with a Z value of 2.29). In the SDHD group, the mean succinate concentration was 2.078 ± 0.491 mmol/L (COV of 0.24); this value was higher (P < .05) than in the apparently sporadic PHEO/PGL group. The NF1 PHEOs showed a similar mean succinate concentration (0.364 ± 0.165 mmol/L; COV of 0.454) to apparently sporadic PHEOs/PGLs (Table 2).
Table 2.
Succinate and Fumarate Concentrations and Succinate-to-Fumarate Ratio in PHEO/PGL
| Sample No. | Succinate (mmol/L) | Fumarate (mmol/L) | Succinate-to-Fumarate Ratio |
|---|---|---|---|
| Sporadic | |||
| 1 | 0.18 | 0.02 | 7.35 |
| 2 | 0.22 | 0.03 | 7.24 |
| 3 | 0.20 | 0.02 | 8.43 |
| 4 | 0.17 | 0.06 | 2.90 |
| 5 | 0.15 | 0.04 | 3.93 |
| 6 | 0.22 | 0.04 | 5.86 |
| 7 | 0.37 | 0.07 | 5.03 |
| 8 | 0.28 | 0.03 | 8.35 |
| 9 | 0.19 | 0.03 | 5.60 |
| 10 | 0.21 | 0.02 | 8.58 |
| Mean | 0.219 ± 0.066 | 0.038 ± 0.016 | 6.3 ± 2.0 |
| SDHB | |||
| 11 | 2.79 | 0.01 | 203.11 |
| 12 | 7.92 | 0.01 | 1165.31 |
| 13 | 3.28 | 0.03 | 109.16 |
| 14 | 1.42 | 0.01 | 127.34 |
| 15 | 2.60 | 0.02 | 137.48 |
| 16 | 1.30 | 0.01 | 175.47 |
| 17 | 1.73 | 0.01 | 132.95 |
| 18 | 2.88 | 0.02 | 128.30 |
| 19 | 1.15 | 0.01 | 89.50 |
| 20 | 1.85 | 0.02 | 117.30 |
| Mean | 2.692 ± 1.979 | 0.015 ± 0.007 | 238.6 ± 327.2 |
| NF1 | |||
| 21 | 0.48 | 0.07 | 6.82 |
| 22 | 0.25 | 0.04 | 5.59 |
| SDHD | |||
| 23 | 1.75 | 0.03 | 50.70 |
| 24 | 2.27 | 0.02 | 116.75 |
| 25 | 2.26 | 0.04 | 62.13 |
| 26 | 1.42 | 0.09 | 14.95 |
| 27 | 2.68 | 0.05 | 56.59 |
| Mean | 2.078 ± 0.491 | 0.046 ± 0.029 | 60.24 ± 36.58 |
Fumarate concentration
In SDHB-related PGLs, the results showed a mean fumarate concentration of 0.015 ± 0.007 mmol/L with the COV of 0.46, in SDHD-related PGLs 0.046 ± 0.029 (COV of 0.62), and in apparently sporadic PHEOs/PGLs 0.038 ± 0.016 mmol/L (COV of 0.43). Fumarate concentrations were significantly lower in the SDHB-related compared with SDHD-related and apparently sporadic PHEOs/PGLs (P = .005, P = .0008, respectively). The NF1 PHEO group showed a mean fumarate concentration of 0.057 ± 0.018 mmol/L (COV of 0.324) (Table 2).
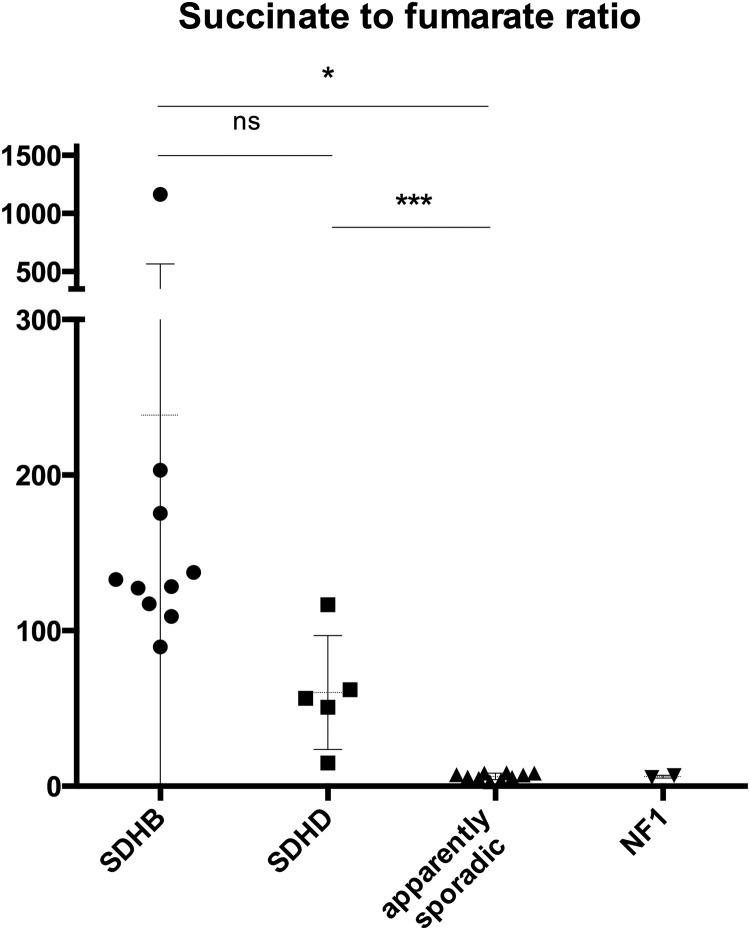
Succinate-to-fumarate ratio
The mean succinate-to-fumarate ratio was high in SDHB- and SDHD-related PGLs, 238.6 ± 327.2 (COV of 1.37) and 60.24 ± 36.58 (COV of 0.60), in contrast to apparently sporadic PHEOs/PGLs 6.3 ± 2.0 (COV of 0.31) (P = .0376, P = .0003, respectively) (Table 2, Figure 1). The NF1 PHEOs had a mean succinate-to-fumarate ratio of 6.204 ± 0.87 with the COV of 0.14.
Figure 1.
Succinate-to-fumarate ratio in SDHB, SDHD, apparently sporadic, and NF1 tumors. ns, not significant. *, P < .05; ***, P < .0005.
Succinate-to-fumarate ratio in plasma samples
The plasma mean succinate-to-fumarate ratio showed a slight increase in the SDHB and SDHD groups (3.15 ± 1.63 and 2.75 ± 1.65) compared with the apparently sporadic group (1.61 ± 0.61), but this difference was not significant.
Succinate-to-fumarate ratio in MPC and MTT cells
The succinate-to-fumarate ratio was significantly higher in SDHB-silenced MTT cells compared with control MTT cells; 7.53 vs 2.45 (P = .0115), whereas the small elevation in the SDHB-silenced vs control MPC cells was not significant (1.62 vs 1.16, P = .164) (Table 3).
Table 3.
Mean Succinate and Fumarate Concentrations and Succinate-to-Fumarate Ratio in SDHB-silenced and Control MPC and MTT Cells (n = 5 for each group)
| Succinate (mmol/L) | Fumarate (mmol/L) | Succinate-to-Fumarate Ratio | |
|---|---|---|---|
| MPC | |||
| Control | 1.39 | 1.20 | 1.16 |
| SDHB-silenced | 1.63 | 1.05 | 1.62 |
| MTT | |||
| Control | 2.25 | 1.26 | 2.45 |
| SDHB-silenced | 6.86 | 0.85 | 7.53 |
Culture supernatants were analyzed in the SDHB-silenced and control MPC and MTT cells, but no apparent differences in the succinate-to-fumarate ratio were determined.
The degree of SDHB silencing was 62% in MPC and 63% in MTT cells. (Results are not shown.)
Discussion
In the present study, we found that the tumor tissue succinate-to-fumarate ratio was significantly higher in SDHB- and SDHD-related PGLs in contrast to apparently sporadic and NF1-related PHEOs/PGLs. Furthermore, SDHB-silenced MTT cells showed a similar trend of increased succinate-to-fumarate ratio compared with control MTT cells. These results for the first time suggest that the succinate-to-fumarate ratio can be used as a new metabolic marker for SDHB/D-related PHEOs/PGLs.
SDH is the crucial enzyme in energy metabolism that links the tricarboxylic acid cycle, also called the Krebs cycle, to oxidative phosphorylation (19). In the Krebs cycle, SDH catalyzes the oxidation of succinate to fumarate, whereas as mitochondrial complex II, it transfers electrons to the quinone pool, supporting the reduction of ubiquinone (19).
More than a decade ago, mutations in genes encoding SDH subunits B, C, and D, and more recently mutations in SDHAF2 and SDHA, were discovered to be involved in the pathogenesis of PHEOs/PGLs (2–6). Mutations in these genes result in impaired function of the SDH enzyme associated with succinate accumulation and loss of fumarate (10). Succinate accumulation has been shown to result in the inhibition of prolyl hydroxylases and consequently in the impaired degradation of hypoxia-inducible factor α (HIF1-, 2-α) (20). HIF1-, 2-α stabilization affects the activation of many genes promoting tumorigenesis and cancer development with accelerated aerobic glycolysis (the so-called Warburg effect) (21, 22). Reactive oxygen species, which also accumulate due to SDH mutations, were found to stabilize HIF-α (23, 24). These and other findings suggest that, indeed, SDHx-related PHEOs/PGLs could be viewed as a metabolic disease (25). Thus, the assessment of metabolic intermediates in these tumors could bring new discoveries, including the introduction of novel biomarkers specifically used in the clinical diagnosis of these unique metabolic tumors.
Metabolomics encompasses the characterization of metabolite profiles to genetic or environmental changes in biological samples (26). There are several different separation and detection methods for analytical procedures of the samples, including nuclear magnetic resonance spectroscopy and GC-MS. Metabolomic analysis is fast and reliable in the identification of metabolite changes in specific tissues, including tumors (26).
Genetic testing and immunohistochemistry are currently excellent methods in the diagnosis of SDHx mutations (11, 12). Unfortunately, these methods cannot assess any response of these tumors (eg, to chemo- or radiotherapy, their therapeutic resistance, or follow-up after a therapy is completed). Moreover, these methods cannot detect acute changes in the activity of these tumors; thus, they cannot predict the sudden aggressive behavior and metastatic spread that is often seen in patients with SDHB mutations. In the present study, introducing the succinate-to-fumarate ratio as a new marker in these tumors provides a new opportunity to not only diagnose but also monitor their behavior and therapeutic responses. Currently such monitoring would require a tumor sample to be obtained; we predict that in the near future plasma samples could also be used to assess these tumors as described above. This will be based on large prospective studies, as well as the introduction of more sensitive GC-MS methods. Because the pathogenesis of these tumors is primarily based on mitochondrial damage tightly linked to the Krebs cycle and the Warburg effect, we predict that other important metabolites will soon be introduced and used in clinical assessment with the succinate-to-fumarate ratio.
In the present study assessing SDHB mutations, we therefore particularly focused on the metabolic intermediates succinate and fumarate in various PHEOs/PGLs. We found that in SDHB- and SDHD-related PGLs, the succinate-to-fumarate ratio was significantly higher compared with apparently sporadic and NF1-related PHEOs/PGLs. The obtained results were validated in an in vitro model using SDHB-silenced MTT cells, which showed a higher succinate-to-fumarate ratio compared with control MTT cells.
This study for the first time suggests that the succinate-to-fumarate ratio could be used as a new metabolic marker for the presence of SDHB/D-related PGLs. Our results also strongly call for a large prospective clinical study including other SDH PHEOs/PGLs. Furthermore, on confirmation of our initial results, we predict that tumor and perhaps plasma changes in the succinate-to-fumarate ratio will serve as an important indicator of potential therapies directed toward mutated SDH proteins (27).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- COV
- coefficient of variation
- GC-MS
- gas chromatography-mass spectrometry
- HIF
- hypoxia-inducible factor
- MPC
- mouse pheochromocytoma
- MTT
- mouse tumor tissue
- NF1
- neurofibromatosis 1
- NIH
- National Institutes of Health
- NICHD
- National Institute of Child Health and Human Development
- PGLs
- paragangliomas
- PHEOs
- pheochromocytomas
- SDH
- succinate dehydrogenase
- SDHB
- succinate dehydrogenase subunit B.
References
- 1. Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44:328–333. [DOI] [PubMed] [Google Scholar]
- 2. Baysal BE, Ferrell RE, Willett-Brozick JE, et al. . Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. [DOI] [PubMed] [Google Scholar]
- 3. Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–270. [DOI] [PubMed] [Google Scholar]
- 4. Astuti D, Latif F, Dallol A, et al. . Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hao HX, Khalimonchuk O, Schraders M, et al. . SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burnichon N, Brière JJ, Libé R, et al. . SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gimenez-Roqueplo AP, Favier J, Rustin P et al. ; COMETE Network. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–5621. [PubMed] [Google Scholar]
- 8. Timmers HJ, Kozupa A, Eisenhofer G, et al. . Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with SDHB-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92:779–786. [DOI] [PubMed] [Google Scholar]
- 9. Eisenhofer G, Lenders JW, Siegert G, et al. . Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pollard PJ, Brière JJ, Alam NA, et al. . Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. [DOI] [PubMed] [Google Scholar]
- 11. Pawlu C, Bausch B, Reisch N, Neumann HP. Genetic testing for pheochromocytoma-associated syndromes. Ann Endocrinol (Paris). 2005;66:178–185. [DOI] [PubMed] [Google Scholar]
- 12. van Nederveen FH, Gaal J, Favier J, et al. . An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baysal BE. On the association of succinate dehydrogenase mutations with hereditary paraganglioma. Trends Endocrinol Metab. 2003;14:453–459. [DOI] [PubMed] [Google Scholar]
- 14. Dahia PL, Ross KN, Wright ME, et al. . A HIF1α regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao M, Yang H, Xu W, et al. . Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powers JF, Evinger MJ, Tsokas P, et al. . Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000;302:309–320. [DOI] [PubMed] [Google Scholar]
- 17. Martiniova L, Lai EW, Elkahloun AG, et al. . Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin Exp Metastasis. 2009;26:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pawlosky RJ, Kashiwaya Y, Srivastava S, et al. . Alterations in brain glucose utilization accompanying elevations in blood ethanol and acetate concentrations in the rat. Alcohol Clin Exp Res. 2010;34:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. [DOI] [PubMed] [Google Scholar]
- 20. Selak MA, Armour SM, MacKenzie ED, et al. . Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7:77–85. [DOI] [PubMed] [Google Scholar]
- 21. Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 23. Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N. A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res. 2005;65:203–209. [PubMed] [Google Scholar]
- 24. Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28:718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fiehn O. Metabolomics—the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–171. [PubMed] [Google Scholar]
- 27. Yang C, Matro JC, Huntoon KM, et al. . Missense mutations in the human SDHB gene increase protein degradation without altering intrinsic enzymatic function. FASEB J. 2012;26:4506–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]