Abstract
Tctex1d2 (Tctex1 domain containing 2) is an open reading frame that encodes for a functionally unknown protein that contains a Tctex1 domain found in dynein light chain family members. Examination of gene expression during adipogenesis demonstrated a marked increase in Tctex1d2 protein expression that was essentially undetectable in preadipocytes and markedly induced during 3T3-L1 adipocyte differentiation. Tctex1d2 overexpression significantly inhibited insulin-stimulated glucose transporter 4 (GLUT4) translocation and 2-deoxyglucose uptake. In contrast, Tctex1d2 knockdown significantly increased insulin-stimulated GLUT4 translocation and 2-deoxyglucose uptake. However, acute insulin stimulation (up to 30 min) in 3T3-L1 adipocytes with overexpression or knockdown of Tctex1d2 had no effect on Akt phosphorylation, a critical signal transduction target required for GLUT4 translocation. Although overexpression of Tctex1d2 had no significant effect on GLUT4 internalization, Tctex1d2 was found to associate with syntaxin 4 in an insulin-dependent manner and inhibit Doc2b binding to syntaxin 4. In addition, glucose-dependent insulinotropic polypeptide rescued the Tctex1d2 inhibition of insulin-stimulated GLUT4 translocation by suppressing the Tctex1d2-syntaxin 4 interaction and increasing Doc2b-Synatxin4 interactions. Taking these results together, we hypothesized that Tctex1d2 is a novel syntaxin 4 binding protein that functions as a negative regulator of GLUT4 plasma membrane translocation through inhibition of the Doc2b-syntaxin 4 interaction.
One major action of insulin is to stimulate an increase in glucose uptake in adipocytes through the intracellular trafficking of the glucose transporter 4 (GLUT4) cargo protein from GLUT4 storage vesicles to the plasma membrane (1). In this manner, the increase in the cell surface number of GLUT4 facilitative glucose transporters increases the postprandial absorption of circulating glucose to maintain euglycemia (2). A variety of studies have established the proximal signaling events and plasma membrane fusion proteins required for this translocation process. In particular, insulin stimulates Akt phosphorylation, resulting in the activation of the Akt kinase that in turn phosphorylates and inhibits the GAP activity of AS160 that suppresses GTP activation of Rab10 in adipocytes and Rab8 in a skeletal muscle cell line (3). Although the precise function of Rab10 has not been established, it is thought that activated Rab10 is responsible for the trafficking and/or docking of GLUT4 transport vesicles with the plasma membrane (4). After GLUT4 vesicle docking with the plasma membrane, fusion with the plasma membrane requires a selective subset of soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) attachment proteins and SNARE accessory factors (5). In particular, syntaxin 4 is the required plasma membrane target (t-SNARE), and Doc2b binding provides an accessory function facilitating the fusion events (6).
The mouse Tctex1 domain containing 2 (Tctex1d2) protein is encoded by the Tctex1d2 gene (chromosome 3, 3q29) and consists of 142 amino acids. Tctex1d2 displays a 31% similarity with mouse Tctex-1, consistent with Tctex1d2 belonging to the Tctex-1 gene family. The Tctex1d2 amino acid sequence also displays sequence homology with the Tctex-1 domain of the dynein light chain, but only encodes for the Tctex-1 domain, suggesting a function independent of dynein or microtubules. Currently there are only 2 recent studies reporting that Tctex1d2 is expressed in human spermatozoa and functions as an intra-acrosomal protein in cargo transport (7) and as a candidate ciliopathy gene (8). In the current study, we demonstrate that Tctex1d2 is induced during 3T3-L1 adipocyte differentiation and negatively regulates insulin-stimulated GLUT4 translocation and glucose uptake in 3T3-L1 adipocytes through direct binding to syntaxin 4.
Materials and Methods
Reagents
Tctex1d2 antibody was purchased from Abcam. α-Tubulin, adaptin (aP2), phospho-Akt, Akt, syntaxin 4, Doc2b, and FLAG antibodies were purchased from Sigma-Aldrich (Table 1). Myc antibody was purchased from Merck Millipore. ECL and the ECL Plus Western Blotting Detection System were purchased from GE Healthcare. Anti-mouse and anti-rabbit IgG-horseradish peroxidase (HRP) were obtained from Pierce. Cell culture medium and reagents were from Invitrogen Life Technologies. Short hairpin RNA constructs against Mus musculus Tctex1d2 was purchased from Santa Cruz Biotechnology. Tctex1d2 RNA interference (RNAi) was purchased from Santa Cruz Biotechnology and OriGene. All other chemicals used in this study were purchased from Sigma-Aldrich.
Table 1.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| Tctex1d2 | Anti-Tctex1d2 Ab | Abcam, ab139804 | pAb | ×1000 |
| α-Tubulin | Anti-α-tubulin Ab | Sigma, T5168 | mAb | ×4000 |
| Adaptin | Anti-FABP4 Ab | Sigma, SAB1410359 | pAb | ×2000 |
| Phospho-Akt | Anti-phospho-PKB | Sigma, P4112 | pAb | ×1000 |
| Akt | Anti-pan-Akt Ab | SAB4301170 | pAb | ×1000 |
| Syntaxin 4 | Anti-STX4 Ab | HPA001330 | pAb | ×2000 |
| Doc2b | Anti-Doc2b Ab | SAB1400312 | mAb | ×1000 |
| FLAG | Anti-Flag-M2 | F108 | mAb | ×1000 |
Cell culture
3T3-L1 preadipocytes were cultured in DMEM containing 25 mM glucose and 10% calf serum at 37°C with 8% CO2. Confluent cultures were induced to differentiate into adipocytes as described previously (9).
Transfection of 3T3-L1 adipocytes
3T3-L1 adipocytes were suspended by mild trypsinization and electroporated with a total of 1 mg of plasmid under a low-voltage condition (0.16 kV, 950 μF) as described previously (9, 10). The cells were then allowed to adhere to collagen-coated tissue culture dishes for 30 to 48 hours, and the adipocytes were then serum starved for 4 hours before incubation in the absence or presence of 100 nM insulin at 37°C for the various time periods indicated in each figure.
MBP fusion protein and precipitation
Cytoplasmic portions of syntaxin 4 (amino acids 2–274) were subcloned into the pGEX-4T-1 expression vector (Pharmacia). The maltose-binding protein (MBP) and MBP-syntaxin 4 were prepared, and the fusion proteins were purified as described previously (11). Cell lysates from 3T3-L1 adipocytes were incubated with either MBP alone or with MBP-syntaxin 4 fusion proteins immobilized on amylose-agarose beads for 1 hour at 4°C. The beads were washed extensively 3 times with 1 mL of HNTG (50 mM HEPES [pH 7.4], 150 mM sodium chloride, 1% Triton X-100, 10% glycerol, and 1 mM EDTA) buffer and then 2 times with 1 mL of distilled water. The samples were then resuspended in 1× Laemmli sample buffer and immunoblotted with either a Tctex1d2 or a Doc2b antibody.
Immunoprecipitation and immunoblotting
Scraped frozen cells were rocked for 10 minutes at 4°C with NP-40 lysis buffer (25 mM HEPES [pH 7.4], 10% glycerol, 1% NP-40, 50 mM sodium fluoride, 10 mM sodium phosphate, 137 mM sodium chloride, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonylfluoride, 10 μg/mL aprotinin, 1 μg/mL pepstatin, and 5 μg/mL leupeptin). Insoluble material was separated from the soluble extract by centrifugation for 10 minutes at 4°C, and the total protein amount in the supernatant was determined by the BCA method. Immunoprecipitations were performed by using 2 mg of the cell extracts incubated with 4 μg of a syntaxin 4 polyclonal antibody for 2 hours at 4°C. The samples were then incubated with protein A-agarose for 1 hour at 4°C. Either immunoprecipitated samples or whole-cell lysate samples were resuspended in SDS sample buffer (125 mM Tris-HCl [pH 6.8], 20% [v/v] glycerol, 4% [w/v] SDS, 100 mM dithiothreitol, and 0.1% [w/v] bromphenol blue), and heated at 100°C for 5 minutes. Samples were separated by SDS-PAGE (the glass plate size was 18 cm × 24 cm) and electrophoretically transferred to polyvinylidene difluoride membranes (Immobilon-P; Merck Millipore). The samples were immunoblotted with a monoclonal- or polyclonal-specific antibody as indicated on each figure.
Enhanced green fluorescent protein (EGFP)-GLUT4 translocation assay (translocation assay)
EGFP-GLUT4 plasmid (50 μg) with 550 μg of interesting plasmids was electroporated to 3T3-L1 adipocytes. The cells were then allowed to adhere to collagen-coated tissue culture dishes for 30 to 48 hours, and the adipocytes were serum starved for 2 hours before incubation in the absence or presence of 100 nM insulin for 15 minutes at 37°C. Transfected adipocytes were washed in PBS and fixed for 10 minutes in PBS containing 4% paraformaldehyde and 0.2% Triton X-100. The samples were mounted on glass slides with DAKO fluorescent mounting medium (DAKO Corporation). Cells were imaged using a confocal fluorescence microscope (model MRC-1024; Bio-Rad Laboratories) (9). The relative extent of GLUT4 translocation was then determined by counting the number of cells with readily discernible circumscribed cell surface fluorescence.
Quantification of GLUT4 translocation (colorimetric assay)
The translocation assay described above estimates the cell surface localization of GLUT4 by single-cell observation. To eliminate potential subjective bias in the translocation assay, we also used a biochemical quantitative GLUT4 translocation (colorimetric assay) as previously described (9, 10). In brief, 3T3-L1 adipocytes were cotransfected with 400 μg of EGFP-cMyc-GLUT4 plus 600 μg of various other cDNAs as indicated on each figure. After basal or hormonal stimulation, the cells were cooled to 4°C and incubated with a myc antibody followed by an HRP-conjugated anti-mouse IgG antibody. The specific cell surface–bound HRP was then determined by incubation with the substrate, o-phenylenediamine dihydrochloride peroxidase.
Quantification of 2-deoxyglucose uptake
Glucose uptake was determined by 2-deoxyglucose uptake with an enzymatic photometric assay by using a 2-deoxyglucose uptake measurement kit (COSMO BIO Co. Ltd.) (12).
GLUT4 internalization
At 48 hours postelectroporation, 3T3-L1 cells were serum starved for 2 hours in DMEM containing 25 mM glucose. The cells were left untreated or stimulated with 100 nM insulin for 30 minutes. To remove the bound insulin from the insulin receptor, the cells were then washed in an acidic buffer (140 mM NaCl and 5 mM sodium acetate [pH 5.0]) followed by a PBS rinse and incubation at 37°C for an additional 2 hours in fresh starvation medium. Then a colorimetric assay was performed to access the remaining amount of GLUT4 on the cell surface (13, 14).
Statistical analysis
All values are expressed as means ± SD of the mean. Data were evaluated for statistical significance using ANOVA and the Turkey-Kramer multiple comparison test. The minimum level of significance was set at a P value of <.05. The InStat 2 program was used for statistical analysis.
Results
Tcetx1d2 is induced during adipocyte differentiation
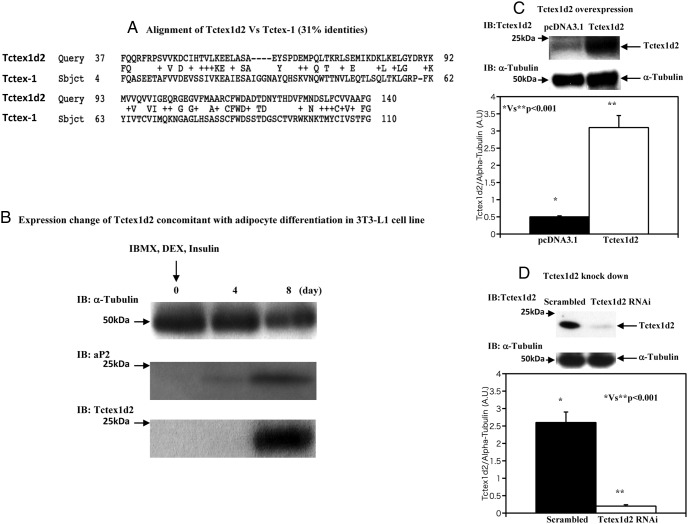
During our analysis of differentially expressed proteins in 3T3-L1 preadipocytes vs differentiated 3T3-L1 adipocytes, we observed the induction of the Tctex1d2 protein. An amino acid sequence comparison between mouse Tctex1d2 and mouse Tctex-1 is shown in Figure 1A. There is a 31% sequence similarity between Tctex1d2 and Tctex-1. Because Tctex1d2 has a unique amino-terminal 21 amino acids and Tctex1d2 cDNA (genomic location is 3q29) is completely different from the dynein light chain coding cDNA (genomic location is 12q24.23), we speculated that Tctex1d2 might have a physiological function distinct from that of dynein. Tctex1d2 protein induction during 3T3-L1 adipocytes differentiation is shown in Figure 1B. The increase in Tctex1d2 protein is similar to the induction of the established adipocyte-specific marker aP2 (9, 10). As a loading control, the protein levels of α-tubulin remained relatively constant during 3T3-L1 adipocyte differentiation.
Figure 1. Tcetx1d2 is expressed during adipocyte differentiation and design of overexpression and knockdown of Tctex1d2 by electroporation.
A, Comparison of amino acid sequences between mouse Tctex1d2 and mouse Tctex-1. The overall sequence similarity is 31%. B, 3T3–L1 cells were differentiated as described under Materials and Methods, and cell extracts at the indicated times were immunoblotted (IB) for α-tubulin, aP2, and Tctex1d2. These are representative immunoblots performed independently 3 times. IBMX, 3-isobutyl-1-methylxanthine; DEX, dexamethasone. C, 3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Tctex1d2 as described under Materials and Methods. Cell extracts were prepared and immunoblotted for Tctex1d2 and α-tubulin. D, 3T3–L1 adipocytes were transfected either with a scrambled RNAi or a Tctex1d2-specific RNAi as described under Materials and Methods. Cell extracts were prepared and immunoblotted for Tctex1d2 and α-tubulin. A.U., arbitrary units.
Design of overexpression and knockdown of Tctex1d2 by electroporation
As previously reported using CsCl double-banded cDNA, we routinely obtain a transfection efficiency of 3T3-L1 adipocytes of 50% to 80% (9–11). Under these conditions, Tctex1d2 was overexpressed approximately 6-fold over the endogenous Tctex1d2 levels (Figure 1C). Thus, under these conditions, the average expression per cell will be 7.5- to 12-fold higher in 50% to 80% of the cells in the population. Knockdown of Tctex1d2 using either scrambled RNAi or Tctex1d2-specific RNAi reduced the levels of the endogenous Tctex1d2 protein to 10% to 20% (Figure 1D).
Tctex1d2 negatively regulates insulin-stimulated GLUT4 translocation and glucose uptake
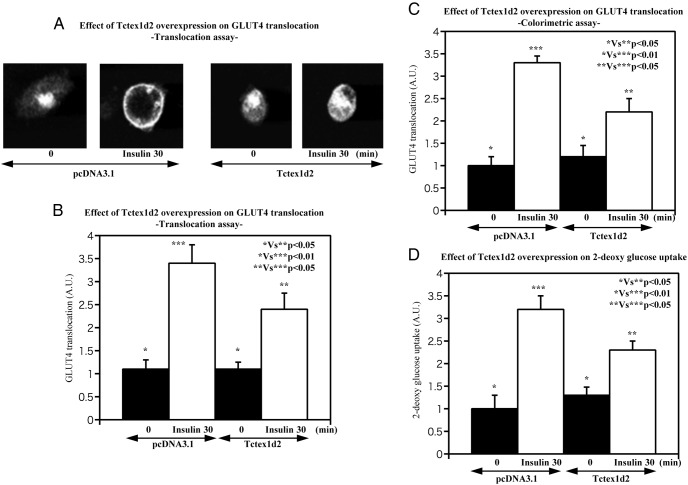
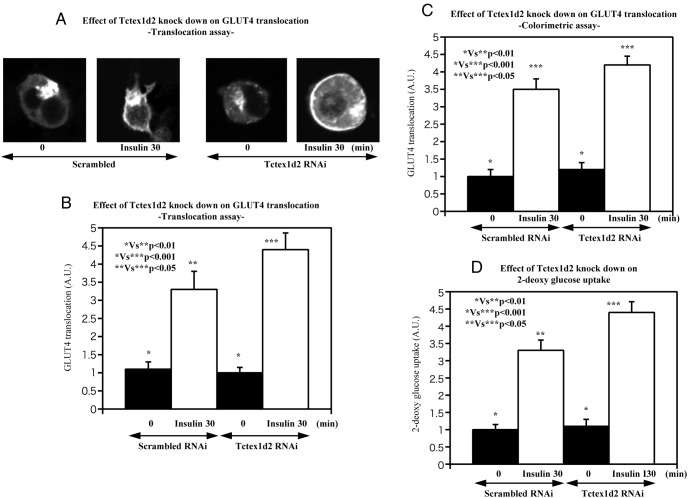
It was previously reported that the related Tctex1d4 plays a role in the regulation of vesicle transport (7), we examined the potential role of Tctex1d2 in modulating GLUT4 translocation in adipocytes. As shown in Figure 2A to C, overexpression of Tctex1d2 inhibited insulin-stimulated GLUT4 translocation. Concomitant with the reduction in GLUT4 translocation, overexpression of Tctex1d2 resulted in a parallel decrease of insulin-stimulated glucose uptake (Figure 2D). Consistent with Tctex1d2 overexpression, knockdown of Tctex1d2 resulted in increased insulin-stimulated GLUT4 translocation (Figure 3A–C) and in a parallel increase in insulin-stimulated glucose uptake compared with that in scrambled RNAi-transfected cells (Figure 3D). These data directly demonstrate that Tctex1d2 is a suppressor of insulin-stimulated GLUT4 translocation and glucose uptake.
Figure 2. Effect of Tctex1d2 overexpression on insulin-stimulated GLUT4 translocation and glucose uptake.
3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Tctex1d2 as described under Materials and Methods. The cells were then either left untreated or incubated with 100 nM insulin for 30 minutes as indicated. GLUT4 translocation (A–C) and glucose (2-deoxyglucose) uptake (D) were determined as described under Materials and Methods. These data are from 4 independent determinations expressed as the mean ± SD. A.U., arbitrary units.
Figure 3. Effect of Tctex1d2 knockdown on insulin-stimulated GLUT4 translocation and glucose uptake.
The cells were either left untreated or incubated with 100 nM insulin for 30 minutes as indicated. GLUT4 translocation (A–C) and glucose (2-deoxyglucose) uptake (D) were determined as described under Materials and Methods. These data are from 4 independent determinations expressed as the mean ± SD. A.U., arbitrary units.
Tctex1d2 and Akt and Erk phosphorylation
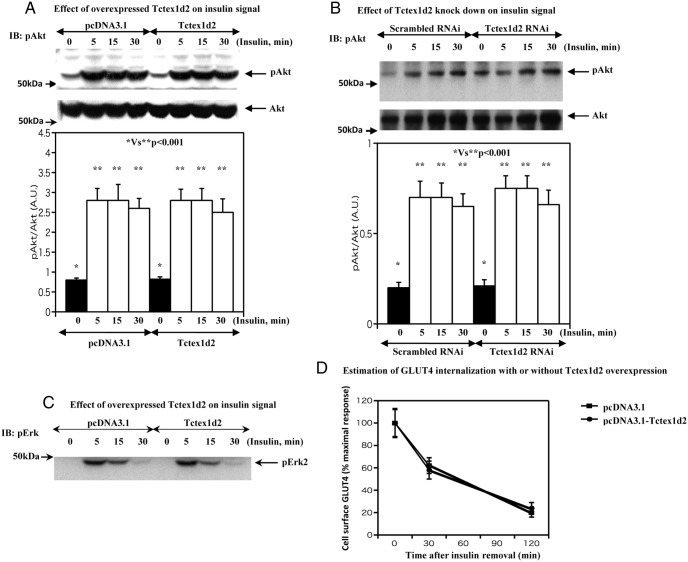
To examine the to potential mechanism responsible for the inhibition of insulin-stimulated GLUT4 translocation by Tctex1d2, we assessed the impact on insulin signaling. In vector-transfected control adipocytes, 100 nM insulin treatment for 5 minutes resulted in an increase in Akt phosphorylation that remained phosphorylated over the next 30 minutes (Figure 4A). In Tctex1d2-transfected cells, essentially an identical extent and time dependence of Akt phosphorylation were observed (Figure 4A). Consistent with Tctex1d2 having no insulin signal leading to Akt activation, Tctex1d2 knockdown cells also display the same extent and time dependence of Akt phosphorylation compared with that of scrambled RNAi-transfected cells (Figure 4B). Similarly, there was no significant alteration in the time dependence or extent of Erk2 phosphorylation (Figure 4C). These data indicate that Tctex1d2 functions downstream of Akt activation.
Figure 4. Tctex1d2 and Akt and Erk phosphorylation and GLUT4 internalization.
A, 3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Tctex1d2 as described under Materials and Methods. The cells were then either left untreated or incubated with 100 nM insulin for 5, 15, and 30 minutes as indicated. Cell extracts were prepared and immunoblotted (IB) for total Akt protein (Akt1/2/3, bottom panel) and Akt phosphorylation (Ser473, top panel). These are representative immunoblots performed independently 3 times. B, Scrambled or Tctex1d2-specific RNAi-transfected 3T3–L1 adipocytes were then either left untreated or incubated with 100 nM insulin for 5, 15, and 30 minutes as indicated. Cell extracts were prepared and immunoblotted for total Akt protein (Akt1/2/3, bottom panel) and Akt phosphorylation (Ser473, top panel). These are representative immunoblots performed independently 3 times. C, 3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Tctex1d2 as described under Materials and Methods. The cells were then either left untreated or incubated with 100 nM insulin for 5, 15, and 30 minutes as indicated. Cell extracts were prepared and immunoblotted for p42 Erk phosphorylation (Thr202/Thr204). These are representative immunoblots performed independently 3 times. D, 3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Tctex1d2 as described under Materials and Methods. The cells were incubated with 100 nM insulin for 30 minutes. Then cells were washed as described under Materials and Methods to remove insulin. The cell surface GLUT4 amount was estimated as described under Materials and Methods. These data are from 8 independent determinations expressed as the mean ± SD. A.U., arbitrary units.
Tctex1d2 does not affect GLUT4 internalization
Dynein has been reported to be an important regulator of GLUT4 endocytosis (13, 14) and because Tctex1d2 displays a similarity with Tctex1, a component of the dynein light chain, we tested the hypothesis that Tctex1d2 alone might increase the clearance of plasma membrane GLUT4 protein. After insulin stimulation, insulin was removed, and the time-dependent decrease in cell surface GLUT4 was determined. Over the time frame observed, there was no significant difference in the amount of cell surface GLUT4 protein with and without Tctex1d2 overexpression (Figure 4D). These data indicate that Tctex1d2 itself has no effect on the rate of GLUT4 internalization.
Tctex1d2 binds to syntaxin 4
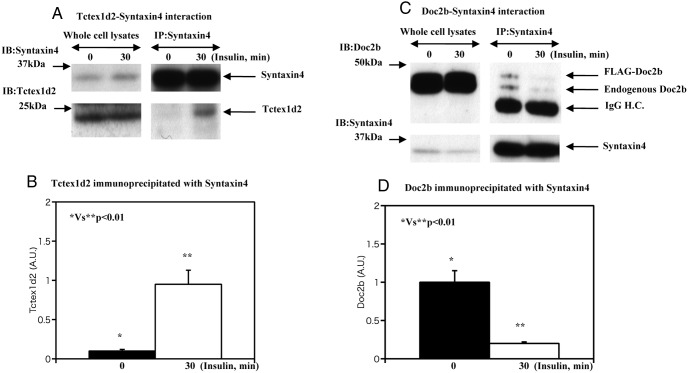
Syntaxin 4 is part of the core SNARE protein complex that also contains SNAP23 and VAMP2 required for the fusion of GLUT4 transport vesicles with the plasma membrane (15). In addition, several syntaxin 4 binding modulators such as MUC18c, Synip, and Doc2b regulate syntaxin 4 function (15). For example, Doc2b is a necessary positive regulator required for the binding of VAMP2-containing GLUT4 vesicles with syntaxin 4 (6). Because this interaction of syntaxin 4, Doc2b, and VAMP2 occurs independent of Akt activation, we tested the possibility that Tctex1d2 may be a modulator of these syntaxin 4 interactions. In the basal state, there was a relatively low amount of Tctex1d2 that was coimmunoprecipitated with syntaxin 4 (Figure 5A). However, following insulin stimulation, the amount of Txtex1d2 coimmunoprecipitated with syntaxin 4 markedly increased. Quantification indicated an approximate 4.8-fold increase in the amount of Tctex1d2 associated with syntaxin 4 (Figure 5B).
Figure 5. Estimation of Doc2b-syntaxin 4 and Tctex1d2-syntaxin 4 interaction.
A and B, 3T3–L1 adipocytes were either untreated or incubated with 100 nM insulin for 30 minutes as indicated. Cell extracts were prepared and immunoblotted (IB) for syntaxin 4 and Tctex1d2. In parallel, syntaxin 4 was immunoprecipitated (IP), and the resultant precipitates were immunoblotted for syntaxin 4 and Tctex1d2. This is a representative immunoblot performed independently 3 times. C and D, 3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Doc2b as described under Materials and Methods. Exogenous Doc2b expression was adjusted to a as similar extent with endogenous Doc2b expression. The cells were either untreated or incubated with 100 nM insulin for 5, 15, and 30 minutes as indicated. Cell extracts were prepared and immunoblotted for Doc2b and syntaxin 4. In parallel, syntaxin 4 was immunoprecipitated, and the resultant precipitates were immunoblotted for Doc2b and syntaxin 4. These are representative immunoblots performed independently 3 times. H.C., heavy chain; A.U., arbitrary units.
In contrast, insulin stimulation decreases the amount of Doc2b binding. To determine whether this also occurs with overexpressed Doc2b, we expressed a FLAG epitope-tagged Doc2b (FLAG-Doc2b) at levels similar to those of the endogenous Doc2b protein. As expected, insulin stimulation resulted in a decrease in the amount of endogenous and transfected Doc2b that was coimmunoprecipitated with syntaxin 4 (Figure 5, C and D). As the total levels of syntaxin 4 and Doc2b did not change over this time frame, these data indicate that insulin stimulates the dissociation of both the endogenous Doc2b and the transfected FLAG-Doc2b from syntaxin 4.
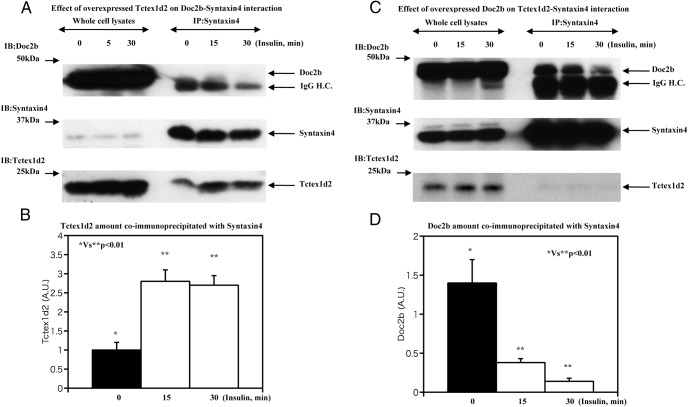
Because the binding of Tctex1d2 and Doc2b displayed an inverse binding to syntaxin 4, we next overexpressed Tctex1d2 and examined its effect on Doc2b-syntaxin 4 interactions. Overexpressed Tctex1d2 coimmunoprecipitated with syntaxin 4 in the basal state was increased approximately 3-fold after insulin stimulation (Figure 6, A and B). In the cells overexpressing Tctex1d2, there was no significant coimmunoprecipitation of Doc2b with syntaxin 4 (Figure 6A), suggesting a direct binding competition between Tctex1d2 and Doc2b for syntaxin 4. To confirm this, we overexpressed Doc2b that was coimmunoprecipitated with syntaxin 4 and prevented the coimmunoprecipitation of Tctex1d2 (Figure 6, C and D).
Figure 6. Estimation of Doc2b-syntaxin 4 with Tctex1d2 overexpression and Tctex1d2-syntaxin 4 interaction with Doc2b overexpression.
A and B, 3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Tctex1d2 as described under Materials and Methods. The cells were either untreated or incubated with 100 nM insulin for 5, 15, and 30 minutes as indicated. Cell extracts were prepared and immunoblotted (IB) for Doc2b, syntaxin 4, and Tctex1d2. In parallel, syntaxin 4 was immunoprecipitated (IP), and the resultant precipitates were immunoblotted for Doc2b, syntaxin 4, and Tctex1d2. These are representative immunoblots independently performed 3 times. C and D, 3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Doc2b as described under Materials and Methods. The cells were either untreated or incubated with 100 nM insulin for 5, 15, and 30 minutes as indicated. Cell extracts were prepared and immunoblotted for Doc2b, syntaxin 4, and Tctex1d2. In parallel, syntaxin 4 was immunoprecipitated, and the resultant precipitates were immunoblotted for Doc2b, syntaxin 4, and Tctex1d2. These are representative immunoblots performed independently 3 times. H.C., heavy chain; A.U., arbitrary units.
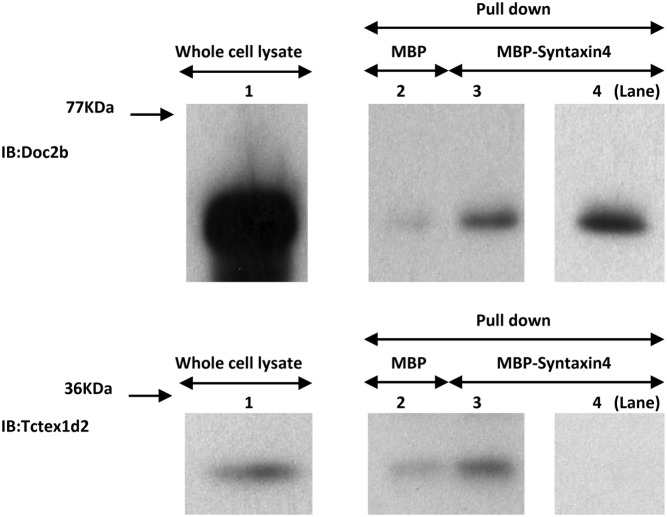
To confirm the apparent direct competition between Tctex1d2 and Doc2b for binding to syntaxin 4, we prepared cell extracts from Tctex1d2- and from Doc2b-overexpressing cells that were incubated with either purified MBP or MBP-syntaxin 4 fusion proteins. Incubation of either cell extract with MBP resulted in a low level of Doc2b or Tctex1d2 pulldown (Figure 7, lane 2 in the top and bottom panels). On the other hand, incubation of MBP-syntaxin 4 with either extract resulted in increased amounts of Doc2b and Tctex1d2 that were precipitated (Figure 7, lane 3 in the top and bottom panels, respectively). In contrast, mixing of the cell lysates with a 4-fold excess of Doc2b over Tctex1d2 resulted in increased precipitation of Doc2b (Figure 7, lane 4 in the top panel) and a complete loss of Tctex1d2 in the MBP-syntaxin 4 pulldown (Figure 7, lane 4 in the bottom panel).
Figure 7. Estimation of competition between Tctex1d2 and Doc2b on Synatxin4 binding.
Cell lysates from 3T3–L1 adipocytes expressing either Tctex1d2 or Doc2b were incubated either with MBP alone or with MBP-syntaxin 4 fusion proteins immobilized on amylose-agarose beads for 1 hour at 4°C. The beads were washed extensively. The samples were then resuspended in 1× Laemmli sample buffer and immunoblotted (IB) with either a Tctex1d2 or Doc2b antibody. Lane 1 shows the input amount of Doc2b (top panel) and Tctex1d2 (bottom panel). Pulldown by MBP protein alone is shown in lane 2. The top panel shows the Doc2b amount pulled down by MBP alone, and the bottom panel shows the Tctex1d2 amount pulled down by MBP alone. In lane 3, MBP-syntaxin 4 (approximately 10 fmol was added) pulldown data for Doc2b and Tctex1d2 (lane 3 in the top and bottom panels) are shown. In lane 4, the Doc2b amount was increased (approximately 4 times more than Tctex1d2), and then MBP-syntaxin 4 (approximately 10 fmol was added) pulldown was performed. The amount of Doc2b is shown in lane 4 in the top panel, and the amount of Tctex1d2 is shown in lane 4 in the bottom panel. These are representative immunoblots performed independently 4 times.
Gastric inhibitory peptide (GIP) reverses Tctex1d2 inhibition
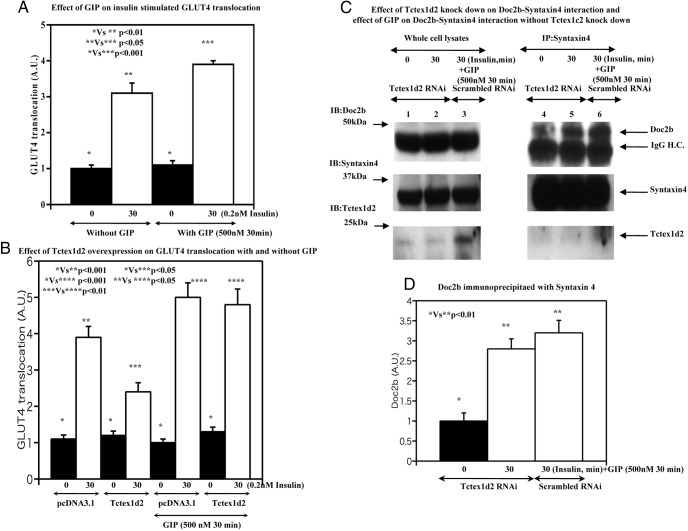
In adipocytes, GIP does not increase glucose uptake but potentiates insulin-stimulated GLUT4 translocation and glucose uptake through activation of both the cAMP/protein kinase A/cAMP response element-binding protein and p110β phosphoinositide-3′ kinase signaling pathways (16). We also were unable to detect any significant effect of GIP on the basal level of cell surface GLUT4 protein but GIP did potentiate insulin-stimulated GLUT4 translocation (Figure 8A). As observed previously, overexpression of Tctex1d2 suppressed insulin-stimulated GLUT4 translocation that was rescued by the addition of GIP (Figure 8B). Tctex1d2 knockdown (Figure 8C, lanes 1, 2, 4, and 5) resulted in an increased amount of Doc2b coimmunoprecipitated with syntaxin 4 (Figure 8D), consistent with the increase in insulin-stimulated GLUT4 translocation and glucose uptake shown in Figure 3. Moreover, GIP stimulation also increased the amount of Doc2b associated with syntaxin 4 (Figure 8C, lane 6 in the upper right panel) similar to the effect of Tctex1d2 knockdown. In addition, GIP stimulation decreased the amount of Tctex1d2 not coimmunoprecipitated with syntaxin 4. These data demonstrate that GIP provides a signal that modulates the relative distribution of Doc2b and Tctex1d2 bound to syntaxin 4 and thereby potentiates the action of insulin in regulating GLUT4 translocation in adipocytes.
Figure 8. Effect of GIP stimulation on GLUT4 translocation with or without Tctex1d2 overexpression and knockdown.
A, 3T3–L1 adipocytes were untreated or incubated with 0.2 nM insulin for 30 minutes as indicated. In addition, cells were untreated or incubated with 0.2 nM insulin for 30 minutes accompanied with 500 nM GIP for 30 minutes as indicated. Thereafter, the GLUT4 translocation amount was quantitatively estimated as described under Materials and Methods. These data are from 4 independent determinations expressed as the mean ± SD. B, 3T3–L1 adipocytes were transfected with either the empty vector (pcDNA3.1) or pcDNA3.1-Tctex1d2 as described under Materials and Methods. Cells were either untreated or incubated with 100 nM insulin for 30 minutes as indicated. The other set of cells were either untreated or incubated with 0.2 nM insulin for 30 minutes as indicated and incubated with 500 nM GIP for 30 minutes as indicated. Thereafter, the GLUT4 translocation amount was quantitatively estimated as described under Materials and Methods. These data are from 7 independent determinations expressed as the mean ± SD. C and D, scrambled (lanes 3 and 6) or Tctex1d2-specific RNAi (lanes 1, 2, 4, and 5)–transfected 3T3–L1 adipocytes were either left untreated (lanes 1 and 4) or incubated with 0.2 nM insulin for 30 minutes (lane 2 and 5) or 0.2 nM insulin for 30 minutes accompanied with 500 nM GIP for 30 minutes (lanes 3 and 6) as indicated. Cell extracts were prepared and immunoblotted for Doc2b, syntaxin 4, and Tctex1d2. In parallel, syntaxin 4 was immunoprecipitated, and the resultant precipitates were immunoblotted for Doc2b, syntaxin 4, and Tctex1d2. These are representative immunoblots performed independently 3 times. H.C., heavy chain; A.U., arbitrary units.
Discussion
Tctex1d2 contains 142 amino acids that encode for a Tctex1 domain. Tctex1 itself is a dynein light chain involved in cargo binding (17) encoded by DYNLL1 (genomic location is 12q24.23), whereas Tctex1d2 is encoded by a separate gene located on chromosome 3 (3q29). Therefore, we speculated that Tctex1d2 might have a physiological function distinct from that of the dynein light chain. Thus, to examine a potential physiological function for Tctex1d2, we initially compared the endogenous Tctex1d2 expression level between preadipocytes and differentiated adipocytes. Surprisingly, in cultured 3T3-L1 cells, Tctex1d2 was selectively expressed in differentiated adipocytes but not in preadipocytes.
In differentiated adipocytes, Tctex1d2 appears to play a role in the regulation of insulin-stimulated GLUT4 translocation and glucose uptake at the level of GLUT4 vesicle fusion with the plasma membrane. This conclusion is based on findings that Tctex1d2 overexpression had no significant effect on the proximal signaling events (Akt phosphorylation) that are known to regulate GLUT4 vesicle trafficking to the plasma membrane. Despite the apparent normal level of insulin signaling, both GLUT4 translocation and glucose uptake were reduced in cells overexpressing Tctex1d2 and enhanced in adipocytes with reduced levels of Tctex1d2.
The assay used for determining insulin-stimulated GLUT4 translocation is based on the exofacial appearance of an myc epitope tag present in the large extracellular loop of GLUT4 (9, 10). Thus, we speculated that Tctex1d2 might regulate the plasma membrane docking/fusion step of GLUT4 vesicles. It has been established that GLUT4 vesicle fusion with the plasma membrane is dependent on the interaction of GLUT4 vesicle v-SNARE (VAMP2) with the plasma membrane t-SNAREs syntaxin 4 and SNAP23 (5). Several accessory proteins have been reported to modulate this interaction, in particular, the syntaxin 4 binding protein Doc2b (6). Our data demonstrate that Tctex1d2 functionally competes for Doc2b association with syntaxin 4, suggesting that this may be the basis for Tctex1d2 inhibition of GLUT4 translocation and glucose uptake. Consistent with this conclusion, knockdown of Tctex1d2 increased the amount of Doc2b associated with syntaxin 4. Interestingly, although Doc2b is bound to syntaxin 4 in the basal state, we have observed an insulin-stimulated decrease in binding. Whether this part of the SNARE assembly/membrane fusion mechanisms or the SNARE complex disassembly/recycling pathway is involved remains to be determined.
Although how Tctex1d2 associates with syntaxin 4 has yet to be addressed, our preliminary data suggest that the Tctex1d2 phosphorylation status might attenuate the Tctex1d2-syntaxin 4 interaction. Because the S42 residue of Tctex1d2 is a putative phosphorylation site for protein kinase A, which is activated by GIP (18), in fact GIP stimulation abolished the Tctex1d2 inhibitory effect on insulin-stimulated GLUT4 translocation. Further studies are now needed to determine whether Tctex1d2 is a bona fide protein kinase A substrate and whether this phosphorylation alters Tctex1d2 binding to syntaxin 4. Nevertheless, the data presented in this study demonstrate that Tctex1d2 is a new syntaxin 4 binding partner specifically expressed in adipocytes that acts as a repressor of insulin-stimulated GLUT4 translocation and glucose uptake. In this context, GIP signal impairment would lead to insulin resistance, and GIP could be another treatment means to rescue insulin resistance of adipocytes.
In addition to the cognate SNARE proteins that interact with syntaxin 4 to drive membrane fusion, previous reports have identified several interacting regulatory proteins that include Munc18c, Synip, and Doc2b. Although the interrelationship between these accessory factors has yet to be resolved, this is an important issue for the control of membrane fusion events. In the case of Munc18c and Synip, these regulatory factors are bound to syntaxin 4 in the basal state but undergo insulin-stimulated dissociation (1, 3, 9). Previously we suggested that the Munc18c-syntaxin 4 and Synip-syntaxin 4 complexes consist of 2 different populations (19); however, the physiological and functional consequences of these 2 distinct populations remain enigmatic. In contrast, the data presented in this study indicate that in the absence of insulin Tctex1d2 does not interact with syntaxin 4 but functions as a negative regulator of Doc2b binding after insulin stimulation. Further studies are needed to distinguish whether distinct Doc2b-syntaxin 4, Munc18c-syntaxin 4, and Synip-syntaxin 4 complexes exist and the potential effect of Tctex1d2 in modulating these complex interactions.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EGFP
- enhanced green fluorescent protein
- GIP
- glucose-dependent insulinotropic polypeptide
- GLUT4
- glucose transporter 4
- HRP
- horseradish peroxidase
- MBP
- maltose-binding protein
- RNAi
- RNA interference
- SNARE
- soluble N-ethylmaleimide-sensitive factor attachment receptor
- Tctex1d2
- Tctex1 domain containing 2.
References
- 1. Govers R. Molecular mechanisms of GLUT4 regulation. Diabetes Metab. 2014;40:400–410. [DOI] [PubMed] [Google Scholar]
- 2. Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. [DOI] [PubMed] [Google Scholar]
- 3. Klip A, Sun Y, Chiu TT, Foley KP. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol. 2014;306:C879–C886. [DOI] [PubMed] [Google Scholar]
- 4. Stöckli J, Fazakerley DJ, James DE. GLUT4 exocytosis. J Cell Sci. 2011;124:4147–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. [DOI] [PubMed] [Google Scholar]
- 6. Fukuda N, Emoto M, Nakamori Y, et al. . Doc2B: a novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes. Diabetes. 2009;58:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freitas MJ, Korrodi-Gregório L, Morais-Santos F, Cruz e Sliva E, Fardiha M. Tctex1d4 interactome in human testis: unraveling the function of dynein light chain in spermatozoa. OMICS. 2014;18:242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asante D, Stevenson NL, Stephens DJ. Subunit composition of the human cytoplasmic dynein-2 complex. J Cell Sci. 2014;127:4774–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamada E, Okada S, Saito T, et al. . Akt2 phosphorylates Synip to regulate docking and fusion of GLUT4-containing vesicles. J Cell Biol. 2005;168:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okada S, Mori M, Pessin JE. Introduction of DNA into 3T3–L1 adipocytes by electroporation. Methods Mol Med. 2003;83:93–96. [DOI] [PubMed] [Google Scholar]
- 11. Okada S, Matsuda M, Anafi M, Pawson T, Pessin JE. Insulin regulates the dynamic balance between Ras and Rap1 signaling by coordinating the assembly states of the Grb2-SOS and CrkII-C3G complexes. EMBO J. 1998;17:2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamada E, Saito T, Okada S, et al. . Synip phosphorylation is required for insulin-stimulated Glut4 translocation and glucose uptake in podocyte. Endocr J. 2014;61:523–527. [DOI] [PubMed] [Google Scholar]
- 13. Kao AW, Yang C, Pessin JE. Functional comparison of the role of dynamin 2 splice variants on GLUT-4 endocytosis in 3T3L1 adipocytes. Am J Physiol Endocrinol Metab. 2000;278:E825–E831. [DOI] [PubMed] [Google Scholar]
- 14. Huang J, Imamura T, Olefsky JM. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc Natl Acad Sci USA. 2001;98:13084–13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grusovin J, Macaulay SL. Snares for GLUT4—mechanisms directing vesicular trafficking of GLUT4. Front Biosci. 2003;8:620–641. [DOI] [PubMed] [Google Scholar]
- 16. Mohammad S, Ramos LS, Buck J, Levin LR, Rubino F, McGraw TE. Gastric inhibitory peptide controls adipose insulin sensitivity via activation of cAMP-response element-binding protein and p110β isoform of phosphatidylinositol 3-kinase. J Biol Chem. 2011;286:43062–43070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chuang JZ, Yeh TY, Bollati F, et al. . The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell. 2005;9:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramalingam L, Yoder SM, Oh E, Thurmond DC. Munc18c: a controversial regulator of peripheral insulin action. Trends Endocrinol Metab. 2014;25:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Umahara M, Okada S, Yamada E, et al. . Tyrosine phosphorylation of Munc18c regulates platelet-derived growth factor-stimulated glucose transporter 4 translocation in 3T3L1 adipocytes. Endocrinology. 2008;149:40–49. [DOI] [PubMed] [Google Scholar]