Abstract
Background
Aedes albopictus is one of the most invasive human disease vectors. Its control has been largely based on insecticides, such as the larvicide temephos. Temephos resistance has been associated with the up-regulation, through gene amplification, of two carboxylesterase (CCE) genes closely linked on the genome, capable of sequestering and metabolizing temephos oxon, the activated form of temephos.
Principal findings
Here, we investigated the occurrence, geographical distribution and origin of the CCE amplicon in Ae. albopictus populations from several geographical regions worldwide. The haplotypic diversity at the CCEae3a locus revealed high polymorphism, while phylogenetic analysis showed an absence of correlation between haplotype similarity and geographic origin. Two types of esterase amplifications were found, in two locations only (Athens and Florida): one, previously described, results in the amplification of both CCEae3a and CCEae6a; the second is being described for the first time and results in the amplification of CCEae3a only. The two amplification events are independent, as confirmed by sequence analysis. All individuals from Athens and Florida carrying the CCEae3a-CCEae6a co-amplicon share a common haplotype, indicating a single amplification event, which spread between the two countries.
Significance
The importance of passive transportation of disease vectors, including individuals carrying resistance mechanisms, is discussed in the light of efficient and sustainable vector control strategies.
Author summary
Control of mosquito borne diseases is being seriously challenged by the ongoing development of insecticide resistance. Resistance of Aedes albopictus, a major arbovirus vector, to the organophosphate larvicide temephos was recently associated with the up-regulation, through gene amplification, of two carboxylesterases; CCEae3a and CCEae6a. Here we investigated the worldwide distribution and origin of the amplified esterases, which is of great value for designing and implementing efficient vector control programs. Individuals with amplification of both esterases were found in Greece and Florida (U.S.A), representing a single amplification event that spread between the two countries, highlighting the importance of passive transportation of disease vectors carrying resistance mechanisms, which is mainly facilitated by human activities. In addition, individuals with amplification of the CCEae3a only, but not the CCEae6a, representing a second and independent amplification event were found in Florida. The worldwide haplotypic diversity obtained for CCEae3a is consistent with the highly invasive nature of the Aedes albopictus.
Introduction
Aedes albopictus is vector of several important arboviruses, and has recently become a major threat to human health [1,2]. Its role in several disease outbreaks has been documented, for example dengue in Hawaii (2001–02), Gabon (2007) and Japan (2014)[3–5]; chikungunya in Italy (2007) and Reunion Island (2005–06) [6,7]; Zika in Gabon (2007) [8]. This mosquito species has also drawn attention by being one of the most invasive human disease vectors worldwide. It originated from India and South-East Asia, and quickly invaded almost all continents [9]. Its presence was first reported in Europe (Albania) in 1979 [10], in USA (Texas) in 1985 [11] and in Africa (Cape Town) in 1989 [12]. The successful spread of Ae. albopictus throughout the globe has been facilitated by human activities, like trade of tires and other goods, as well as tourism [2]; these indeed allow transportation of desiccated eggs and larvae to new places. Its ability to adapt to different environments has also been associated with specific biological traits, such as the production of diapausing eggs, which enables its survival at cooler temperatures [9].
The control of Ae. albopictus is largely based on habitat management campaigns [13], repellents (spatial or personal) and insecticides (larvicides and adulticides) [14], while the use of alternative means such as Wolbachia, Sterile Insect Techniques and genetic manipulation approaches are also currently being investigated [15,16]. Among a limited number of mosquito larvicides (including bacterial toxins and insect growth regulators—IGRs), temephos is an organophosphate (OP) that has been used extensively for the control of Aedes mosquitoes (Ae. aegypti and the often sympatric Ae. albopictus) in several continents and countries [17]. However, resistance against this insecticide has been reported [18,19]. We recently showed [20] that resistance against temephos in an Ae. albopictus population from Greece is associated with the upregulation, through gene amplification (i.e. multiple gene copies) of two carboxylesterase genes (CCEs), namely CCEae3a and CCEae6a, which are closely located on the genome [21]. Notably, the same orthologous genes (CCEae3a and CCEae6a) have also been associated with temephos resistance in Ae. aegypti, the primary dengue and yellow fever vector worldwide [22]. CCEae3a protein was shown to be localized in malpighian tubules (MT) and nerve tissues of the Ae. albopictus larvae, as well as being able to sequester and metabolize temephos oxon, the activated form of temephos [23].
OP resistance based on sequestration and enhanced metabolism resulting from CCE gene amplifications has also been described in other insects and mosquito species, such as the aphid Myzus persicae [24] and members of the mosquito Culex pipiens complex [25,26]. At the world scale, only a few CCE genes have been recorded amplified in cases of resistance in insects, which tends to indicate that advantageous mutations could be limiting. Although the mechanism by which esterase genes are amplified has not been established yet, it has been suggested that certain genome regions are probably a "hot spot" for recombination and amplification. Regions showing homology with repetitive elements have been found in DNA flanking the amplified CCEs, suggesting that these may have a role in the amplification process, as they may be functionally related to transposable elements [27].
Resistance to OPs in Culex mosquitoes has been shown to occur via over-expression, through gene amplification, of two esterase loci, Est-2 and Est-3, which may be amplified singly (e.g. the estβ1 gene) or more commonly are co-amplified as allelic pairs in resistant mosquitoes [27,28]. However, the amplified alleles can differ, which indicates that the amplification process happened several times independently. These amplified CCE alleles have been described in different geographical places. Some of them remained localized in a relatively limited area and appeared as independent events. Others have spread to distant regions from a single evolutionary origin; the same common haplotypes are indeed found in mosquitoes from different continents [29–32]. It appears that once amplification has occurred, it can easily reach other geographic areas by migration, and then invade thanks to local insecticide selection [33]. For example, the worldwide most common allele is Ester2 (or estα2-estβ2 co-amplicon), which occurs in >80% of insecticide resistant strains [34], suggesting that it may confer higher fitness than other allelic variants [33,35].
The distribution and origin of amplified CCEs associated with insecticide resistance has not been studied in Aedes mosquitoes. Here, we investigated the occurrence, frequency and geographical distribution, as well as the phylogenetic relationship and origin of the CCEae3a-CCEae6a amplicon(s)/loci in Ae. albopictus populations from 16 different places across the globe.
Materials and methods
Sampling and species ID verification
Ae. albopictus field mosquitoes used in this study were collected from Mexico (Apocada, Reynosa and Tapachula), U.S.A (Florida and Atlanta), Brazil (Rio de Janeiro), Belize (Orange walk town), Gabon (Franceville, Cocobeach, Lope), Switzerland (Ticino), France (Montpellier), Italy (Lombardy), Greece (Agios Stefanos, Koronida), Taiwan (Taipei), China (Beijing), Sri Lanka (Peradeniya), Australia (Hammond), Bangladesh (Panchagarh), Lebanon (Beirut) and Japan (Tokyo). In addition individuals from two laboratory colonies were used: i) the Tem-GR strain, derived from an Ae. albopictus population collected in 2010 in Athens (Greece) and selected with temephos using standard WHO larval bioassays [20], and ii) the Malaysia-Lab strain, a susceptible laboratory strain originally collected in Malaysia [36].
Ae. albopictus adults or larvae stored in ethanol were first dried, and then genomic DNA was extracted from each individual using the Cethyl Trymethil Ammonium Bromide (CTAB) method described in Navajas et al. [37]. The DNA pellet was dissolved in 20μl of sterile water. Individuals were identified to species based on a species ID PCR [38]. In each PCR reaction, reference Ae. albopictus and Ae.aegypti samples were used as controls.
Detection of esterase gene amplification
CCEae3a and CCEae6a gene copy number variation (CNV) was assessed using quantitative PCR (qPCR) on individual Ae. albopictus specimens (S1 Table). Amplification reactions (25μl final volume) were performed on a MiniOpticon Two-Color Real-Time PCR Detection System (BioRad) using 2μl of genomic DNA (diluted 5 times), 0.4μM primers (two different primer pairs per target gene) (S2 Table) and Kapa SYBR FAST qPCR Master Mix (Kapa-Biosystems). Two housekeeping genes, histone3 (NCBI: XM_019696438.1) and the ribosomal protein L34 (NCBI: XM_019677758.1), were used as reference genes for normalization[39]. Fivefold dilution series of pooled genomic DNA from the temephos susceptible Malaysia-Lab strain and the temephos selected TemGR strain were used to assess the efficiency of the qPCR reaction for each gene specific primer pair. A no-template control (NTC) was included to detect possible contamination and a melting curve analysis was performed to check the presence of a unique PCR product. Differences in CCEae3a and CCEae6a gene copy numbers were estimated relative to the temephos susceptible Malaysia-Lab strain, following Pfaffl [40].
Sequencing of CCEae3a intronic regions
CCEae3a (Vector base, AALF007796) is predicted to encompass three exons and two introns. To identify the most variable part of the gene the full intron1 was amplified using forward primer 5’-ACGGTCCTCGATACATAGTG-3’ and reverse primer 5’-TAGCCTCATTGCTGGTTAGC-3’ (hybridizing respectively at the end of exon1 and at the beginning of exon2) and the full intron2 was amplified using forward primer 5’-AGAGTGCGTTACGGATCAAG-3’ and reverse primer 5’-CACTGGCTTCCAGGAGATAC-3’ (hybridizing respectively at the end of exon2 and at the beginning of exon3). The PCR reactions (25μl final volume) were performed using 2μl genomic DNA from individual Ae. albopictus mosquitoes, 0.4μM primers, 0.2mM dNTPs, 5μl of 10X buffer and 1U of Kapa Taq DNA Polymerase (KAPABIOSYSTEMS). The PCR conditions were 95°C for 5min followed by 29 cycles of 94°C for 30sec, 48°C for 30sec, 72°C for 1min and a final extension of 72°C for 10min. PCR products were purified using a PCR purification kit (Macherey Nagel) and sent for sequencing using the forward primer (Macrogen Sequencing Facility, Amsterdam).
To assess the diversity of CCEae3a, the 709bp fragment of the gene (including the last 314bp of exon1, the whole intron1 and the first 192bp of exon2) was sequenced. PCR products from homozygous individuals were sequenced directly using the forward primer (5’-ACGGTCCTCGATACATAGTG-3’); for heterozygotes, the PCR products were cloned using the pGEM-Teasy vector (Promega) according to manufacturer’s instructions to separate the different alleles, and six clones for each individual were sent for sequencing (Macrogen sequencing facility, Amsterdam), with the T7 universal primer. Sequences were examined and aligned using the BioEdit software.
Phylogenetic tree construction
Phylogenetic relationships between the different CCEae3a haplotype sequences were determined using the Phylogeny.fr platform (‘‘one click mode”) [41]. Briefly, sequences were aligned using the MUSCLE 3.8.31 algorithm, and alignment was then refined using the Gblocks 0.91b software to exclude poorly aligned parts. Subsequently the PhyML 3.1/3.0 (aRLT) software was used to assess the clade support, by computing the maximum likelihood tree and aLRT test (approximate Likelihood Ratio Test) [42]. Finally the tree was drawn using the TreeDyn 198.3 software [43].
Ethics statement
The work described in this manuscript is in no way linked (directly or indirectly) to ethical concerns. No data from humans have been collected. All research activities respect fundamental ethics principles, including those reflected in the Charter of Fundamental Rights of the European Union (2000/C 364/01). The work is compatible with EU and international law, as a number of entomological monitoring activities (and transport of gDNA in Ethanol) is contacted worldwide and in Europe (European Mosquito Control Association, http://www.emca-online.eu).
Results
Geographic distribution of CCEae3a and CCEae6a gene amplification
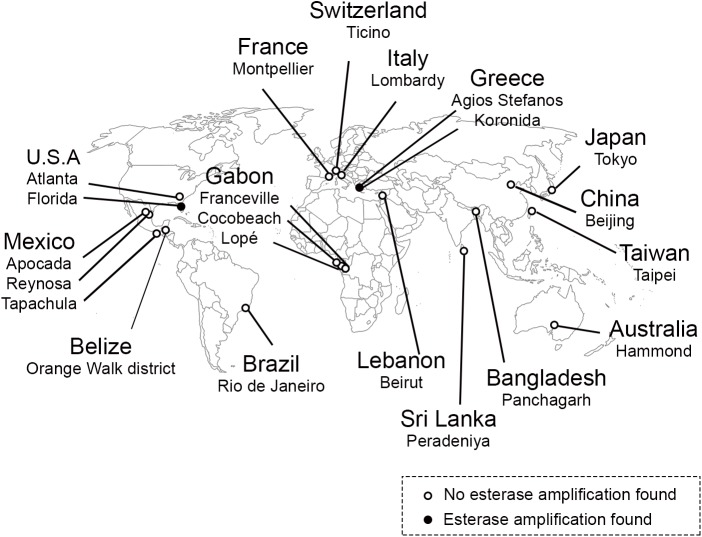
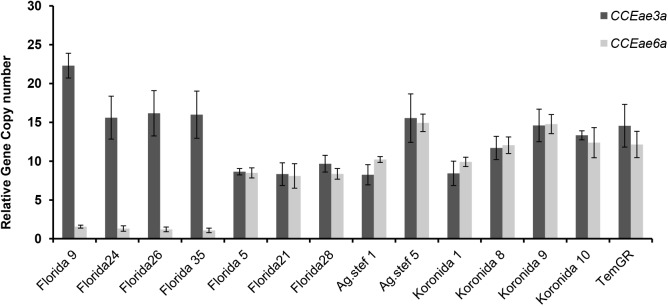
A total of 385 mosquitoes from 16 countries and 22 different collection sites (Fig 1 and S1 Table) were first confirmed to be Ae. albopictus (species ID PCR) and subsequently tested for CCEae3a and CCEae6a CNV via qPCR. Out of 35 individuals tested from Florida, three showed amplification of both CCEae3a and CCEae6a (Florida 5, 21 and 28), while four showed amplification of CCEae3a only (Florida 9, 24, 26 and 35) (Fig 2). Amplification of both esterases was also detected in Greece: two individuals out of 10 from Agios Stefanos (Ag.stef 1, 2), and four out of 10 from Koronida (Koronida 1, 8, 9, 10). Amplification of both esterases was also confirmed in all tested individuals of the temephos resistant TemGR strain (Fig 2). None of the 330 individuals tested from the remaining 14 countries showed amplification of CCEae3a or CCEae6a (S1 File).
Fig 1. Sample collection map.
The countries of origin for the 385 individuals screened for CCEae3a and CCEae6a gene amplification are indicated. Black and white circles respectively represent places were amplification was or not detected.
Fig 2. CCEae3a and CCEae6a gene copy number variation in individuals from Florida and Greece.
Quantitative real time PCR values are represented relatively to the values of reference individuals from the Lab strain. Error bars represent the standard error of the mean from at least three technical replicates. Histone 3 and rpl34 have been used as reference genes.
Phylogenetic relationships of CCE3ae locus/amplicon: At least two independent amplification events have occurred
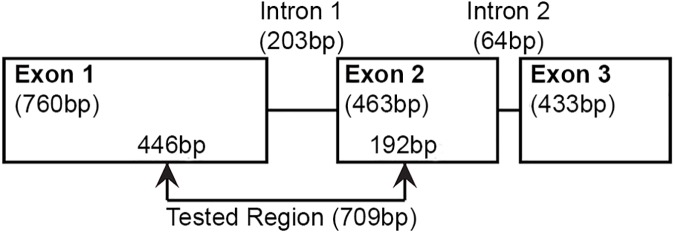
The two predicted introns of the CCEae3a locus were sequenced using individuals from the TemGR and Malaysia-Lab strains. Intron1 sequences were longer and more variable between individuals from these two strains, while intron2 sequences were identical. A 709 bp region including part of exon1, the whole intron1 and part of exon2 (Fig 3) was thus used to examine the haplotype diversity between individuals from the sampled countries, with and without CCE amplification (1–14 individuals per collection site, S1 Table).
Fig 3. CCEae3a gene structure.
CCEae3a consists of three exons and two introns, represented by boxes and lines respectively, with their size in base pairs (bp). The region selected for the phylogenetic analysis is indicated by the arrows.
Sequence alignment revealed several SNPs throughout the amplified region, both in introns and exons, plus some insertions and deletions in the intronic sequence (S2 File). The diversity was higher in the intron sequences (mean distance = 0.041 substitution/site) than in the exons (0.012 and 0.014 for exon1 and exon2, respectively)[44]. A total of 45 different haplotypes, differing by at least one mutation, were identified from the 49 individuals tested (S2 File).
Haplotypes did not cluster based on geographic proximity. While most haplotypes were found in a single individual, some sequences from individuals collected in distant geographic locations were indeed identical: for example H3 was present in individuals from Atlanta, Italy, Greece (Agios Stefanos), Florida and Mexico (Apocada), H7 in individuals from Belize, Italy, Florida and Switzerland, H9 in individuals from Belize, Florida and Mexico (Tapachula), H12 in individuals from China and Lebanon, and H13 in individuals from China, Lebanon and Mexico (Reynosa). Moreover, sequences obtained from individuals collected in the same area often showed a great variability and were found in different clades in the tree, to the point that two sequences found in a single heterozygous individual could be quite distant (e.g. Bangladesh4A and B or Brazil14A and B). In particular, haplotypes obtained from individuals with no esterase amplification originating from Florida and Greece were dispersed throughout the phylogenetic tree (Fig 4), clustering with haplotypes from distant areas. In contrast, all individuals displaying amplified esterases clustered in only two highly supported clades. In the first, all the individuals from Florida (U.S.A) showing amplification of only CCEae3a shared a common haplotype (H29), which was found in no other individual in the dataset. The second clade clustered all the individuals showing amplification of both CCEae3a and CCEae6a, whether from Florida (U.S.A) or from Greece (Agios Stephanos and Koronida), including the reference strain TemGR. They also shared a common haplotype (H30), which again was found in no other individual in the dataset. In addition, this second clade was closer to haplotypes obtained from individuals without esterase amplification (e.g Brazil 14B and Taiwan 1B) than to the first clade (i.e. individuals with amplification of CCEae3a only).
Fig 4. CCEae3a diversity.
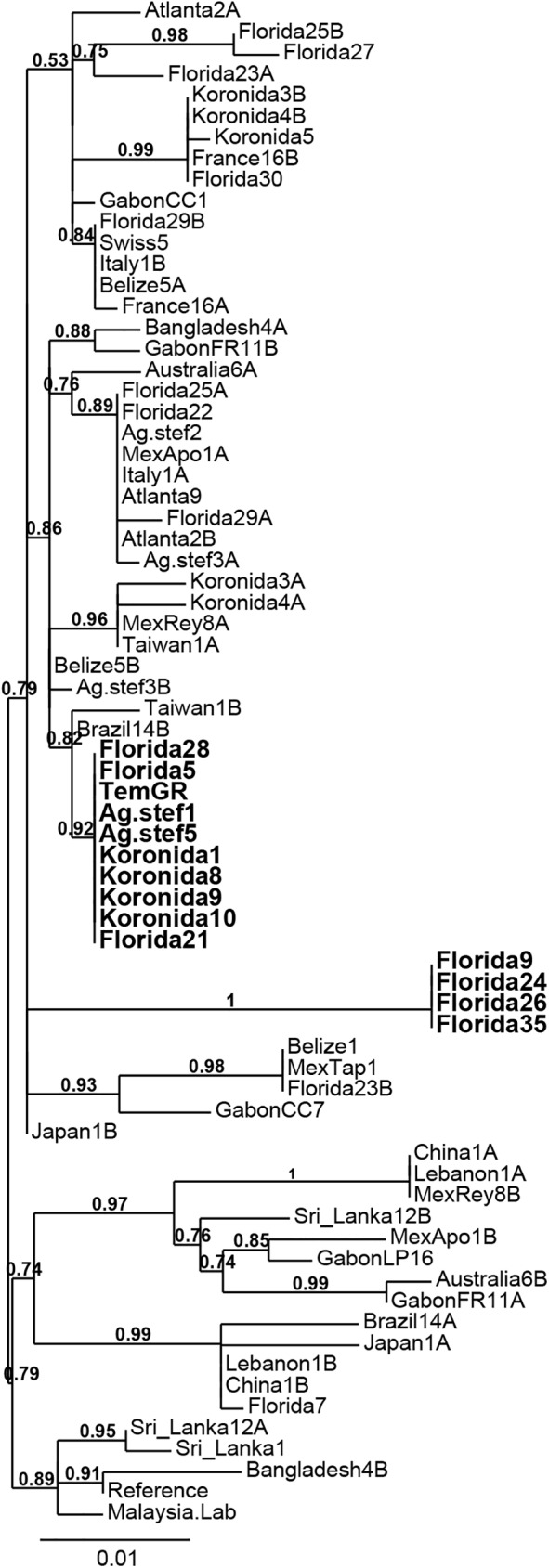
An unrooted maximum likelihood tree was built to represent the phylogenetic distances between the 45 CCEae3a haplotypes identified worldwide (see also S1 Table). Distances are expressed in substitutions/site. The numbers on tree branches represent the supporting probability of each node, based on the aLRT statistical test. Individuals with copy number variation are shown with bold.
Discussion
Two carboxylesterase genes (CCEs), CCEae3a and CCEae6a have recently been shown in Ae. albopictus to be implicated in OP resistance through gene amplification [20]. To understand the origin and spread of these resistance alleles, we assessed the haplotypic diversity at the CCEae3a locus. Analysis revealed that this gene is polymorphic in non-amplified alleles (S2 File): 45 different haplotypes were identified in 49 individuals collected around the globe, with only five (H3, H7, H9, H12 and H13) shared between two or more individuals. Moreover, for these five haplotypes, the individuals came from distant areas, on different continents. The phylogenetic tree further confirmed the absence of correlation between haplotype similarity and geographic origin, as individuals from the same collection area clustered mostly in different clades of the tree (this was even observed for the two haplotypes of a single heterozygote). This observation echoes previous studies on mitochondrial genes, microsatellites and other nuclear genetic markers [45–47] that showed that Ae. albopictus populations have been repeatedly transported from their original range (South-East Asia) to different areas around the globe; progenies of mosquitoes originally from the same locality can thus be found in different continents. The frequent exchange of goods at an international level and travelling of people around the world indeed facilitate the passive transportation of mosquitoes, which are often found in aircrafts and ships [48]. These studies also showed that the non-Asiatic populations often result from a mix of several independent invasions. This mechanism, promoting genetic diversity, is often proposed as a key factor contributing to successful establishment of a species in new areas [45,49].
We then addressed the worldwide occurrence, distribution and diversity of esterase amplification in Ae. albopictus populations in response to OP selection: our study evidenced that at least two types of esterase amplifications are currently segregating. The first one, resulting in amplification of both CCEae3a and CCEae6a, had been previously described [20], while the second; resulting in amplification of CCEae3a only, is being described for the first time. In contrast to the high CCEae3a haplotypic diversity in non-amplified alleles, the two amplified genotypes associated with OP resistance displayed no diversity. It also revealed that the two amplification events were independent: individuals with CCEae3a and CCEae6a co-amplification displayed identical sequences and thus clustered together, far apart from individuals with CCEae3a-only amplification, which also displayed identical sequences. The appearance of independent amplification events at the same genomic region suggests the presence of favoring features, which promote unequal crossing-overs and/or transposition [27]. For example, a repetitive element Juan (possibly related to transposable elements) was found close to the amplified esterase locus in Cx. quinquefasciatus [50]. The Ae. albopictus genome is also known to carry many transposable elements, and 68% of its genome is occupied by repetitive sequences [21]. If one of those is close to the CCEae3a locus, it could facilitate its repeated and independent duplications. In addition, these mechanisms might also act after the first amplification event resulting in further variation in copy numbers. This has been hypothesized in Cx.pipiens [51] and might also explain the differences observed in the relative gene copy numbers among individuals from Florida and Greece. The fact that all individuals carrying co-amplifications of both CCEae3a and CCEae6a, from Greece and Florida U.S.A (two regions hosting many millions of tourists every year), share a common haplotype reveals that a single amplification event took place, and then spread between the two countries. This again outlines the importance of passive transportation of disease vectors, including individuals carrying resistance mechanisms, which unfortunately promotes resistance spread at the world scale, as has been described in Cx. pipiens mosquitoes with OP-resistant amplified esterases [30,52]. However, the establishment of a resistance mechanism in a new area largely depends on the local selective advantage it offers in these new environmental conditions [33]. The repetitive use of temephos [53] or other organophosphate insecticides, which could show cross resistance, like the adulticide naled commonly used in Florida [54] probably facilitated the establishment of CCEae3a amplified haplotypes in Ae. albopictus populations from both countries. It also ensured that these haplotypes were selected and reached high frequencies.
It is actually surprising that the resistance allele found in individuals from the TemGR strain, originally collected in 2010, is still present in Greece 2016 collection: temephos has been officially banned in Europe in 2007, and resistance mechanisms are usually (but not always) associated with fitness costs [55,56]. The persistence of the resistant allele throughout these years suggests either the lack of a significant fitness cost for individuals carrying them or the presence of a current selection source, either from non officially approved vector control activities, or from other substances (e.g. originating for example from agriculture) [57].
In any case, the presence of two independently amplified OP-resistant alleles in Ae. albopictus already segregating in distant places in the world (i.e. Athens—Greece and Florida–U.S.A) raises concerns about the future control of this species. Although the levels of insecticide resistance in Ae. albopictus are low at present (i.e. resistance mechanisms/alleles are less frequent) compared to Ae. aegypti [18,58], our study shows that resistance can be selected and spread rapidly around the globe also in this species and possibly compromise control activities. As only a limited number of mosquito larvicides are available on the market, temephos resistance is an important consideration for many countries, where this active ingredient is still in use. Moreover, striking resistance mutations have been found in other insect species (Plutella xylostella) that completely inactivate the IGR diflubenzuron, now one of the most important mosquito larvicides in Europe and other regions [59]. This further raises serious concerns for regions that have banned the use of temephos, such as Europe, as it is certainly a potential reliable resource for emergency epidemics, new invasion cases, or lack of alternative efficient mosquito control solutions.
Supporting information
(DOCX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We would like to thank all colleagues who kindly provided samples: Ilias Kioulos, Dafni company, Athens;; Philip Mccall, Liverpool School of Tropical Medicine, UK; Parakrama Karunaratne, University of Peradeniya, Sri Lanka; Maria Goreti Rosa Freitas, Instituto Oswaldo Cruz FIOCRUZ Rio de Janeiro, Brazil; Diego Ayala, MIVEGEC- Centre IRD Montpellier, France and International Centrer for Medical Research, Gabon; Gonzalo M. Vazquez-Prokopec, Emory University, Atlanta USA; Audrey Lenhart, Division of Parasitic Diseases and Malaria Center for Global Health CDC, Atlanta U.S.A; Pie Mueller, Swiss Tropical and Public Health Institute Basel, Switzerland; Tobias Suter, Swiss Tropical and Public Health Institute Basel, Switzerland; Ju-Chun Hsu, National University of Taiwan, Taiwan; Guadalupe del Carmen Reyes Solis, Universidad Autónoma de Yucatán, Mexico; Nicole L. Achee, University of Notre Dame, U.S.A; Mike Osta, American University of Beirut, Lebanon; Nigel Beebe, University of Queensland, Australia; Hirotaka Kanuka, The Jikei University School of Medicine Tokyo, Japan; Deok Ho Kwon, Seoul National University, Korea; Charles Wondji, Liverpool School of Tropical Medicine, UK; Gong Cheng, Tsinghua University, China; Xiao-Guang Chen, Southern Medical University Guang Zhou, China. We would also like to thank Natassa Kampouraki and Filippia Strati, University of Crete, Biology Department for technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research received funding from the European Union FP7 (2013) under grant agreement no 228421 to INFRAVEC (http://www.infravec.eu/) and a bilateral Greece–France Cooperation Program (no 1641) (www.gsrt.gr). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D (2009) Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect 11: 1177–1185. doi: 10.1016/j.micinf.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 2.Gratz NG (2004) Critical review of the vector status of Aedes albopictus. Med Vet Entomol 18: 215–227. doi: 10.1111/j.0269-283X.2004.00513.x [DOI] [PubMed] [Google Scholar]
- 3.Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, et al. (2005) Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis 11: 742–749. doi: 10.3201/eid1105.041063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leroy EM, Nkoghe D, Ollomo B, Nze-Nkogue C, Becquart P, et al. (2009) Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis 15: 591–593. doi: 10.3201/eid1504.080664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutsuna S, Kato Y, Moi ML, Kotaki A, Ota M, et al. (2015) Autochthonous dengue fever, Tokyo, Japan, 2014. Emerg Infect Dis 21: 517–520. doi: 10.3201/eid2103/141662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S (2007) A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 3: e201 doi: 10.1371/journal.ppat.0030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson R (2007) Europe witnesses first local transmission of chikungunya fever in Italy. BMJ 335: 532–533. doi: 10.1136/bmj.39332.708738.DB [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, et al. (2014) Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 8: e2681 doi: 10.1371/journal.pntd.0002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonizzoni M, Gasperi G, Chen XG, James AA (2013) The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends in Parasitology 29: 460–468. doi: 10.1016/j.pt.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedict MQ, Levine RS, Hawley WA, Lounibos LP (2007) Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis 7: 76–85. doi: 10.1089/vbz.2006.0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore CG, Mitchell CJ (1997) Aedes albopictus in the United States: ten-year presence and public health implications. Emerg Infect Dis 3: 329–334. doi: 10.3201/eid0303.970309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornel AJ, Hunt RH (1991) Aedes albopictus in Africa? First records of live specimens in imported tires in Cape Town. J Am Mosq Control Assoc 7: 107–108. [PubMed] [Google Scholar]
- 13.CDC (2012) Dengue and the Aedes albopictus mosquito. http://wwwcdcgov/dengue/resources/30Jan2012/albopictusfactsheetpdf.
- 14.WHO (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. [PubMed]
- 15.Lees RS, Gilles JRL, Hendrichs J, Vreysen MJB, Bourtzis K (2015) Back to the future: the sterile insect technique against mosquito disease vectors. Current Opinion in Insect Science 10: 156–162. [DOI] [PubMed] [Google Scholar]
- 16.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, et al. (2014) Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop 132 Suppl: S150–163. [DOI] [PubMed] [Google Scholar]
- 17.George L, Lenhart A, Toledo J, Lazaro A, Han WW, et al. (2015) Community-Effectiveness of Temephos for Dengue Vector Control: A Systematic Literature Review. PLoS Negl Trop Dis 9: e0004006 doi: 10.1371/journal.pntd.0004006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vontas J, Kioulos E, Pavlidi N, Morou E, della Torre A, et al. (2012) Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pesticide Biochemistry and Physiology 104: 126–131. [Google Scholar]
- 19.Ranson H, Burhani J, Lumjuan N, Black W (2010) Insecticide resistance in dengue vectors. TropIKAnet. [Google Scholar]
- 20.Grigoraki L, Lagnel J, Kioulos I, Kampouraki A, Morou E, et al. (2015) Transcriptome Profiling and Genetic Study Reveal Amplified Carboxylesterase Genes Implicated in Temephos Resistance, in the Asian Tiger Mosquito Aedes albopictus. PLoS Negl Trop Dis 9: e0003771 doi: 10.1371/journal.pntd.0003771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen XG, Jiang X, Gu J, Xu M, Wu Y, et al. (2015) Genome sequence of the Asian Tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc Natl Acad Sci U S A 112: E5907–5915. doi: 10.1073/pnas.1516410112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poupardin R, Srisukontarat W, Yunta C, Ranson H (2014) Identification of Carboxylesterase Genes Implicated in Temephos Resistance in the Dengue Vector Aedes Aegypti. Plos Neglected Tropical Diseases 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigoraki L, Balabanidou V, Meristoudis C, Miridakis A, Ranson H, et al. (2016) Functional and immunohistochemical characterization of CCEae3a, a carboxylesterase associated with temephos resistance in the major arbovirus vectors Aedes aegypti and Ae. albopictus. Insect Biochem Mol Biol 74: 61–67. doi: 10.1016/j.ibmb.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 24.Field LM, Devonshire AL, Forde BG (1988) Molecular evidence that insecticide resistance in peach-potato aphids (Myzus persicae Sulz.) results from amplification of an esterase gene. Biochem J 251: 309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillemaud T, Makate N, Raymond M, Hirst B, Callaghan A (1997) Esterase gene amplification in Culex pipiens. Insect Molecular Biology 6: 319–327. [PubMed] [Google Scholar]
- 26.Weill M, Berticat C, Raymond M, Chevillon C (2000) Quantitative polymerase chain reaction to estimate the number of amplified esterase genes in insecticide-resistant mosquitoes. Anal Biochem 285: 267–270. doi: 10.1006/abio.2000.4781 [DOI] [PubMed] [Google Scholar]
- 27.Bass C, Field LM (2011) Gene amplification and insecticide resistance. Pest Manag Sci 67: 886–890. doi: 10.1002/ps.2189 [DOI] [PubMed] [Google Scholar]
- 28.Raymond M, Berticat C, Weill M, Pasteur N, Chevillon C (2001) Insecticide resistance in the mosquito culex pipiens: what have we learned about adaptation? Genetica 112–113: 287–296. [PubMed] [Google Scholar]
- 29.Qiao CL, Raymond M (1995) The same esterase B1 haplotype is amplified in insecticide-resistant mosquitoes of the Culex pipiens complex from the Americas and China. Heredity (Edinb) 74 (Pt 4): 339–345. [DOI] [PubMed] [Google Scholar]
- 30.Raymond M, Callaghan A, Fort P, Pasteur N (1991) Worldwide Migration of Amplified Insecticide Resistance Genes in Mosquitos. Nature 350: 151–153. doi: 10.1038/350151a0 [DOI] [PubMed] [Google Scholar]
- 31.Raymond M, Beyssat-Arnaouty V, Sivasubramanian N, Mouches C, Georghiou GP, et al. (1989) Amplification of various esterase B's responsible for organophosphate resistance in Culex mosquitoes. Biochem Genet 27: 417–423. [DOI] [PubMed] [Google Scholar]
- 32.Guillemaud T, Rooker S, Pasteur N, Raymond M (1996) Testing the unique amplification event and the worldwide migration hypothesis of insecticide resistance genes with sequence data. Heredity (Edinb) 77 (Pt 5): 535–543. [DOI] [PubMed] [Google Scholar]
- 33.Labbe P, Lenormand T, Raymond M (2005) On the worldwide spread of an insecticide resistance gene: a role for local selection. J Evol Biol 18: 1471–1484. doi: 10.1111/j.1420-9101.2005.00938.x [DOI] [PubMed] [Google Scholar]
- 34.Hemingway J (2000) The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect Biochem Mol Biol 30: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 35.Labbe P, Sidos N, Raymond M, Lenormand T (2009) Resistance Gene Replacement in the Mosquito Culex pipiens: Fitness Estimation From Long-Term Cline Series. Genetics 182: 303–312. doi: 10.1534/genetics.109.101444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishak IH, Jaal Z, Ranson H, Wondji CS (2015) Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasites & Vectors 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navajas M, Lagnel J, Fauvel G, De Moraes G (1999) Sequence variation of ribosomal internal transcribed spacers (ITS) in commercially important phytoseiidae mites. Experimental and Applied Acarology 23: 851–859. [DOI] [PubMed] [Google Scholar]
- 38.Higa Y, Toma T, Tsuda Y, Miyagi I (2010) A multiplex PCR-based molecular identification of five morphologically related, medically important subgenus Stegomyia mosquitoes from the genus Aedes (Diptera: Culicidae) found in the Ryukyu Archipelago, Japan. Jpn J Infect Dis 63: 312–316. [PubMed] [Google Scholar]
- 39.Reynolds JA, Poelchau MF, Rahman Z, Armbruster PA, Denlinger DL (2012) Transcript profiling reveals mechanisms for lipid conservation during diapause in the mosquito, Aedes albopictus. Journal of Insect Physiology 58: 966–973. doi: 10.1016/j.jinsphys.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36: W465–469. doi: 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol 55: 539–552. doi: 10.1080/10635150600755453 [DOI] [PubMed] [Google Scholar]
- 43.Chevenet F, Brun C, Banuls AL, Jacq B, Christen R (2006) TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7: 439 doi: 10.1186/1471-2105-7-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manni M, Gomulski LM, Aketarawong N, Tait G, Scolari F, et al. (2015) Molecular markers for analyses of intraspecific genetic diversity in the Asian Tiger mosquito, Aedes albopictus. Parasit Vectors 8: 188 doi: 10.1186/s13071-015-0794-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong D, Lo E, Hu R, Metzger ME, Cummings R, et al. (2013) Genetic analysis of invasive Aedes albopictus populations in Los Angeles County, California and its potential public health impact. PLoS One 8: e68586 doi: 10.1371/journal.pone.0068586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamgang B, Ngoagouni C, Manirakiza A, Nakoune E, Paupy C, et al. (2013) Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl Trop Dis 7: e2590 doi: 10.1371/journal.pntd.0002590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivet Y, Marquine M, raymond M (1993) French mosquito populations invaded by A2-B2 esterases causing insecticide resistance. Biological Journal of the Linnean Society 49: 249–255. [Google Scholar]
- 49.Kolbe JJ, Glor RE, Schettino LRG, Lara AC, Larson A, et al. (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431: 177–181. doi: 10.1038/nature02807 [DOI] [PubMed] [Google Scholar]
- 50.Mouches C, Pauplin Y, Agarwal M, Lemieux L, Herzog M, et al. (1990) Characterization of amplification core and esterase B1 gene responsible for insecticide resistance in Culex. Proc Natl Acad Sci U S A 87: 2574–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Callaghan A, Guillemaud T, Makate N, Raymond M (1998) Polymorphisms and fluctuations in copy number of amplified esterase genes in Culex pipiens mosquitoes. Insect Mol Biol 7: 295–300. [DOI] [PubMed] [Google Scholar]
- 52.Raymond M, Chevillon C, Guillemaud T, Lenormand T, Pasteur N (1998) An overview of the evolution of overproduced esterases in the mosquito Culex pipiens. Philos Trans R Soc Lond B Biol Sci 353: 1707–1711. doi: 10.1098/rstb.1998.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connelly CR, Carlson DB (2009) Florida Coordinating Council on Mosquito Control. Florida Mosquito Control: The state of the mission as defined by mosquito controllers, regulators, and environmental managers University of Florida, Institute of Food and Agricultural Sciences, Florida Medical Entomology Laboratory. [Google Scholar]
- 54.Agency USEP Naled for Mosquito Control.
- 55.Rivero A, Magaud A, Nicot A, Vezilier J (2011) Energetic cost of insecticide resistance in Culex pipiens mosquitoes. J Med Entomol 48: 694–700. [DOI] [PubMed] [Google Scholar]
- 56.Guillemaud T, Raymond M, Tsagkarakou A, Bernard C, Rochard P, et al. (1999) Quantitative variation and selection of esterase gene amplification in Culex pipiens. Heredity (Edinb) 83 (Pt 1): 87–99. [DOI] [PubMed] [Google Scholar]
- 57.Milesi P, Lenormand T, Lagneau C, Weill M, Labbe P (2016) Relating fitness to long-term environmental variations in natura. Mol Ecol 25: 5483–5499. doi: 10.1111/mec.13855 [DOI] [PubMed] [Google Scholar]
- 58.Smith LB, Kasai S, Scott JG (2016) Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic Biochem Physiol 133: 1–12. doi: 10.1016/j.pestbp.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 59.Douris V, Steinbach D, Panteleri R, Livadaras I, Pickette JA, et al. (2016) A resistance mutation conserved between insects and mites unravels the benzoylurea insecticide mode of action on chitin biosynthesis PNAS-accepted. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.