Abstract
Background
Q fever is a main zoonotic disease around the world. The aim of this meta-analysis was to estimate the overall seroprevalence of Coxiella burnetii among human and animal population in Iran.
Methods
Major national and international databases were searched from 2005 up to August 2016. We extracted the prevalence of Q fever antibodies (IgG) as the main primary outcome. We reported the prevalence of the seropositivity as point and 95% confidence intervals.
Results
The overall seroprevalence of IgG phase I and II antibodies of Q fever in human was 19.80% (95% CI: 16.35–23.25%) and 32.86% (95% CI: 23.80–41.92%), respectively. The herd and individual prevalence of C. burnetii antibody in goat were 93.42% (95% CI: 80.23–100.00) and 31.97% (95% CI: 20.96–42.98%), respectively. The herd and individual prevalence of Q fever antibody in sheep's were 96.07% (95% CI: 89.11–100.00%) and 24.66% (95% CI: 19.81–29.51%), respectively. The herd and individual prevalence of C. burnetii antibody in cattle were 41.37% (95% CI: 17.88–64.86%) and 13.30% (95% CI: 2.98–23.62%), respectively. Individual seropositivity of Q fever in camel and dog were 28.26% (95% CI: 21.47–35.05) and 0.55% (0.03–2.68), respectively.
Conclusion
Seroprevalence of Q fever among human and domestic animals is considerable. Preventative planning and control of C. burnetii infections in Iran is necessary. Active surveillance and further research studies are recommended, to more clearly define the epidemiology and importance of C. burnetii infections in animals and people in Iran.
Author summary
Q fever is a zoonotic diseases caused by a bacterium so called Coxiella burnetii. Domestic ruminants (primarily cattle, sheep and goats) are the most important reservoir of C. burnetii in the nature. Q fever is mostly asymptomatic in livestock and animals. Clinical manifestations of Q fever in humans includes asymptomatic, acute, chronic to fatigue syndrome. Acute Q fever is defined as primary infection with C. burnetii, and <60% of infected patients may be asymptomatic. Acute Q fever can manifest as a flu-like and self-limited illness. Chronic Q fever is accompanied with endocarditis and vascular infection which is fatal if untreated. The results of this meta-analysis showed the prevalence of IgG phase I and II antibodies of C. burnetii among human in Iran were 19.80% and 32.86%, respectively. The prevalence of Q fever antibodies in cattle, goat and sheep were 13.30%, 31.97% and 24.66% in Iran, respectively. Seroprevalence of Q fever among human and domestic animals is considerable. Preventative planning and control of C. burnetii infections in Iran is necessary. Active surveillance and further research studies are recommended, to more clearly define the epidemiology and importance of C. burnetii infections in animals and people in Iran.
Introduction
Q fever is a zoonosis caused by the intracellular, gram negative bacterium Coxiella burnetii. C. burnetii is an extremely infectious pathogen [1]. The extremely high infectivity, the ability to withstand harsh environmental conditions, and the potential to cause severe disease in man, has deemed this organism to be considered as a biological terrorist agent. It has been listed as a Category B biological warfare agent by the Centre’s of Disease Control and Prevention [2,3].
C. burnetii infects people and a wide range of wild and domesticated animals. Within the environment, C. burnetii survives in arthropod hosts, such as ticks. From these hosts it can spread, and it primarily spreads into ruminants. Domestic ruminants (primarily cattle, sheep and goats) are the most important reservoir of C. burnetii in the nature. Q fever is mostly asymptomatic in livestock and animals, except in some cases, where causes abortion, stillbirth, endometritis or infertility. Infected animals shed C. burnetii into the environment in milk, colostrum, urine, vaginal discharges and especially in birth products [4,5]. High numbers of organisms exist in the amniotic fluids and placenta during birthing (e.g., 109 bacteria/g placenta) [6]. C. burnetii can survive for long periods in the environment, and it is common for aerosols from infected herds to be carried by the wind and cause infection in humans. Q fever outbreaks could be directly connected to the speed and frequency of the wind [7]. Inhalation of infectious aerosol or contaminated dusts containing air-borne bacterium the major route of acquiring the disease in humans, so that a single inhaled organism may produce clinical illness. Nevertheless, the other routes of transmission of this infection to human are consumption of contaminated milks and dairy products, skin or mucosal contact, tick bites, blood transfusion, sexual transmission and embryo transfer [4,5,8].
Clinical manifestations of Q fever in humans includes acute, chronic to fatigue syndrome. The main characteristic of Q fever is its clinical polymorphism. Acute Q fever is defined as primary infection with C. burnetii, and <60% of infected patients may be asymptomatic [9]. However, acute Q fever can manifest as a flu-like and self-limited illness, and major clinical presentations of these patients are fever, headache, coughing, atypical pneumonia, hepatitis, myalgia, arthralgia, cardiac involvement, skin rash and neurologic signs, and 2% of patients with acute disease are hospitalized. The case fatality rate of acute Q fever is reported up to 1–2% [4,8,10]. Approximately 5% of acute Q fever cases go on to develop chronic Q fever. People may become chronically infected without having being previously diagnosed with acute disease, and chronic Q fever may manifest months or years after an acute infection [11]. Chronic Q fever is accompanied with endocarditis, vascular infection, prosthetic joint arthritis, osteoarticular infection and lymphadenitis [4,12,13]. Endocarditis and vascular infection caused by Q fever are fatal if untreated[9].
Human Q fever has been described in countries around the world, New Zealand being the only exception. As it is not a notifiable disease in many countries, the geographical distribution of the organism is extrapolated from serological surveys and investigated outbreaks[3]. In Iran, the first clinical cases of acute Q fever are reported in 1952. From 1970 to 1976, 133 patients with acute Q fever were reported from different parts of Iran [14]. After 1976, Q fever was neglected in Iran, and no human case was reported. At the same time with large outbreak of Q fever in the Netherlands (2007–2010)[15],C. burnetii antibodies were reported in febrile patients in the Kerman province (southeastern Iran), [16]and investigation for Q fever was resumed. After that, various seroepidemiological studies were conducted on animal and human population. The first case of chronic Q fever (endocarditis) was reported in 2013 [17].
We do not have an overall estimation of Q fever infection in Iran. Current studies have reported Q fever seroprevalence in human and domestic animals. The overall estimation of Q fever seroprevalence in the human and animal population will help health policymakers create or modify control and prevention programs for Q fever in Iran. In the present systematic review, we reviewed the local Iranian publications on Q fever and also international publications relating to the disease in Iran. In this report we provide a summary of the more recent data collected on Q fever in Iran.
Methods
Information sources and search
From January 2005 to June 2016, we searched the literature for articles that assessed the prevalence of Q fever infection in human and animals in Iran.
We searched multiple English and Persian electronic data sources including Iranmedex, Scientific Information Database (SID), Magiran, Iranian Research Institute for Information Science and Technology (IRANDOC), Google Scholar, Medline, PubMed, Science Direct, Scopus and Web of science. In addition, the citations of the included articles were reviewed to find other relevant studies. We also looked at the electronic abstract list of congress conducted in Iran and also at the electronic database of students’ thesis. Keywords that we used for our search were “Q fever, Coxiella burnetii and Iran".
Eligibility criteria and study selection
Articles with cross sectional design which were sampling from Iran, published in Persian or English and measured seroposivity by serological assays (just IgG) were eligible to enter meta-analysis.
Exclusion criteria for studies from systematic review were: 1- Lack of access to full article or insufficient data in abstract; 2- Unclear testing methods used to detect studied infection or non-serology test 3- IgM detection4- other study design except cross sectional.
We contacted the corresponding author when we have questions about the eligibility of the article.
Data collection and data items
Data was extracted by two reviewers and checked twice based on the following items: type of study, sample size, location and time of the study, species and prevalence of Q fever. We grouped the studies with species in herd and individual level as sheep, goat, cattle and camel and also human participants as phase I and phase II IgG.
Analytic approach
We conducted meta-analyses in STATA version 12. We did meta-analysis for Q fever prevalence in any species in herd and individual level and in phase I and II for human. The outcome was measured and reported as prevalence, with point and 95% confidence intervals. A Q-test was used to assess heterogeneity. When the heterogeneity test had a p-value less than 0.1, a random-effects model was used; otherwise the fixed-effects model was used to calculate the pooled prevalence. Also by calculating pooled Q fever seroprevalence in each province we mapped prevalence of Q fever using ArcGIS ver. 10.2.
Results
Description of included studies
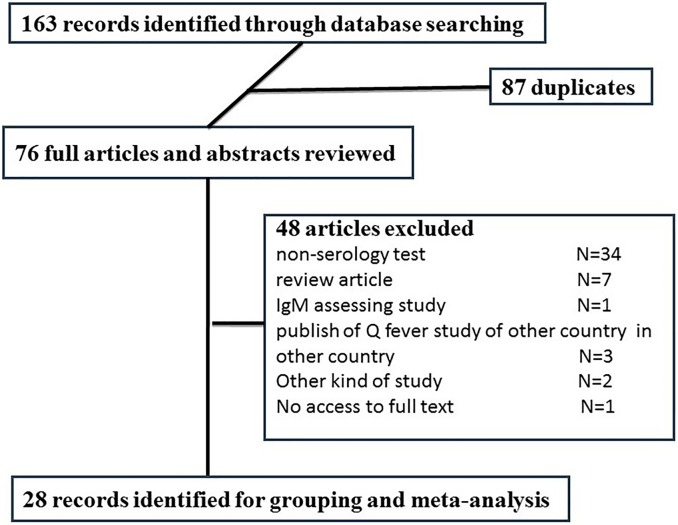
As presented in Fig 1, we found 163 abstracts in our literature review. After removing duplications (n = 87) based on title and abstract, 76 remained for full text review. Of those, 48 articles were excluded for various reasons including non-serology test (n = 34), review article (n = 7), IgM assessing study (n = 1), publish of Q fever study of other country in Iranian journals (n = 3), other kind of study (n = 2) and no access to full text (n = 1) (Fig 1). Characteristics of the final included studies (n = 28) in the systematic review showed in Table 1.
Fig 1. Flow diagram of included and excluded records.
Table 1. Characteristics of the included studies in the systematic review, 2005–2016.
| Group | Level | Sample Size | Time Study | Province | Reference | |
|---|---|---|---|---|---|---|
| 1 | Human | Phase I | 187 | 2011 | Sistan and Baluchistan | [44] |
| Phase II | ||||||
| 2 | Human | Phase I | 75 | 2009 | Kerman | [16] |
| Phase II | ||||||
| 3 | Human | Phase I | 250 | 2011–2012 | Kurdistan | [45] |
| Phase II | ||||||
| 4 | Human | Phase II | 200 | 2014 | Ardabil | [46] |
| Khuzestan | ||||||
| 5 | Human | Phase II | 105 | 2011 | Sistan v Baluchistan | [47] |
| 6 | Human | Phase II | 45 | 2013 | Kerman | [48] |
| 7 | Human | Phase II | 75 | 2010–2011 | Kerman | [49] |
| 8 | Human | Phase II | 121 | 2014 | Kerman | [50] |
| 9 | Human | Phase II | 53 | 2015–2016 | Mazandaran | [51] |
| 10 | Human | Phase II | 92 | 2014 | South Khorasan | [52] |
| 11 | Sheep | Herd | 10 | 2009 | Kerman | [53] |
| Individual | 85 | |||||
| 12 | Sheep | Herd | 29 | 2012 | Khorasan Razavi | [54] |
| Individual | 255 | |||||
| 13 | Sheep | Herd | 10 | 2014 | Hamadan | [41] |
| Individual | 200 | |||||
| 14 | Sheep | Individual | 256 | 2011–2012 | Ardabil | [55] |
| 15 | Sheep | Individual | 235 | 2011–2012 | Fars | [34] |
| 336 | Isfahan | |||||
| 297 | Khorasan Razavi | |||||
| 232 | Markazi | |||||
| 16 | Sheep | Individual | 12 | 2011 | Hormozgan | [35] |
| 37 | 2011 | Kerman | ||||
| 78 | 2011 | Sistan v Baluchistan | ||||
| 17 | Sheep | Individual | 220 | 2010–2011 | Khuzestan | [56] |
| 18 | Sheep | Individual | 253 | 2011–2012 | Mazandaran | [57] |
| 19 | Goat | Herd | 9 | 2008 | Kerman | [58] |
| Individual | 79 | |||||
| 20 | Goat | Herd | 28 | 2012 | Khorasan Razavi | [54] |
| Individual | 205 | |||||
| 21 | Goat | Herd | 10 | 2014 | Hamadan | [41] |
| Individual | 50 | |||||
| 22 | Goat | Individual | 76 | 2011–2012 | Fars | [34] |
| 76 | Isfahan | |||||
| 13 | Khorasan Razavi | |||||
| 15 | Markazi | |||||
| 23 | Goat | Individual | 58 | 2011 | Kerman | [59] |
| 24 | Goat | Individual | 39 | 2011 | Hormozgan | [35] |
| 136 | Kerman | |||||
| 66 | Sistan v Baluchistan | |||||
| 25 | Cattle | Herd | 10 | 2014 | Hamedan | [41] |
| Individual | 120 | |||||
| 26 | Cattle | Herd | 12 | 2008 | Kerman | [58] |
| Individual | 93 | |||||
| 27 | Cattle | Herd | 44 | 2010 | Kerman | [60] |
| 28 | Cattle | Herd | 19 | 2011 | Kerman | [40] |
| Individual | 161 | |||||
| 29 | Cattle | Herd | 19 | 2010 | Khorasan Razavi | [61] |
| Individual | 246 | |||||
| 30 | Cattle | Herd | 34 | 2014 | Kurdistan | [62] |
| 31 | Cattle | Herd | 37 | 2014 | West Azerbaijan | [63] |
| 32 | Cattle | Individual | 86 | 2011 | Khuzestan | [64] |
| 33 | Camel | Individual | 42 | 2012–2013 | Khorasan Razavi | [65] |
| 59 | North Khorasan | |||||
| 66 | South Khorasan | |||||
| 34 | Dog | Individual | 182 | 2013–2014 | Khuzestan | [66] |
Prevalence of seropositivity
Q fever seroprevalence in human
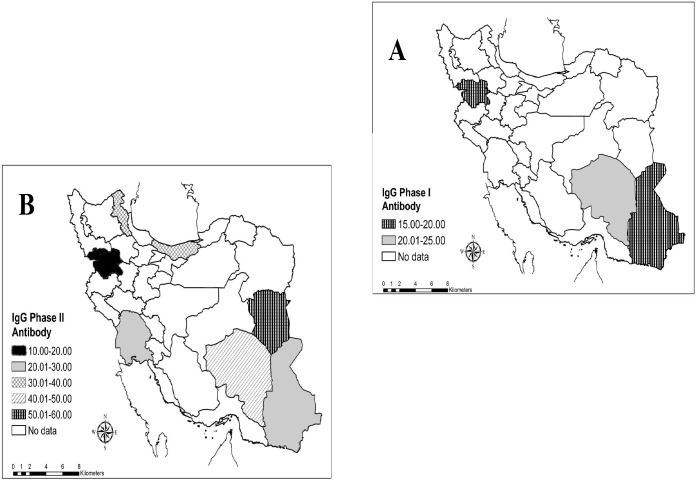
In final, 10 studies were found about seroprevalence of Q fever in different parts of Iran which three studies were about IgG phase I antibody and eighth studies were IgG phase II antibody. The overall seroprevalence of IgG phase I and II antibodies of Q fever in human was 19.80% (95% CI: 16.35–23.25%) and 32.86% (95% CI: 23.80–41.92%), respectively (Table 2). Geographical distribution of Q fever seropositivity was shown in Fig 2. C. burnetii antibodies have been detected in human from 9 provinces. The most prevalence of IgG phases I and II antibodies was seen in Kerman (24%) and South Khorasan (54%) provinces, respectively (Fig 2).
Table 2. Prevalence Q fever antibody among human and domestic animals, 2005–2016.
| Level | Sample size | Number of studies | Pooled estimate (%) | |
|---|---|---|---|---|
| Human | Phase I | 512 | 3 | 19.80 (16.35–23.25) |
| Phase II | 1203 | 10 | 32.86 (23.80–41.92) | |
| Goat | Herd | 47 | 3 | 93.42(80.23–100.00) |
| Individual | 813 | 6 | 31.97 (20.96–42.98) | |
| Sheep | Herd | 49 | 3 | 96.07 (89.11–100.00) |
| Individual | 2496 | 9 | 24.66 (19.81–29.51) | |
| Cattle | Herd | 175 | 7 | 41.37 (17.88–64.86) |
| Individual | 706 | 5 | 13.30 (2.98–23.62) | |
| Camel | Individual | 167 | 1 | 28.26 (21.47–35.05) |
| Dog | Individual | 182 | 1 | 0.55 (0.03–2.68) |
Fig 2.
Geographical distribution of anti- C. burnetii IgG Phase I (A) and IgG Phase II (B) among Iranian people.
Q fever seroprevalence in goat
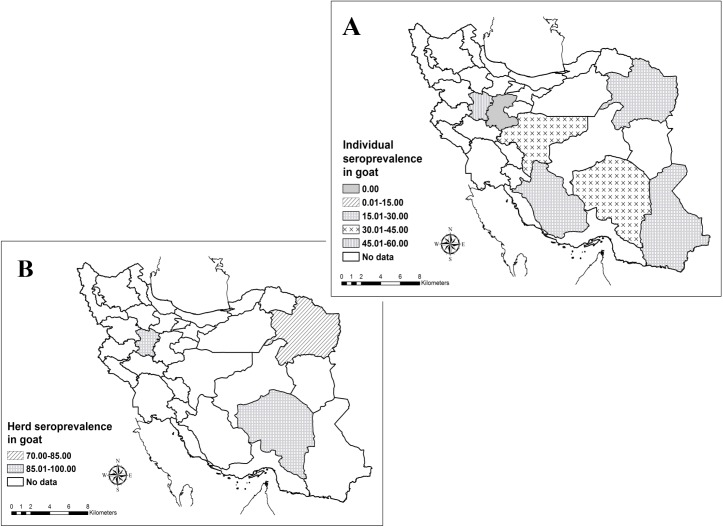
Six studies were conducted about seroprevalence of Q fever in goats which three studies were in herd level and six studies were in individual’s level. The herd and individual prevalence of Q fever antibody in goat were 93.42% (95% CI: 80.23–100.00) and 31.97% (95% CI: 20.96–42.98%), respectively. The higher and lower seroprevalence was seen in Kerman (63.3%) and Markazi (0%) provinces, respectively. Also seroprevalence of Q fever among goats in Iran showed Fig 3.
Fig 3.
Geographical distribution of Q fever seropositivity among goats in individual (A) and herd (B) levels in the Iran.
Q fever seroprevalence in sheep
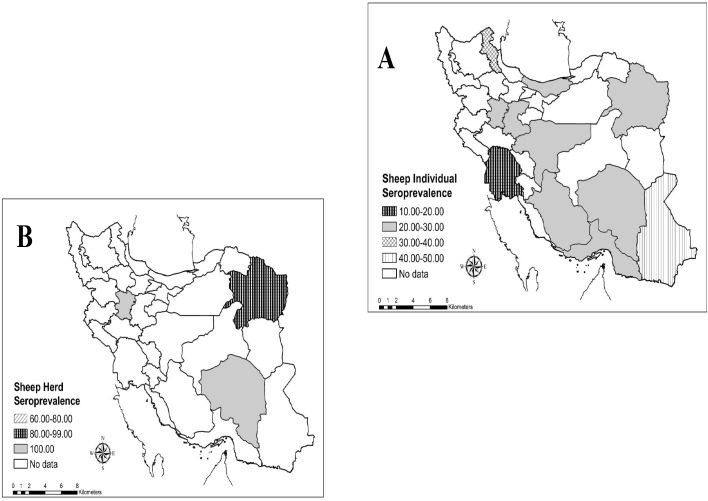
In final, 8 studies were found about seroprevalence of Q fever in sheep’s, which 3 studies were in herd level and 9 studies were in individual’s level. The herd and individual prevalence of Q fever antibody in sheep’s were 96.07% (95% CI: 89.11–100.00%) and 24.66% (95% CI: 19.81–29.51%), respectively. The higher and lower seropositivity of C. burnetii among sheep’s showed in Sistan va Baluchestan (43.6%) and Khorasan Razavi (12.8%) provinces, respectively. Also geographical distribution of Q fever seropositivity in sheep’s was shown in Fig 4.
Fig 4.
Geographical distribution of C. burnetii seroprevalence among sheep’s in individual (A) and herd (B) levels in Iran.
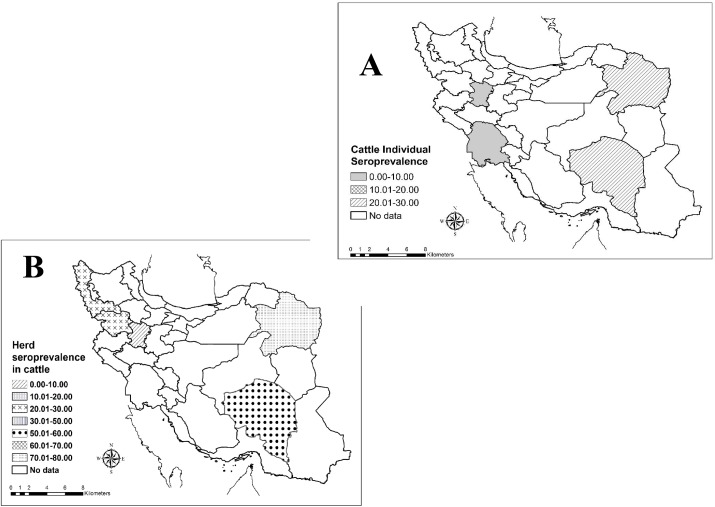
Q fever seroprevalence in cattle
In final, 8 studies had been done about seroprevalence of Q fever in sheep’s, which 7 studies were in herd level and 5 studies were in individual’s level. The herd and individual prevalence of Q fever antibody in cattle were 41.37% (95% CI: 17.88–64.86%) and 13.30% (95% CI: 2.98–23.62%), respectively. The seroprevalence of Q fever among cattle’s in the different parts of Iran showed in Fig 5.
Fig 5.
Geographical distribution of Q fever seropositivity among cattle’s in individual (A) and herd (B) levels in Iran.
Other animals
Only one study found the seroprevalence of dogs and camels in Iran. Individual seropositivity of Q fever in camel and dog were 28.26% (95% CI: 21.47–35.05) and 0.55% (0.03–2.68), respectively.
Discussion
The current systematic review reports the seroprevalence of Q fever among human and domestic animals in Iran. The results of this meta-analysis showed the prevalence of IgG phase I and II antibodies of C. burnetii among human in Iran were 19.80% and 32.86%, respectively. These rates were very high compared with other similar study. As example seropositivity of Q fever in China was reported 10% [18]. In a recent systematic review study, human seroprevalence was reported from 1–32% in Africa [19]. Human seroprevalence of Q fever were reported 3 to 35.8% in Kenya [20], 12.3–32%in Turkey[21,22], 15.3% in Spain [23],5.2%in Australia[24]and 11% in Denmark[25]. Overall seroprevalence for C. burnetii was reported 3.1% among general population in the USA [26]. The differences between countries could be due to varieties in ecologic, social, cultural, behavioral and economical conditions and also levels of animals infections, which affect the exposures of people in each of the regions of the world. In all conducted studies in Iran, C. burnetii antibodies have been detected in human from 9 provinces. Seroprevalence varied in different areas of Iran. The seroprevalence of IgG phase I of Q fever ranged from 18.2% to 24%, which Sistan and Baluchestan and Kerman provinces had lowest and highest seropositivity, respectively. Also the prevalence of IgG phase II antibodies ranged from 14.5% to 68%, which higher and lower seroprevalence showed in Kerman and Sistan and Baluchestan provinces, respectively [17,18]. According to the findings of this study, it is highly recommended that physicians and health care workers are informed about bacteria circulating in Iran.
Goats are important sources of C. burnetii infection in people and seven serosurveys were conducted on goats in Iran between 2005 and 2016. According to the results of this meta-analysis, the seroprevalence of Q fever in goats was 31.97%. Also, 93.42% of the goat’s herds were seropositive in Iran. In similar studies, goats seropositivity were 13% to 23% in Africa [19],20% to 46% in Kenya [20] and 0.8% to 60.6% in China [18]. Antibodies against C. burnetii in Netherland, Bulgaria and Bangladesh were 7.8%, 13.7% and 9.52%, respectively [27–29]. Our study showed C. burnetii antibodies have been detected in goats from 7 provinces. The higher and lower seroprevalence was seen in Kerman (65.8%) and Markazi (0%) provinces, respectively. From 2007 to 2010, more than 4,000 human cases were diagnosed in Netherlands. The outbreaks in humans were mainly related area with intensive dairy goat farming [15]. A recent (2012–2014) human outbreak of Q fever in the Australia was linked to an intensive goat and sheep dairy farm and also seroprevalence in goats was 15% [30]. Due to the recent outbreaks of Q fever, it seems that the goat is very strong role in human infections. In Iran, due to the high seroprevalence antibodies to C. burnetii in goats, this case can be possible, but more studies are needed to prove this point in the future.
Large human Q fever outbreaks related to sheep in published studies around the world included Bulgaria (2009), Croatia (2008),France (2007), Germany (2006, 2008), Italy (2004) and Switzerland (1987) [31]. Therefore, sheep is considered as an important factor for human Q fever infection. The results of this meta-analysis demonstrated that were 24.66% of sheep’s had antibody of C. burnetii in Iran. In total, seroprevalence of Q fever was 96.07% among sheep’s herds. Different rates of sheep seroprevalence were reported in other countries, so that the seropositivity was 2.4% in Netherlands [32], 5% in China [18], 11.6% in Bulgaria [28], 20% in Turkey [33] and 11% to 33% in Africa [19]. The studies of Q fever seroprevalence found in 12 provinces in Iran. The higher and lower seropositivity of C. burnetii among sheep’s showed in Sistan and Baluchestan (43.6%) and Khorasan Razavi (12.8%) provinces, respectively [34,35].
In cattle's like other main reservoirs (sheep and goat) of Q fever, C. burnetii is shed by birth products (placenta, birth fluids), but may also be shed by vaginal mucus, milk, and faeces, urine and semen[36].Contact with these contaminated materials can lead to human infection. According to the results of this meta-analysis, the seroprevalence of Q fever in cattle was 13.30%. Also, 41.37% of the cattle’s herds were seropositive in Iran. In other countries, seroprevalence of Q fever was different rates among cattle, for example: 6.2% in Northern Ireland[37], 8.5% in in Bulgaria [28], 15% in China [18], 16.0% in Netherlands [38] and 30.4% in Cameroon [39]. In all conducted studies in Iran, C. burnetii antibodies have been detected in human from 7 provinces. The higher and lower seroprevalence of Q fever was seen in Kerman (29.2%) and Hamadan (0.8%) provinces, respectively[40,41]. The lower seroprevalence in cattle’s compared to the two other main reservoirs (sheep and goat) in Iran, this can be caused by difference in strains circulating in Iran and other areas and genotyping studies can be helpful in support of this subject. Therefore, it seems that there are differences in geographic prevalence of the disease among cattle in Iran and it is recommended to be done in the future a comprehensive study to determine the case in all provinces of Iran.
The seropositivity of Q fever among camel was 28.26% in Iran. Antibodies to C. burnetii were reported in 51.6% and 80% of camels in Saudi Arabia and Chad, respectively [42,43]. Also, the seroprevalence of Q fever was 0.55% among dogs in Iran, but only one study conducted in Iran. For better judgment on this issue needs more studies in the future.
Although human and animal infections of Q fever are known to occur and endemic in Iran, but the Q fever is not a reportable disease in the country and clinical cases are probably largely unrecognizable by health system. There is a need for information on the epidemiology of C. burnetii in Iran as well as many other issues such as distribution, pathogenesis and molecular typing. The data from the studies to date in Iran provide only a basic picture of Q fever in the country. Active case finding and further research studies are recommended, to more clearly define the epidemiology and importance of C. burnetii infections in animals and people in Iran. This will enable the formulation and implementation of locally applicable control methods for Q fever which can be implemented by animal and human healthcare workers.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Supporting information
(PDF)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE (2013) Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nature Reviews Microbiology 11: 561–573. doi: 10.1038/nrmicro3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madariaga MG, Rezai K, Trenholme GM, Weinstein RA (2003) Q fever: a biological weapon in your backyard. The Lancet infectious diseases 3: 709–721. [DOI] [PubMed] [Google Scholar]
- 3.Oyston P, Davies C (2011) Q fever: the neglected biothreat agent. Journal of medical microbiology 60: 9–21. doi: 10.1099/jmm.0.024778-0 [DOI] [PubMed] [Google Scholar]
- 4.Angelakis E, Raoult D (2010) Q fever. Veterinary microbiology 140: 297–309. doi: 10.1016/j.vetmic.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 5.Raoult D, Marrie T, Mege J (2005) Natural history and pathophysiology of Q fever. The Lancet infectious diseases 5: 219–226. doi: 10.1016/S1473-3099(05)70052-9 [DOI] [PubMed] [Google Scholar]
- 6.Bouvery NA, Souriau A, Lechopier P, Rodolakis A (2003) Experimental Coxiella burnetii infection in pregnant goats: excretion routes. Veterinary research 34: 423–433. doi: 10.1051/vetres:2003017 [DOI] [PubMed] [Google Scholar]
- 7.Tissot-Dupont H (2004) Wind in November, Q fever in December. Emerging Infectious Disease 10: 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker NR, Barralet JH, Bell AM (2006) Q fever. The Lancet 367: 679–688. [DOI] [PubMed] [Google Scholar]
- 9.Anderson A, Bijlmer H, Fournier P- E, Graves S, Hartzell J, et al. (2013) Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep 62: 1–30. [PubMed] [Google Scholar]
- 10.Frankel D, Richet H, Renvoisé A, Raoult D (2011) Q fever in France, 1985–2009. Emerg Infect Dis 17: 350–356. doi: 10.3201/eid1703.100882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenollar F, Fournier P-E, Carrieri MP, Habib G, Messana T, et al. (2001) Risks factors and prevention of Q fever endocarditis. Clinical Infectious Diseases 33: 312–316. doi: 10.1086/321889 [DOI] [PubMed] [Google Scholar]
- 12.Raoult D (2012) Chronic Q fever: expert opinion versus literature analysis and consensus. Journal of Infection 65: 102–108. doi: 10.1016/j.jinf.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 13.Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, et al. (2017) From Q fever to Coxiella burnetii infection: a paradigm change. Clinical Microbiology Reviews 30: 115–190. doi: 10.1128/CMR.00045-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mostafavi E, Rastad H, Khalili M (2012) Q fever: an emerging public health concern in Iran. Asian Journal of Epidemiology 5: 66–74. [Google Scholar]
- 15.Dijkstra F, van der Hoek W, Wijers N, Schimmer B, Rietveld A, et al. (2012) The 2007–2010 Q fever epidemic in The Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunology & Medical Microbiology 64: 3–12. [DOI] [PubMed] [Google Scholar]
- 16.Khalili M, Shahabi-Nejad N, Golchin M (2010) Q fever serology in febrile patients in southeast Iran. Transactions of the Royal Society of Tropical Medicine and Hygiene 104: 623–624. doi: 10.1016/j.trstmh.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 17.Yaghmaie F, Esmaeili S, Francis SA, Mostafavi E (2015) Q fever endocarditis in Iran: A case report. Journal of infection and public health 8: 498–501. doi: 10.1016/j.jiph.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 18.El-Mahallawy H, Lu G, Kelly P, Xu D, Li Y, et al. (2015) Q fever in China: a systematic review, 1989–2013. Epidemiology and infection 143: 673–681. doi: 10.1017/S0950268814002593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanderburg S, Rubach MP, Halliday JE, Cleaveland S, Reddy EA, et al. (2014) Epidemiology of Coxiella burnetii infection in Africa: a OneHealth systematic review. PLoS Negl Trop Dis 8: e2787 doi: 10.1371/journal.pntd.0002787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Njeru J, Henning K, Pletz M, Heller R, Neubauer H (2016) Q fever is an old and neglected zoonotic disease in Kenya: a systematic review. BMC public health 16: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gozalan A, Rolain J, Ertek M, Angelakis E, Coplu N, et al. (2010) Seroprevalence of Q fever in a district located in the west Black Sea region of Turkey. European journal of clinical microbiology & infectious diseases 29: 465–469. [DOI] [PubMed] [Google Scholar]
- 22.Kilic S, Yilmaz GR, Komiya T, Kurtoglu Y, Karakoc EA (2008) Prevalence of Coxiella burnetii antibodies in blood donors in Ankara, Central Anatolia, Turkey. New Microbiol 31: 527–534. [PubMed] [Google Scholar]
- 23.Cardenosa N, Sanfeliu I, Font B, Munoz T, Nogueras MM, et al. (2006) Seroprevalence of human infection by Coxiella burnetii in Barcelona (northeast of Spain). The American journal of tropical medicine and hygiene 75: 33–35. [PubMed] [Google Scholar]
- 24.Tozer S, Lambert S, Sloots T, Nissen M (2011) Q fever seroprevalence in metropolitan samples is similar to rural/remote samples in Queensland, Australia. European journal of clinical microbiology & infectious diseases 30: 1287–1293. [DOI] [PubMed] [Google Scholar]
- 25.Bosnjak E, Hvass A, Villumsen S, Nielsen H (2010) Emerging evidence for Q fever in humans in Denmark: role of contact with dairy cattle. Clinical microbiology and infection 16: 1285–1288. doi: 10.1111/j.1469-0691.2009.03062.x [DOI] [PubMed] [Google Scholar]
- 26.Anderson AD, Kruszon-Moran D, Loftis AD, McQuillan G, Nicholson WL, et al. (2009) Seroprevalence of Q fever in the United States, 2003–2004. The American journal of tropical medicine and hygiene 81: 691–694. doi: 10.4269/ajtmh.2009.09-0168 [DOI] [PubMed] [Google Scholar]
- 27.Van den Brom R, Moll L, Van Schaik G, Vellema P (2013) Demography of Q fever seroprevalence in sheep and goats in The Netherlands in 2008. Preventive veterinary medicine 109: 76–82. doi: 10.1016/j.prevetmed.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 28.Martinov S (2007) Contemporary state of the problem Q fever in Bulgaria. Biotechnology & Biotechnological Equipment 21: 353–361. [Google Scholar]
- 29.Rahman MA, Alam MM, Islam MA, Bhuiyan A, Rahman A (2016) Serological and Molecular Evidence of Q Fever in Domestic Ruminants in Bangladesh. Veterinary Medicine International 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bond K, Vincent G, Wilks C, Franklin L, Sutton B, et al. (2016) One Health approach to controlling a Q fever outbreak on an Australian goat farm. Epidemiology and infection 144: 1129–1141. doi: 10.1017/S0950268815002368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van den Brom R, van Engelen E, Roest H, van der Hoek W, Vellema P (2015) Coxiella burnetii infections in sheep or goats: an opinionated review. Veterinary microbiology 181: 119–129. doi: 10.1016/j.vetmic.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 32.Van den Brom R, Vellema P (2009) Q fever outbreaks in small ruminants and people in the Netherlands. Small Ruminant Research 86: 74–79. [Google Scholar]
- 33.Kennerman E, Rousset E, Gölcü E, Dufour P (2010) Seroprevalence of Q fever (coxiellosis) in sheep from the Southern Marmara Region, Turkey. Comparative immunology, microbiology and infectious diseases 33: 37–45. doi: 10.1016/j.cimid.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 34.Asadi J, Kafi M, Khalili M (2013) Seroprevalence of Q fever in sheep and goat flocks with a history of abortion in Iran between 2011 and 2012. Vet Ital 49: 163–168. [PubMed] [Google Scholar]
- 35.Ezatkhah M, Alimolaei M, Khalili M, Sharifi H (2015) Seroepidemiological study of Q fever in small ruminants from Southeast Iran. Journal of infection and public health 8: 170–176. doi: 10.1016/j.jiph.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 36.Guatteo R, Beaudeau F, Berri M, Rodolakis A, Joly A, et al. (2006) Shedding routes of Coxiella burnetii in dairy cows: implications for detection and control. Veterinary Research 37: 827–833. doi: 10.1051/vetres:2006038 [DOI] [PubMed] [Google Scholar]
- 37.McCaughey C, Murray L, McKenna J, Menzies F, McCullough S, et al. (2010) Coxiella burnetii (Q fever) seroprevalence in cattle. Epidemiology and infection 138: 21–27. doi: 10.1017/S0950268809002854 [DOI] [PubMed] [Google Scholar]
- 38.Muskens J, Van Engelen E, Van Maanen C, Bartels C, Lam T (2011) Prevalence of Coxiella burnetii infection in Dutch dairy herds based on testing bulk tank milk and individual samples by PCR and ELISA. The veterinary record 168: 79 doi: 10.1136/vr.c6106 [DOI] [PubMed] [Google Scholar]
- 39.Scolamacchia F, Handel IG, Fèvre EM, Morgan KL, Tanya VN, et al. (2010) Serological patterns of brucellosis, leptospirosis and Q fever in Bos indicus cattle in Cameroon. PLoS One 5: e8623 doi: 10.1371/journal.pone.0008623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalili M, Sakhaee E, Babaei H (2012) Frequency of anti-Coxiella burnetii antibodies in cattle with reproductive disorders. Comparative clinical pathology 21: 917–919. [Google Scholar]
- 41.Edalati-Shokat H, Abbasi-Doulatshahi E, Hajian-Bidar H, Gharekhani J, Rezaei A-A (2015) Q fever in domestic ruminants: A Seroepidemiological survey in Hamedan, Iran. Int J Curr Microbiol App Sci 4: 589–596. [Google Scholar]
- 42.Hussein MF, Alshaikh MA, Al-Jumaah RS, GarelNabi A, Al-Khalifa I, et al. (2015) The Arabian camel (Camelus dromedarius) as a major reservoir of Q fever in Saudi Arabia. Comparative Clinical Pathology 24: 887–892. [Google Scholar]
- 43.Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P, et al. (2003) Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Preventive veterinary medicine 61: 279–293. [DOI] [PubMed] [Google Scholar]
- 44.Esmaeili S, Naddaf SR, Pourhossein B, Shahraki AH, Amiri FB, et al. (2016) Seroprevalence of Brucellosis, Leptospirosis, and Q Fever among Butchers and Slaughterhouse Workers in South-Eastern Iran. PloS one 11: e0144953 doi: 10.1371/journal.pone.0144953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esmaeili S, Pourhossein B, Gouya MM, Amiri FB, Mostafavi E (2014) Seroepidemiological survey of Q fever and brucellosis in Kurdistan Province, western Iran. Vector-Borne and Zoonotic Diseases 14: 41–45. doi: 10.1089/vbz.2013.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khayyat KM, Asadi J, Khalili M, Abiri Z (2016) The First Serological Study of Coxiella burnetii among Pregnant Women in Iran. Iranian journal of public health 45: 523 [PMC free article] [PubMed] [Google Scholar]
- 47.Metanat M, RAD NS, Alavi-Naini R, Shahreki S, Sharifi-Mood B, et al. (2014) Acute Q fever among febrile patients in Zahedan, southeastern Iran. Turkish journal of medical sciences 44: 99–103. [DOI] [PubMed] [Google Scholar]
- 48.Naderipour Z, Golchin M, Khalili M (2014) Design of an ELISA kit for detection human acute Q fever. Iranian Journal of Medical Microbiology 8: 28–34. [Google Scholar]
- 49.Khalili M, Mosavi M, Diali HG, Mirza HN (2014) Serologic survey for Coxiella burnetii phase II antibodies among slaughterhouse workers in Kerman, southeast of Iran. Asian Pacific journal of tropical biomedicine 4: S209–S212. doi: 10.12980/APJTB.4.2014C1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalili M, Qorbani A, Sharifi H, Golchin M (2015) Prevalence and risk factor of Q fever among veterinary students in Iran. Tropical Biomedicine 32: 704–709. [PubMed] [Google Scholar]
- 51.Ghasemian R, Mostafavi E, Esmaeili S, Najafi N, Arabsheybani S (2016) A Survey of Acute Q Fever among Patients with Brucellosis-Like and Atypical Pneumonia Symptoms Who Are Referred to Qaemshahr Razi Hospital in Northern Iran (2014–2015). Global Journal of Health Science 9: 225–232. [Google Scholar]
- 52.Khalili M, Aflatoonian MR, Rahanjam M, Golchin M, Sharifi H, et al. (2016) Frequency of Seropositivity for anti-Coxiella Burnetii (Phase II) among Veterinary Staff in Southern Khorasan, Iran, in 2014. Journal of Kerman University of Medical Sciences 23: 164–173. [Google Scholar]
- 53.Sakhaee E, Khalili M (2010) The first serologic study of Q fever in sheep in Iran. Tropical animal health and production 42: 1561–1564. doi: 10.1007/s11250-010-9606-2 [DOI] [PubMed] [Google Scholar]
- 54.Keyvani Rad N, Azizzadeh M, Taghavi Razavizadeh A, Mehrzad J, Rashtibaf M (2014) Seroepidemiology of coxiellosis (Q fever) in sheep and goat populations in the northeast of Iran. Iranian Journal of Veterinary Research 15: 1–6. [Google Scholar]
- 55.Esmaeili S, Bagheri Amiri F, Mostafavi E (2014) Seroprevalence survey of Q fever among sheep in northwestern Iran. Vector-Borne and Zoonotic Diseases 14: 189–192. doi: 10.1089/vbz.2013.1382 [DOI] [PubMed] [Google Scholar]
- 56.PourMahdi M, Gharibi D, Gorani-Nejad S, Zamiri S (2013) Seroprevalence of coxiellosis in Ahvaz sheep. Iranian Veterinary Journal 9: 11–18. [Google Scholar]
- 57.Esmaeili S, Mostafavi E, Shahdordizadeh M, Mahmoudi H (2013) A seroepidemiological survey of Q fever among sheep in Mazandaran province, northern Iran. Annals of Agricultural and Environmental Medicine 20. [PubMed] [Google Scholar]
- 58.Khalili M, Sakhaee E (2009) An update on a serologic survey of Q fever in domestic animals in Iran. The American journal of tropical medicine and hygiene 80: 1031–1032. [PubMed] [Google Scholar]
- 59.Banavand R (2012) Detection of antibodies against to Coxiella burnetii in goat milk samples. Kerman: Shahid Bahoonar. [Google Scholar]
- 60.Khalili M, Sakhaee E, Aflatoonian MR, Shahabi-Nejad N (2011) Herd–prevalence of Coxiella burnetii (Q fever) antibodies in dairy cattle farms based on bulk tank milk analysis. Asian Pacific journal of tropical medicine 4: 58–60. doi: 10.1016/S1995-7645(11)60033-3 [DOI] [PubMed] [Google Scholar]
- 61.Azizzadeh M, Mohammadi GR, Haghparast AR, Heidarpour-Bami M (2014) Seroepidemiology of Coxiella Burnetii in commercial dairy herds in northeast of Iran. The Iranian Journal of Veterinary Science and Technology 3: 33–40. [Google Scholar]
- 62.Shajiee A (2014) Prevalence of antibodies against Coxiella burnetii (Q fever) in dairy herds of Sanandaj. Kerman: Shahid Bahoonar [Google Scholar]
- 63.Akbari S (2013) Prevalence of Coxiella burnetii antibodies in Miandoab dairy herds Kerman: Shahid Bahoonar. [Google Scholar]
- 64.Alipour Z (2011) The prevalence of Q fever in dairy cows referred to the veterinary hospital in Ahvaz by ELISA and polymerase chain reaction. Ahvaz: Shahid Chamran. [Google Scholar]
- 65.Janati Pirouz H, Mohammadi G, Mehrzad J, Azizzadeh M, Shirazi MHN (2015) Seroepidemiology of Q fever in one-humped camel population in northeast Iran. Tropical animal health and production 47: 1293–1298. doi: 10.1007/s11250-015-0862-z [DOI] [PubMed] [Google Scholar]
- 66.Rezaei A, Gharibi D, Pourmahdi Borujeni M, Mosallanejad B (2016) Seroprevalence of Lyme disease and Q fever in referred dogs to Veterinary Hospital of Ahvaz. Iranian Veterinary Journal 11: 34–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.