Abstract
After the sequential successes of catheter ablation for the treatment of pre-excitation syndromes (WPW), junctional reentry (AVNRT) atrial flutter (AFL) and ventricular arrhythmias, clinical electrophysiologists have focused on the myocardial basis of atrial fibrillation (AF). Thus, the strategy for ablation of drug and cardioversion refractory AF was to isolate the myocardial connections from the focal firing pulmonary veins (PVs) in addition to altering the atrial substrate maintaining AF. However, the overall success rates have not achieved those of the other types of ablation procedures. In this review we have summarized the favorable aspects and drawbacks of pulmonary vein isolation (PVI). As for the role of the Intrinsic Cardiac Autonomic Nervous System (ICANS), both basic and clinical evidence has shown that ganglionated plexi (GP) stimulation promotes initiation and maintenance of AF, and that GP ablation reduces recurrence of AF following catheter or surgical ablation of these structures. Based on these findings, the GP Hyperactivity Hypothesis has been proposed to explain, at least in part, the mechanistic basis for the focal form of AF. For example, PV isolation may not always be necessary for elimination of AF, as in the early stages of paroxysmal AF. GP ablation alone, in these cases, may suffice for focal AF termination. In the persistent and long standing persistent forms the substrate for AF may be more extensive and therefore require GP ablation plus PV isolation and/or CFAE ablations. Clinical reports, both catheter based as well as minimally invasive surgical procedures, which include PVI plus GP ablation have shown relatively long-term success rates much closer to or equal to those achieved by myocardial ablation procedures in patients with WPW, AVNRT and AFL.
Historical Background
Clinical Electrophysiology, a subspecialty of Cardiology, had its beginnings in the 1960s and 1970s with the development of new intra-cardiac electrical recordings techniques[1,2] and procedures for provocative pacing of the atria and ventricles.[3] These diagnostic tools provided more precise insights as to the mechanisms of various cardiac arrhythmias[4-6] than previously achieved by the use of the electrocardiogram alone. Another milestone occurred in 1981 with the use of atrio-ventricular (A-V) junctional ablation to control the rapid ventricular response in a patient with atrial fibrillation.[7] The use of radiofrequency energy[8] became the mainstay for catheter ablation as a curative approach for a number of cardiac arrhythmias which were previously treated, most not effectively, by drugs.
Subsequently, Kuck et al[9] and Jackman et al.[10] used radiofrequency energy for catheter ablation of accessory pathways, and the A-V junction. These procedures were followed in rapid succession by radiofrequency ablation of the slow A-V nodal pathway to cure A-V junctional reentrant tachycardia[11,12] and the ablation of the inferior vena cava-tricuspid valve isthmus to terminate atrial flutter.[13] The factor common to this non-surgical, non-pharmacological therapy was the use of radiofrequency lesions to interrupt a reentrant circuit which served as the substrate for these cardiac arrhythmias.
Based on the 95-99% success rates resulting from their previous successes, clinical electrophysiologists confronted the most common and most vexing of the cardiac arrhythmias, atrial fibrillation (AF). The initial attempts followed the suc cess of the surgical Maze technique.[14] Insteadm of cut and sew, Swartz et al[15] used radiofrequency catheter ablation to induce bi-atrial linear lesion sets. Others,[16] using similar approaches in an attempt to mimic the surgical procedure but with the use of radio-frequency energy, had low success rates and unexpected complications.
A seminal discovery was made just before the turn of the century with the observation by the Bordeaux group, that patients with drug and cardioversion resistant paroxysmal AF consistently manifested focal firing arising from the myocardial sleeves of the pulmonary veins.[17,18] This breakthrough observation represented a radical departure from the prevailing view that the mechanism responsible for AF was based on multiple reentrant wavelets continuously encircling the atria.[19,20] Initially, the clinical strategy adopted was to locate the focal firing sites within the PV(s) and ablate them with radiofrequency current.[21] Although this strategy was effective in treating this form of AF, 62% success over the short term, several factors caused this approach to be abandoned: 1) The danger of PV stenosis, 2) The finding that all the PVs were potential arrhythmogenic sites and 3) In approximately 15% of this AF subpopulation non-PV focal firing sites could be identified.[22,23]
Pulmonary Vein Isolation (PVI)
These factors engendered the strategy of PV isolation (PVI) which was undertaken to not only prevent the escape of the PV triggers[24] but also to markedly reduce the affected substrate maintaining AF. In regard to the latter, somewhat different procedures were devised including left atrial circumferential ablation [LACA];[25] wide area circumferential ablation [WACA];[26] and pulmonary vein antrum isolation [PVAI].[27] All of these, performed as a single procedure, have resulted in success rates as high as 84% for paroxysmal and persistent AF[28] but relatively lower success rates for long standing persistent AF.
Another important catheter based technique for ablation of AF was reported by Nademanee et al[29] who specifically targeted sites showing low level complex fractionated atrial electrograms (CFAE) during AF. They reported, of the 121 patients treated, 110 (91%) patients were free of arrhythmia and symptoms, 92 (76%) after one procedure and an additional 18 after two procedures, with a follow-up of 1 year. More recent studies have combined techniques in order to increase success rates particularly in patients with long standing persistent forms of AF. In a recent study by Elayi et al[30] in 144 patient with long standing persistent AF, after a mean follow-up of 16 months, these investigators reported that a hybrid technique consisting of pulmonary vein antrum ablation (PVAI) plus ablation of CFAE provided a better outcome than either circumferential pulmonary vein ablation (CPVA) or PVAI with the highest success rates seen with the hybrid approach after 2 procedures (94%).
Consequences of PVI
The PVI procedure could require 100+ radiofrequency applications.[25] Even those procedures which targeted CFAE,[29] without PVI, as few as 40 or as many as 140 radiofrequency applications were delivered to achieve the ablation endpoint. A recent consensus statement reported serious complications in 6% of more than 8700 cases including cardiac tamponade, PV stenosis, phrenic nerve injury, esophageal injury/left atrial fistula, thrombo-embolism, among others.[28] Early on atrogenic consequences were reported with PVI procedures in the form of recurrent tachyarrhythmias. The lesion sets induced to isolate PV firing by encircling lesion sets tend to establish a large channel which favors the induction of a macro-reentrant circuit and subsequent atrial tachycardias. In order to prevent such occurrences additional lesion sets were introduced: a left atrial roof line and a mitral isthmus line. More recently other procedures have been introduced as well, such as ablation of area showing CFAE and areas within the coronary sinus[31] or within the superior vena cava.[32] These additional lesion sets further increase the risk of gaps which in and of themselves provide channels that can allow macro-reentrant atrial tachycardias or left atrial flutters to develop. These recurrences have, therefore, necessitated repeat or even multiple procedures to close gaps and terminate the iatrogenically induced arrhythmias.
Once the ablation within or at the ostia of the PV was abandoned most centers adopted the circum ferential approach proposed by Pappone et al.[25] The rationale for the strategy of PVI was basically to isolate the rapid ectopic firing arising within or at the ostium of the PV without incurring, PV stenosis. However the results, albeit positive (70- 80% success), were somewhat counter-intuitive. Pappone et al.[25] found that “isolation of PV foci may not be the sole mechanism responsible for the AF cure, as suggested by our finding of no significant relationship between lesion completeness and clinical outcome… PV isolation might have interrupted pathways crucial in the genesis of AF located at the PV-LA junction…Finally, atrial debulking and/or denervation may have contributed to suppression of AF.” Subsequent studies further amplified the questions raised by Pappone et al. For example, Stabile et al., using the same anatomic approach with circumferential lesion sets, found that PVI was “not crucial in determining clinical success”.[33] Cappato et al[34] found that, after the first procedure, clinical success was observed in “32% of patients despite the presence of late conduction recurrence across the disconnecting line of one or both superior PVs …in particular, 11 (79%) such patients had conduction recurrences in both superior PVs”. Cappato et al. state, “Causes accounting for this effect may include occasional ablation of the culprit arrhythmogenic focus and severe impairment of conductive PV tissue crucial for arrhythmia generation…” Whether such an explanation can be applied to other findings suggesting that clinical success of PVI electrical disconnection can also be achieved, at least for some time, despite conduction recurrence[34-36] remains moot. Other possibilities that may account for these findings are presented below.
The Hyperactive GP Hypothesis: The PVI Paradox
Intrinsic Cardiac Autonomic Nervous System
The role of the intrinsic cardiac autonomic nervous system (ICANS) under physiological and pathological circumstances has been of interest for the past 40 years.[37] To paraphrase Ardell[38] the intrinsic neural network on the heart and within the pericardium, serves as more than a relay station for the extrinsic projections of the vagosympathetic system from the brain and spinal cord to the heart. It functions as an integrative system which acts cooperatively with the extrinsic innervations but can act independently to modulate numerous cardiac functions, e.g., automaticity, contractility, conduction etc. Early basic studies showed the relationship between the ICANS and cardiac arrhythmias.[39,40]
Anatomy of ICANS
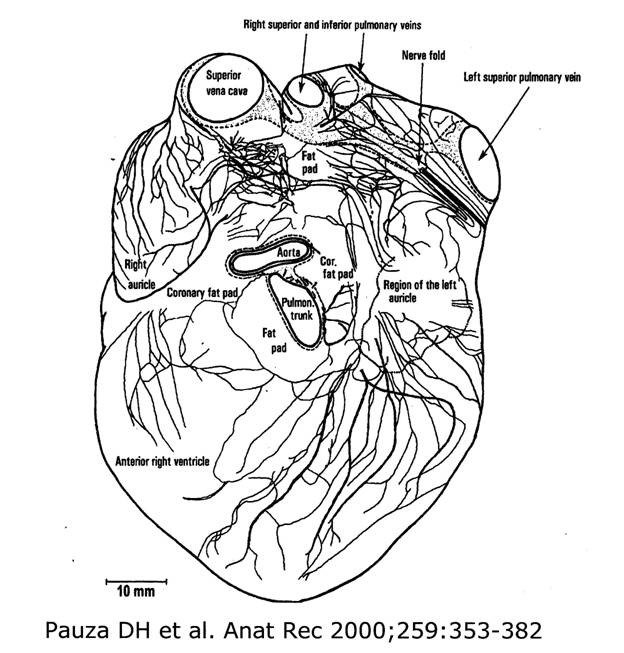
Armour et al[41] provided a comprehensive anatomic study of the ICANS in the human heart by delineating the locations of the major ganglionated plexi and their axonal fields and peripheral ganglia. This study demonstrated that the ICANS is “distributed more extensively than previously considered.” Further elaboration of the anatomy of ICANS has been published by Pauza et al. In essence these studies revealed that there is an extensive neural network covering, not only the atria but also both ventricles [[Figure 1]]. The major modulating centers reside in the clusters of neuronal bodies collectively housed in ganglionated plexi (GP), which, in turn, are located within fat pads. Of interest, 4 of these GP lie adjacent to the four PVs and have been reported to contain 200 or more neuronal cell bodies[41] On the other hand, as many as 1500 ganglia have been estimated to be found on the atrial and ventricular epicardium.[42]
Figure 1. A diagrammatic representation of the neural network that extends over the right and left atria as well as on the epicardial surface of the ventricles. This illustration is the drawing taken from the publication by Pauza et al. showing the course of nerves which were seen after acetycholinesterase staining. In this study, ganglia within fat pads are not depicted although as many as 4300 intrinsic neurons were estimated to be found in the adult human heart. (Reproduced by permission from Pauza et al. Anatomic Record 2000;259:353-382.).
[Figure 2A] illustrates the “right” fat pads associated with the right pulmonary veins (RSPV, RIPV) in a patient undergoing thorascopically guided surgery. The anterior right (AR) GP is found within the large fat pad lying between the right PVs, whereas the inferior right (IR) GP is located within the smaller fat pad close to the inferior junction of the right and left atria. It should be noted that the ARGP and IRGP are to the left of Waterston’s groove (also known as the sulcus terminalis) which marks the boundary between the left and right atria. Although the right PVs are seen through a right thoracotomy their entrance into the left atrial chamber confirms the location of these PV and their associated GP as left atrial structures.
Figure 2. View of the fat pads (panel A) on the human heart as seen through a right thorascopic port. The fat pads (which contain the anterior and inferior right GP) are shown within the demarcated areas (dashed lines) lying between the right superior and right inferior pulmonary veins (RSPV, RIPV). Panel B. A thorascopic view from a left sided port showing the left superior and left inferior, LSPV, LIPV as well as the ligament of Marshall (LOM). The superior left GP is located at the junction of the LSPV and the pulmonary artery while the left inferior GP is located inferior and posterior to the LIPV.

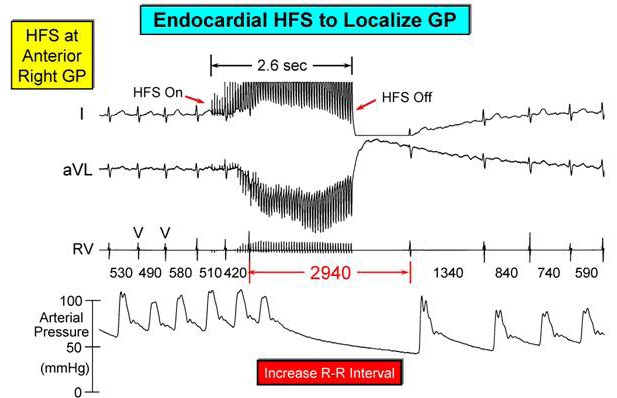
A thorascopic view of the left side [[Figure 2B]], locates the superior left (SL) GP and inferior left (IL) GP in fat pads at the LSPV/pulmonary artery junction and inferior posterior LIPV border, respec tively. These GP can be located during endocardial catheterization procedures for AF ablation by electrical activation with high frequency stimulation (20 Hz). This results in marked slowing of the ventricular response during AF, at least a ≥ 50% increase in the R-R interval [[Figure 3]]. In this way the major locations of the GP can be delineated on a CARTO map [[Figure 4]]. It should be mentioned that other GP can be found on the heart itself, e.g., within the ligament of Marshall[43] as well as on the large vessels within the pericardium, e.g., the right pulmonary artery[44] and at the base of the aorta/pulmonary artery intersection.[38]
Figure 3. CARTO map showing the localization of the GP adjacent to the 4 PVs by high frequency stimulation (HFS) from an electrode catheter placed endocardially subjacent to each of the epicardial fat pads containing the GP. Note that the encircled red dots indicate the sites at which a marked slowing of the ventricular rate was observed during HFS applied at that site (see [Figure 4]).
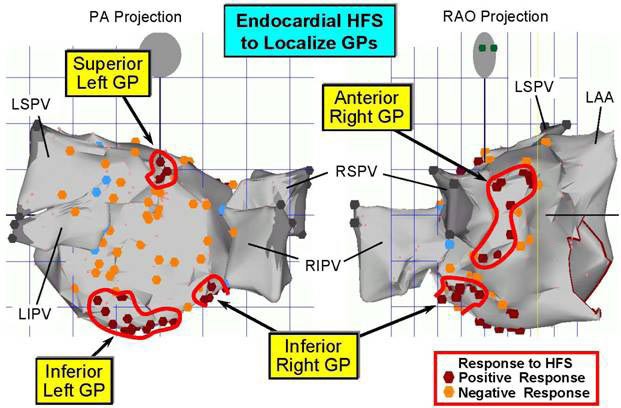
Figure 4. A typical response to HFS at a GP site during ongoing AF which consists of a marked slowing of the ventricular response due to an initial strong parasympathetic effect causing suppression of A-V conduction for about 3 seconds. With termination of GP stimulation the ventricular rate is quickly restored.
Role of ICANS in Relation to AF-Basic Studies
An early report by Sharifov et al[45] implicated autonomic neurohumors, which were injected into the sino-atrial artery, in the initiation of AF. Subsequent basic studies addressed some of the fundamental questions arising from the clinical breakthrough findings that patients with paroxysmal AF have focal firing that arose from the myocardial sleeves which invest the PVs.[18] As mentioned previously, another key finding was reported by Nademanee et al[29] describing the distribution of CFAE in the atria in patients with AF. The abnormal PV firing was thought to provide the triggers for AF, whereas the CFAE was apparently an important constituent of the substrate for this form of AF since ablation at these sites was associatedwith a high rate of termination of AF. From these observations three critical questions arise.
Question 1. How does the focal firing in the PVs become converted into AF and not just manifest as atrial tachycardia? Scherlag et al[46] demonstrated that the number of stimulated impulses applied to the PV would not induce AF unless there was simultaneous activation of the GP adjacent to that PV. Of importance, GP activation is achieved with electrica stimulation using high frequency (20 Hz) and very short stimuli duration (0.1 ms). During sinus rhythm, these stimulation parameters, which slow the heart rate, are delivered at a voltage that does not excite the atrium but does activate the neuronal clusters found in the fat pads on the heart.[47]
Question 2. What is the mechanism whereby the PVs rather than other atrial regions become the sites of focal firing in those patients with AF resistant to drugs and cardioversion? Po et al.[48] caused focal firing in either the right or left superior PV after injecting the neurotransmitter acetylcholine (Ach) into the GP anatomically adjacent to those PV. Furthermore, additional studies by Patterson et al[49,50] provided additional evidence suggesting that PV myocytes show distinctive cellular electrophysiological differences from adjacent atrium, particularly, a shorter action potential duration (APD). Moreover, the PV tissue exhibited greater sensitivity to both cholinergic and adrenergic stimulation than adjacent atrial tissue. Thus, local stimulation of nerve endings in the PV induced release of acetylcholine which further shortened APD while release of the adrenergic neurotransmitters induced early after depolarizations (EADs) leading to rapid, triggered firing. The underlying mechanism for the EADs relates to the temporal disproportionality between the very short APD and the longer lasting calcium transient in the PV myocytes. Under autonomic stimulation these differences are further exacerbated so that the effects on the sodium-calcium exchanger favors excess calcium entry thereby leading to EAD formation,[50] i.e., triggered PV firing. Lemola et al[51] performed PV isolation in dogs while preserving the GP and then ablated the GP while leaving the PV intact. Using vagal induced AF in both cases they concluded, “ it is the PV associated ganglia not the PV themselves that are important in vagally mediated AF promotion.”
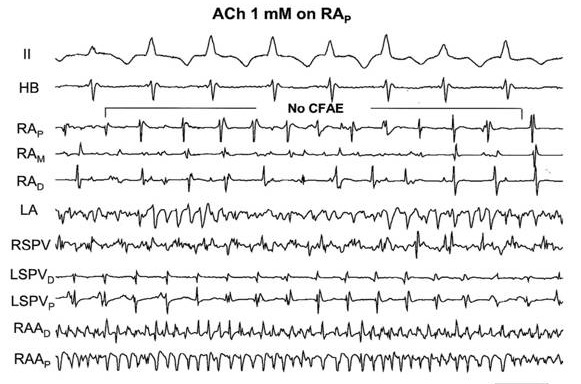
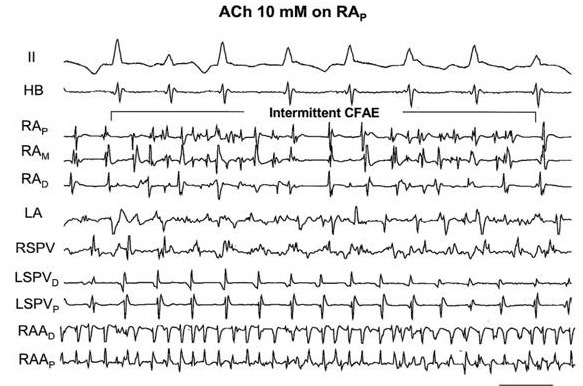
Question 3. In regard to substrate alterations, it has been suggested that PV isolation affects the substrate for AF maintenance. Yet, what exactly constitutes the AF substrate has not been clearly defined. The most specific substrate feature that has been identified has been the prevalence of CFAE whose ablation resulted in at least a 20% increase in AF ablation success[29] compared to PVI alone. As such this approach has been incorporated into the stepwise ablation procedures used in many centers (see below). However, the mechanistic basis for this electrogram characteristic had not been elaborated. To test the hypothesis that autonomic factors might be responsible for CFAE, Lin et al[52] applied different concentrations of Ach to local atrial sites during sustained AF. These sites were chosen specifically because the bipolar electrograms manifested stable Type I potentials, i.e., regular, rapid activation separated by diastolic isoelectric intervals [[Figure 5A]]. Local application of 1 mM Ach to this electrogram site showed little, if any change in the electrogram morphology [[figure 5B]]. On the other hand, local application of 10 mM [[Figure 5C]] and 100 mM [[figure 5D]] resulted in a change from Type I to intermittent CFAE and continuous CFAE, respectively.
Figure 5. The effect of locally applied acetylcholine (ACH) on the conversion of a Type I electrogram to one showing various forms of fractionation, i.e., complex fractionated atrial electrogram, (CFAE). Traces include ECG lead II, His bundle recording (HB), bipolar electrograms from the right (R) and left (L) atrial (A) free walls, R and L pulmonary veins (PVs) and right atrial appendage (RAA). Panel A. The trace labeled RAp represents a bipole on an electrode catheter which showed a Type I electrogram during AF (no CFAE). It was chosen to be locally painted with various concentrations of Ach. Panel B. There was no change when Ach, 1mM was applied to this bipole (no CFAE). Panel C. However, when 10mM Ach was applied to this site intermittent CFAE was noted. Panel D. The subsequent local application of 100mM Ach resulted in the appearance of continuous CFAE. See text for further discussion. (Reproduced with permission from Lin et al J Cardiovasc Electrophysiol 2007;18:1197-1205).

Role of ICANS in Relation to AF-Clinical Studies
Ablation Strategies not Involving PVI
A report from Platt et al[53] described the identification of the GP at the PV-atrial junctions by applying high frequency stimuli to these nerve clusters, endocardially. In patients with persistent forms of AF, the response was a marked slowing of the ventricular response (≥ 50%) during AF. Ablation of these GP terminated the persistent AF in the 23/26 patients who had a complete study with an overall success rate of 96% during a 6 month follow-up. Lemery et al[54] concluded, “Ganglionated plexuses can be precisely mapped using high-frequency stimulation and are located predominantly in the path of (PVI) lesions delivered during ablation of AF.” More recent studies have reported wide ranging results after ablation of GP alone. Scanavacca et al.[55] studied 7 patients with vagotonic AF in whom GP were identified by electrical stimulation (epicardially or endocardially) followed by GP ablation. Five of the seven patients showed AF recurrences over a follow up period ranging from 5-15 months. These authors concluded that ablation of GP may prevent AF recurrences in “selected” patients with apparent vagal induced paroxysmalAF. Katritsis et al[] compared the results of GP ablation alone in 19 patients with paroxysmal AF and 19 age and gender matched patients who had circumferential pulmonary vein ablations. It should be pointed out that, in this study, GP ablation was performed based on anatomic identification of GP sites. No high frequency electrical stimulation was used to identify the GP or determine that they were ablated after radiofrequency applications. Nevertheless, arrhythmia recurrence was found in 14 of 19 (74%) with GP ablation vs, 7 of 19 (37%) with circumferential ablation during a 1 year follow-up. In contrast, Pokushalov et al[57] also used an anatomic approach to identify the location of the GP and then applied radiofrequency energy to ablate these sites. After a 1 year follow-up in 58 patients with persistent and long standing persistent AF (75%) and paroxysmal AF (25%) they reported an overall success rate of 86% during a short follow-up of 7 months. Danik et al.[58] reported on a series of 18 patients whose AF duration averaged 5 years despite various drug regimens. These investigators were able to induce AF with burst pacing after acute GP ablation in 17 of 18 patients but after a 1 year follow-up freedom from AF recurrence was 94% in this same group.
Given the diverse outcomes reported by several investigators, it is important to establish some criteria for GP localization so that the optimal number of GP are effectively ablated in order to obtain results equivalent to PVI or better if PVI and GP ablation are combined (see below). A clinical example of partial GP ablations can be seen from Scanavacca et al.[55] [[figure 3]]. Both epicardial and endocardial sites showing a “vagal” response before but not after ablation were relegated to the posterior wall of the left atrium. It would appear that the anterior aspect of the left atrium, where the largest of the GP is located [[figure 1]], was not ablated although a parasympathetic response was elicited at this site. The high AF recurrence rate of patients in this study may have been due to partial ablation of the major GP located at the PV atrial junctions.
Combination of GP Ablation and PVI Procedures
The first clinical study showing the relatively long term success of a combination of GP ablation and PVI was reported by Pappone et al.[59] In a nonrandomized study of 297 patients with paroxysmal AF, undergoing left atrial circumferential ablation to isolate the pulmonary veins, these investigators found that some 34% showed marked slowing of the ventricular response along with hypotension during the application of radiofrequency energy to 4 specific areas adjacent to the PVs. Continued energy application consistently terminated this “vagal reflex.” In a 12 month follow-up, those 102 patients showed a 99% freedom from AF, whereas the others had a success rate of 85% over the same follow-up period. These workers were obviously impressed by these results, so much so, that their closing suggestion was: “Vagal reflexes can be elicited in several specific sites around all PV ostia and should be specifically targeted to cure paroxysmal AF.” Subsequent studies from this group have not indicated that this advice has been followed. However, there have been other studies using either endocardial catheter ablation or surgical approaches which have performed both PVI and GP ablation. For example, in the small series reported by Scanavacca et al.[55] in which GP ablation alone accounted for a success rate of 25%, the addition of PV isolation showed a 100% success during a follow up of 250 days. In the study by Danik et al.,[58] even though, with GP ablation, in 18/19 patients AF was acutely inducible, the same group with both GP ablation and PVI after a 1 year follow-up had only one recurrence of AF; a success rate of 94%. In a larger series of 83 patients with paroxysmal and persistent AF, Nakagawa et al[60] reported that the freedom from symptomatic AF and AT at 22 months was 86% after a single procedure targeting both GP and performing an antral type PVI.
Surgical Reports Combining PVI and GP Ablations
Using minimally invasive surgical techniques, the results of combined PVI and GP ablation have been more consistent and more encouraging. McClelland et al[61] over a 1 year follow-up, had 14/16 patients showing and overall success rate of 87.5%. Mehall et al.[62] using the same techniques found 14 /15 patient free of AF after a short 6 month follow- up. Matsutani et al.[63] reported the results of a combined Japan-United States experience using a “thorascopic mini-Maze” procedure for bilateral PVI plus ablation of the epicardial GP. They found 18 patients (90%) were free of AF over a mean follow- up of 17 months. Some surgical groups have been applying the combined PVI plus GP ablation approach in order to prevent post operative AF. Onorati et al.[64] compared two groups of patients with AF undergoing mitral valve surgery. Group A (44 patients) underwent left and right mini-Maze procedure, i.e., PVI using a bipolar clamp radiofrequency device; whereas, Group B (31 patients) had the PVI plus fat pad resection along the Waterston’s Groove, left pulmonary veins and Marshall’s ligament. GP were intra-operatively mapped and fat pad specimens sectioned and analyzed for presence of GP. At 13 months of follow-up, free freedom from atrial fibrillation or atrial tachycardia without anti-arrhythmic drugs was 73% in Group A and 93% in Group B. Doll et al 65 studied 12 patients who had valve or coronary artery bypass graft procedures but also had AF. The average duration of AF was 4.5 years, although 5 patients had the paroxysmal form. After a 1 year follow up, 83% were in sinus rhythm and there were no recurrences of AF in the 5 with the paroxysmal form.
The GP Hyperactivity Hypothesis: Evidence that GP Stimulation Promotes AF Initiation and Maintenance
Based on the presently accumulated data, the approach, either endocardial or surgical, combining PVI and GP ablation shows a markedly increased success rate compared to PVI or GP ablation alone. As previous investigators[19,20] have surmised, self sustaining AF would require both an inducing trigger and an appropriate substrate for maintenance. For the macro-reentrant or multiple wavelet form of AF, atrial premature beats (triggers), a markedly shortened and dispersed refractory period found in the remodeled atria, would provide the substrate for AF maintenance.[66] Of interest this form of AF has been shown to be readily terminated by multiple class drug therapy.[67] On the other hand, in the drug resistant form of AF, we postulate that the trigger for focal firing at PV[17,18] or non-PV sites[22][23] is caused by hyperactivity of the major GP adjacent to PV ostia 48 or those associated with the myocardial sleeve into the superior vena cava 68 or within the ligament of Marshall.[23] Intermittent bursts of neural activity in these hyperactive GP release high concentrations of cholinergic and adrenergic neurotransmitters at susceptible sites, shortening refractory periods and inducing EADs as described above 48-50 thereby, providing the basis for triggered firing and subsequent AF. The same GP hyperactivity can extend through the inter- connected neural network 69 causing excessive release of neurotransmitters at multiple nerve endings leading to CFAE,[52] the latter serving, in large part, as the substrate for maintenance of AF.
Possible Mechanism Underlying GP Pathology
Invoking the GP hyperactivity hypothesis engenders the question regarding the pathologic basis underlying the development of this dysautonomia. Preliminary evidence from basic studies[70,71] indicate that the extrinsic autonomic input to the heart, i.e., from the brain and spinal cord, exerts an inhibitory control over the ICANS suggesting that attenuation or loss of this control would allow the GP to become independently hyperactive. Presumptive evidence in support of this hypothesis can be inferred from the recent reports describing the incidence of AF in patients with heart transplants. Khan et al.[72] did a retrospective analysis of 923 patients who underwent orthotopic heart transplantation. This group was age, gender and body mass index matched versus a coronary artery bypass graft group. The differences in the onset of AF over a 3-7 year period were 0.3% for the former and 21% for the latter cohort. All the transplants, except 3, were done using the biatrial technique, whereby the major portion of the recipients atria are left and a remnant of the two atria from the donor are sutured to the recipient’s atria. Thus the major GP and their extrinsic innervation remain intact allowing control by the higher centers over the ICANS. The authors’ state, “…we did observe that the only cases of AF were all in patients who had bicaval anastomosis.” These three patients lost connection and supposedly control between the extrinsic autonomic innervation (from recipient) and GP of the donor heart. Since the PV in both types of anastomoses are isolated the possible source of the triggering for AF might well arise from the superior vena caval myocardial sleeve due to hyperactivity of the adjacent GP.[68]
Progression of AF: From Paroxysmal to Long Standing Persistent Forms
The stochastic nature of GP firing 37 is consistentwith the episodic nature of paroxysmal AF. Insofar as AF progression from paroxysmal to persistent and long standing persistent forms, the same remodeling mechanisms described for the progression of the multiple wavelet form of AF would come into play as the paroxysmal AF burden increased. In addition, more and prolonged episodes of AF have also been shown to result in au autonomic remodeling which manifests as a greater propensity for AF inducibility.[73] It is likely, that both electrophysiological and autonomic remodeling factors are involved in the “AF begets AF” phenomenon, thereby allowing the coexistence of the neurally based drug resistant focal AF and the myocardial based macro- or multiple reentrant forms of AF.[74] Indeed, this coexistence, previously predicted[19,20] could explain the findings of Danik et al.[58] who induced AF after acute GP ablation in 17 of 18 patients but showed long term (1 year follow-up) freedom from AF recurrence in 94%. Also, others have reported that drugs that were ineffective prior to GP ablation could be used to maintain sinus rhythm in patients still inducible after GP ablation and PVI.[75]
The PVI Paradox
Finally, another reported, apparently paradoxical, effect of PVI may be explained by the hyperactive GP hypothesis. Numerous investigators[33-36] have concluded, “Complete electrical isolation of the PVs is not a requirement for a successful outcome after LACA”.[25] It should be noted, that although the major GP, containing hundreds of neurons, are situated close to the PVs, there are many other GP with few neurons throughout the atria.[41] The interruption of axons from these hyperactive GP to PVs may have also contributed to PV focal firing. A recent experimental study showed that myocardial conduction block could be achieved across the atrial appendages but that subsequent application of Ach to the appendage could cause focal firing arising from the PV via unblocked neural connections.[76] The converse can also be predicted, if one accepts the existence of both a neural as well as a myocardial conduction system throughout the heart[41] [[Figure 1]]. It seems possible that in some cases, neural connections can be interrupted from atria to PV while myocardial conduction may return.
By recognizing these diverse pathologies developing within this dual cardiac conduction system, i.e., neural as well as myocardial, new insights into the diagnosis of various cardiac arrhythmias, besides AF, may emerge and provide potential new therapeutic approaches to their prevention or termination.
Conclusions
In this review we have summarized the favorable aspects and drawbacks of pulmonary vein isolation (PVI). As for the role of the ganglionated plexi (GP), found adjacent to the PV atrial entrances, both basic and clinical evidence has shown that GP stimulation promotes initiation and maintenance of AF, and that GP ablation reduces recurrence of AF following catheter or surgical ablation of these structures. Based on these findings, the GP Hyperactivity Hypothesis has been proposed to explain, at least in part, the mechanistic basis for the focal form of AF. In addition, the co-existence of both a myocardial and neural conduction system in the atrium can aid in understanding the greater success for AF ablation by the combined use of PVI and GP ablations.
Disclosures
None.
References
- 1.GIRAUD G, PUECH P, LATOUR H. [The physiological electrical activity of Tawara's node and of His' bundle in man. Endocavitary electrocardiographic registration]. Bull. Acad. Natl. Med. 1960 May 10;144 ():363–6. [PubMed] [Google Scholar]
- 2.Scherlag B J, Lau S H, Helfant R H, Berkowitz W D, Stein E, Damato A N. Catheter technique for recording His bundle activity in man. Circulation. 1969 Jan;39 (1):13–8. doi: 10.1161/01.cir.39.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Wellens H J. Value and limitations of programmed electrical stimulation of the heart in the study and treatment of tachycardias. Circulation. 1978 May;57 (5):845–53. doi: 10.1161/01.cir.57.5.845. [DOI] [PubMed] [Google Scholar]
- 4.Narula O S. Atrioventricular conduction defects in patients with sinus bradycardia. Analysis by His bundle recordings. Circulation. 1971 Dec;44 (6):1096–110. doi: 10.1161/01.cir.44.6.1096. [DOI] [PubMed] [Google Scholar]
- 5.Castellanos A, Castillo C, Cunha D, Myerburg R J. Value of his bundle recordings in the evaluation of the pre-excitation (Wolff-Parkinson-White) syndrome. Proc K Ned Akad Wet C. 1972;75 (5):402–21. [PubMed] [Google Scholar]
- 6.Rosen K M, Bauernfeind R A, Swiryn S, Strasberg B, Palileo E V. Dual AV nodal pathways and AV nodal reentrant paroxysmal tachycardia. Am. Heart J. 1981 May;101 (5):691–5. doi: 10.1016/0002-8703(81)90249-0. [DOI] [PubMed] [Google Scholar]
- 7.Scheinman M M, Morady F, Hess D S, Gonzalez R. Catheter-induced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. JAMA. 1982 Aug 20;248 (7):851–5. [PubMed] [Google Scholar]
- 8.Huang S K, Bharati S, Graham A R, Lev M, Marcus F I, Odell R C. Closed chest catheter desiccation of the atrioventricular junction using radiofrequency energy--a new method of catheter ablation. J. Am. Coll. Cardiol. 1987 Feb;9 (2):349–58. doi: 10.1016/s0735-1097(87)80388-1. [DOI] [PubMed] [Google Scholar]
- 9.Kuck K H, Kunze K P, Schlüter M, Geiger M, Jackman W M. Ablation of a left-sided free-wall accessory pathway by percutaneous catheter application of radiofrequency current in a patient with the Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. 1989 Oct;12 (10):1681–90. doi: 10.1111/j.1540-8159.1989.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 10.Jackman W M, Wang X Z, Friday K J, Roman C A, Moulton K P, Beckman K J, McClelland J H, Twidale N, Hazlitt H A, Prior M I. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N. Engl. J. Med. 1991 Jun 06;324 (23):1605–11. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- 11.Haissaguerre M, Gaita F, Fischer B, Commenges D, Montserrat P, d'Ivernois C, Lemetayer P, Warin J F. Elimination of atrioventricular nodal reentrant tachycardia using discrete slow potentials to guide application of radiofrequency energy. Circulation. 1992 Jun;85 (6):2162–75. doi: 10.1161/01.cir.85.6.2162. [DOI] [PubMed] [Google Scholar]
- 12.Jackman W M, Beckman K J, McClelland J H, Wang X, Friday K J, Roman C A, Moulton K P, Twidale N, Hazlitt H A, Prior M I. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow-pathway conduction. N. Engl. J. Med. 1992 Jul 30;327 (5):313–8. doi: 10.1056/NEJM199207303270504. [DOI] [PubMed] [Google Scholar]
- 13.Cosio F G, López-Gil M, Goicolea A, Arribas F, Barroso J L. Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter. Am. J. Cardiol. 1993 Mar 15;71 (8):705–9. doi: 10.1016/0002-9149(93)91014-9. [DOI] [PubMed] [Google Scholar]
- 14.Cox J L, Boineau J P, Schuessler R B, Ferguson T B, Cain M E, Lindsay B D, Corr P B, Kater K M, Lappas D G. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991 Oct 09;266 (14):1976–80. [PubMed] [Google Scholar]
- 15.Swartz JF, Pellersels G, Silvers J, Patten L, Cervantez D. A catheter-based curative approach to atrial fibrillation in humans.(abstract). Circulation. 1994;90:0–335. [Google Scholar]
- 16.Haïssaguerre M, Jaïs P, Shah D C, Gencel L, Pradeau V, Garrigues S, Chouairi S, Hocini M, Le Métayer P, Roudaut R, Clémenty J. Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 1996 Dec;7 (12):1132–44. doi: 10.1111/j.1540-8167.1996.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 17.Jaïs P, Haïssaguerre M, Shah D C, Chouairi S, Gencel L, Hocini M, Clémenty J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997 Feb 04;95 (3):572–6. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 18.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 19.Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn Ther. 1962;140:183–188. [Google Scholar]
- 20.llessie M, Lammers WJEP, Bonke FIM, Hollen J. Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In Zipes DP, Jalife (eds) Cardiac Electr physiology and Arrhythmias. Grune and Stratton, New York. 1985;0:265–275. [Google Scholar]
- 21.Jaïs P, Shah D C, Haïssaguerre M, Hocini M, Garrigue S, Clémenty J. Atrial fibrillation: role of arrhythmogenic foci. J Interv Card Electrophysiol. 2000 Jan;4 Suppl 1 ():29–37. doi: 10.1023/a:1009857926782. [DOI] [PubMed] [Google Scholar]
- 22.Tsai C F, Tai C T, Hsieh M H, Lin W S, Yu W C, Ueng K C, Ding Y A, Chang M S, Chen S A. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000 Jul 04;102 (1):67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 23.Hwang C, Wu T J, Doshi R N, Peter C T, Chen P S. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000 Apr 04;101 (13):1503–5. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 24.Haïssaguerre M, Shah D C, Jaïs P, Hocini M, Yamane T, Deisenhofer I, Chauvin M, Garrigue S, Clémenty J. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000 Nov 14;102 (20):2463–5. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- 25.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabrò M P, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001 Nov 20;104 (21):2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 26.Cha YM, Munger TM, Asirvatham SJ, Friedman PA, Monahan KH, Packer DL. Impact of left atrial size on the outcome of wide area circumferential ablation vs lasso-guided pulmonary vein isolation. Heart Rhythm. 2005;2:0–0. [Google Scholar]
- 27.Verma Atul, Marrouche Nassir F, Natale Andrea. Pulmonary vein antrum isolation: intracardiac echocardiography-guided technique. J. Cardiovasc. Electrophysiol. 2004 Nov;15 (11):1335–40. doi: 10.1046/j.1540-8167.2004.04428.x. [DOI] [PubMed] [Google Scholar]
- 28.Calkins Hugh, Brugada Josep, Packer Douglas L, Cappato Riccardo, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, Haines David E, Haissaguerre Michel, Iesaka Yoshito, Jackman Warren, Jais Pierre, Kottkamp Hans, Kuck Karl Heinz, Lindsay Bruce D, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Natale Andrea, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Ruskin Jeremy N, Shemin Richard J. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007 Jun;9 (6):335–79. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 29.Nademanee Koonlawee, McKenzie John, Kosar Erol, Schwab Mark, Sunsaneewitayakul Buncha, Vasavakul Thaveekiat, Khunnawat Chotikorn, Ngarmukos Tachapong. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004 Jun 02;43 (11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 30.Elayi Claude S, Verma Atul, Di Biase Luigi, Ching Chi Keong, Patel Dimpi, Barrett Conor, Martin David, Rong Bai, Fahmy Tamer S, Khaykin Yaariv, Hongo Richard, Hao Steven, Pelargonio Gemma, Dello Russo Antonio, Casella Michela, Santarelli Pietro, Potenza Domenico, Fanelli Raffaele, Massaro Raimondo, Arruda Mauricio, Schweikert Robert A, Natale Andrea. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008 Dec;5 (12):1658–64. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill Mark D, Jaïs Pierre, Takahashi Yoshihide, Jönsson Anders, Sacher Frédéric, Hocini Mélèze, Sanders Prashanthan, Rostock Thomas, Rotter Martin, Pernat Andrej, Clémenty Jacques, Haïssaguerre Michel. The stepwise ablation approach for chronic atrial fibrillation--evidence for a cumulative effect. J Interv Card Electrophysiol. 2006 Sep;16 (3):153–67. doi: 10.1007/s10840-006-9045-1. [DOI] [PubMed] [Google Scholar]
- 32.Arruda M, Natale A. The adjunctive role of non pulmonary venous ablation in the cure of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:0–0. [Google Scholar]
- 33.Stabile Giuseppe, Turco Pietro, La Rocca Vincenzo, Nocerino Pasquale, Stabile Eugenio, De Simone Antonio. Is pulmonary vein isolation necessary for curing atrial fibrillation? Circulation. 2003 Aug 12;108 (6):657–60. doi: 10.1161/01.CIR.0000086980.42626.34. [DOI] [PubMed] [Google Scholar]
- 34.Cappato Riccardo, Negroni Silvia, Pecora Domenico, Bentivegna Stefano, Lupo Pier Paolo, Carolei Adriana, Esposito Cristina, Furlanello Francesco, De Ambroggi Luigi. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003 Sep 30;108 (13):1599–604. doi: 10.1161/01.CIR.0000091081.19465.F1. [DOI] [PubMed] [Google Scholar]
- 35.Lemola Kristina, Oral Hakan, Chugh Aman, Hall Burr, Cheung Peter, Han Jihn, Tamirisa Kamala, Good Eric, Bogun Frank, Pelosi Frank, Morady Fred. Pulmonary vein isolation as an end point for left atrial circumferential ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2005 Sep 20;46 (6):1060–6. doi: 10.1016/j.jacc.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 36.Pratola Claudio, Baldo Elisa, Notarstefano Pasquale, Toselli Tiziano, Ferrari Roberto. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008 Jan 15;117 (2):136–43. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 37.Randall WC. Changing perspectives concerning neural control of the heart. In: Neurocardiology. Eds: Armour JA, Ardell JL. Oxford University Press, New York, NY. 1994;0:0–0. [Google Scholar]
- 38.Ardell JL. Structure and Function of the Mammalian Intrinsic Cardiac Neurons. In: Neurocardiology. Eds: Armour JA, Ardell JL. Oxford University Press, New York, NY. 1994;0:0–0. [Google Scholar]
- 39.Armour J A, Hageman G R, Randall W C. Arrhythmias induced by local cardiac nerve stimulation. Am. J. Physiol. 1972 Nov;223 (5):1068–75. doi: 10.1152/ajplegacy.1972.223.5.1068. [DOI] [PubMed] [Google Scholar]
- 40.Hageman G R, Goldberg J M, Armour J A, Randall W C. Cardiac dysrhythmias induced by autonomic nerve stimulation. Am. J. Cardiol. 1973 Nov;32 (6):823–30. doi: 10.1016/s0002-9149(73)80012-8. [DOI] [PubMed] [Google Scholar]
- 41.Armour J A, Murphy D A, Yuan B X, Macdonald S, Hopkins D A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 1997 Feb;247 (2):289–98. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 42.Pauza D H, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat. Rec. 2000 Aug 01;259 (4):353–82. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 43.Lin Jiaxiong, Scherlag Benjamin J, Lu Zhibing, Zhang Ying, Liu Shaowen, Patterson Eugene, Jackman Warren M, Lazzara Ralph, Po Sunny S. Inducibility of atrial and ventricular arrhythmias along the ligament of marshall: role of autonomic factors. J. Cardiovasc. Electrophysiol. 2008 Sep;19 (9):955–62. doi: 10.1111/j.1540-8167.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 44.Chiou C W, Eble J N, Zipes D P. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation. 1997 Jun 03;95 (11):2573–84. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- 45.Sharifov Oleg F, Fedorov Vadim V, Beloshapko Galina G, Glukhov Alexey V, Yushmanova Anna V, Rosenshtraukh Leonid V. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J. Am. Coll. Cardiol. 2004 Feb 04;43 (3):483–90. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 46.Scherlag Benjamin J, Yamanashi William, Patel Utpal, Lazzara Ralph, Jackman Warren M. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J. Am. Coll. Cardiol. 2005 Jun 07;45 (11):1878–86. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 47.Lazzara R, Scherlag B J, Robinson M J, Samet P. Selective in situ parasympathetic control of the canine sinoatrial and atrioventricular nodes. Circ. Res. 1973 Mar;32 (3):393–401. doi: 10.1161/01.res.32.3.393. [DOI] [PubMed] [Google Scholar]
- 48.Po Sunny S, Scherlag Benjamin J, Yamanashi William S, Edwards Jeff, Zhou Jing, Wu Richard, Geng Ning, Lazzara Ralph, Jackman Warren M. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm. 2006 Feb;3 (2):201–8. doi: 10.1016/j.hrthm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Patterson Eugene, Po Sunny S, Scherlag Benjamin J, Lazzara Ralph. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005 Jun;2 (6):624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Patterson Eugene, Lazzara Ralph, Szabo Bela, Liu Hong, Tang David, Li Yu-Hua, Scherlag Benjamin J, Po Sunny S. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J. Am. Coll. Cardiol. 2006 Mar 21;47 (6):1196–206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 51.Lemola Kristina, Chartier Denis, Yeh Yung-Hsin, Dubuc Marc, Cartier Raymond, Armour Andrew, Ting Michael, Sakabe Masao, Shiroshita-Takeshita Akiko, Comtois Philippe, Nattel Stanley. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008 Jan 29;117 (4):470–7. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- 52.Lin Jiaxiong, Scherlag Benjamin J, Zhou Jing, Lu Zhibing, Patterson Eugene, Jackman Warren M, Lazzara Ralph, Po Sunny S. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE). J. Cardiovasc. Electrophysiol. 2007 Nov;18 (11):1197–205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 53.Platt M, Mandapati R, Scherlag BJ, Yamanashi WS, Nakagawa H, Lazzara R, Jackman WM. Limiting the number and extent of radiofrequency applications to terminate atrial fibrillation and subsequently prevent its inducibility. (abstract). Heart Rhythm. 2004;1:0–11. [Google Scholar]
- 54.Lemery Robert, Birnie David, Tang Anthony S L, Green Martin, Gollob Michael. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006 Apr;3 (4):387–96. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Scanavacca Mauricio, Pisani Cristiano F, Hachul Denise, Lara Sissy, Hardy Carina, Darrieux Francisco, Trombetta Ivani, Negrão Carlos Eduardo, Sosa Eduardo. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006 Aug 29;114 (9):876–85. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 56.Katritsis Demosthenes, Giazitzoglou Eleftherios, Sougiannis Demetrios, Goumas Nicolaos, Paxinos George, Camm A John. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am. J. Cardiol. 2008 Aug 01;102 (3):330–4. doi: 10.1016/j.amjcard.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 57.Pokushalov Evgueny, Turov Alex, Shugayev Pavel, Artyomenko Sergey, Romanov Alex, Shirokova Natalya. Catheter ablation of left atrial ganglionated plexi for atrial fibrillation. Asian Cardiovasc Thorac Ann. 2008 Jun;16 (3):194–201. doi: 10.1177/021849230801600304. [DOI] [PubMed] [Google Scholar]
- 58.Danik Stephan, Neuzil Petr, d'Avila Andre, Malchano Zachary J, Kralovec Stepan, Ruskin Jeremy N, Reddy Vivek Y. Evaluation of catheter ablation of periatrial ganglionic plexi in patients with atrial fibrillation. Am. J. Cardiol. 2008 Sep 01;102 (5):578–83. doi: 10.1016/j.amjcard.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 59.Pappone Carlo, Santinelli Vincenzo, Manguso Francesco, Vicedomini Gabriele, Gugliotta Filippo, Augello Giuseppe, Mazzone Patrizio, Tortoriello Valter, Landoni Giovanni, Zangrillo Alberto, Lang Christopher, Tomita Takeshi, Mesas Cézar, Mastella Elio, Alfieri Ottavio. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004 Jan 27;109 (3):327–34. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa H, Yokoyama K, Scherlag BJ, Katari V, Aoyama H, Foresti S, Jackman WM. Ablation of autonomic ganglia In: A Practical Approach to Catheter Ablation of Atrial fibrillation. Eds: Calkins H, Jais P, Steinberg JS. W. olters Kluwer/Lippincott Williams & Wilkins. Philadelphia. 2008;0:0–0. [Google Scholar]
- 61.McClelland James H, Duke David, Reddy Ramakota. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007 Dec;18 (12):1289–95. doi: 10.1111/j.1540-8167.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 62.Mehall John R, Kohut Robert M, Schneeberger E William, Taketani Tsuyoshi, Merrill Walter H, Wolf Randall K. Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann. Thorac. Surg. 2007 Feb;83 (2):538–41. doi: 10.1016/j.athoracsur.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 63.Matsutani Noriyuki, Takase Bonpei, Ozeki Yuichi, Maehara Tadaaki, Lee Richard. Minimally invasive cardiothoracic surgery for atrial fibrillation: a combined Japan-US experience. Circ. J. 2008 Mar;72 (3):434–6. doi: 10.1253/circj.72.434. [DOI] [PubMed] [Google Scholar]
- 64.Onorati Francesco, Curcio Antonio, Santarpino Giuseppe, Torella Daniele, Mastroroberto Pasquale, Tucci Luigi, Indolfi Ciro, Renzulli Attilio. Routine ganglionic plexi ablation during Maze procedure improves hospital and early follow-up results of mitral surgery. J. Thorac. Cardiovasc. Surg. 2008 Aug;136 (2):408–18. doi: 10.1016/j.jtcvs.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 65.Doll Nicolas, Pritzwald-Stegmann Patrick, Czesla Markus, Kempfert Joerg, Stenzel Monika Anna, Borger Michael A, Mohr Friedrich-Wilhelm. Ablation of ganglionic plexi during combined surgery for atrial fibrillation. Ann. Thorac. Surg. 2008 Nov;86 (5):1659–63. doi: 10.1016/j.athoracsur.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 66.Wijffels M C, Kirchhof C J, Dorland R, Power J, Allessie M A. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation. 1997 Nov 18;96 (10):3710–20. doi: 10.1161/01.cir.96.10.3710. [DOI] [PubMed] [Google Scholar]
- 67.Wijffels M C, Dorland R, Allessie M A. Pharmacologic cardioversion of chronic atrial fibrillation in the goat by class IA, IC, and III drugs: a comparison between hydroquinidine, cibenzoline, flecainide, and d-sotalol. J. Cardiovasc. Electrophysiol. 1999 Feb;10 (2):178–93. doi: 10.1111/j.1540-8167.1999.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 68.Niu G, Scherlag BJ, Lu Z, Ghias M, Lazzara R, Jackman WM, Po SS. Inducibility of atrial fibrillation in the myocardial sleeve of the superior vena cava is mediated by the ganglionated plexi on the right pulmonary artery. Circulation. 2007;116:0–40. [Google Scholar]
- 69.Hou Yinglong, Scherlag Benjamin J, Lin Jiaxiong, Zhou Jing, Song Jianguo, Zhang Ying, Patterson Eugene, Lazzara Ralph, Jackman Warren M, Po Sunny S. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm. 2007 Jan;4 (1):56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Gagliardi M, Randall W C, Bieger D, Wurster R D, Hopkins D A, Armour J A. Activity of in vivo canine cardiac plexus neurons. Am. J. Physiol. 1988 Oct;255 (4 Pt 2):H789–800. doi: 10.1152/ajpheart.1988.255.4.H789. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Scherlag BJ, Lu Z, Niu GD, Yamanashi WS, Hogan C, Fields J, Ghias M, Lazzara R, Jackman WM, Po S. Comparison of atrial fibrillation inducibility by electrical stimulation of either the extrinsic or the intrinsic autonomic nervous systems. J Interv Card Electrophysiol. 2008;0:0–0. doi: 10.1007/s10840-008-9297-z. [DOI] [PubMed] [Google Scholar]
- 72.Khan Mohammed, Kalahasti Vidyasagar, Rajagopal Vivek, Khaykin Yaariv, Wazni Oussama, Almahameed Soufian, Zuzek Ryan, Shah Tehmeena, Lakkireddy Dhanumjaya, Saliba Walid, Schweikert Robert, Cummings Jennifer, Martin David O, Natale Andrea. Incidence of atrial fibrillation in heart transplant patients: long-term follow-up. J. Cardiovasc. Electrophysiol. 2006 Aug;17 (8):827–31. doi: 10.1111/j.1540-8167.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 73.Lu Z, Scherlag BJ, Lin J, Niu G, Fung KM, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H, Po SS. Atrial Fibrillation Begets Atrial Fibrillation: Autonomic Mechanism for Atrial Electrical Remodeling Induced by Short-Term Rapid Atrial Pacing Circulation: Arrhythmia and Electrophysiology. 2008;1:184–192. doi: 10.1161/CIRCEP.108.784272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scherlag BJ, Hou Y, Lin J, Lu Z, Po SS. , , , , . Neurally based focal and myocardial based macroreentrant atrial fibrillation: Two acute models of sustained atrial fibrillation in a single preparation. (abstract). J Cardiovasc Electrophysiol. 2007;0:0–0. [Google Scholar]
- 75.Scherlag Benjamin J, Nakagawa Hiroshi, Jackman Warren M, Yamanashi William S, Patterson Eugene, Po Sunny, Lazzara Ralph. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005 Aug;13 Suppl 1 ():37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 76.Scherlag Benjamin J, Hou Ying-Long, Lin Jiaxiong, Lu Zhibing, Zacharias Soni, Dasari Tarun, Niu Guodong, Ghias Muhammad, Patterson Eugene, Jackman Warren M, Lazzara Ralph, Po Sunny S. An acute model for atrial fibrillation arising from a peripheral atrial site: evidence for primary and secondary triggers. J. Cardiovasc. Electrophysiol. 2008 May;19 (5):519–27. doi: 10.1111/j.1540-8167.2007.01087.x. [DOI] [PubMed] [Google Scholar]


