Abstract
Background: Atrial Fibrillation is the most common cardiac arrhythmia. It increases the risk of thromboembolic events and many atrial fibrillation patients suffer quality of life impairment due to disturbed heart rhythm. Pulmonary vein isolation using radiofrequency catheter ablation treatment is aimed at maintaining sinus rhythm ultimately improving quality of life. Randomized clinical trial have shown that catheter ablation is more effective than antiarrhythmic drugs for the treatment of atrial fibrillation, but its impact on quality of life and cost-effectiveness has not been widely studied.
Aims: To assess the cost-effectiveness of radiofrequency ablation (RFA) vs. antiarrhythmic drug (AAD) treatment, among symptomatic atrial fibrillation patients not previously responding to AAD.
Methods: A decision-analytic Markov model was developed to assess costs and health outcomes in terms of quality adjusted life years (QALYs) of RFA and AAD over a lifetime time horizon. We conducted a literature search and used data from several sources as input variables of the model. One-year rates of atrial fibrillation with RFA and AAD, respe tively, were available from published randomized clinical trials. Other data sources were published papers and register data.
Results: The RFA treatment strategy was associated with reduced costs and an incremental gain in QALYs compared to the AAD treatment strategy. The results were sensitive to whether long-term quality of life improvement is maintained for the RFA treatment strategy and the risk of stroke in the different atrial fibrillation health states.
Conclusion: This study shows that the short-term improvement in atrial fibrillation associated with RFA is likely to lead to long-term quality of life improvement and lower costs indicating that RFA is cost-effective compared to AAD.
Keywords: Cost, Cost-Effectiveness, Decision-Analytic Model, Ablation, Atrial Fibrillation, Cardiovascular Disease
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and occurs in 2 % of adults aged 65 to 75 years. Its prevalence increases with age; 5 % of adults above 75 years old and 14 % of adults above 85 years old.[1] Furthermore, AF increases the risk of thromboembolic events and many AF patients perceive/suffer quality of life (QoL) impairment in the form of palpitations and shortness of breath due to the disturbed heart rhythm. Pulmonary vein isolation using radiofrequency catheter ablation (RFA) is aimed at maintaining sinus rhythm. Randomized clinical trials have shown that RFA is more effective than antiarrhythmic drug treatment (AAD) in maintaining sinus rhythm, but its cost-effectiveness has not been widely studied. Cost comparison studies of RFA versus AAD were identified.[2-3] Though, we could only identify two studies assessing both costs and benefits (effects) of RFA treatment.[4-5]
Medical management for AF involves the use of a combination of different medications. Rhythm control management often involves the use of AAD, foremost amiodarone or flecainide, aimed at maintaining sinus rhythm and at avoiding relapses. Sotalol and propafenone are less commonly used for rhythm control management in Sweden. Many AF patients do not tolerate long-term AAD treatment, particularly with amiodarone, without side effects. In the event of side effects or lack of efficacy the use of a non-pharmaceutical treatment, such as pacemaker implantation followed by AV-node ablation or RFA might be considered. RFA is already an established treatment strategy for different types of arrhythmias including WPW-syndrome, AV-nodal reentrytachycardia, atrial flutter and focal atrial tachycardia. However, RFA, aiming at pulmonary vein isolation, for AF patients has only been used during the last decade and is usually considered as an alternative treatment strategy only after medical management has caused side effects or had no or insufficient effect.
AF has traditionally been divided into paroxysmal, persistent and permanent AF. According to international ACC/AHA/ESC 2006 guidelines patients with severe symptomatic paroxysmal and persistent AF are eligible for RFA treatment. The RFA treatment strategy for the treatment of symptomatic AF has been debated in the current Swedish National Guidelines for Heart Disease and is now recommended for symptomatic AF patients with paroxysmal or persistent AF not responding well to AAD treatment.[6] Patients with permanent AF, on the other hand are not eligible for RFA and are excluded in the analysis.
In this analysis, we assess the lifetime costs and health outcomes of radiofrequency ablation (RFA) treatment compared to antiarrhythmic drug treatment (AAD) alone. The population used in our analysis consists of symptomatic patients with paroxysmal or persistent AF, not responding well to AAD treatment and eligible for RFA i.e. as a second-line treatment strategy.
Methods
Overall Analytical Approach
A decision-analytic model was developed to estimate costs, health outcomes and incremental costeffectiveness of RFA compared to AAD treatment for AF for a lifetime time horizon. In the absence of long-term data, decision-analytic models can be used to estimate costs and health outcomes of health interventions beyond the follow-up of clinical trials. Data from several sources were used to populate the model with best available evidence. The outcome measure used in the analysis was quality-adjusted life years (QALYs). Probability distributions were defined for the model parameters reflecting the uncertainty in evidence/of the information available. A Swedish societal perspective was taken, and both costs and health outcomes were discounted at 3 % per annum, respectively. All costs are in 2006 prices and have been converted to USD using purchasing power parities (PPPs).
Model Structure And Underlying Assumptions
A two-part model structure was used, a decision tree for the initial year in which the RFA procedure is assumed to take place, and a long-term Markov structure for subsequent years (see [Figures 1a] and [1b]). The short-term model provides the proportion of patients entering the long-term model health states after accounting for non-stroke mortality and stroke risk. Short-term clinical endpoints i.e. freedom of AF at 12 months were used in the model. If the patient suffers a clinically significant relapse into AF, a second RFA procedure is usually offered as a standard in Sweden and was assumed to take place during the initial year.
Figure 1A. Short-term model structure.
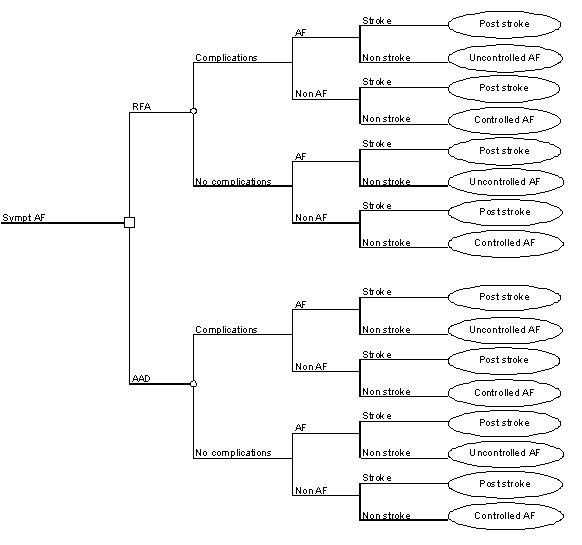
Figure 1B. Long-term Markov model structure.
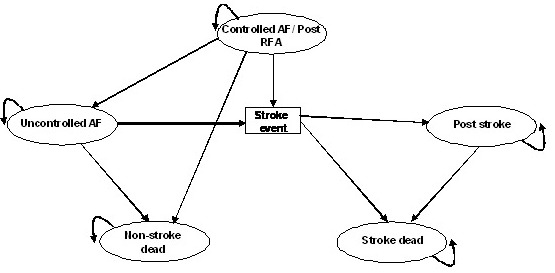
A Markov model structure was developed to extrapolate the lifetime costs and QALYs of the two intervention strategies. In a Markov structure a hypothetical cohort of patients reside in mutually exclusive health states during intervals of equal length referred to as Markov cycles. The model consists of health states for controlled AF, uncontrolled AF, stroke and death. Separate health states for death were used; whether caused by stroke or other cause mortality. Annual Markov cycles were applied.
Successful treatment implies that the hypothetical patients enter the controlled AF health state. If the treatment strategy is not successful, the cohort of patients enters the uncontrolled AF health state. In case of a stroke event, the cohort may enter the stroke dead or post stroke health states. The ´post stroke´ health state implying an elevated mortality risk and reduced QoL. Patients face a risk of non-stroke mortality and may at any stage make the transition to the non-stroke dead health state.
A summary of base-case model inputs are given in [Table 1].
Table 1. Summary of model inputs.
* Average cost for RFA procedure includes; 3-4 hospitalization days, diagnostic examinations e.g. ultrasound and/or CT and MR and catheters. § Anticoagulation treatment (warfarin) consists of; monitoring at specialist dept. (58 %), average cost per unit, 200SEK (22USD); number of visits per annum, 16.25; monitoring at primary care unit average cost per unit 545SEK (60USD) (42 % average cost 509SEK (56USD) of which 10 % at home, average cost 861SEK (94USD), number of visits, 13.75; travel, 42SEK (5USD); loss of production, 26SEK (3USD) and medication 576SEK (63USD).
| Variable | Value | Distribution |
|---|---|---|
| Probability of AF free at 12 months | ||
| RFA | 0.780 | |
| AAD | 0.090 | |
| Rate of AF in AAD | 2.4423 | Gamma(24, 0.10) |
| Risk ratio RFA vs AAD | 0.1017 | Log normal |
| Stroke risk, AF (%) | 1.5 | Gamma(1, 0.02) |
| Stroke risk, free from AF (%) | 1.5 | Gamma(1, 0.02) |
| Complication with RFA | 0.030 | Beta(14, 448) |
| Cost items | Mean costs; SEK (USD) | |
| RFA procedure, single event* | 90 000 (9 860) | Gamma(36, 2500) |
| Complication cost | 20 000 (2 190) | Gamma(2, 11250) |
| AAD treatment, annual | 15 000 (1 640) | Gamma(9, 1667) |
| Anticoagulation treatment, annual§ | 7 000 (770) | Gamma(8, 875) |
| Cost of stroke (yr 1) | 175 000 (19 180) | Gamma(77, 2286) |
| Cost of stroke (>yr 1, per annum)) | 40 000 (4 380) | Gamma(16, 2500) |
| QALY-weights for males in normal population Age | QALY-weights | |
| >69 | 0.830 | Beta(812, 166) |
| 70-79 | 0.800 | Beta(394, 99) |
| 80< | 0.740 | Beta(103, 36) |
| Decrement for AF | 0.100 | Gamma(11, 0.01) |
| Decrement for stroke | 0.250 | Gamma(69, 0.00) |
| Annual discount rate Costs | 0.03 | |
| Effects | 0.03 |
Model Inputs
Clinical Effectiveness
We conducted a literature search to find data to populate our model. Clinical studies have shown a success rate for RFA, measured as freedom from AF relapses at 12 months, between 70 to 80 %, assuming that the intervention is repeated within a year in case of clinically significant relapse into AF or atrial tachycardia.[7-8] Five randomized controlled clinical trials reporting efficacy of RFA compared with AAD were identified.[9-13] One study was excluded as it only consider RFA as first-line treatment i.e. the patients did not receive AAD treatment prior to RFA.[10] One of the randomized clinical trial found showing 56 % free from AF relapses during a follow-up period of 12 months of RFA treatment after a single procedure i.e. not repeated if failed.[11] After a follow-up period of 12 months, 91 % (63/69) patients still using AAD had at least one AF recurrence with the AAD treatment strategy.[11]
The probability used for the decision-tree was based on the assumption that the intervention is repeated within the first year in case of relapse into AF, the standard procedure in Sweden. The yearly rate of AF and relative risk ratios for both a first and a second RFA procedure were estimated using randomized controlled clinical trial data. An average of 1.4 procedures per patient is needed to successfully isolate the pulmonary veins based on Swedish clinical data.[14]
Mortality and Stroke Risks
All AF patients with at least one risk factor for stroke (CHADS2) benefit from anticoagulation treatment to reduce thromboembolic events. No evidence was found to indicate different stroke risks in the controlled AF and uncontrolled AF health states. The baseline risk of stroke was assumed to be 1.5 % for AF and non-AF on anticoagulation treatment using a conservative assumption.[1] The age-dependant standard mortality rates were based on the Swedish national data.[15]
Costs
The short-term decision tree considered the costs associated with the RFA procedure. It was assumed that the RFA procedure was repeated within a year if not successful implying an additional cost for the repeated procedure in the short-term decision tree. A single RFA procedure was costed at 90 000 SEK (9 860 USD).[16-17] This cost includes 3 to 4 hospitalization days and diagnostic examinations e.g. ultrasound, CT /or MR and disposables such as catheters. This cost was thus multi plied by 1.4 procedures in the Markov model.
Serious complications associated with the RFA procedure include; tamponade, bleeding, pulmonary vein stenosis, stroke and oesophageal fistulas.[18] Deaths have been reported in some cases in connection with pulmonary vein stenosis and oesophageal fistulas. In the Swedish national catheter ablation register information on complicationsassociated with RFA treatment was available. The probability of a major complication was assumed to be 3 % using Swedish register data, no deaths were reported.[19] All complications used in our model were treated as costs.
Medical management for AF often involves the use of a combination of different medications. Both the RFA and AAD treatment strategy involves the use of AAD. The annual cost of AAD treatment has been estimated to 15 000 SEK (1 640 USD). This cost includes hospitalisation, AAD medication and consultation; hospitalisation being the major cost driver for AAD.[20] In the long-term model, continued use of AAD after the initial year, was assumed in the case RFA did not eliminate AF i.e. not free from AF.
The average cost of monitoring AF patients using warfarin (anticoagulation) was 375 SEK (41 USD) per visit and 15 times a year, totalling to 6 052 SEK (663 USD) per annum.[21] This cost includes the cost for monitoring at either a specialist department or primary care unit and actual medication. The cost of medication was estimated to 575 SEK (63 USD) per annum. The post stroke health state is associated with increased cost and the annual cost of stroke was assumed to be greater during the first year, based on the incidence of first-time stroke.[22]
Quality-Adjusted Life Years
No studies were found measuring QoL improvement on AF patients in a way that could readily be used for QALY weights. However, several studies have shown improved quality of life (QoL) after RFA treatment.[23,8] For instance, QoL, measured by the SF-36 instrument, improved significantly in all eight health dimensions after RFA treatment.[23] In order to estimate QALY weights for different health states, age-adjusted QALY weights based on a Swedish general population were applied for patients in the controlled AF state, and used as reference points. The QALY weights used in the model was 0.83, 0.81 and 0.74 for individuals aged >69, 70-79 and >80. Decrements were applied to the general population utility weight for the uncontrolled AF state and the post stroke state. A decrement of 0.1 for uncontrolled AF and 0.25 for stroke was applied to the baseline utility in the controlled AF state.
Analysis
The model was evaluated using second-order Monte Carlo simulation. The cohort was simulated during Markov cycles until all hypothetical patients were assumed to be in the ‘dead’ health state during sixty-one Markov cycles. The total accumulated costs and health outcomes for each Markov cycle were summarized for the hypothetical cohort of symptomatic AF patients. The results were presented in two ways. First, mean lifetime costs and QALYs showing the incremental cost-effectiveness ratios (ICERs) of the compared treatment strategies are shown in [Table 2] illustrating the additional costs needed per additional gained QALY. Second, decision uncertainty of the probabilistic analysis is plotted in the cost-effectiveness plane. The model was programmed and analyzed using Microsoft Office Excel.
Table 2. Quality adjusted life years and incremental cost effectiveness ratios for RFA compared with AAD treatment.
ICER, Incremental cost-effectiveness ratio; QALY, quality adjusted life years; RFA, radiofrequency ablation treatment; AAD, antiarrhythmic drug treatment. Dom, dominant.
| Intervention | QALY | Cost SEK (USD) | ICER SEK (USD)/ QALY) |
|---|---|---|---|
| Probabilistic base-case analysis | |||
| RFA | 9.46 | 232 300 (25 460) | |
| AAD | 8.68 | 277 700 (30 440) | Dom |
| Annual probability of reversion to uncontrolled AF after RFA | |||
| 5 %RFA | 9.06 | 318 600 (34 920) | |
| AAD | 8.55 | 279 700 (30 660) | 75 500 (8 280) |
| 10 % RFA | 8.91 | 366 400 (40 160) | |
| AAD | 8.55 | 279 700 (30 660) | 241 400 (26 460) |
| 15 % RFA | 8.81 | 395 300 (43 330) | |
| AAD | 8.55 | 279 700 (30 660) | 440 800 (48 310) |
Results
Base-Case Analysis
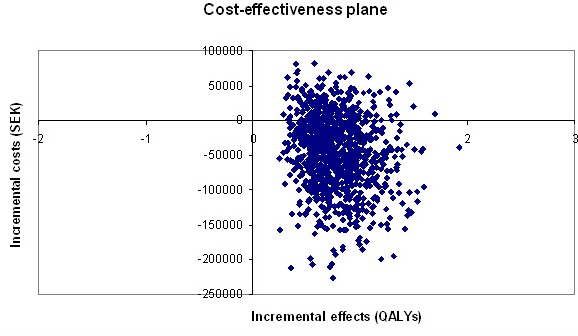
The base-case results show that the RFA treatment strategy was associated with an incremental gain in QALYs and reduced costs compared to the AAD strategy in the lifetime analysis. The model was run probabilistically and the results of the 1000 simulations are shown in [Figure 2]. The vertical axis represents the difference in costs and the horizontal axis the difference in health outcomes for the two treatment strategies. The plotted results imply that most of the ICERs are more effective and less costly in the SE quadrant and more costly in the NE quadrant. If the benefits of the RFA treatment strategy are sustained during a lifetime, the RFA treatment strategy would be the optimal one.
Figure 2. Cost-effectiveness plane of probabilistic base-case analysis of RFA vs. AAD.
Alternative Scenarios
One-way deterministic sensitivity analysis was preformed to assess whether the results were affected by changes in the model assumptions. The results of the analysis are dependant whether the long-term positive effect of RFA is maintained over a lifetime period i.e. patients remaining free from AF. In the absence of data beyond a 12 month follow- up period, we considered annual reversion rates back to uncontrolled AF after RFA of 5 %, 10 % and 15 % annually, in the alternative scenarios ([Table 2]). The results of the analyses were sensitive to reversion back into AF, implying both decreasing QALYs and higher costs for the RFA treatment strategy. Even though the results were sensitive to reversion back into AF, the costs of RFA are only slightly higher compared to the AAD treatment strategy. The benefits (QALYs) of RFA are always higher than that of the AAD strategy in the alternative scenarios. In spite of higher costs and decreasing QALYs for the RFA strategy, [Table 2] is to be interpreted, by combining both costs and benefits in the ICER column. For all values tested, the ICERs were below the so called threshold value for what is considered cost-effective (ranging from dominant to 440 800 SEK (48 310 USD).
Little is known whether the elevated stroke risk in the AF health state is eliminated with the RFA treatment strategy. In the base-case analysis an estimate of 1.5 % was used for both controlled and uncontrolled AF. In the sensitivity analysis the stroke risk was varied in the uncontrolled AF health state. There are more patients in the AF state in the AAD treatment strategy. An elevated stroke risk for the AF state will decrease health outcomes in the AAD treatment strategy. As there are more AF patients in the AAD strat egy, the AAD treatment strategy is disfavoured.
Discussion
Our results, based on a modelling approach, indicate that the RFA treatment strategy is cost-effective. We assessed lifetime costs and effects using relevant randomized controlled trials, different published papers and Swedish register data as input variables for both treatment strategies. Using probabilistic analysis allows uncertain parameters to vary randomly within predefined distributions reflecting the overall level of uncertainty of model parameters.
There are several sources of uncertainty to be considered when interpreting the results associated with methodological aspects and model assumptions. We used a lifetime time horizon to analyse the model. A lifetime time horizon is relevant, as benefits are likely to accrue well beyond the duration of a clinical trial and costs are largely the result of the initial intervention. The main costs for RFA treatment occurs during the first year due to considerably higher intervention costs compared to AAD treatment.
The RFA treatment strategy, when used as a second- line strategy, is the standard procedure in Sweden and in accordance with international guidelines. AAD treatment involves the daily use of medications and is not always well tolerated by the patient. This is also a reason why the RFA might be considered cost-effective compared to AAD. The AAD strategy has often proven non successful and the low efficacy AAD therefore favours the RFA treatment strategy. One could argue that the AAD treatment strategy might be associated with a higher disutility compared to the RFA treatment strategy. We chose to use conservative estimates as not to disfavour the AAD treatment strategy in the base-case scenario.
The two key parameters we found to be most important to examine were the reversion rates of the RFA procedure back to AF and variations in stroke risks in the different AF health states. We found no long-term studies of the sustainability of the RFA treatment strategy i.e. if the QoL benefits are maintained over a lifetime period. We considered different annual reversion rates back to AF in the alternative scenarios, implying both decreasing QALYs and higher costs for the RFA strategy. We used a conservative estimate for the stroke risk in the base-case analysis for the controlled and uncontrolled AF health states. There are more patients in the AF health state in the AAD strategy; therefore the AAD strategy was more sensitive to variations in stroke risk.
Only two studies were found assessing cost-effectiveness of the RFA treatment strategy. 4-5 The US study concludes that RFA treatment is potentially cost-effective for symptomatic AF patients compared to medical management. The benefit of each treatment strategy was driven primarily by stroke risk reduction. A wide range of efficacy rates were explored and there is a risk that the effects of the RFA treatment strategy have been overestimated. Early studies have indicated that RFA is a curative treatment strategy and the US study also refers to the restoration of sinus rhythm. We found no evidence to indicate stroke risk reduction in the controlled AF health state after an RFA procedure. The second study was based on a UK population using a similar model structure to ours.[5] The UK study has considerably higher probabilities for the success rates for both the RFA and AAD treatment strategies compared to our study. The clinical effectiveness input variables in the UK study were based on a meta-analysis. It is unclear whether the high efficacy refers to a mix of first- and second-line treatment.[10] There is also the possibility of bias toward one study with higher efficacy compared to other clinical studies .12 However, both previous cost-effectiveness studies are in line with our results, indicating a cost-effective treatment strategy for RFA if QoL improvement is maintained. Other studies were found comparing catheter ablation treatment with medical management but not in relation to effects. The cost comparison analyses by Khaykin et al. 2- 3 studied RFA versus AAD both as a second-line and first-line treatment strategy. They conclude that the RFA treatment strategy was considered cost-equivalent at 4 years when used as secondline treatment strategy and was cost neutral at 2 years when used as a first-line treatment strategy.
The results were sensitive to whether the longterm QoL benefits are maintained after the initial RFA procedure. Follow-up studies would be important when confirming the sustainability of the RFA treatment strategy. Our modelling approach provides an analytic framework and new parameter estimates can readily be incorporated into the model once more evidence becomes available.
In conclusion, the RFA treatment strategy was associated with reduced cost and an incremental gain in QALYs and was considered a cost-effective treatment strategy compared to the AAD in a lifetime perspective, despite higher initial intervention costs.
Disclosures
None.
References
- 1.Reynolds Matthew W, Fahrbach Kyle, Hauch Ole, Wygant Gail, Estok Rhonda, Cella Catherine, Nalysnyk Luba. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis. Chest. 2004 Dec;126 (6):1938–45. doi: 10.1378/chest.126.6.1938. [DOI] [PubMed] [Google Scholar]
- 2.Khaykin Yaariv, Morillo Carlos A, Skanes Allan C, McCracken Aaron, Humphries Karin, Kerr Charles R. Cost comparison of catheter ablation and medical therapy in atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007 Sep;18 (9):907–13. doi: 10.1111/j.1540-8167.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 3.Khaykin Yaariv, Wang Xiaoyin, Natale Andrea, Wazni Oussama M, Skanes Allan C, Humphries Karin H, Kerr Charles R, Verma Atul, Morillo Carlos A. Cost comparison of ablation versus antiarrhythmic drugs as first-line therapy for atrial fibrillation: an economic evaluation of the RAAFT pilot study. J. Cardiovasc. Electrophysiol. 2009 Jan;20 (1):7–12. doi: 10.1111/j.1540-8167.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 4.Chan Paul S, Vijan Sandeep, Morady Fred, Oral Hakan. Cost-effectiveness of radiofrequency catheter ablation for atrial fibrillation. J. Am. Coll. Cardiol. 2006 Jun 20;47 (12):2513–20. doi: 10.1016/j.jacc.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 5.McKenna C, Palmer S, Rodgers M, Chambers D, Hawkins N, Golder S, Van Hout S, Pepper C, Todd D, Woolacott N. Cost-effectiveness of radiofrequency catheter ablation for the treatment of atrial fibrillation in the United Kingdom. Heart. 2009 Apr;95 (7):542–9. doi: 10.1136/hrt.2008.147165. [DOI] [PubMed] [Google Scholar]
- 6.Swedish National Guidelines for Heart Disease 2008. The National Board of Health and Welfare. Socialstyrelsens riktlinjer för hjärtsjukvård 2008. Beslutsstöd för prioriteringar, Socialstyrelsen. 0;0:0–0. [Google Scholar]
- 7.Pappone C, Oreto G, Lamberti F, Vicedomini G, Loricchio M L, Shpun S, Rillo M, Calabrò M P, Conversano A, Ben-Haim S A, Cappato R, Chierchia S. Catheter ablation of paroxysmal atrial fibrillation using a 3D mapping system. Circulation. 1999 Sep 14;100 (11):1203–8. doi: 10.1161/01.cir.100.11.1203. [DOI] [PubMed] [Google Scholar]
- 8.Khaykin Yaariv. Cost-effectiveness of catheter ablation for atrial fibrillation. Curr. Opin. Cardiol. 2007 Jan;22 (1):11–7. doi: 10.1097/HCO.0b013e32801129d7. [DOI] [PubMed] [Google Scholar]
- 9.Krittayaphong Rungroj, Raungrattanaamporn Ongkarn, Bhuripanyo Kiertijai, Sriratanasathavorn Charn, Pooranawattanakul Sukanya, Punlee Kesaree, Kangkagate Charuwan. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation. J Med Assoc Thai. 2003 May;86 Suppl 1 ():S8–16. [PubMed] [Google Scholar]
- 10.Wazni Oussama M, Marrouche Nassir F, Martin David O, Verma Atul, Bhargava Mandeep, Saliba Walid, Bash Dianna, Schweikert Robert, Brachmann Johannes, Gunther Jens, Gutleben Klaus, Pisano Ennio, Potenza Dominico, Fanelli Raffaele, Raviele Antonio, Themistoclakis Sakis, Rossillo Antonio, Bonso Aldo, Natale Andrea. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005 Jun 01;293 (21):2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 11.Stabile Giuseppe, Bertaglia Emanuele, Senatore Gaetano, De Simone Antonio, Zoppo Franco, Donnici Giovanni, Turco Pietro, Pascotto Pietro, Fazzari Massimo, Vitale Dino Franco. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur. Heart J. 2006 Jan;27 (2):216–21. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 12.Pappone Carlo, Augello Giuseppe, Sala Simone, Gugliotta Filippo, Vicedomini Gabriele, Gulletta Simone, Paglino Gabriele, Mazzone Patrizio, Sora Nicoleta, Greiss Isabelle, Santagostino Andreina, LiVolsi Laura, Pappone Nicola, Radinovic Andrea, Manguso Francesco, Santinelli Vincenzo. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J. Am. Coll. Cardiol. 2006 Dec 05;48 (11):2340–7. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Jaïs Pierre, Cauchemez Bruno, Macle Laurent, Daoud Emile, Khairy Paul, Subbiah Rajesh, Hocini Mélèze, Extramiana Fabrice, Sacher Fréderic, Bordachar Pierre, Klein George, Weerasooriya Rukshen, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008 Dec 09;118 (24):2498–505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 14.Department of Cardiology. Linköping University Hospital, Sweden. 0;0:0–0. [Google Scholar]
- 15.Statistics Sweden. Life Tables. 2008. (accessed 28 Feb 2008). http://www.scb.se/ statistik/BE/BE0101/2006MO2/be0101Livslängdstabeller eng. xls. 0;0:0–0. [Google Scholar]
- 16.Samverkansnämnden Sydöstra sjukvårdsregionen. Prislista år 2005. 2005 (accessed 28 Aug 2008). http://www.lio.se/upload/16047/Prislista. 0;0:0–0. [Google Scholar]
- 17.SBU Alert, Kateterburen ablationsbehandling vid förmaksflimmer, 2005-02, 2005. SBU ALERT-Rapport nr. 0;0:0–0. [Google Scholar]
- 18.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Packer Douglas, Skanes Allan. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005 Mar 08;111 (9):1100–5. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 19.Swedish national catheter ablation register. Swedish. 2007;0:0–0. [Google Scholar]
- 20.Weerasooriya Rukshen, Jaïs Pierre, Le Heuzey Jean-Yves, Scaveé Christophe, Choi Kee-Joon, Macle Laurent, Raybaud Florence, Hocini Mélèze, Shah Dipen C, Lavergne Thomas, Clémenty Jacques, Haïssaguerre Michel. Cost analysis of catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2003 Jan;26 (1 Pt 2):292–4. doi: 10.1046/j.1460-9592.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 21.Davidson T, Levin L-Å. Kostnaden för förmaksflimmer Östergötland, CMT-rapport, 2006:5. Linköpings universitet. 2006;0:0–0. [Google Scholar]
- 22.Ghatnekar Ola, Persson Ulf, Glader Eva-Lotta, Terént Andreas. Cost of stroke in Sweden: an incidence estimate. Int J Technol Assess Health Care. 2004;20 (3):375–80. doi: 10.1017/s0266462304001217. [DOI] [PubMed] [Google Scholar]
- 23.Weerasooriya Rukshen, Jaïs Pierre, Hocini Mélèze, Scavée Christophe, MacLe Laurent, Hsu Li Fern, Sandars Prashanthan, Garrigue Stephane, Clémenty Jacques, Haïssaguerre Michel. Effect of catheter ablation on quality of life of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2005 Jun;2 (6):619–23. doi: 10.1016/j.hrthm.2005.02.1037. [DOI] [PubMed] [Google Scholar]


