Abstract
Purpose
Electrical neurostimulation enhances tear secretion, and can be applied to treatment of dry eye disease. Using a chronic implant, we evaluate the effects of stimulating the anterior ethmoid nerve on the aqueous, lipid, and protein content of secreted tears.
Methods
Neurostimulators were implanted beneath the nasal mucosa in 13 New Zealand white rabbits. Stimulations (2.3–2.8 mA pulses of 75–875 μs in duration repeated at 30–100 Hz for 3 minutes) were performed daily, for 3 weeks to measure changes in tear volume (Schirmer test), osmolarity (TearLab osmometer), lipid (Oil-Red-O staining), and protein (BCA assay, mass spectrometry).
Results
Stimulation of the anterior ethmoid nerve in the frequency range of 30 to 90 Hz increased tear volume by 92% to 133% (P ≤ 0.01). Modulating the treatment with 50% duty cycle (3 seconds of stimulation repeated every 6 seconds) increased tear secretion an additional 23% above continuous stimulation (P ≤ 0.01). Tear secretion returned to baseline levels within 7 minutes after stimulation ended. Tear film osmolarity decreased by 7 mOsmol/L, tear lipid increased by 24% to 36% and protein concentration increased by 48% (P ≤ 0.05). Relative abundance of the lacrimal gland proteins remained the same, while several serum and corneal proteins decreased with stimulation (P ≤ 0.05).
Conclusions
Electrical stimulation of the anterior ethmoid nerve increased aqueous tear volume, reduced tear osmolarity, added lipid, and increased the concentration of normal tear proteins. Human studies with an intranasal stimulator should verify these effects in patients with aqueous- and lipid-deficient forms of dry eye disease.
Keywords: neurostimulation, dry eyes, meibominan gland, lacrimal gland, tear film
The tear film, including the lipid, aqueous, and mucin layers, protects the eye against infectious intruders and harsh environmental conditions, facilitates oxygen and nutrient transport to the avascular cornea, and carries away cellular debris. It also maintains a clear optical path and smooth refractive surface, and prevents damage from the mechanical forces during blinking and eye movements. Decreased aqueous or lipid secretions lead to dry eye and ocular surface disease, where high tear osmolarity and inflammation desensitize corneal sensory nerves, cripple the neural feedback control of ocular glands, and further reduce tear secretion. This inflammatory cycle may result in ocular pain and irritation. In severe cases, corneal opacification and vascularization may lead to a significant loss of vision.
Aqueous deficiency occurs in approximately 50% of patients with dry eye disease,1 and can result from damage to the neural components of the lacrimal functional unit or a diseased lacrimal gland (i.e., Sjogren's syndrome). The aqueous layer includes water, proteins, and electrolytes provided by the lacrimal and accessory glands to sustain epithelial cells exposed to the extracorporeal environment. Parasympathetic nerves from the sphenopalatine ganglion control aqueous tear secretion,2 while sympathetic nerves from the superior cervical ganglion regulate tear proteins and electrolytes.3–5
In 86% of patients with dry eye, an evaporative form of the disease originates from meibomian gland dysfunction.1 Meibomian glands release lipid along the eyelid margin to stabilize the tear film and reduce evaporation. Sensory, parasympathetic, and sympathetic nerves innervate the meibomian acini and regulate meibomian production and possibly secretion.6–8 Contraction of the orbicularis oculi during a blink may also squeeze lipid out of the excretory duct to the ocular surface.8 Other mammals, including rabbits, secrete lipid from the orbital harderian gland.9
Several soluble and membrane-bound glycoproteins compose the mucous layer.10 Goblet cells in the epithelium secrete mucin 5AC, which, together with membrane bound mucins, spread the aqueous tears evenly across the hydrophobic epithelial surface. Mucin secretion by goblet cells in response to corneal damage is mediated via parasympathetic nerves.10–12
Current treatment options for dry eye disease fail to provide adequate relief in the majority of cases. Lubricating eye drops and ointments provide limited relief before nasolacrimal drainage. Topical cyclosporine and lifitegrast inhibit some of the inflammatory aspects of dry eye disease, but only a subset of patients respond to the treatment, and many report side effects of burning or an unpleasant taste.13–16 Punctal plugs block tear drainage to increase the retention of aqueous volume, but they also prevent clearance of inflammatory cytokines and cellular debris from the tear film.17,18 Heat therapy in combination with mechanical massage of the eye lids helps to clear obstructed meibomian glands in patients with reduced lipid outflow.19,20
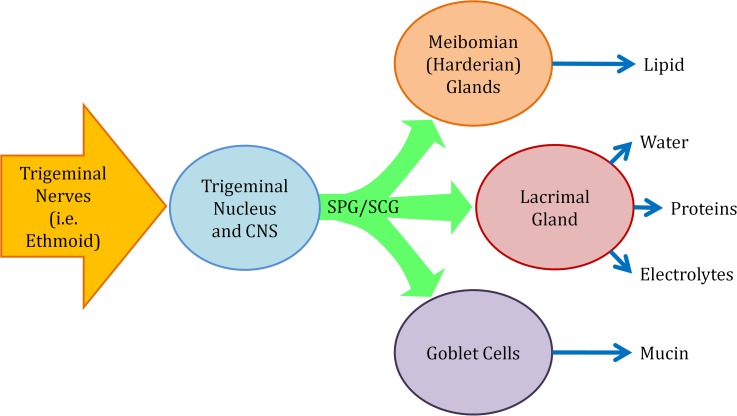
Electrical stimulation of the lacrimal nerve,21 lacrimal gland,22 anterior ethmoid nerve,22,23 and cornea24 increase tear volume. A recent clinical trial demonstrated reduction in dry eye symptoms using intranasal electrical stimulation of the anterior ethmoid nerve at least four times a day.23 Direct stimulation of the lacrimal gland efferent nerves increases tear volume and secretion of the gland proteins and electrolytes, which drain through the nasolacrimal ducts within minutes.25 However, stimulation of the afferent pathways (i.e., anterior ethmoid) could recruit other ocular glands and cells to provide lipid and mucin, thereby enabling a longer lasting relief (Fig. 1).
Figure 1.
Neural control of the tear film. Neural pathways from the sphenopalatine ganglion (SPG) and superior cervical ganglion (SCG) control meibomian glands, lacrimal gland, and goblet cells.2–12 Electrical stimulation of the anterior ethmoid increases aqueous tears22,23 and could also affect production and secretion of lipid and mucin.
We explore the effects of anterior ethmoid nerve stimulation parameters on aqueous and lipid secretion, tear osmolarity, and protein content in rabbits.
Methods
All experimental procedures with animals were conducted in accordance with the Stanford University institutional guidelines and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision research. Thirteen New Zealand white rabbits (male, 3.5–4.5 kg; Western Oregon Rabbit Co., Philomath, OR, USA) were used: six for the pulse frequency and duty cycle modulation studies, and seven for the post stimulation tear volume measurements. To measure meibum, protein, and osmolarity, paired samples (i.e., treatment and no treatment) were collected from animals belonging to either group.
Tear fluid and meibum samples were collected after all the tear volume tests were completed: beginning on day 8 or 14 after implantation, depending on which tear volume tests were performed. Sham or treatment samples were collected in the morning, within an hour of the previous day's sampling.
The purpose of the study was to assess the volume and composition of tears elicited by electrical stimulation rather than measure changes in the baseline over the 3-week follow-up period. For this reason, the tear samples for osmolarity, protein, and meibum were collected immediately following stimulation or sham.
Surgery
Rabbits were anesthetized with ketamine (35–50 mg/kg, intramuscular), xylazine (3 mg/kg, intramuscular), and glycopyrrolate (0.01 mg/kg, intramuscular). Buprenorphine (0.05–0.1 mg/kg, subcutaneous every 6–12 hours) was given for analgesia before and up to 24 hours after surgery.
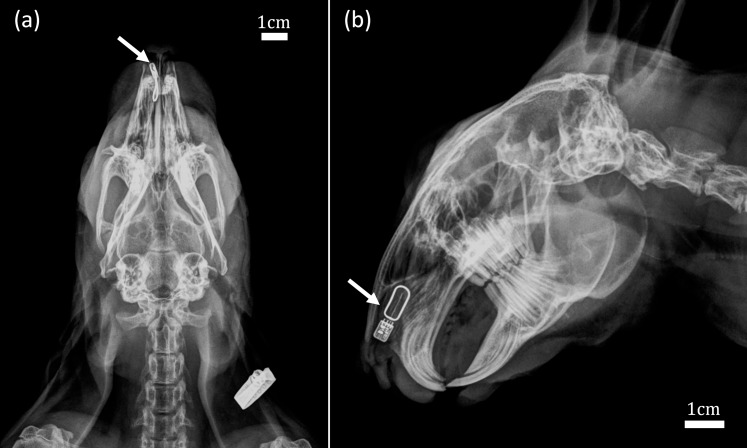
Bilateral incisions opened each nostril so that the distal end of the mucosa could be easily visualized. The neurostimulator was placed unilaterally as follows: a 5- to 10-mm vertical incision was made near the caudal end of the septal mucosa; the mucosa was dissected from the septal cartilage using a Cottle elevator in a subperichondrial plane; the neurostimulator (Oculeve, South San Francisco, CA, USA) (Fig. 2) was carefully inserted with the active electrode facing the mucosa, approximately 3-cm deep and along the dorsal rim of the septum (Fig. 3); finally, a suture was placed through the mucosa and septal cartilage to close the incision and secure the device in place. Figure 3 shows the dorsal-ventral and lateral radiographs of the implanted neurostimulator. Optimal device placement was correlated with a sneeze reflex at the onset of stimulation. Animals without a sneeze reflex still responded to stimulation, but they required longer pulses.
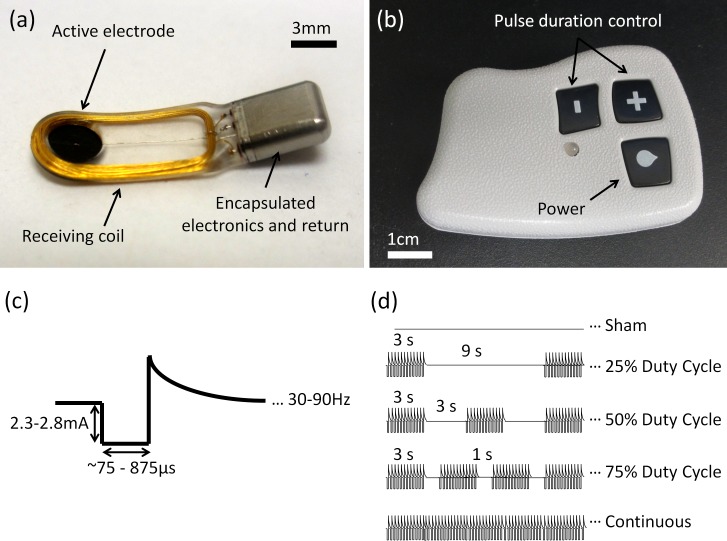
Figure 2.
Implantable neurostimulator and controller. The implantable neurostimulator (a) was powered wirelessly using an external controller (b). The device delivered cathodic-first, biphasic, charge-balanced pulses of 2.3 to 2.8 mA (c). Pulse duration and frequency, as well as the on-off duty cycle (d), were adjusted using the external controller.
Figure 3.
Implanted neurostimulator. Dorsal-ventral (a) and lateral (b) radiographs showing the neurostimulator (white arrows) implanted between the nasal septum and mucosa, with the active electrode approximately 3-cm deep, facing the mucosa.
Measurements of the tear volume and composition were performed during 3-weeks post implantation, after which animals were euthanized with an intravenous injection of beuthanasia-D (120 mg/kg).
Electrical Stimulation
Stimulations began 4 days after implantation by first finding the lowest pulse duration at which the animal gently responded by moving its head at the stimulus onset. In the absence of stimulation, rabbits sat calmly and a slight movement at the stimulus onset was easily detected. Measurements of the tear volume or collection of various tear samples were performed once or twice a day over the next 2 to 3 weeks. On average, animals received approximately 10 total stimulations to set initial pulse durations, measure tear volume, and collect tear samples. Implants delivered cathodic-first, biphasic charge–balanced pulses of electric current with amplitude of 2.3 to 2.8 mA (Fig. 2c). Pulse duration, ranging from 75 to 875 μs, frequency, and on-off duty cycle modulation were set with the external controller (Figs. 2b, 2d). Unless stated otherwise, stimulation was applied continuously at 50 Hz for 3 minutes using the animal-specific pulse durations. As a control, similar procedures were performed with no radio-frequency power transmission from the controller—referred to as “sham stimulation” below.
Tear Volume
Tear volume was measured using the 5-minute Schirmer test. The cornea and conjunctiva were anesthetized with 1 to 2 drops of proparacaine administered 5 minutes prior to the Schirmer test. Three minutes before the Schirmer test, blank Schirmer strips were used to soak any proparacaine remaining in the conjunctival sac. Electrical or sham stimulation began immediately after placing the Schirmer test strip (Eagle Vision, Memphis, TN, USA).
Pulse Frequency Dependence.
In this part of the study, six animals were stimulated with 30, 50, 70, or 90 Hz, or with pulses separated by random intervals within a 10- to 33-ms range, for 3 minutes. The order was randomized and two stimulations were performed per day. To avoid effects of reflex desensitization, the pulse duration began at the level found on the first day (level at which the animal responded to stimulation with slight head movement) and then increased one level (∼90 μs, on average) every 30 seconds for 2 minutes, after which the pulse duration remained constant for the final minute. In four animals, a strong, repeated sneeze reflex occurred before 2 minutes passed. In the case of a strong sneeze reflex, pulse duration was not increased further, but kept constant for the remainder of the treatment.
Poststimulation Tearing.
To assess the longevity of tear secretion, we performed the Schirmer tests during stimulation (3 minutes at 50 Hz) and 7 minutes later, without stimulation.
Duty Cycle Modulation.
To measure the effect of duty cycle on tearing, we used the maximum pulse duration reached for each animal in the frequency-dependence study. Stimulation at 50 Hz was modulated on and off for a total of 20 cycles. The ‘on' time per cycle was 3 seconds and was followed by ‘off' times of 0 (100%), 1 (75%), 3 (50%), and 9 seconds (25% duty cycle), as shown in Figure 2d.
Lipid Content
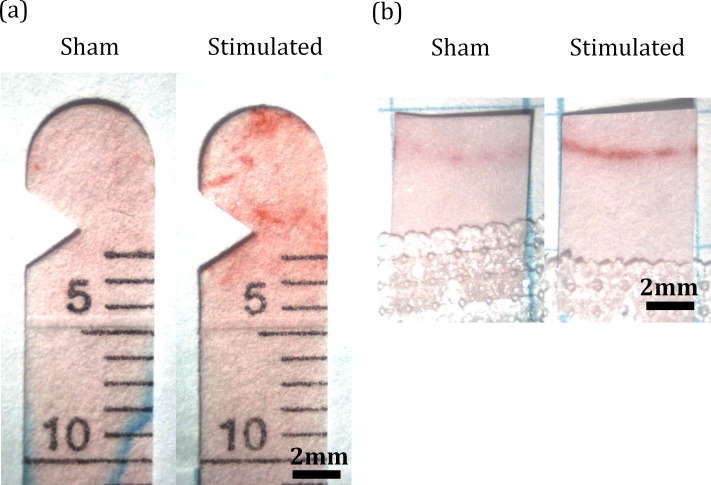
To detect lipid, the Schirmer strips from the tear volume studies were air-dried, stained with Oil-Red-O (Sigma) for 10 minutes, rinsed in 60% isopropyl alcohol for 1 minute and then in distilled water for several minutes (Fig. 4a). The strips were imaged and analyzed in ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). Lipid secretion was quantified as the total amount of red color up to the 3-mm marking on the strips. Secretion of lipid from the meibomian glands was measured by placing a 5 × 7-mm piece of filter paper gently on the inferior lid margin for 30 seconds without or following stimulation (Fig. 4b). These impressions were stained in Oil-Red-O, imaged and analyzed in a similar way.
Figure 4.
Oil-Red-O staining. Filter paper from Schirmer tests (a) and meibomian impressions (b) were stained with Oil-Red-O, imaged, and analyzed using ImageJ software.
Osmolarity
Rabbits would shut their eye or retract their head when the TearLab osmometer (TearLab, San Diego, CA, USA) was brought near their eye. It was less stressful to sample tears (less than 1 μL) with microcapillary tubes (1–5 μL; Drummond Scientific, Broomhall, PA, USA). To limit evaporation, the osmolarity was promptly measured by extruding the sample onto the tip of the TearLab osmometer. The average of three tests per animal at each condition (before and immediately following stimulation) was used for statistical analysis.
Protein Content
Stimulated and nonstimulated tear samples (5 μL) were collected from the same eye, at the same time on consecutive days, using siliconized (Sigmacote; Sigma-Aldrich Corp., St. Louis, MO, USA) 1- to 5-μL capillary tubes (Drummond Scientific). Proparacaine HCl (25 μl, 0.5%; Bausch & Lomb, Rochester, NY, USA) was applied 3 minutes before the sample collection. Samples were diluted in tris-buffered saline with protease inhibitors (cOmplete-EDTA free; Roche, Basel, Switzerland), split into two vials and immediately frozen at −20°C. To one sample, 2% SDS was added to remove lipid interference, and protein concentration was measured using the Pierce micro-BCA assay (Thermo Fisher Scientific, Waltham, MA, USA). Samples from six animals were stored at −80°C and then submitted for proteomic analysis (Vincent Coates Foundation Mass Spectrometry Laboratory, Stanford University, Stanford, CA, USA). Protein samples of 20 μg were analyzed, and the relative amount of the 28 most abundant proteins was calculated by averaging spectral counts. Amino-acid sequences with uncertainty less than 0.01 were included and identified using the uniprot (oryctolagus cuniculus) database. For unidentified proteins, homology with human proteins was inferred using the uniprot basic local alignment search tool (BLAST) with bit errors less than 10−99 and an identity score of 73% or above.26
Statistical Analysis
Data from each animal were averaged together and significance was determined using the paired Student's t-test. Error bars shown are the standard error of the mean (SEM, N = 6 or 7).
Results
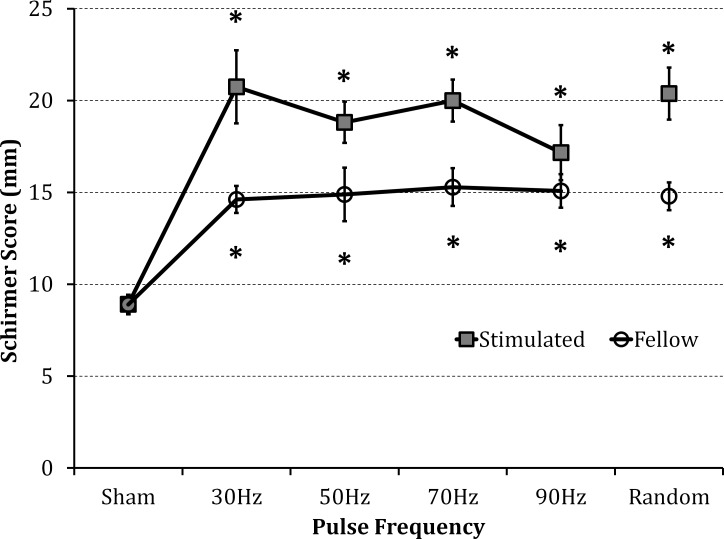
Ethmoid nerve stimulation increased tear volume above the sham (external controller off) by a similar amount for repetition rates of 30 Hz (133 ± 22%), 50 Hz (111 ± 13%), 70 Hz (124 ± 13%), and using pulses with random delays within 10 to 33 ms (128 ± 16%) (Fig. 5). The 90-Hz stimulation improved tear volume the least (92 ± 17% increase).
Figure 5.
The effect of pulse frequency on tear volume. Stimulation at 30, 50, and 70 Hz, and with pulses of random delays within 10 to 33 ms, increased tearing similarly (2.3–2.8 mA, 75–875 μs pulses). At 90 Hz, the tear volume enhancement was lower, but not significantly different from the other frequencies. Tearing significantly increased in the fellow eye, independent of frequency. *P ≤ 0.01 compared with sham (n = 6).
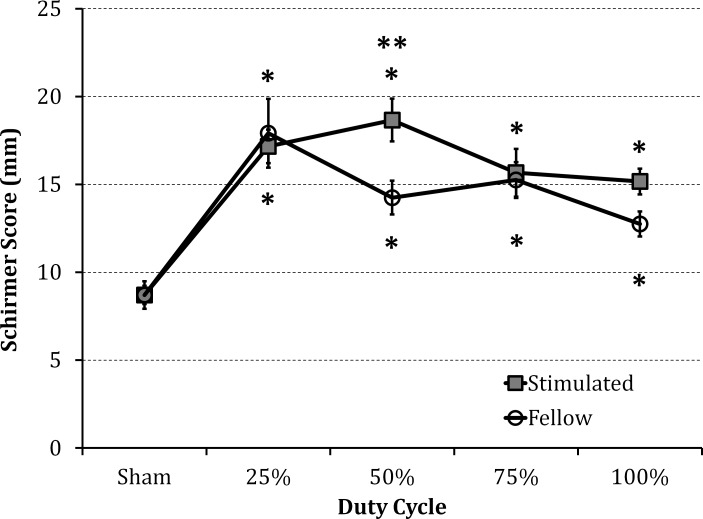
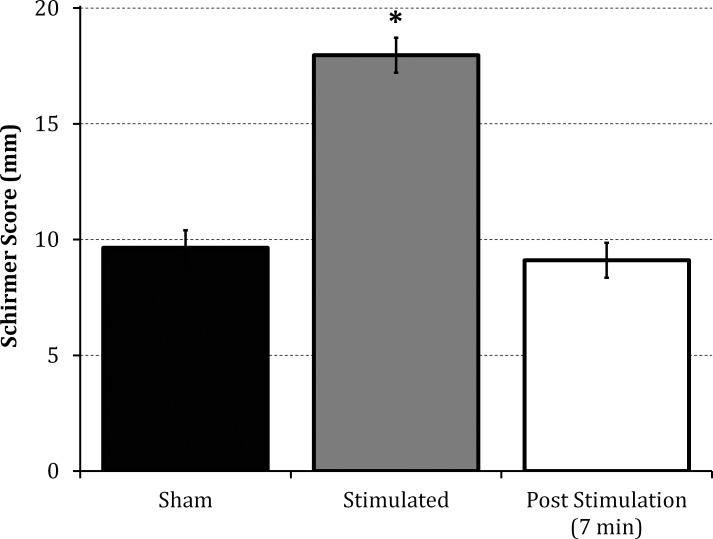
Only the 50% duty cycle (3 seconds of stimulation followed by a 3-second break) significantly improved tearing (23%) above the continuous stimulation (100% duty cycle), as shown in Figure 6. Tear secretion returned to baseline levels within 7 minutes after stimulation (Fig. 7).
Figure 6.
The effect of duty cycle on tear volume. Treatments included twenty bursts of 3 seconds (2.3–2.8 mA, 96–875 μs pulses at 50 Hz) interrupted by various delays (0 for 100%, 1 second for 75%, 3 second for 50%, and 9 second delay for 25%). Tearing increased for all duty cycles, but for the 50% duty cycle (3 seconds ‘ON' followed by 3 seconds ‘OFF') tearing significantly improved compared with continuous (100% duty cycle) stimulation; P ≤ 0.01 compared with sham (*) and 100% (**) (n = 6).
Figure 7.
Extent of post stimulation secretion. Tear volume without stimulation (sham), during stimulation, and 7 minutes after stimulation. Tear secretion returned to baseline in less than 7 minutes; *P < 0.001 compared with sham or post stimulation (n = 7).
Increase in tear volume resulted in small reduction (2.4%) in tear osmolarity: from 297 to 290 mOsmol/L (P < 0.05, N = 7).
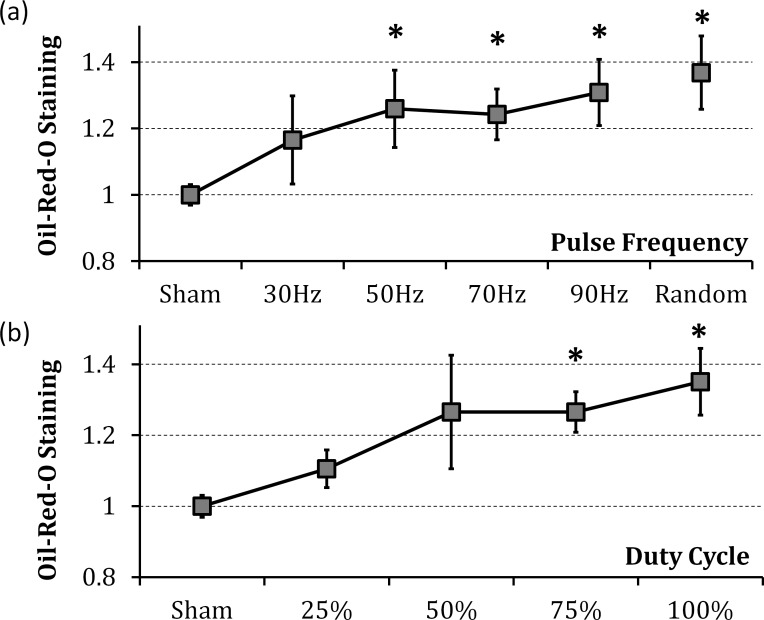
Oil-Red-O staining on Schirmer test strips increased 24% to 36% in response to stimulation at frequencies of 50 Hz and above. Continuous, randomly pulsed stimulation elicited the most significant increase (36%, Fig. 8). Oil-Red-O staining of the meibomian gland secretions also increased with stimulation by 27%, as compared with the sham (P < 0.05, N = 7).
Figure 8.
Lipid secretion in response to stimulation. Lipid staining increased with stimulation (a) frequency and (b) duty cycle; *P ≤ 0.05 (n = 6).
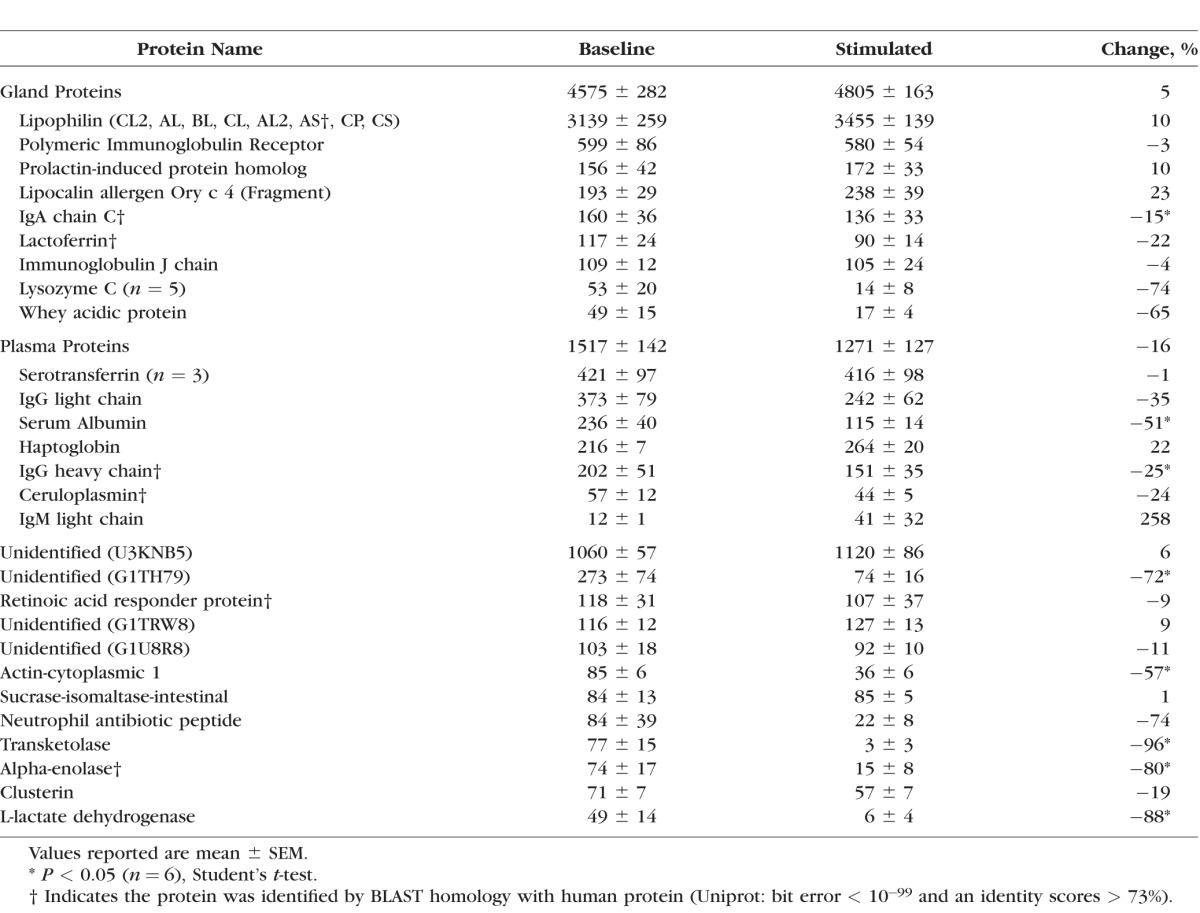
Tear protein concentration increased with stimulation by 48%, from 10.5 to 15.7 mg/mL (P < 0.05, N = 7). Lipophilin was the most abundant protein, and in baseline samples it was responsible for 38% of all spectral counts. Significant changes in relative abundance occurred in only 8 of 28 most prevalent proteins, as listed in the Table. A few proteins secreted by the lacrimal gland slightly increased: lipophilin (10%), prolactin-induced protein (10%), and lipocalin (23%), but not significantly. Immunoglobulin A (IgA) significantly decreased with stimulation (15%). All together, the spectral counts of the gland proteins only slightly increased (5%, not significant). Proteins from the blood plasma generally decreased in relative abundance (16%, not significant), with significant decreases in serum albumin (51%) and IgG heavy chain (35%). Other proteins, including an unidentified protein (uniprot: G1TH79, 72%), cytoplasmic-1 actin (57%), transketolase (96%), alpha-enolase (80%), and L-lactate dehydrogenase (88%), significantly decreased.
Table.
Tear Fluid Proteomics: Average Spectral Counts for the 28 Most Abundant Proteins in 20 μg of Protein From Baseline and Stimulated Tears
Discussion
Electrical stimulation of the anterior ethmoid nerve in rabbits more than doubled the tear volume. Pulse frequencies between 30 and 90 Hz increased Schirmer scores by 92% to 133% (9–13 mm above the baseline of 9 mm). If sustainable, such an increase would reclassify severe and moderate dry eye patients as mild.27 Earlier experiments with direct stimulation of the lacrimal gland in rabbits produced smaller enhancement: 5- to 6-mm increase, or 50% above the healthy baseline.22
Clinical studies of nasal neurostimulation in patients with dry eye disease demonstrated a 7-mm increase in Schirmer score, or 60% above baseline.23 This is 3.5 times more than the improvement from 0.05% cyclosporine ophthalmic drops (Restasis), which provide a 2-mm enhancement after several months of treatment.14
We found that the response to stimulation was largely independent of pulse frequency (although 90-Hz stimulation may be less effective than other frequencies). One possible reason for this could be that our pulse amplitude and duration maximized the outflow of tears and obscured any subtle effects of pulse frequency. A strong sneeze reflex in some animals, observed at the lowest pulse durations, supports this hypothesis. Optimal pulse frequencies could probably be identified in experiments with less current (<2 mA). Waveform modulation with 50% duty cycle increased tear secretion compared with the continuous pulse-train waveform, similar to previous observations with lacrimal gland stimulation.22
Although the implantation and stimulation occurred unilaterally, we recorded an increase in tearing from both eyes, albeit the response in the fellow eye was approximately half that on the stimulated side. For a more balanced response, bilateral implants may be required to stimulate both sides of the nasal mucosa.
Large variation in the animal-specific pulse duration is likely due to variability in the implant location relative to the ethmoid nerve that travels along the dorsal rim of the septum. The device tended to shift toward the middle of the mucosa, where the ethmoid nerve branches and the fibers are smaller (smaller fibers require longer pulse durations to reach stimulation threshold). Because we are targeting a sensory pathway for tear secretion, we selected pulse durations corresponding to the onset of sensation in each animal. Adjustment of the stimulus strength based on sensory feedback is very common in electroneural interfaces.
Stimulated tear secretion reduced tear osmolarity by 7 mOsmol/L, nearly as much as osmolarity increased in dry eye patients compared with healthy subjects (9–24 mOsmol/L).28,29 Consequently, nasal stimulation may not only wash out inflammatory markers and cellular debris, but may also normalize the tear film osmolarity in dry eye patients.
While an increase in aqueous volume benefits approximately half of patients with dry eye syndrome, increased lipid secretion could benefit as much as 85% of dry eye patients.1 We found that stimulation of afferent nerves not only increases aqueous volume but recruits other glands (i.e., meibomian) to stabilize the tear film by increased secretion of lipid. Though not tested directly in this study, stimulation may activate accessory glands (i.e., glands of Zeis, Moll, Wolfring), which also secrete tear fluid and lipid.
We found that lipid secretion increased at higher pulse frequencies and duty cycles. If tear volume continues to decrease above 90 Hz, lipid deficiency could be treated using higher frequency stimulation, without creating an overflow of aqueous tears.
Unlike humans, where meibomian glands provide lipid for the tear film, in rabbits both the harderian and meibomian glands secrete lipids. Lipid increase with stimulation was observed with both the Schirmer strips absorbing the lipid from the tear film and with the filter paper impressions of the meibomian excretory ducts (Fig. 4). The punctuated Oil-Red-O staining on the filter paper illustrates meibomian secretion from the meibomian ducts. Because the orbicularis muscle did not appear to constrict with stimulation, meibum may be secreted by stimulation of a neural pathway. Electromyography could be used to verify the muscle activity in the future studies.
Phospholipids stabilize the tear film by preventing its evaporation, and their deficiency results in dry eye disease.30–32 Our observations in rabbits support the recent clinical evidence that nasal reflex tears have more phospholipids than baseline tears.33 Enhanced lipid secretion by electrical stimulation may stabilize the tear film and reduce aqueous evaporation in patients with lipid deficiency.
Protein concentration in tear fluid increased with electrical stimulation. Earlier studies demonstrated a similar increase in the lacrimal gland protein concentration in pharmacologically induced tears.34 However, others have shown that the total protein concentration decreased with mechanical stimulation of the anterior ethmoid nerve in humans.35,36 This discrepancy may result from different collection techniques or animal species. We administered 25 μL of proparacaine solution before collecting tear samples (to prevent a reflex tear) and probably washed away some protein in the tear film.
Overall, we found that the relative abundance of individual proteins remained the same, except for a decrease in immunoglobulins (IgA chain C, IgG heavy chain), serum albumin, some common corneal proteins (transketolase, cytoplasmic-1 actin, alpha-enolase),37 and an unidentified protein (uniprot: G1TH79) that may be homologous to the IgG Fc-binding domain. Even though these proteins comprise a smaller fraction of the stimulated protein profile, because the total protein concentration increased with stimulation, the concentration of these proteins in the tear fluid remained the same. Furthermore, there does not appear to be a pattern of increase or decrease of specific classes of protein. For example, there was an increase of IgM light chain, but a decrease in IgG light chain. Overall, these results suggest that stimulation of the anterior ethmoid nerve supplies fluid with an increased concentration of normal lacrimal gland proteins.
The lacrimal gland supplies epidermal growth factor (EGF) to the tear film and increases its secretion with reflex tearing.38–40 Therefore, it is likely that electrical stimulation of the anterior ethmoid nerve increases EGF secretion, which could play a role in healing of the corneal surface. However, we did not detect EGF in our proteomic study, possibly due to our technique. Protein quantification methods more sensitive than mass spectrometry (i.e., ELISA) could be used to confirm an EGF increase with electrical stimulation.
Neural pathways control mucin release from goblet cells,12 and a clinical study demonstrated that the number of degranulated goblet cells increases with nasal stimulation for both healthy and dry eye subjects (Gumus K, et al. IOVS 2016;57:ARVO E-Abstract 2864). However, our attempts to image the goblet cells with periodic acid-Schiff and alcian blue mucin staining in conjunctiva biopsy did not reveal a change in the number of goblet cells before and after stimulation (data not shown). We were unable to replicate the staining method of conjunctival impressions with enough clarity to delineate ruptured and intact goblet cells described in human studies (Gumus K, et al. IOVS 2016;57:ARVO E-Abstract 2864). An alternative approach could be to detect mucin in tear fluid using an ELISA.
Dry eye patients using an intranasal stimulator only four times a day showed improved symptoms and ocular surface health.23 Because the tear secretion in our experiments was found to return to baseline levels within several minutes, the other effects from stimulation, such as increased concentration of protein, lipid and possibly mucin, are likely responsible for stabilizing the tear film and improving symptomatic relief and corneal healing.25 Increase in aqueous, lipid and protein in healthy rabbits may not directly translate to human patients with diseased glands, such as Sjögren's syndrome or meibomian gland dysfunction. Therefore, clinical studies should verify the benefits of the nasal stimulation in patients with diseased glands.
Finite-element modeling of the implanted stimulator (not shown) indicates that tissue heating due to stimulation was negligible, on the order of 0.1°C, and maximum current density was below the electroporation threshold reported for retina41 (about 100 mA/cm2). With the larger electrodes used in the clinical nasal stimulator,23 both these values are further reduced.
Although a nonimplanted intranasal stimulator has the benefit of being minimally invasive, an implantable neurostimulator could enable treatment without insertion of the intranasal leads, which may be more acceptable socially, especially for patients leading busy, interactive lives. However, our treatment period was limited to 3 weeks due to erosion of the mucosa near the elevated edges of the implant. The implant had a curved shape because it was initially designed for stimulation of the lacrimal gland. For nasal stimulation, a thinner flat shape would be more appropriate, but we could not redesign and manufacture a different set of implants for these experiments.
In summary, electrical stimulation of the anterior ethmoid nerve increases the aqueous tear volume, reduces osmolarity, adds lipid to stabilize the tear film, and increases the concentration of the naturally secreted proteins. An implanted or an external intranasal neurostimulator could provide on-demand secretion of tears, with enhanced concentration of lipid and protein for patients suffering from both the aqueous- and the lipid-deficient forms of dry eye disease.
Acknowledgments
The authors thank Roopa Dalal, Peter Egbert, Ira Schachar, and Stephen Pflugfelder for their advice regarding the goblet cells and mucin imaging and detection. Tear proteomics were performed by the staff at the Vincent Coates Foundation Mass Spectrometry Laboratory at Stanford University.
Supported by the National Institutes of Health (R01 EY023259; Bethesda, MD, USA).
Disclosure: M. Brinton, None; A.L. Kossler, None; Z.M. Patel, None; J. Loudin, P; M. Franke, Allergan (C), P; C.N. Ta, None; D. Palanker, P
References
- 1. Lemp MA,, Crews LA,, Bron AJ,, Foulks GN,, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012; 31: 472–478. [DOI] [PubMed] [Google Scholar]
- 2. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009; 28, 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dartt DA,, Rose PE,, Dicker DM,, Ronco LV,, Hodges RR. Alpha 1-adrenergic agonist-stimulated protein secretion in rat exorbital lacrimal gland acini. Exp Eye Res. 1994; 58: 423–429. [DOI] [PubMed] [Google Scholar]
- 4. Bromberg BB,, Cripps MM,, Welch MH. Sympathomimetic protein secretion by young and aged lacrimal gland. Curr Eye Res. 1986; 5: 217–223. [DOI] [PubMed] [Google Scholar]
- 5. Ding C,, Walcott B,, Keyser KT. Sympathetic neural control of the mouse lacrimal gland. Invest Ophthalmol Vis Sci. 2003; 44: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 6. LeDoux MS,, Zhou Q,, Murphy RB,, Greene ML,, Ryan P. Parasympathetic innervation of the meibomian glands in rats. Invest Ophthalmol Vis Sci. 2001; 42: 2434–2441. [PubMed] [Google Scholar]
- 7. Kirch W,, Horneber M,, Tamm ER. Characterization of Meibomian gland innervation in the cynomolgus monkey (Macaca fascicularis). Anat Embryol (Berl). 1996; 193: 365–375. [DOI] [PubMed] [Google Scholar]
- 8. Knop E,, Knop N,, Millar T,, Obata H,, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011; 52: 1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Payne AP. The harderian gland: a tercentennial review. J Anat. 1994; 185: 1–49. [PMC free article] [PubMed] [Google Scholar]
- 10. Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004; 78: 173–185. [DOI] [PubMed] [Google Scholar]
- 11. Kessler TL,, Dartt DA. Neural stimulation of conjunctival goblet cell mucous secretion in rats. Adv Exp Med Biol. 1994; 350: 393–398. [DOI] [PubMed] [Google Scholar]
- 12. Kessler TL,, Mercer HJ,, Zieske JD,, McCarthy DM,, Dartt DA. Stimulation of goblet cell mucous secretion by activation of nerves in rat conjunctiva. Curr Eye Res. 1995; 14: 98–992. [DOI] [PubMed] [Google Scholar]
- 13. Barber LD,, Pflugfelder SC,, Tauber J,, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology. 2005; 112: 1790–1794. [DOI] [PubMed] [Google Scholar]
- 14. Sall K, Stevenson OD, Mundorf TK, Reis BL; for the CsA Phase 3 Study Group. . Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000; 107: 631–639. [DOI] [PubMed] [Google Scholar]
- 15. Sheppard JD,, Torkildsen GL,, Lonsdale JD,, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014; 121: 475–483. [DOI] [PubMed] [Google Scholar]
- 16. Tauber J,, Karpecki P,, Latkany R,, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 Study. Ophthalmology. 2015; 122: 2423–2431. [DOI] [PubMed] [Google Scholar]
- 17. Yen MT,, Pflugfelder SC,, Feuer WJ. The effect of punctal occlusion on tear production, tear clearance, and ocular surface sensation in normal subjects. Am J Ophthalmol. 2001; 131: 314–323. [DOI] [PubMed] [Google Scholar]
- 18. Ervin AM,, Wojciechowski R,, Schein O. Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev. 2010: 9:CD006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goto E,, Monden Y,, Takano Y,, et al. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol. 2002; 86: 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olson MC,, Korb DR,, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens. 2003; 29: 96–99. [DOI] [PubMed] [Google Scholar]
- 21. Kossler AL,, Wang J,, Feuer W,, Tse DT. Neurostimulation of the lacrimal nerve for enhanced tear production. Ophthal Plast Reconstr Surg. 2015; 31: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brinton M,, Chung JL,, Kossler A,, et al. Electronic enhancement of tear secretion. J Neural Eng. 2016; 13: 016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedman NJ,, Butron K,, Robledo N,, Loudin J,, Baba SN,, Chayet A. A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease. Clin Ophthalmol. 2016; 10: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yasui T,, Karita K,, Izumi H,, Tamai M. Correlation between vasodilatation and secretion in the lacrimal gland elicited by stimulation of the cornea and facial nerve root of the cat. Invest Ophthalmol Vis Sci. 1997; 38: 2476–2482. [PubMed] [Google Scholar]
- 25. Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998; 17: 565–596. [DOI] [PubMed] [Google Scholar]
- 26. Pearson WR. An introduction to sequence similarity (“homology”) searching. Curr Protoc Bioinformatics. 2013; Chapter 3: Unit 3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. AAO Cornea/External Disease PPP Panel. Dry Eye Syndrome Preferred Practice Patterns - 2013. Available at: https://www.aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp—2013. [Google Scholar]
- 28. Versura P,, Profazio V,, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010; 35: 553–564. [DOI] [PubMed] [Google Scholar]
- 29. Tomlinson A,, Khanal S,, Ramaesh K,, Diaper C,, McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006; 47: 4309–4315. [DOI] [PubMed] [Google Scholar]
- 30. Shine WE,, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998; 116: 849–852. [DOI] [PubMed] [Google Scholar]
- 31. Yamada M,, Mochizuki H,, Kawashima M,, Hata S. Phospholipids and their degrading enzyme in the tears of soft contact lens wearers. Cornea. 2006; 25: S68–S72. [DOI] [PubMed] [Google Scholar]
- 32. Nagyová B,, Tiffany JM. Components responsible for the surface tension of human tears. Curr Eye Res. 1999; 19: 4–11. [DOI] [PubMed] [Google Scholar]
- 33. Rohit A,, Stapleton F,, Brown SH,, Mitchell TW,, Willcox MD. Comparison of tear lipid profile among basal, reflex, and flush tear samples. Optom Vis Sci. 2014; 91: 1391–1395. [DOI] [PubMed] [Google Scholar]
- 34. Dartt DA,, Botelho SY. Protein in rabbit lacrimal gland fluid. Invest Ophthalmol Vis Sci. 1979; 18: 1207–1209. [PubMed] [Google Scholar]
- 35. Fullard RJ,, Snyder C. Protein levels in nonstimulated and stimulated tears of normal human subjects. Invest Ophthalmol Vis Sci. 1990; 31: 1119–1126. [PubMed] [Google Scholar]
- 36. Fullard RJ,, Tucker DL. Changes in human tear protein levels with progressively increasing stimulus. Invest Ophthalmol Vis Sci. 1991; 32: 2290–2301. [PubMed] [Google Scholar]
- 37. Dyrlund TF,, Poulsen ET,, Scavenius C,, et al. Human cornea proteome: identification and quantitation of the proteins of the three main layers including epithelium, stroma, and endothelium. J Proteome Res. 2012; 11: 4231–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson SE,, Lloyd SA,, Kennedy RH. Epidermal growth factor messenger RNA production in human lacrimal gland. Cornea. 1991; 10: 519–524. [DOI] [PubMed] [Google Scholar]
- 39. Chen L,, Hodges RR,, Funaki C,, et al. Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells. Am J Physiol Cell Physiol. 2006; 291: C946–C956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Setten GB. Epidermal growth factor in human tear fluid: increased release but decreased concentrations during reflex tearing. Curr Eye Res. 1990; 9: 79–83. [DOI] [PubMed] [Google Scholar]
- 41. Butterwick A,, Vankov A,, Huie P,, Freyvert Y,, Palanker D. Tissue damage by pulsed electrical stimulation. IEEE Trans Biomed Eng. 2007; 54: 2261–2267. [DOI] [PubMed] [Google Scholar]