Abstract
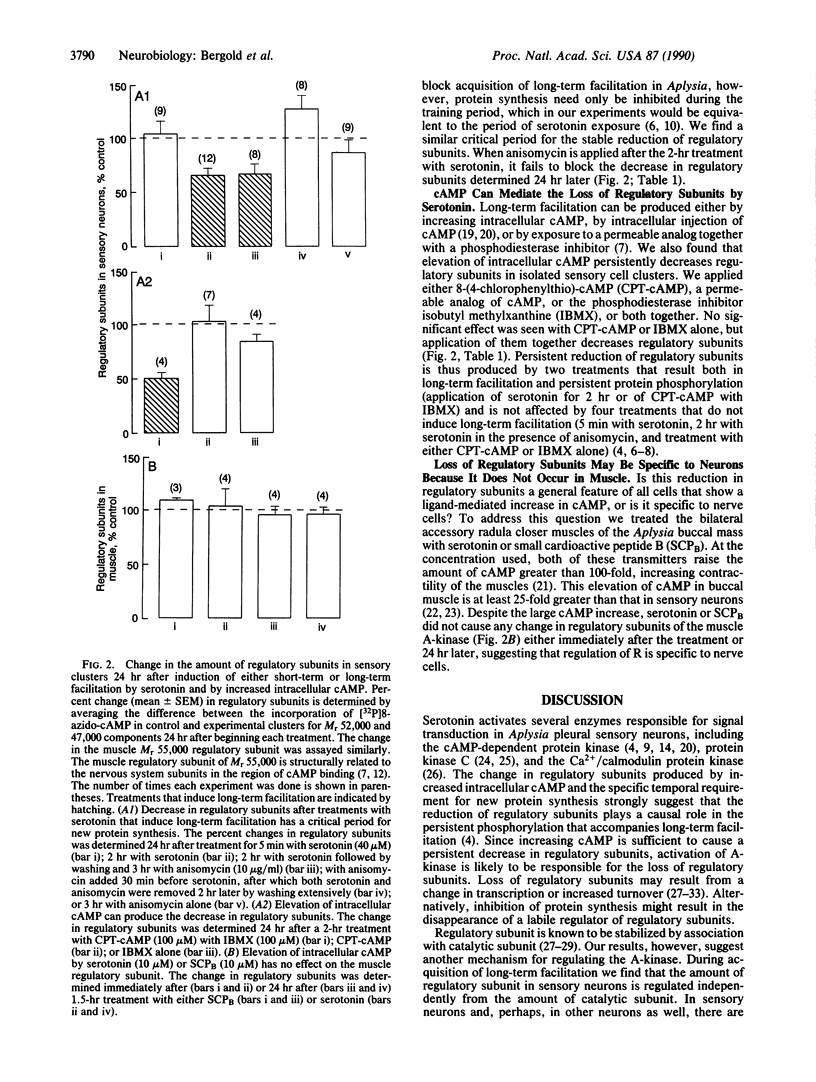
Depending on the number or the length of exposure, application of serotonin can produce either short-term or long-term presynaptic facilitation of Aplysia sensory-to-motor synapses. The cAMP-dependent protein kinase, a heterodimer of two regulatory and two catalytic subunits, has been shown to become stably activated only during long-term facilitation. Both acquisition of long-term facilitation and persistent activation of the kinase is blocked by anisomycin, an effective, reversible, and specific inhibitor of protein synthesis in Aplysia. We report here that 2-hr exposure of pleural sensory cells to serotonin lowers the concentration of regulatory subunits but does not change the concentration of catalytic subunits, as assayed 24 hr later; 5-min exposure to serotonin has no effect on either type of subunit. Increasing intracellular cAMP with a permeable analog of cAMP together with the phosphodiesterase inhibitor isobutyl methylxanthine also decreased regulatory subunits, suggesting that cAMP is the second messenger mediating serotonin action. Anisomycin blocked the loss of regulatory subunits only when applied with serotonin; application after the 2-hr treatment with serotonin had no effect. In the Aplysia accessory radula contractor muscle, prolonged exposure to serotonin or to the peptide transmitter small cardioactive peptide B, both of which produce large increases in intracellular cAMP, does not decrease regulatory subunits. This mechanism of regulating the cAMP-dependent protein kinase therefore may be specific to the nervous system. We conclude that during long-term facilitation, new protein is synthesized in response to the facilitatory stimulus, which changes the ratio of subunits of the cAMP-dependent protein kinase. This alteration in ratio could persistently activate the kinase and produce the persistent phosphorylation seen in long-term facilitated sensory cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzilai A., Kennedy T. E., Sweatt J. D., Kandel E. R. 5-HT modulates protein synthesis and the expression of specific proteins during long-term facilitation in Aplysia sensory neurons. Neuron. 1989 Jun;2(6):1577–1586. doi: 10.1016/0896-6273(89)90046-9. [DOI] [PubMed] [Google Scholar]
- Bernier L., Castellucci V. F., Kandel E. R., Schwartz J. H. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3':5'-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982 Dec;2(12):1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Blumenfeld H., Goelet P., Kandel E. R. Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J Neurobiol. 1989 Jan;20(1):1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- Crow T. Cellular and molecular analysis of associative learning and memory in Hermissenda. Trends Neurosci. 1988 Apr;11(4):136–147. doi: 10.1016/0166-2236(88)90138-5. [DOI] [PubMed] [Google Scholar]
- Dale N., Kandel E. R., Schacher S. Serotonin produces long-term changes in the excitability of Aplysia sensory neurons in culture that depend on new protein synthesis. J Neurosci. 1987 Jul;7(7):2232–2238. doi: 10.1523/JNEUROSCI.07-07-02232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppler C. M., Bayley H., Greenberg S. M., Schwartz J. H. Structural studies on a family of cAMP-binding proteins in the nervous system of Aplysia. J Cell Biol. 1986 Jan;102(1):320–331. doi: 10.1083/jcb.102.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Greenberg S. M., Castellucci V. F., Bayley H., Schwartz J. H. A molecular mechanism for long-term sensitization in Aplysia. Nature. 1987 Sep 3;329(6134):62–65. doi: 10.1038/329062a0. [DOI] [PubMed] [Google Scholar]
- Jahnsen T., Hedin L., Kidd V. J., Beattie W. G., Lohmann S. M., Walter U., Durica J., Schulz T. Z., Schiltz E., Browner M. Molecular cloning, cDNA structure, and regulation of the regulatory subunit of type II cAMP-dependent protein kinase from rat ovarian granulosa cells. J Biol Chem. 1986 Sep 15;261(26):12352–12361. [PubMed] [Google Scholar]
- Kalderon D., Rubin G. M. Isolation and characterization of Drosophila cAMP-dependent protein kinase genes. Genes Dev. 1988 Dec;2(12A):1539–1556. doi: 10.1101/gad.2.12a.1539. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lloyd P. E., Kupfermann I., Weiss K. R. Evidence for parallel actions of a molluscan neuropeptide and serotonin in mediating arousal in Aplysia. Proc Natl Acad Sci U S A. 1984 May;81(9):2934–2937. doi: 10.1073/pnas.81.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Montarolo P. G., Goelet P., Castellucci V. F., Morgan J., Kandel E. R., Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986 Dec 5;234(4781):1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Müller U., Spatz H. C. Ca2(+)-dependent proteolytic modification of the cAMP-dependent protein kinase in Drosophila wild-type and dunce memory mutants. J Neurogenet. 1989 Nov;6(2):95–114. doi: 10.3109/01677068909107104. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Byrne J. H. Membrane responses and changes in cAMP levels in Aplysia sensory neurons produced by serotonin, tryptamine, FMRFamide and small cardioactive peptideB (SCPB). Neurosci Lett. 1985 Apr 9;55(2):113–118. doi: 10.1016/0304-3940(85)90004-7. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Tabata M., Byrne J. H. Stimuli that produce sensitization lead to elevation of cyclic AMP levels in tail sensory neurons of Aplysia. Brain Res. 1986 Apr 16;371(1):190–192. doi: 10.1016/0006-8993(86)90828-0. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Walters E. T., Byrne J. H. Associative conditioning analog selectively increases cAMP levels of tail sensory neurons in Aplysia. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2548–2552. doi: 10.1073/pnas.82.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten A. D., McKnight G. S. Overexpression of the type II regulatory subunit of the cAMP-dependent protein kinase eliminates the type I holoenzyme in mouse cells. J Biol Chem. 1989 Dec 5;264(34):20255–20260. [PubMed] [Google Scholar]
- Palazzolo M., Katz F., Kennedy T. E., Schwartz J. H. Multiple cAMP-binding proteins in Aplysia tissues. J Neurobiol. 1989 Dec;20(8):746–761. doi: 10.1002/neu.480200807. [DOI] [PubMed] [Google Scholar]
- Sacktor T. C., Schwartz J. H. Sensitizing stimuli cause translocation of protein kinase C in Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1990 Mar;87(5):2036–2039. doi: 10.1073/pnas.87.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Serotonin alters the subcellular distribution of a Ca2+/calmodulin-binding protein in neurons of Aplysia. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6708–6712. doi: 10.1073/pnas.80.21.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S., Castellucci V. F., Kandel E. R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988 Jun 17;240(4859):1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Byrne J. H. Intracellular injection of cAMP induces a long-term reduction of neuronal K+ currents. Science. 1988 Jun 17;240(4859):1664–1666. doi: 10.1126/science.2837826. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Castellucci V. F., Kandel E. R. Functioning of identified neurons and synapses in abdominal ganglion of Aplysia in absence of protein synthesis. J Neurophysiol. 1971 Nov;34(6):939–953. doi: 10.1152/jn.1971.34.6.939. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Greenberg S. M. Molecular mechanisms for memory: second-messenger induced modifications of protein kinases in nerve cells. Annu Rev Neurosci. 1987;10:459–476. doi: 10.1146/annurev.ne.10.030187.002331. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Agard D. A. Turnover of regulatory subunit of cyclic AMP-dependent protein kinase in S49 mouse lymphoma cells. Regulation by catalytic subunit and analogs of cyclic AMP. J Biol Chem. 1981 Nov 10;256(21):10731–10734. [PubMed] [Google Scholar]
- Sweatt J. D., Kandel E. R. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989 May 4;339(6219):51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]
- Weiss K. R., Mandelbaum D. E., Schonberg M., Kupfermann I. Modulation of buccal muscle contractility by serotonergic metacerebral cells in Aplysia: evidence for a role of cyclic adenosine monophosphate. J Neurophysiol. 1979 May;42(3):791–803. doi: 10.1152/jn.1979.42.3.791. [DOI] [PubMed] [Google Scholar]