Abstract
Atrial fibrillation (AF) is certainly the most common arrhythmia encountered in clinical practice, reaching epidemic proportions in occidental society. Nowadays, transcatheter ablation using radiofrequency (RF) has become a popular technique in the treatment of drug-resistant AF. Since ectopic beats originating from the pulmonary veins (PVs) have been shown to be the main trigger initiating AF, electrical isolation of these venous structures has become the goal when performing this procedure.
Introduction
Atrial fibrillation (AF) is certainly the most common arrhythmia encountered in clinical practice, reaching epidemic proportions in occidental society. Nowadays, transcatheter ablation using radiofrequency (RF) has become a popular technique in the treatment of drug-resistant AF. Since ectopic beats originating from the pulmonary veins (PVs) have been shown to be the main trigger initiating AF, electrical isolation of these venous structures has become the goal when performing this procedure.[1-2] Traditionally, RF PV isolation uses a point by point technique which makes it difficult to create continuous lines and allows conduction gaps to develop which may facilitate recurrence of the arrhythmia.[3] Furthermore, this approach is depending on the operator’s dexterity and the learning curve is still too long.[4] Finally, even though AF ablation is widely performed, the procedure itself is nowadays associated with a significant amount of complications such as PV stenosis, thrombo-embolism, pericardial tamponade, and the dreaded atrio-oesophageal fistula.[4]
Recently, catheters designed as balloons have radically changed the approach to PV isolation. In fact not only do these tools offer fast and effective electrical isolation in comparison to the focal “point by point” approach but by their own design they are able to achieve complete circular lesions around the PVs with virtually a single application.[5] The novel Cryoballoon (Arctic Front, Medtronic , USA) using cryothermal energy ablation has proven very effective in achieving PV isolation.[5] Additionally, the latter might also lead to a lower rate of adverse events. In fact, cryothermal energy has been proposed as a safe and reliable energy source having been demonstrated to be less thrombogenic and associated with a lower risk of PV stenosis than RF energy.[6,7,8] Although mainly, if not only, designed to approach paroxysmal AF, cryoballoon ablation might lead more and more electrophysiology laboratories around the world to start performing AF ablation, due to its relatively simpler and more straightforward technique to achieve PV isolation.
The Cryoballoon
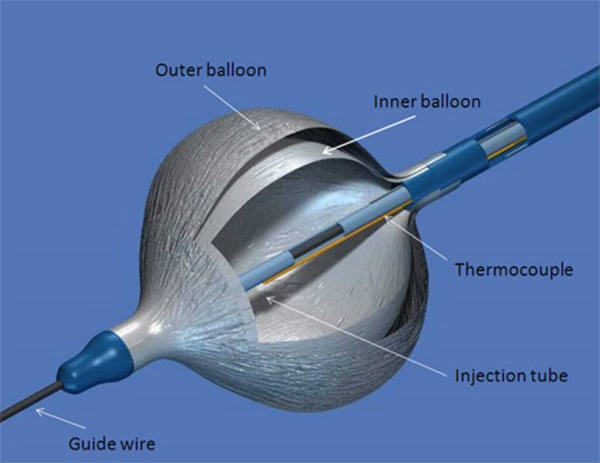
The cryoballoon catheter [[Fig 1]] conversely to the focal tip cryocatheter, represents a much more appealing system to perform PV isolation as it can achieve the goal of electrical isolation with potentially one single application. Mainly designed to approach drug resistant paroxysmal AF, the cryoballoon therapy apparatus includes the Arctic Front® doubled-walled cryoballoon catheter [[Fig 2]], which inflates and fills with coolant (N2O) to ablate between the pulmonary veins and the left atrium; the FlexCath® Steerable Sheath, which helps deliver and position the cryocatheter in the LA and the CryoConsole®, which houses the coolant, electrical and mechanical components that run the catheter during a cryotherapy procedure. The refrigerant N2O is delivered into the balloon where it undergoes a liquid-to-gas phase change, resulting in cooling temperature to approximately -80°C. As other cryothermal tools the endocardial lesion created by the cryoballoon occurs in three main phases. The first phase is characterized by the immediate effect of freezing on the cardiac tissue by the formation of ice crystals in both the extracellular and intracellular space. When reaching temperatures lower than -20/-25°C ice crystal formation occurs in the extracellular space. Further cryoablation reaching temperatures lower than -40°C leads to cellular damage by the means of intracellular ice crystal formation. This phenomenon provokes destruction of cytoplasmic organelles and architectural subversion of the plasmatic membrane. Irreversible cellular death will occur when temperatures reach values lower than -70°C. When cryothermal energy is stopped, a rapid vasodilatation and a state of augmented vascular permeability lead to hemorrage and edema with further tissue injury. This phase is also characterized by microthrombus formation in the tissue, leading to further damage. During the latter, tissue reparation occurs by collagen deposits, fatty infiltrations and neovascularisation. The fibrotic scar will first develop from the peripheric areas of the lesion eventually reaching the centre.[9,10]
Figure 1. The cryoballoon catheter.
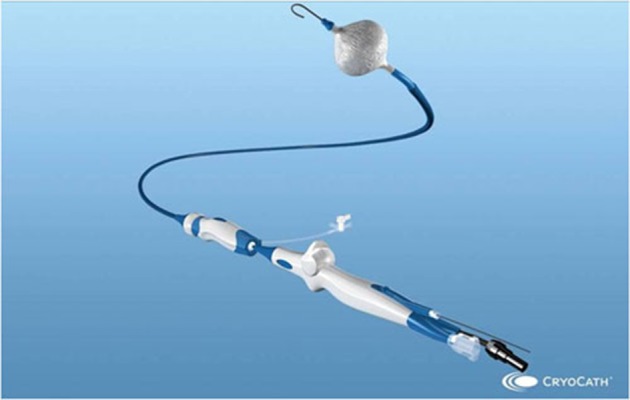
Figure 2. Detailed view of the double-walled balloon principal components.
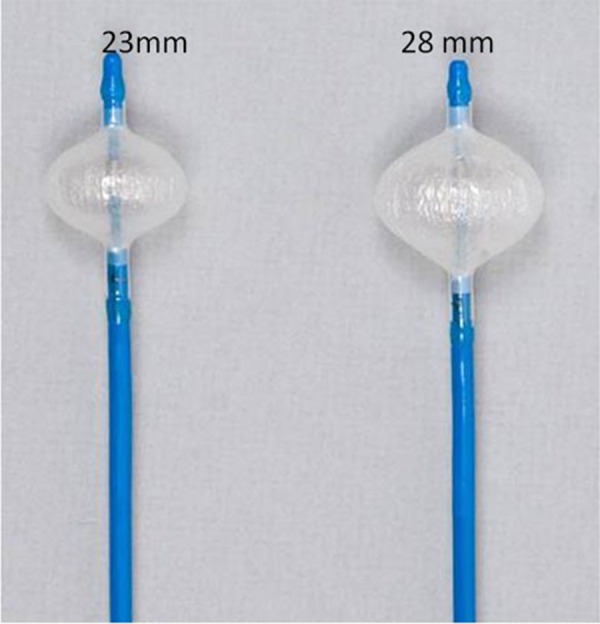
The cryoballoon catheter has a central lumen, which can be used for different scopes such as the insertion of the guide wire, the injection of contrast medium (diluted 1:1 ratio with 0.9% saline) for PV angiograms and eventually the insertion of a circular mapping catheter to evaluate PV isolation during cryothermal energy application.[8] Navigation of the balloon inside the LA is simplified using the ‘over-the-wire’ technique in conjunction with the steerable sheath (12 F, FlexCath, Medtronic CryocathTM, USA). In addition, the cryoballoon itself is steerable thanks to a pull wire mechanism integrated in the handle of the catheter itself. Today two different balloon diameters are available (23 and 28 mm) [[fig 3]].
Figure 3. The 2 cryoballoon diameters: 23 and 28 mm.
Ablation Procedure
Usually a single transseptal puncture is performed using the right femoral venous approach. In some centers, a double transseptal puncture is achieved in order to have simultaneous electrical information during the freezing phase. After gaining left atrial access, a 70 UI/Kg heparin organelles bolus is given and afterwards repeated to maintain the activation clotting time between 250 and 300 s. Selective PV angiograms are then performed. Immediately after, a 0,032’’ 260 cm long guide-wire (Emerald, Cordis, Johnson & Johnson, Diamond Bar, California, USA) is advanced in the left superior PV (LSPV) and a steerable 15 F over-the-wire sheath (FlexCath, Cryocath, Medtronic, USA) is positioned in the LA. A circular mapping catheter (Lasso, Biosense Webster, Inc., Diamond Bar CA, USA) is then advanced in each PV ostium to obtain baseline electrical information. After withdrawing the mapping catheter, a 23 or 28 mm double walled cryoballoon (Arctic Front, Medtronic, USA) is advanced over the wire up to the LA, inflated and positioned in the PV ostium of each vein. Optimal vessel occlusion is considered to have been achieved when selective contrast injection show total contrast retention with no flow to the atrium. For each vein, cryoablation usually consists of a minimum of 2 applications lasting 5 minutes each. Whenever possible, the operator tries to engage with the guide-wire 2 different branches of the same vein and to orient the balloon differently, in the attempt of covering a wider ostial surface. However, if successful occlusion could only be obtained in one branch, both applications are delivered by leaving the guide-wire in the same branch. Usually, the left superior PV (LSPV) [[Fig 4A]] is treated first, followed by the left inferior PV (LIPV) [[Fig 4B]], right superior PV (RSPV) [[Fig 4C]] and right inferior PV (RIPV) [[Fig 4D]]. The degree of balloon occlusion obtained by injection of 50% diluted contrast medium into the PV is evaluated using a semiquantitative grading, as previously described5: grade 4 = excellent (full retention of contrast medium without visible outflow) to grade 1 = very poor (immediate rapid outflow from the PV). In order to avoid phrenic nerve palsy, a complication observed during cryoballoon ablation in the right pulmonary veins, a quadripolar catheter is inserted in the superior vena cava in order to pace phrenic nerve with a 1000 ms cycle and a 12 mA pulse output. Diaphragmatic contraction during pacing will indicate correct phrenic capture. If this contraction suddenly stops or weakens, the cryoablation is immediately interrupted. The reason of pacing at such a slow rate is to prevent catheter displacement in the early phases of application due to diaphragmatic contraction. After ablation in all PVs, the circular mapping catheter (Lasso, Biosense Webster, Inc., Diamond Bar CA, USA) is then placed in each ostium to evaluate isolation [[Fig 5]]. In case of persistence of PV potentials extra cryoballoon applications were performed until complete PVI. In case of failure of the cryoballoon, a focal radiofrequency or cryothermal energy catheter is used to complete PV isolation.
Figure 4A. Left superior PV.
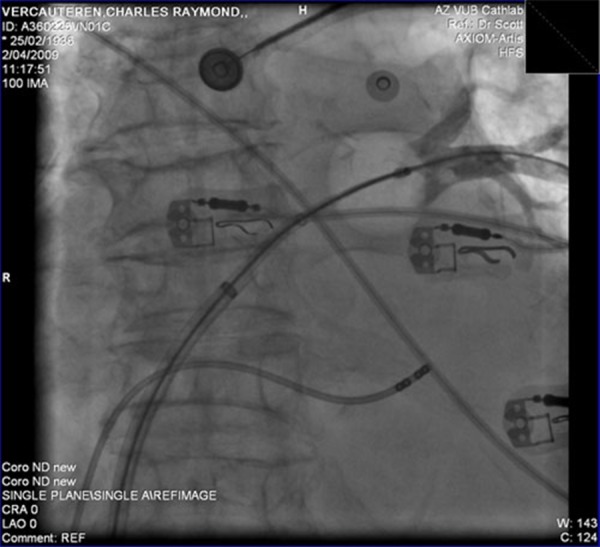
Figure 4B. Left inferior PV.
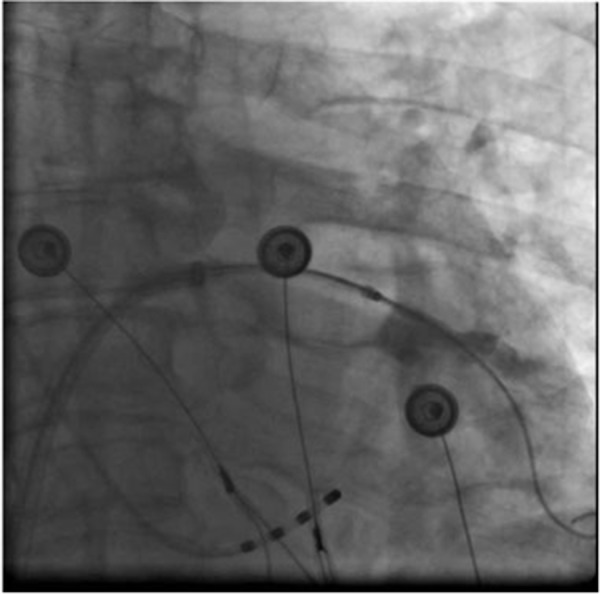
Figure 4C. Right upper PV.
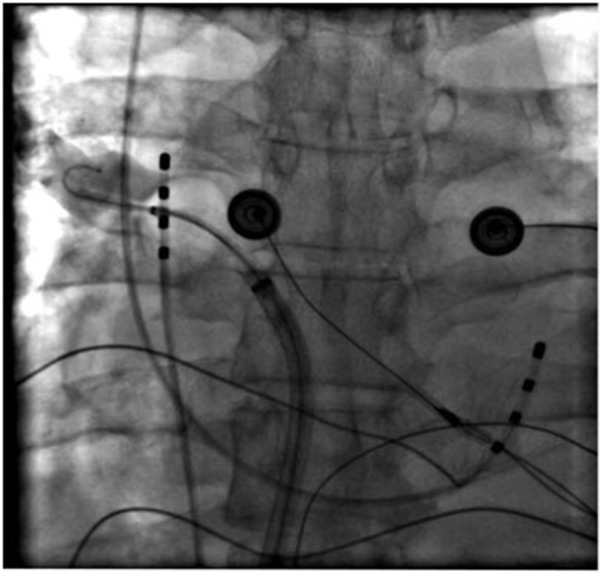
Figure 4D. Right inferior PV.
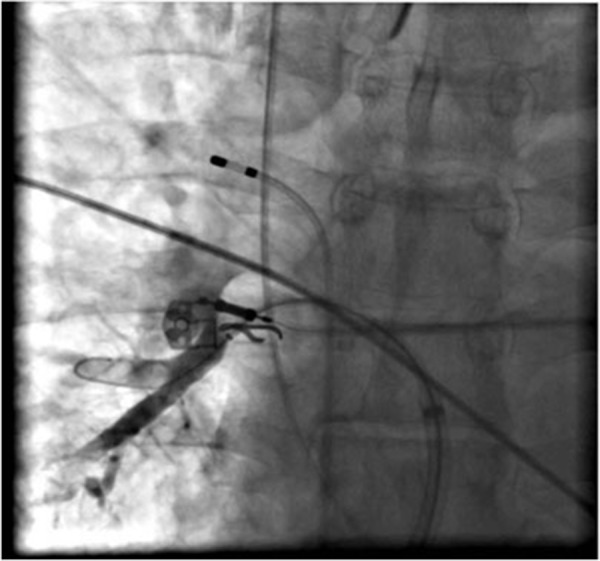
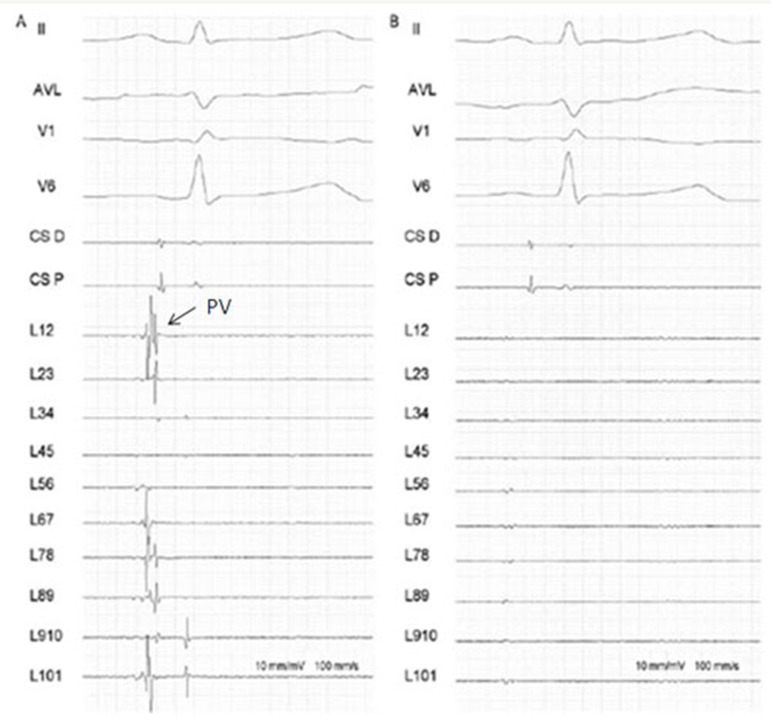
Figure 5. Pulmonary Vein isolation following cryoballoon ablation. Panel A: electrogram recordings from the Lasso circular mapping catheter (L1,2 to L 9,10) from a PV ostium before cryoballoon ablation. Notice the sharp pulmonary vein potentials (PVs).
In clinical practice isolating the inferior pulmonary veins can be particularly challenging in comparison to the superior ones.[11] Specifically, occluding and isolating the RIPV is often quite tricky due to the proximity of this vein to the site of transatrial access and its posterior orientation, especially in patients with relatively small left atria (LA diameter < 35 mm). Various ablation techniques aiming at overcoming these anatomical obstacles have been reported : the “hockey stick” technique, the “pull-down” technique and the “big loop” technique for the RIPV. The “hockey stick” technique is applied in patients with an early branching inferior PV and consists of a maximal bending of the sheath towards the superior-posterior LA, allowing the cryoballoon to be pushed and to adhere to inferior part of the PV ostium. The right inferior PV can be addressed using 2 different approaches: the “pull-down” technique consists of freezing at the ostium of the PV in starting, regardless of the incomplete occlusion and when the latter adheres to the interpulmonary carina (typically at -20°C) to pull down all the cryoballoon apparatus, in order to close the inferior gap. Finally the “big loop” technique, is achieved by maximally bending and directing the balloon towards the lateral posterior LA, allowing the guide wire to advance along the posterior mitral annulus until the ostium of the right inferior PV is reached.
Pulmonary Vein Isolation
According to recent publications PV isolation can be demonstrated in 70% to 98% of veins following cryoballoon ablation [[Table 1]]. If this goal cannot be achieved, a focal tip catheter will be used to close the conduction gaps and obtain complete isolation. Successful PV isolation with the cryoballoon is highly dependent on a perfect contact to the atrial wall at the level of the PV ostia. Indeed, lack of blood back-flow allows the cryoballoon to obtain lower temperatures with the result of achieving more deeper lesions.[12] Complete contact is usually easier to achieve using with the smaller 23 mm diameter cryoballoon with the risk, however, to cause a more distal PV lesion and to be associated with a potentially higher risk of right phrenic nerve palsy when dealing with the RSPV.[5] Anatomical features are the main reason for the inability of this technique to obtain complete PV isolation; Van Belle et al. reported that oval shaped PV ostia or veins inserting into the LA with a sharp angulation are the most difficult to occlude.[12] However like in all other techniques, the learning curve and the level of the operator’s experience will condition the efficacy of cryoballoon ablation. Our group also tested the degree of PV isolation obtained by the cryoballoon technology, administering intravenous boluses of adenosine 15 minutes following acute PV isolation.[13] Recent studies have demonstrated that intravenous injection of adenosine after AF ablation can induce transient reconduction in isolated PVs, due to incomplete lesions.[14-15] In our series of patients, transient PV-LA reconnection occurred only in 4, 6% PVs, a result somewhat surprising seen that following radiofrequency AF ablation, adenosine caused reappearance of atriovenous conduction in roughly 30% of veins. A hypothetical explanation of these findings is that cryoballoon ablation might achieve more homogeneous lesions than traditional RF point by point circumferential PV isolation.
Table 1. Long term success rates (follow-up = 12 months) following cryoballoon ablation.
* one month blanking period
| Authors | |
|---|---|
| Neumann 2008 | 74% AF free (Acute PV isolation: 97%) |
| STOP AF Trial | 69,9% AF free (Acute PV isolation: 98,2%) |
| Linhart 2010 | 50% AF free (Acute PV isolation: 70%)* |
| Kunhe 2010 | 71% AF free (Acute PV isolation: 84%) |
The mid-term permanency of PV isolation using a cryoballoon has also been investigated. Indeed, Fürnkranz and colleagues[16] analyzed the sites of PV reconnection in 26 patients undergoing repeat ablation for recurrent symptomatic AF or atrial tachycardia after initial cryoballoon ablation for paroxysmal AF. They found a high rate of PV reconnection (around 50%) at a median follow-up of about 6 months. Reconnection mostly occurred in the inferior segments of the inferior PVs and on the LAA-LSPV ridge. The authors also observed that lower temperatures were achieved during cryoballoon ablation of the superior PVs compared to the inferior PVs. They postulated, in accordance to the previous findings described by others.[12] That a straighter alignment of the balloon with the superior PVs as compared with the inferior ones provides more contact force on the LA wall and lower temperatures. Accordingly to these findings, Ahmed and coworkers evidenced at 8-12 weeks months follow up that 98% of PVs previously ablated with the cryoablation therapy were still isolated and that reconnections occurred mainly in the LIPV and in the inferior aspects of each vein.[17]
Both groups of authors underlined the importance of full PV occlusion during cryoablation. To this aim, imaging-guided positioning of the cryoballoon in the antrum might improve success. Intracardiac echocardiography[18] on one hand, and transesophageal echocardiography[19] on the other hand, have demonstrated to be helpful during the manipulation of the cryoballoon in achieving PV occlusion by allowing a better identification of the PV-LA junction. Furthermore, Color Doppler documented PV occlusion during cryoballoon ablation has demonstrated to be able to predict effective electrical isolation. Pressure recording at the distal tip of the cryoballoon catheter have also been used to confirm PV occlusion with reliable results.[20]
Follow-Up
Unfortunately, data on follow-up of patients having undergone AF ablation is extremely heterogeneous and varies immensely from center to center. This is mainly due to the fact that this process has not yet been standardized. Thus the success rates following AF ablation on the long-term should be interpreted cautiously. However, data available in the literature reports promising results [[Table 1]]. Klein et al.[21] were among the first to report the success rate of cryoballoon ablation at 6 months. In their study up to 86% of patients were AF free on a short term follow-up. Chun et al.[11] demonstrated a rate of AF recurrence of 30% at about 1 year of follow up after cryoballoon ablation, applying a blanking period of 3 months, while Neumann obtained at 1 year a success rate of 74%. Kühne et al.[22] reported a success rate of 71% at 1 year follow up too. Linhart and colleagues[23] found a success rate of about 50% at 1 year of follow-up, a value significantly inferior to the expectations: they explained this finding with the fact that the blanking period in their study was of only 4 weeks.
Adverse Events
Cryoablation is historically known as a safe ablative energy source. In May 2010, the STOP AF (Sustained Treatment of Paroxysmal Atrial Fibrillation) clinical trial data was presented by Packer at the American College of Cardiology 2010.[24] The aim of the trial was to compare cryoablation of AF with antiarrythmic drugs in terms of success rates and safety, by randomly assigning patients to either a cryoablation procedure or anti-arrhythmic drug therapy. Acute procedural success was defined as demonstration of electrical isolation in 3 or more PVs at the conclusion of the first cryoablation procedure. Using this definition, acute procedural success was achieved in 98.2 percent (160/163) of cryoablation patients. Chronic treatment failure was defined as the occurrence of detectable AF after a 90-day blanking period and/or the occurrence of an AF intervention or the use of a non-protocol AF drug anytime during the 12-month follow up. At 12 months, approximately 70 percent of cryoablation patients were free of AF compared to 7.3 percent of anti-arrhythmic drug patients. The 2 primary safety outcome measures were Cryoablation Procedure Events (CPEs) in cryoablation subjects and Major Atrial Fibrillation Events (MAFEs) in both study groups. The data indicate that both primary safety outcomes were met. Cryoablation patients with one or more CPE(s) was 3.1 percent, which was significantly less than the prespecified 14.8 percent (p< 0.001). No treatment-related deaths or atrioesophageal fistulas were reported.
In addition, 96.9 percent of cryoablation patients were free from MAFE(s), compared to 91.5 percent of anti-arrhythmic drug patients (p < 0.001, non-inferiority). Phrenic nerve palsy, a recognized adverse event with this technology, occurred after 11.2 percent of all cryoablation procedures in 228 patients. At 12 months, 98.2 % of patients that underwent cryoablation were free of any effects related to phrenic nerve injury. An apparent discordance in the list of possible procedural complications has been found regarding the incidence of PV stenosis. In fact, the rate of PV stenosis was 3.1% versus a complete absence of this complication in the already published reports. This difference might be explained by the fact that in the STOP AF trial the PV stenosis was intensively investigated during the follow-up and that the definition of PV stenosis was quite strict.
The results emerging from this trial in terms of rate of procedural complications are concordant to the results reported in previous studies. For example, In a recent publication,[23] comparing cryoballoon technology with RF ablation in a group of patients affected by paroxysmal AF, right phrenic nerve palsy occurred in about 11% of patients undergoing cryoballoon ablation and all these cases resolved spontaneously. Interestingly, all of them were associated with the use of a 23 mm cryoballoon. This could be explained by the fact that the smaller cryoballoon might achieve a more distal lesion occurring closer to the phrenic nerve’s anatomical course. More interestingly, atrio-oesophageal fistula has never been described among procedural complications of cryoballoon ablation. Esophageal lesions following cryoballoon ablation is another matter of debate. Whereas Fürnkranz et al.[25] demonstrated that PV isolation with the cryoballoon was not associated with thermal esophageal lesions in any of 38 studied patients, Ahmed et al.[26] on the contrary showed appearance of transient esophageal ulcerations in 17% of the patients following this procedure. Interestingly, a correlation with temperature changes inside the esophageal lumen was found by the authors.
Pericardial effusion is another potentially life threatening complication observed associated toAF ablation. Our group[27] compared the incidence and clinical outcome of pericardial effusion following cryoballoon ablation and RF ablation. A total of 133 consecutive patients with paroxysmal AF were included in this study. Pericardial effusion was detected in 14.2% of the 133 patients. Sixteen patients presented mild effusion, one moderate effusion, and 2 pericardial tamponades(related to transseptal puncture). Interestingly, there was no significant difference in the incidence of pericardial effusion between the cryoballoon and the RF group (11 vs. 16%). Fortunately, pericardial effusion was mostly mild and asymptomatic, with benign clinical outcome not requiring additional hospitalization days.
Conclusions
Data in the literature convincingly indicate that Cryoballoon ablation is a very promising technique to treat AF. However, although cryoballoon technology potentially represents a very quick, simple and safe approach to the treatment of paroxysmal atrial fibrillation, we still are relatively far from that reality. Unfortunately, the cryoballoons currently available do not always adapt themselves well to the anatomical variants and do not systematically isolate the pulmonary veins in one application (sometimes requiring “touch up” lesions with a traditional ablation catheter). Also, there continues to be the significant risk of phrenic nerve paralysis. Ideally, a softer and more compliant balloon which would snugly fit into most PVs might lead to a more effective procedure. Future technological improvements will certainly overcome some of the abovementioned issues and might lead a larger number of electrophysiologists to adopt such a technique. However, although very promising, a direct comparison with the “traditional” point by point isolation is needed in order to assess the real efficacy on this technology. Large randomized trials such as the ongoing Freeze AF trial[28] will certainly provide valuable answers to this important issue.
Disclosures
None.
References
- 1.Natale Andrea, Raviele Antonio, Arentz Thomas, Calkins Hugh, Chen Shih-Ann, Haïssaguerre Michel, Hindricks Gerhard, Ho Yen, Kuck Karl Heinz, Marchlinski Francis, Napolitano Carlo, Packer Douglas, Pappone Carlo, Prystowsky Eric N, Schilling Richard, Shah Dipen, Themistoclakis Sakis, Verma Atul. Venice Chart international consensus document on atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2007 May;18 (5):560–80. doi: 10.1111/j.1540-8167.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang Feifan, Bänsch Dietmar, Ernst Sabine, Schaumann Anselm, Hachiya Hitoshi, Chen Minglong, Chun Julian, Falk Peter, Khanedani Afsaneh, Antz Matthias, Kuck Karl-Heinz. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004 Oct 12;110 (15):2090–6. doi: 10.1161/01.CIR.0000144459.37455.EE. [DOI] [PubMed] [Google Scholar]
- 4.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan, Ambrogi Federico, Biganzoli Elia. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 5.Neumann Thomas, Vogt Jürgen, Schumacher Burghard, Dorszewski Anja, Kuniss Malte, Neuser Hans, Kurzidim Klaus, Berkowitsch Alexander, Koller Marcus, Heintze Johannes, Scholz Ursula, Wetzel Ulrike, Schneider Michael A E, Horstkotte Dieter, Hamm Christian W, Pitschner Heinz-Friedrich. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J. Am. Coll. Cardiol. 2008 Jul 22;52 (4):273–8. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Khairy Paul, Chauvet Patrick, Lehmann John, Lambert Jean, Macle Laurent, Tanguay Jean-François, Sirois Martin G, Santoianni Domenic, Dubuc Marc. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003 Apr 22;107 (15):2045–50. doi: 10.1161/01.CIR.0000058706.82623.A1. [DOI] [PubMed] [Google Scholar]
- 7.Tse Hung-Fat, Reek Sven, Timmermans Carl, Lee Kathy Lai-Fun, Geller J Christoph, Rodriguez Luz-Maria, Ghaye Benoit, Ayers Gregory M, Crijns Harry J G M, Klein Helmut U, Lau Chu-Pak. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J. Am. Coll. Cardiol. 2003 Aug 20;42 (4):752–8. doi: 10.1016/s0735-1097(03)00788-5. [DOI] [PubMed] [Google Scholar]
- 8.Tang Min, Kriatselis Charalampos, Nedios Sotiris, Ye Gang, Roser Mathias, Fleck Eckard, Gerds-Li Jin-Hong. A novel cryoballoon technique for mapping and isolating pulmonary veins: a feasibility and efficacy study. J. Cardiovasc. Electrophysiol. 2010 Jun 01;21 (6):626–31. doi: 10.1111/j.1540-8167.2009.01668.x. [DOI] [PubMed] [Google Scholar]
- 9.Atienza Felipe, Almendral Jesús, Sánchez-Quintana Damián, Zaballos Matilde, Murillo Margarita, Jimeno Concepción, Parra Verónica, Fernández-Avilés Francisco. Cryoablation time-dependent dose-response effect at minimal temperatures (-80 degrees C): an experimental study. Europace. 2009 Nov;11 (11):1538–45. doi: 10.1093/europace/eup310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubuc M, Roy D, Thibault B, Ducharme A, Tardif J C, Villemaire C, Leung T K, Talajic M. Transvenous catheter ice mapping and cryoablation of the atrioventricular node in dogs. Pacing Clin Electrophysiol. 1999 Oct;22 (10):1488–98. doi: 10.1111/j.1540-8159.1999.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 11.Chun Kyoung-Ryul Julian, Schmidt Boris, Metzner Andreas, Tilz Roland, Zerm Thomas, Köster Ilka, Fürnkranz Alexander, Koektuerk Buelent, Konstantinidou Melanie, Antz Matthias, Ouyang Feifan, Kuck Karl Heinz. The 'single big cryoballoon' technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur. Heart J. 2009 Mar;30 (6):699–709. doi: 10.1093/eurheartj/ehn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Belle Yves, Janse Petter, Rivero-Ayerza Maximo J, Thornton Andrew S, Jessurun Emile R, Theuns Dominic, Jordaens Luc. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur. Heart J. 2007 Sep;28 (18):2231–7. doi: 10.1093/eurheartj/ehm227. [DOI] [PubMed] [Google Scholar]
- 13.Chierchia Gian Battista, Yazaki Yoshinao, Sorgente Antonio, Capulzini Lucio, de Asmundis Carlo, Sarkozy Andrea, Duytschaever Matthias, De Ponti Roberto, Brugada Pedro. Transient atriovenous reconnection induced by adenosine after successful pulmonary vein isolation with the cryothermal energy balloon. Europace. 2009 Dec;11 (12):1606–11. doi: 10.1093/europace/eup339. [DOI] [PubMed] [Google Scholar]
- 14.Arentz Thomas, Macle Laurent, Kalusche Dietrich, Hocini Mélèze, Jais Pierre, Shah Dipen, Haissaguerre Michel. "Dormant" pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2004 Sep;15 (9):1041–7. doi: 10.1046/j.1540-8167.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 15.Tritto Massimo, De Ponti Roberto, Salerno-Uriarte Jorge A, Spadacini Giammario, Marazzi Raffaella, Moretti Paolo, Lanzotti Marcelo. Adenosine restores atrio-venous conduction after apparently successful ostial isolation of the pulmonary veins. Eur. Heart J. 2004 Dec;25 (23):2155–63. doi: 10.1016/j.ehj.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Fürnkranz Alexander, Chun K R Julian, Nuyens Dieter, Metzner Andreas, Köster Ilka, Schmidt Boris, Ouyang Feifan, Kuck Karl-Heinz. Characterization of conduction recovery after pulmonary vein isolation using the "single big cryoballoon" technique. Heart Rhythm. 2010;7 (2):184–90. doi: 10.1016/j.hrthm.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed Humera, Neuzil Petr, Skoda Jan, D'Avila Andre, Donaldson David M, Laragy Margaret C, Reddy Vivek Y. The permanency of pulmonary vein isolation using a balloon cryoablation catheter. J. Cardiovasc. Electrophysiol. 2010 Jul;21 (7):731–7. doi: 10.1111/j.1540-8167.2009.01703.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt Martin, Daccarett Marcos, Marschang Harald, Ritscher Guido, Turschner Oliver, Brachmann Johannes, Rittger Harald. Intracardiac echocardiography improves procedural efficiency during cryoballoon ablation for atrial fibrillation: a pilot study. J. Cardiovasc. Electrophysiol. 2010 Nov;21 (11):1202–7. doi: 10.1111/j.1540-8167.2010.01796.x. [DOI] [PubMed] [Google Scholar]
- 19.Siklódy Claudia Herrera, Minners Jan, Allgeier Martin, Allgeier Hans-Jürgen, Jander Nikolaus, Weber Reinhold, Schiebeling-Römer Jochen, Neumann Franz-Josef, Kalusche Dietrich, Arentz Thomas. Cryoballoon pulmonary vein isolation guided by transesophageal echocardiography: novel aspects on an emerging ablation technique. J. Cardiovasc. Electrophysiol. 2009 Nov;20 (11):1197–202. doi: 10.1111/j.1540-8167.2009.01524.x. [DOI] [PubMed] [Google Scholar]
- 20.Siklódy Claudia Herrera, Minners Jan, Allgeier Martin, Allgeier Hans-Jürgen, Jander Nikolaus, Keyl Cornelius, Weber Reinhold, Schiebeling-Römer Jochen, Kalusche Dietrich, Arentz Thomas. Pressure-guided cryoballoon isolation of the pulmonary veins for the treatment of paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2010 Feb;21 (2):120–5. doi: 10.1111/j.1540-8167.2009.01600.x. [DOI] [PubMed] [Google Scholar]
- 21.Klein Gunnar, Oswald Hanno, Gardiwal Ajmal, Lüsebrink Ulrich, Lissel Christoph, Yu Hong, Drexler Helmut. Efficacy of pulmonary vein isolation by cryoballoon ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2008 Jun;5 (6):802–6. doi: 10.1016/j.hrthm.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Kühne Michael, Schaer Beat, Ammann Peter, Suter Yves, Osswald Stefan, Sticherling Christian. Cryoballoon ablation for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Swiss Med Wkly. 2010 Apr 17;140 (15-16):214–21. doi: 10.4414/smw.2010.12977. [DOI] [PubMed] [Google Scholar]
- 23.Linhart Markus, Bellmann Barbara, Mittmann-Braun Erica, Schrickel Jan W, Bitzen Alexander, Andrié René, Yang Alexander, Nickenig Georg, Lickfett Lars, Lewalter Thorsten. Comparison of cryoballoon and radiofrequency ablation of pulmonary veins in 40 patients with paroxysmal atrial fibrillation: a case-control study. J. Cardiovasc. Electrophysiol. 2009 Dec;20 (12):1343–8. doi: 10.1111/j.1540-8167.2009.01560.x. [DOI] [PubMed] [Google Scholar]
- 24.D Packer. Abstract presented at. ACC. 2010 [Google Scholar]
- 25.Fürnkranz Alexander, Chun K R Julian, Metzner Andreas, Nuyens Dieter, Schmidt Boris, Burchard Andre, Tilz Roland, Ouyang Feifan, Kuck Karl Heinz. Esophageal endoscopy results after pulmonary vein isolation using the single big cryoballoon technique. J. Cardiovasc. Electrophysiol. 2010 Aug 01;21 (8):869–74. doi: 10.1111/j.1540-8167.2010.01739.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed Humera, Neuzil Petr, d'Avila Andre, Cha Yong-Mei, Laragy Margaret, Mares Karel, Brugge William R, Forcione David G, Ruskin Jeremy N, Packer Douglas L, Reddy Vivek Y. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm. 2009 Jul;6 (7):962–9. doi: 10.1016/j.hrthm.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 27.Chierchia Gian Battista, Capulzini Lucio, Droogmans Steven, Sorgente Antonio, Sarkozy Andrea, Müller-Burri Andreas, Paparella Gaetano, de Asmundis Carlo, Yazaki Yoshinao, Kerkhove Dirk, Van Camp Guy, Brugada Pedro. Pericardial effusion in atrial fibrillation ablation: a comparison between cryoballoon and radiofrequency pulmonary vein isolation. Europace. 2010 Mar;12 (3):337–41. doi: 10.1093/europace/eup422. [DOI] [PubMed] [Google Scholar]
- 28.Luik Armin, Merkel Matthias, Hoeren Danielle, Riexinger Tobias, Kieser Meinhard, Schmitt Claus. Rationale and design of the FreezeAF trial: a randomized controlled noninferiority trial comparing isolation of the pulmonary veins with the cryoballoon catheter versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation. Am. Heart J. 2010 Apr;159 (4):555–560.e1. doi: 10.1016/j.ahj.2010.01.008. [DOI] [PubMed] [Google Scholar]