Abstract
Ex vivo CD34+ selected T-cell depletion (TCD) has been developed as a strategy to reduce the incidence of graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Clinical characteristics, treatment responses, and outcomes of patients developing acute (a-) and chronic (c-) GVHD after TCD allo-HSCT have not been well established. We evaluated 241 consecutive patients (median age 57 years) with acute leukemia (n = 191, 79%) or myelodysplastic syndrome (MDS) (n = 50, 21%) undergoing CD34+ selected TCD allo-HSCT without post-HCST immunosuppression in a single institution. Cumulative incidences of grade II–IV and III–IV aGVHD at 180 days were 16% (95% CI:12–21) and 5% (95% CI:3–9), respectively. The skin was the most frequent organ involved, followed by the GI tract. Patients were treated with topical corticosteroids, poorly absorbed corticosteroids (Budesonide), and/or systemic corticosteroids. The overall day 28 treatment response was high at 82%. Cumulative incidence of any cGVHD at 3 years was 5% (95% CI:3–9), with a median time of onset of 256 days (range 95–1645). The 3-year transplant-related mortality, relapse, overall survival and disease-free survival were 24% (95%CI: 18–30), 22% (95% CI:17–27), 57% (95% CI:50–64) and 54% (95% CI:47–61), respectively. The 1-year and 3-years probability of cGVHD-free/relapse-free survival (CRFS) were 65% (95% CI:59–71) and 52% (95% CI:45–59), respectively. Our findings support the use of ex vivo CD34+ selected TCD allograft as a calcineurin inhibitor-free intervention for the prevention of GVHD in patients with acute leukemia and MDS.
Keywords: T-cell depleted transplant, acute GVHD, chronic GVHD
INTRODUCTION
Graft-versus-host disease (GVHD) contributes significantly to transplant-related morbidity and mortality (TRM) after allogeneic hematopoietic stem cell transplantation (allo-HSCT)1. Investigators have therefore addressed this issue by improving selection of patients at lower risk for GVHD2, using biomarkers to facilitate early diagnosis and treatment3–5, and proving efficacy of innovative prophylactic6–10 and therapeutic strategies11,12. Depletion of T cells in the graft through ex vivo CD34+ cell positive selection represents one of the approaches to prevent GVHD. Although investigators have documented the clinical characteristics of GVHD, therapeutic approaches, and outcomes of patients developing acute GVHD (aGVHD) and chronic GVHD (cGVHD) after unmodified grafts for allo-HSCT13–17, there are no comprehensive studies specifically addressing these issues in the CD34+ hematopoietic progenitor cells selected graft setting18. This type of graft manipulation has unique features, which should lead to distinct incidences, clinical presentations, and outcomes of patients with and without acute or chronic GVHD. We therefore investigated the clinical characteristics of acute and chronic GVHD and the risk factors associated with aGVHD, in a homogeneous cohort of patients diagnosed with acute leukemia and myelodysplastic syndrome (MDS) undergoing ex vivo CD34+ selected allo-HSCT.
METHODS
Patient and Graft Characteristics
This analysis included patients who underwent allo-HSCT at Memorial Sloan Kettering Cancer Center (MSKCC) between January 1st 2008 and May 31st 2014. Patients eligible for this analysis included all consecutive adult recipients of first allografts transplanted for the treatment of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), or MDS. Eligibility for this study required that acute leukemia patients be in complete remission, and MDS patients had to have ≤ 5% blasts in pre-transplant bone marrows. Disease risk was assessed using the Disease Risk Index for allo-HSCT19. Patients with donor-recipient HLA-match < 7/8 were excluded from the analysis. Fourteen patients enrolled in the CMX001 clinical trial for the management of cytomegalovirus were also excluded, because this drug is associated with a higher incidence of gastrointestinal aGVHD20. All patients provided written informed consent for transplantation according to the principles of the Declaration of Helsinki, and transplantation outcome analysis was approved by the MSKCC Institutional Review and Privacy Board. Patients included in this analysis were transplanted on trials NCT01746849, NCT01596257, NCT01119066, NCT00629798, NCT00201240, NCT00582933 at ClinicalTrials.gov.
HLA typing used high-resolution, DNA sequence-specific oligonucleotide typing for HLA-A, -B, -C, -DRB1, -DQ (5 allele level). Donor selection used matching at 10 HLA-alleles. For the purpose of this analysis, however, only 8 HLA-allele matching at -A, -B, -C, and -DRB1 was considered. Assessment of comorbidities and calculation of the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) followed standard recommendations21,22.
All patients received CD34+ selected grafts from granulocyte-colony stimulating factor (G-CSF) mobilized peripheral blood stem cells. CD34+ hematopoietic progenitor cells were selected using the Isolex 300i Magnetic Cell Separator (Baxter, Deerfield, IL), followed by additional T cells rosetting with neuraminidase-treated sheep erythrocytes23, or using the CliniMACS® CD34+ Reagent System (Miltenyi Biotech, Gladbach, Germany)24. The Isolex system was only used in allo-HSCT from years 2008–2010 since it became commercially unavailable. The CD34+ selected, TCD graft was infused within the first 48 hours after manipulation.
Conditioning Regimens and Supportive Care
Pre-transplant conditioning included either cyclophosphamide (120 mg/kg), thiotepa (10 mg/kg), and hyperfractionated TBI (1375 cGy), or intravenous busulfan (9.6 mg/kg), melphalan (140 mg/m2), and fludarabine (125 mg/m2). A few patients enrolled in specific protocols received other conditioning regimens (Table 1). G-CSF (5 mcg/kg/day) was given to all patients from day 7 until absolute neutrophil count recovery of > 2.0 × 109/L.
Table 1a.
Patient demographics (n = 241)
| Characteristic | Patients |
|---|---|
|
| |
| Median age (range) | 57 (20–73) |
|
| |
| Male gender, n (%) | 134 (56) |
|
| |
| Donor/Recipient gender, n (%) | |
| Female to male | 46 (19) |
| Other | 195 (81) |
|
| |
| Donor*, n (%) | |
| MRD | 90 (37) |
| MMRD | 3 (1) |
| MUD | 100 (42) |
| MMUD | 48 (20) |
|
| |
| Patient CMV serostatus, n (%) | |
| Seronegative | 105 (44) |
| Seropositive or equivocal | 136 (56) |
|
| |
| Diagnosis, n (%) | |
| Acute myeloid leukemia | 162 (67) |
| Acute lymphoblastic leukemia | 29 (12) |
| Myelodysplastic syndrome | 50 (21) |
|
| |
| Disease risk, n (%) | |
| Low | 5 (2) |
| Intermediate | 207 (81) |
| High | 43 (17) |
|
| |
| Disease status for acute leukemia, n (%)+ | |
| CR1 | 155 (81) |
| CR2–3 | 36 (19) |
|
| |
| HCT-CI, n (%) | |
| 0 | 41 (17) |
| 1–2 | 88 (37) |
| ≥3 | 112 (46) |
|
| |
| Conditioning regimen, n (%) | |
| Bu/Mel/Flu | 147 (61) |
| Cy/Thiotepa/TBI (1375cGy) | 77 (32) |
| Clo/Mel/Thio | 11 (5) |
| Flu/Thiotepa/TBI (1375cGy) | 6 (2) |
|
| |
| Year of HSCT, n (%) | |
| 2008–2010 | 97 (40) |
| 2011–2014 | 144 (60) |
Abbreviations: n, number; MRD, matched-related donor; MMRD, mismatched-related donor; MUD, matched-unrelated donor; MMUD, mismatched-related donor; CMV, cytomegalovirus; CR, complete remission; HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index; Cy, cyclophosphamide; Flu, fludarabine; TBI, total body irradiation; Clo, clofarabine; Mel, melphalan.
At 8 allele level (-A,-B,-C and -DRB1).
Percentage calculated based on acute leukemia patients only.
All patients were hospitalized in high-efficiency particulate air (HEPA)-filtered rooms and received similar supportive care. Management of early toxicities, engraftment, and infections was performed according to standard clinical practice as previously described25–27.
GVHD Prophylaxis, Diagnosis and Treatment
All patients received anti-thymocytic globulin (ATG) pre-HSCT for the prevention of allograft rejection. The dose of ATG was 2.5mg/kg/day on days -3 and -2 except in 20 7/8 HLA-matched and 3 HLA-DQ mismatched recipients who received one additional day of ATG treatment from days -3 to -1. Patients did not receive calcineurin inhibitor or any other immunosuppressive prophylaxis post-HSCT.
aGVHD and cGVHD were diagnosed clinically with histological confirmation as required when clinically appropriate. Treatment of aGVHD followed institutional and national guidelines28. Patients received either topical corticosteroids, poorly absorbed corticosteroids, or systemic corticosteroids according to organ involvement and clinical GVHD severity at the time of diagnosis. Calcineurin inhibitor was used frequently in patients with visceral aGVHD involvement who required systemic corticosteroids therapy.
Study Definitions
The International Bone Marrow Transplant Registry classification guided the aGVHD grading, except grades A–D were labeled grades I–IV. GVHD with purely acute features was graded accordingly, even if it occurred after day 10029. Grading was reviewed by a transplant clinician panel to reach consensus of maximum aGVHD grade. cGVHD was defined according to National Institutes of Health consensus criteria, and these criteria were used to assess GVHD severity for classical and overlap cGVHD30. While patients without neutrophil engraftment (> 0.5/mm3 × 109/L) and those who died before day 100 were not considered evaluable for acute and chronic GVHD, these patients are included in all outcome analyses and are considered censored or as having had a competing risk as appropriate.
Responses to aGVHD therapy were assessed on day 28 of therapy31 using published definitions32: Complete response (CR) required the complete resolution of all aGVHD symptoms in all organs without secondary GVHD therapy. Very good partial response (VGPR) required near resolution of GVHD symptoms, justifying a continuous tapering of corticosteroids. Partial response (PR) meant improvement in GVHD stage in all initially affected organs without complete resolution, and without worsening in other GVHD target organs or requiring secondary GVHD therapy. Progression was worsening of GVHD in at least 1 organ. Patient with no response to therapy maintained the same grade of GVHD and did not meet criteria for PR or progression.
Relapse was defined as recurrence of hematologic malignancy post allo-HSCT whereas TRM was defined as death from any cause in continued remission with the exception of one patient who died due to recurrence of a solid tumor malignancy post-HSCT. Overall survival (OS) and disease-free survival (DFS) were defined following standard criteria and causes of death were described according to Copelan algorithm33.
Statistical Analysis
The MSKCC Adult BMT database, verified by primary source documents, provided data on patient characteristics and transplant-related outcomes. Significant differences in categorical variables were determined by Chi-square or Fisher’s exact test as appropriate, while differences in continuous variables were determined by the Wilcoxon rank-sum test. Date of neutrophil recovery was defined as the first of 3 consecutive days with a sustained absolute neutrophil count (ANC) > 0.5 × 109/l. Primary graft failure was the lack of donor-derived neutrophil recovery by day 28 or death prior to day 28 without neutrophil recovery. Cumulative incidence functions were used to estimate neutrophil engraftment, GVHD, and TRM. The competing risks for each outcome were death for engraftment, death or relapse for GVHD, and relapse for TRM. DFS, OS and CRFS were estimated using the Kaplan-Meier method, with relapse, death, or onset of cGVHD requiring systemic therapy considered as an event for CRFS34. In order to evaluate the effect of aGVHD on transplant outcomes, a landmark analysis was conducted at day 180. Univariate and multivariate cause-specific Cox regression analyses were used to ascertain associations between patient or graft characteristics and aGVHD. Potential risk factors evaluated in the Cox models included patient gender, age (< median vs. > median years), patient CMV serostatus, type of donor (related vs. unrelated), HCT-CI (0 vs.1–2 vs. ≥ 3), conditioning regimen (TBI-based vs. chemo-based), number of CD3+ and CD34+ cells (> median), donor-recipient HLA-match (8/8 vs. 7/8 HLA-match), and TCD method (Isolex vs. CliniMACS®). All analyses were completed using R 3.2.4.
RESULTS
Patient Demographics and Graft Characteristics
We evaluated 241 patients. The median day to neutrophil recovery was 10 days (range 8–19). The cumulative incidence of neutrophil engraftment was 99.6% (95% CI:95–100) with one patient having primary graft failure. Table 1a summarizes patients’ characteristics. The median age at transplantation was 57 years (range 20–73). Most patients received allo-HSCT for acute leukemia in first remission from HLA-matched related or unrelated donors. About half of the patients had an HCT-CI score ≥ 3. The majority of patients received chemotherapy without radiation as myeloablative conditioning pre-transplant. Twenty-six patients received donor lymphocyte infusion for mixed chimerism (n = 16), malignant disease relapse (n = 7), poor immune reconstitution (n = 1), secondary graft failure (n = 1), and Epstein Barr virus post-transplant lymphoproliferative disorder (n = 1). The median follow-up for survivors was 34 months (range 12–84).
Table 1b summarizes the main graft characteristics. The median infused CD34+ cell dose and CD3+ cell dose were 7.7 × 106/kg (range 1.5–28.4) and 2.3 × 103/kg (range 0.3–37), respectively. HLA compatibility between donor and recipients was 8/8 in the majority of patients. CD34+ selection was mostly performed by using the CliniMACS® CD34 Reagent System.
Table 1b.
Graft characteristics.
| Characteristic | |
|---|---|
|
| |
| Infused cell dose, median (range) | |
| TNC × 108/kg | 7.9 (1.6–28.6) |
| CD34+ × 106/kg | 7.7 (1.5–28.4) |
| CD3+ × 103/kg | 2.3 (0.3–37) |
|
| |
| Donor-recipient HLA-match, n (%)* | |
| 8/8 | 200 (83) |
| 7/8 | 41 (17) |
|
| |
| CD34+ selection method, n (%) | |
| Isolex | 70 (29) |
| CliniMACS® | 171 (71) |
TNC indicates total nucleated cells; n, number; HLA, human leukocyte antigen Kg, kilogram.
Considering -A, -B, -C and -DRB1 HLA loci.
Acute GVHD
A total of 68 patients developed aGVHD features (grade I–IV) after HSCT at a median onset of 68 days (range 15–347). Fifty-six patients (82%) presented with grade I–IV aGVHD features before day 180 whereas 12 (18%) patients developed grade I–IV aGVHD features after day 180.
ACUTE GVHD BY DAY 180
Incidence and Manifestations
The cumulative incidences of grade I–IV, II–IV and III–IV aGVHD at day 180 were 23% (95% CI:18–29), 16% (95% CI:12–21), and 5% (95% CI:3–9), respectively (Figure 1). The skin was the most commonly affected organ, followed by the GI tract, whereas only 3 patients had hepatic aGVHD. The most frequent disease manifestations were skin alone, GI tract alone, and the combination of skin and GI tract disease. None of the patients presented with isolated liver involvement. Of the 28 patients who had GI tract aGVHD, 15 had upper GI tract, 6 had lower GI tract, and 7 had both upper and lower GI tract involvement.
Figure 1. Cumulative incidence of grade I–IV, II–IV and III–IV aGVHD.
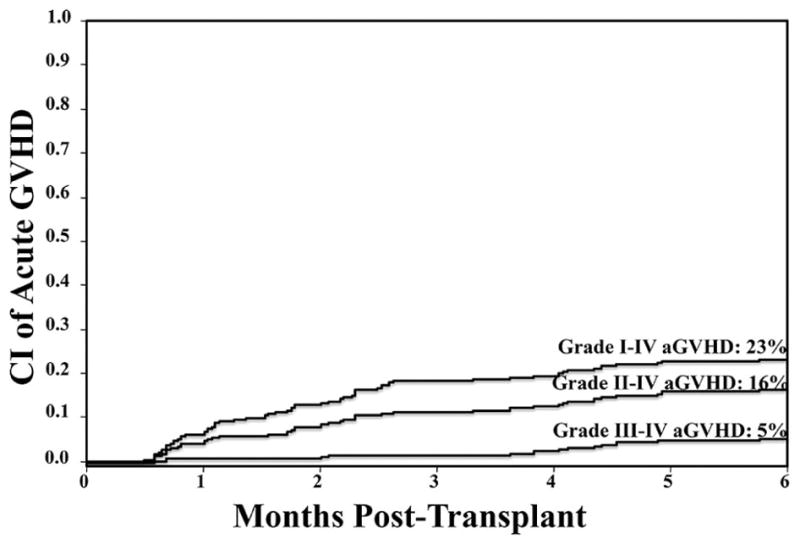
The day 180 analysis shows an overall low incidence of aGVHD.
Organ involvement and aGVHD severity were assessed according to time of onset (< day 100 vs. day 101–180). The skin was the organ most commonly affected in patients who had aGVHD either before or after day 100, while GI involvement was more frequent in patients with aGVHD onset > day 100. Patients developing aGVHD > day 100 had more aGVHD grade III–IV (9 of 12) than patients with aGVHD onset ≤ day 100 (4 of 44), p < 0.001.
Acute GVHD Therapy and Treatment Responses
aGVHD therapy in the 56 patients with grade I–IV aGVHD ≤ day 180 is summarized in Table 2. One patient relapsed with leukemia shortly after GVHD diagnosis and did not receive any GVHD-specific therapy.
Table 2.
Acute GVHD characteristics and treatment response by day 180 (n = 56).
| Characteristic | Patients |
|---|---|
|
| |
| Time to aGVHD onset, n (%) | |
| ≥ 100 days | 44 (79) |
| 101–180 days | 12 (21) |
|
| |
| Organ involvement, n (%) | |
| Skin alone | 28 (50) |
| GI | 28 (50) |
| Skin/GI | 6 (11) |
| GI alone | 19 (34) |
| GI/liver | 1 (2) |
| GI/liver/skin | 2 (3) |
| Liver alone | 0 |
|
| |
| aGVHD grade at treatment onset, n (%) | |
| I | 17 (30.5) |
| II | 26 (46.5) |
| III | 13 (23) |
| IV | 0 |
|
| |
| aGVHD treatment, n (%) | |
| Topical corticosteroids | 27 (49) |
| Budesonide | 15 (27) |
| Systemic corticosteroids | 13 (24) |
| No treatment* | 1 (2) |
aGVHD indicates acute graft-versus-host disease; n, number; GI, gastrointestinal.
One patient with aGVHD ≤ day 100 presented leukemia relapse shortly after GVHD diagnosis and did not received any GVHD therapy.
The majority of the patients treated for skin aGVHD with topical corticosteroids achieved day 28 treatment response of CR/VGPR or PR. All 15 patients treated with oral Budesonide had isolated stage I GI aGVHD, and responded by day 28 of therapy. Thirteen patients were treated with systemic corticosteroids, and of those, 10 received concomitant calcineurin inhibitor. The systemic corticosteroids group had more severe aGVHD (9, 69% grade III–IV aGVHD, and visceral involvement. By day 28 of therapy, the majority achieved CR/PR. Of the 4 patients who failed to achieve at least a PR by day 28, 2 of them eventually had therapy response by day 56. Analysis of type of treatment and therapy response by time of presentation indicated that patients developing aGVHD ≤ 100 days were more often treated with topical than systemic corticosteroids (including Budesonide) (88% vs. 33%, p < 0.001). They also were more likely to achieve a CR response by day 28 (59% vs. 25%) of aGVHD treatment, compared with patients with aGVHD onset > day 100 who were more likely to achieve a VGPR/PR (42% vs. 27%) or < PR (33% vs. 14%) responses (p = 0.08), consistent with a higher incidence of more severe aGVHD after day 100.
Ten patients required second therapeutic agent for the treatment of aGVHD. The most common agent used was mycophenolate mofetil (n = 5), followed by mycophenolate mofetil combined with sirolimus (n = 3). One patient received mesenchymal stem cells, and one patient had etanercept and Rituximab. Of these patients, 7 died of GVHD.
Analyses of Risk Factors for Day 180 Grade II–IV and III–IV aGVHD
We evaluated risk factors for grade II–IV and III–IV aGVHD through day 180 (Table 3). In the univariate analyses the only variable associated with a lower risk for grade II–IV aGVHD was the use of Isolex as TCD method compared with the CliniMACS® CD34+ Reagent System (HR 0.4 [95% CI:0.2–1.0], p = 0.05). There was no association with patient gender, age, recipient CMV serostatus, donor (related vs. unrelated), diagnosis (acute leukemia vs. MDS), pre-transplant HCT-CI score (0 vs. 1–2 vs. > 3), or conditioning regimen (chemotherapy vs. high-dose TBI). When focusing in grade III–IV aGVHD, the only variable associated with a lower risk in the univariate analyses was recipient CMV seropositive status (HR 0.2 [95% CI:0.1–0.9], p = 0.028). None of the other pre-transplant variables tested were associated with grade III–IV aGVHD, including the CD34+ selection method (Isolex HR 1.1 [95% CI:0.3–3.6], p = 0.850). An additional analysis evaluation the CD3+ content by TCD method used showed that while the CD3+ cell content was low irrespective of the method, the content of 0.012 × 103/kg (0–0.267) in the Isolex group was lower when compared to 0.025 × 103/kg (0–0.379) in the CliniMACS group (p = <0.001).
Table 3.
Univariate analyses for the development of grade II–IV and III–IV aGVHD and multivariate analysis for the development of grade II–IV aGVHD
| Patient and Graft Characteristics | Univariate analysis Grade II–IV aGVHD | Univariate analysis Grade III–IV aGVHD | Multivariate analysis Grade II–IV aGVHD | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratioa | P value | Hazard Ratioa | P value | Hazard Ratioa | P value | |
|
| ||||||
| Gender | ||||||
| Male (n = 134) | Reference | 0.654 | Reference | 0.340 | - | - |
| Female (n = 107) | 0.9 (0.5–1.6) | 0.6 (0.2–1.8) | ||||
|
| ||||||
| Age (years)b | ||||||
| < 56.7 (n =120 ) | Reference | 0.631 | Reference | 0.409 | - | - |
| ≥ 56.7 (n =121 ) | 0.9 (0.5–1.6) | 0.6 (0.2–1.9) | ||||
|
| ||||||
| Recipient CMV | ||||||
| Seronegative (n = 105) | Reference | 0.563 | Reference | 0.028 | Reference | 0.665 |
| Seropositive (n = 136) | 0.8 (0.4–1.6) | 0.2 (0.1–0.9) | 0.9 (0.5–1.6) | |||
|
| ||||||
| Donor | ||||||
| Related (n = 93) | Reference | 0.436 | Reference | 0.962 | - | - |
| Unrelated (n = 148) | 1.3 (0.7–2.5) | 1.0 (0.3–3.1) | ||||
|
| ||||||
| HCT-CI | ||||||
| 0 (n = 41) | Reference | Reference | 0.848 | |||
| 1–2 (n = 88) | 1.2 (0.4–3.0) | 0.870 | 0.6 (0.1–2.9) | - | - | |
| ≥ 3 (n = 112) | 1.3 (0.5–3.2) | 0.8 (0.2–3.2) | ||||
|
| ||||||
| Conditioning regimen | ||||||
| Chemotherapy (n = 158) | Reference | 0.572 | Reference | 0.125 | - | - |
| TBI (n = 83) | 1.2 (0.6–2.3) | 2.3 (0.8–7.0) | ||||
|
| ||||||
| CD3+ × 103/kg dose* | ||||||
| < 2.2 (n = 117) | Reference | 0.201 | Reference | 0.601 | - | - |
| > 2.2 (n = 120 | 1.5 (0.8–2.9) | 1.4 (0.4–4.3) | ||||
|
| ||||||
| CD34+ × 106/kg dose** | ||||||
| < 7.74 (n = 119) | Reference | 0.264 | Reference | 0.979 | - | - |
| ≥ 7.74 (n = 120) | 0.7 (0.4–1.3) | 1.0 (0.3–3.1) | ||||
|
| ||||||
| Donor-recipient HLA-match | ||||||
| 8/8 (n = 200) | Reference | 0.322 | Reference | 0.196 | Reference | 0.173 |
| 7/8 (n = 41) | 1.5 (0.7–3.1) | 2.2 (0.7–7.1) | 1.7 (0.5–3.6) | |||
|
| ||||||
| TCD method | ||||||
| CliniMACS (n = 171) | Reference | 0.050 | Reference | 0.850 | Ref. | 0.038 |
| Isolex (n = 70) | 0.4 (0.2–1.0) | 1.1 (0.3–3.6) | 0.4 (0.2–0.9) | |||
includes 95% confidence interval.
Age cut-off based on the median.
aGVHD indicates acute graft-versus-host disease; CMV, cytomegalovirus; HLA, human-leukocyte antigen; TNC, total nucleated cell; kg, kilogram.
Data missing in 4 patients,
data missing in 2 patients.
A multivariate analysis was performed to take potential confounding variables into account in grade II–IV aGVHD (Table 3). In this analysis, Isolex as the CD34+ selection method was associated with lower risk of grade II–IV aGVHD (HR 0.4 [95% CI:0.2–0.9], p = 0.038). Recipient CMV serostatus and donor-recipient HLA-match did not correlate with the development of grade II–IV aGVHD (p = 0.665 and p = 0.173, respectively). Multivariate analyses of risk factors for grade III–IV aGVHD was not obtained due to the low number of events (n = 13).
Chronic GVHD
Thirteen patients had cGVHD features at a median time of 256 days (range 95–1645) for a cumulative incidence of cGVHD at 3 years of 5% (95% CI:3–9) (Figure 3). Nine patients had classical cGVHD (6 interrupted, 3 de novo), and 4 additional patients presented with overlap syndrome (2 with prior late onset aGVHD). Organ involvement in these 13 patients with cGVHD included skin (n = 4), oral cavity (n = 9), ocular (n = 4), lungs (n = 4), liver (n = 1), and joints and fascia (n = 1). Most patients had mild (n = 5, 38%) or moderate (n = 6, 46%) disease, whereas severe cGVHD was least common (n = 2, 15%). Upfront treatment for cGVHD included topical corticosteroids, oral prednisone, and/or the addition of calcineurin inhibitor. Four patients required second therapeutic agent. One patient received mycophenolate mofetil, 1 had mycophenolate mofetil and Rituximab, and 2 patients had Etanercept.
Figure 3. Kaplan-Meier estimate of 1-year CRFS.
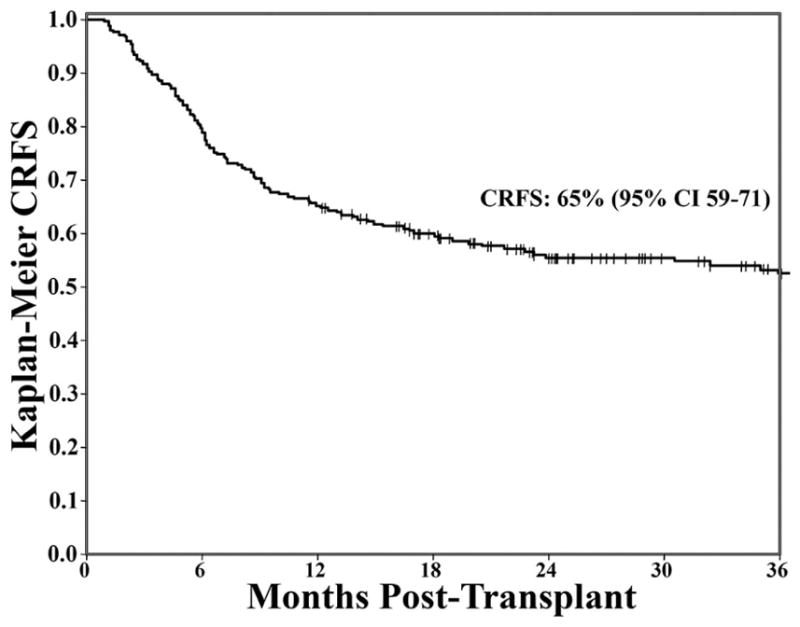
Landmark analysis for patients engrafted and disease-free at 100 days after allo-HSCT. The 1-year post allo-HSCT CRFS was high at 65%.
Causes of Death and Transplant Outcomes
For the whole cohort, 57 patients had died of TRM at last follow-up for a cumulative incidence of 3-year TRM of 24% (95% CI: 18–30). A day 180 landmark analysis showed that the 3-year TRM was higher in patients experiencing grade II–IV aGVHD (31% [95% CI: 15–49]) compared to those with only grade I or no aGVHD (15% [95% CI: 9–21]), p = 0.034. The most common cause of TRM was infection (n = 24, 42%), followed by treatment toxicity/organ failure (n = 15, 26%), GVHD (n = 11, 19%), primary or secondary graft failure (n = 3, 5%), secondary malignancy (n = 2, 3.5%), and other (n = 2, 3.5%). The median time from aGVHD diagnosis to death was 191 days (range 14–1336), and 9 of the 11 patients who died of GVHD had visceral involvement. Forty-four patients died of malignant relapse, and 1 of relapse of previous solid tumor malignancy in remission at the time of allo-HSCT. The 3-year relapse, OS and DFS were 22% (95% CI:17–27), 57% (95% CI:50–64) and 54% (95% CI:47–61), respectively.
The 1-year and 3-year probability of CRFS was 65% (95% CI:59–71) and 52% (95% CI:45–59), respectively (Figure 3). Patients receiving allo-HSCT from 8/8 HLA-matched compared with 7/8 HLA-matched donors showed similar probabilities of CRFS (3-year CRFS: 54% [95% CI:46–61] vs. 46% [95% CI:29–61], p = 0.314).
DISCUSSION
CD34+ selection is a T-cell depletion strategy ex vivo that allows the infusion of a stem cell graft without need for calcineurin inhibitor-based GVHD prophylaxis. Our analyses showed a low incidence of grade I–IV aGVHD at day 180, with the majority of the patients having mild disease manifestation. This incidence and severity compared favorably with other studies of unmodified allografts in a similar patient population8,35. A significant proportion of patients in our study developed aGVHD after day 100, confirming that in the CD34+ selection setting as with unmodified allografts, the traditional distinction of acute and chronic GVHD based on the time of onset before and after day 100 is no longer valid. Additionally, we detected differences between patients developing aGVHD before and after day 100. As previously shown in allo-HSCT with unmodified grafts36, patients diagnosed with aGVHD after day 100 had features of more aggressive GVHD including a higher aGVHD grade and a higher need for systemic therapy than patients diagnosed before day 100.
Our results also showed that patients with aGVHD had a promising response to first line treatment. For instance, more than two thirds of the patients with grade II–IV aGVHD treated with systemic corticosteroids achieved at least a PR on day 28, all patients with stage I GI aGVHD treated with Budesonide achieved a PR/CR, and only a small proportion of patients required second-line agent for the treatment of aGVHD. These results compare favorably with those reported in patients receiving unmodified allografts37,38, and suggest that CD34+ selected allografts recipients have at least a similar response to aGVHD therapy. A group of patients received upfront systemic corticosteroids in combination with calcineurin inhibitor in visceral grade II–IV aGVHD. While additional evaluations are needed to assess its efficacy, this approach has become our institutional standard aiming to decrease the cumulative corticosteroids dose exposure.
The incidence of cGVHD at 3 years in this series was low, with only 2 patients having severe disease. These results compare favorably with similar populations receiving PBSC mobilized unmodified grafts, even with the use of ATG, in which cGVHD occurs in nearly 30% of the patients39. Our patient’s incidence of cGVHD is also markedly lower than what has been reported in the setting of unmodified bone marrow allografts35. However, since all patients received ATG to promote engraftment we cannot determine if this drug added further protection to the ex vivo T cell depletion strategy against GVHD. In any case, we have shown that the transplant platform used leads to a low incidence of acute and chronic GVHD, supporting the sparing of post allo-HSCT immunosuppressive drugs in these patients, making this approach especially interesting in patients at high risk of intolerance to calcineurin inhibitor, including those with renal insufficiency, hypertension or receiving medications with high risk of pharmacological interactions.
In the multivariate analysis, the only factor associated with the development of grade II–IV aGVHD was the use of CliniMACS® as the method for CD34+ selection, although this association was not found for grade III–IV aGVHD. We hypothesize that the higher median CD3+ cell/kg content observed using the CliniMACS system could explain these differences. However, the clinical significance of this finding is uncertain since the observed increase in grade II–IV aGVHD is predominantly explained by an increase in stage 2 skin, overall grade II aGVHD by IBMTR criteria. In the univariate analysis, recipient CMV seronegative status was associated with the development of grade III–IV aGVHD. However, this finding could not be confirmed in a multivariate analysis due to the low number of events. Similarly, the low number of cGVHD events precluded evaluating risk factors for cGVHD. These discrepancies in the evaluation of risk factors for GVHD may reflect the difficulty of conducting risk factor analyses in a population with such low incidence of GVHD, despite the relatively large cohort evaluated in this series. Further efforts are needed in this direction, possibly in the setting of collaborative multicenter studies with a large numbers of patients receiving CD34+ selected grafts.
Nonetheless, with the progressive increase in survival after allo-HSCT40, development of endpoints that reflect survival without ongoing morbidity in the field of HSCT has become a priority. One such endpoint is CRFS that reflects survival in the absence of both disease relapse and moderate or severe cGVHD. The high CRFS probability demonstrated in this analysis it most likely reflects the very low number of patients with moderate to severe cGVHD and no increase risk of relapse in our TCD cohort. These data are therefore pertinent to physicians’ decisions and discussions with patients when considering different approaches for allo-HSCT. However, this study has several limitations including the heterogeneity in the patient cohort and the relatively long duration of the study might have carried out an improvement in supportive care that could have had an impact on the transplant results overtime. Additionally, the lack of a control group of patients receiving unmodified grafts during the same period, precludes the comparisons of the results of this approach with a more widely used approach with unmodified grafts. Notwithstanding, other studies with a control group have also shown a low incidence of GVHD without an increase in disease relapse when compared with unmodified graft recipients27,41,42.
A prospective multicenter, randomized trial is currently underway comparing two calcineurin inhibitor-free strategies with CD34+ selected TCD PBSCT allografts, and post-transplant cyclophosphamide after unmodified bone marrow allografts vs. tacrolimus/methotrexate for unmodified bone marrow grafts (BMT CTN 1301, NCT02345850). These results will further elucidate the role of calcineurin inhibitor-free strategies for the prevention of GVHD and maintenance of relapse-free survival. Our data support the use of ex vivo CD34+ selection to reduce the incidences of acute and chronic GVHD and to therefore ensure a high CRFS rate in patients with acute leukemia and MDS.
Figure 2. Cumulative incidence of cGVHD at 3-years.
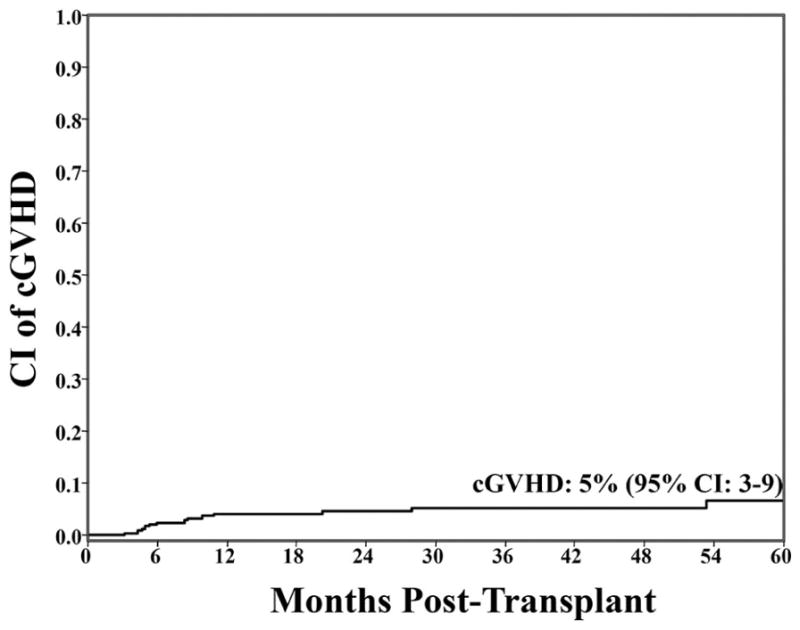
The cumulative incidence of cGVHD was low (5%) which includes 9 patients with classical cGVHD and 4 patients with overlap syndrome.
Highlights.
The day 180 incidence of grade II–IV acute GVHD and the 3-year incidence of chronic GVHD were low after ex vivo CD34+ selected T-cell depletion allogeneic HSCT.
Acute GVHD therapy response was high in the ex vivo CD34+ selected T-cell depletion allograft recipients.
The 1-year and 3-year probabilities of chronic GVHD-free/relapse-free survival (CRFS) were high after ex vivo CD34+ selected T-cell depletion allogeneic HSCT.
Ex vivo CD34+ selected T-cell depletion is a calcineurin inhibitor-free strategy for the prevention of acute and chronic GVHD in patients with acute leukemia and MDS.
Acknowledgments
This work was supported in part by the National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748by the NCI Core Grant Core Grant (P30 CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PB was supported by a grant Innovacion tecnologica 2015 from the Sociedad Española de Hematologia y Hemoterapia. We would like to acknowledge the nursing staff, transplant coordinators and Laboratory Medicine staff who greatly contributed to this work.
Footnotes
Author Contributions: P.B. and M.A.P. interpreted the data and wrote the manuscript. D.M.P designed the study, interpreted the data and wrote the manuscript. P.H. and S.M.D. analyzed the data and wrote the manuscript. M.M., D.D., J.N., M.D.G., J.S., and C.C. collected the data. J.N.B, N.A.K., H.C.M, A.A.J, G.K., E.B.P., S.P., C.S., R.T., M.R.M.vdB., S.T.A., R.M., R.J.O’R., J.D.G., J.W.Y., S.G. wrote the manuscript.
Conflict of Interest: The authors have no relevant conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. [PubMed] [Google Scholar]
- 2.Sorror ML, Martin PJ. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014;124(2):287–295. doi: 10.1182/blood-2014-01-550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113(2):273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine JE, Braun TM, Harris AC, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: A multicenter study. Lancet Haematol. 2015;2(1):e21–e29. doi: 10.1016/S2352-3026(14)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponce DM, Hilden P, Mumaw C, et al. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. 2015;125(1):199–205. doi: 10.1182/blood-2014-06-584789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30(26):3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367(2):135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. doi: 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy GA, Varelias A, Vuckovic S, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15(13):1451–1459. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- 11.Jagasia M, Greinix H, Robin M, et al. Extracorporeal photopheresis versus anticytokine therapy as a second-line treatment for steroid-refractory acute GVHD: a multicenter comparative analysis. Biol Blood Marrow Transplant. 2013;19(7):1129–1133. doi: 10.1016/j.bbmt.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsohn DA, Kurland BF, Pidala J, et al. Correlation between NIH composite skin score, patient-reported skin score, and outcome: results from the Chronic GVHD Consortium. Blood. 2012;120(13):2545–2552. doi: 10.1182/blood-2012-04-424135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuzmina Z, Eder S, Bohm A, et al. Significantly worse survival of patients with NIH-defined chronic graft-versus-host disease and thrombocytopenia or progressive onset type: results of a prospective study. Leukemia. 2012;26(4):746–756. doi: 10.1038/leu.2011.257. [DOI] [PubMed] [Google Scholar]
- 18.Urbano-Ispizua A, Rozman C, Pimentel P, et al. Risk factors for acute graft-versus-host disease in patients undergoing transplantation with CD34+ selected blood cells from HLA-identical siblings. Blood. 2002;100(2):724–727. doi: 10.1182/blood-2001-11-0057. [DOI] [PubMed] [Google Scholar]
- 19.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121(15):2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. Characteristics of CliniMACS(R) System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant. 2012;18(5):690–697. doi: 10.1016/j.bbmt.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(9):1335–1342. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg JD, Linker A, Kuk D, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(2):208–213. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50(4):493–498. doi: 10.1038/bmt.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavletic SZ, Fowler DH. Are we making progress in GVHD prophylaxis and treatment? Hematology Am Soc Hematol Educ Program. 2012;2012:251–264. doi: 10.1182/asheducation-2012.1.251. [DOI] [PubMed] [Google Scholar]
- 29.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 30.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Levine JE, Logan B, Wu J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16(12):1693–1699. doi: 10.1016/j.bbmt.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin PJ, Bachier CR, Klingemann HG, et al. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a joint statement. Biol Blood Marrow Transplant. 2009;15(7):777–784. doi: 10.1016/j.bbmt.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13(12):1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 34.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–1338. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora M, Cutler CS, Jagasia MH, et al. Late Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(3):449–455. doi: 10.1016/j.bbmt.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mielcarek M, Furlong T, Storer BE, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100(6):842–848. doi: 10.3324/haematol.2014.118471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Socié G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 40.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


