Abstract
Purpose
MicroRNAs (miRNAs/miRs) are involved in a large number of biological functions and diseases, such as cancer, cardiovascular diseases, and diabetes. MiR-21 has been reported to target Sprouty homolog 1 (SPRY1), SMAD7, and PTEN. In this study, we examined the underlying role of miR-21 in the regulation of prorenin receptor (PRR)-mediated induction of vascular endothelial growth factor (VEGF) expression via targeting SMAD7, SPRY1, and PTEN in a hyperglycemic condition.
Methods
PRR-mediated induction of VEGF under a hyperglycemic condition (high glucose, 33mM) was studied by treating ARPE-19 cells with perindopril (10 µmol/l), which inhibits angiotensin II-mediated signaling. ARPE-19 cells exposed to normal glucose (NG, 5.5 mM) were considered as the control. To examine the role of miR-21 in the regulation of SPRY1, SMAD7, PTEN, and VEGF, ARPE-19 cells cultured in NG or high glucose were transfected with scramble negative control (Scr), a miR-21 mimic, or a miR-21 antagomir. To investigate the role of PRR and the small GTP-binding protein RAC1 in the regulation of miR-21, the expression of PRR and RAC1 was silenced by transfecting ARPE-19 cells with their corresponding siRNAs.
Results
Compared with the NG control, high glucose significantly induced the expression of PRR, VEGF, VEGFR2, and miR-21 but significantly suppressed the expression of SPRY1, SMAD7, and PTEN at the transcript and protein levels. In contrast, silencing the expression of PRR significantly abolished the high glucose–induced expression of VEGF, VEGFR2, and miR-21. Knockdown of RAC1 significantly attenuated the high glucose–induced expression of LOX, CTGF, and miR-21, suggesting that PRR and RAC1 are involved in the CTGF/LOX-mediated regulation of miR-21. Furthermore, high glucose dramatically increased the levels of pERK (p44), hypoxia-inducible factor (HIF-1α), and VEGF. However, this effect was antagonized by the miR-21 antagomir, indicative of the involvement of high glucose–induced miR-21 in the regulation of VEGF through ERK signaling.
Conclusions
Our findings, for the first time, showed that the pleiotropic action of miR-21 induced the expression of pERK, HIF-1α, and VEGF in the high glucose condition by simultaneously targeting SPRY1, SMAD7, and PTEN in ARPE-19 cells. Therefore, miR-21 may serve as a potential therapeutic target for diabetes-induced retinal pathology.
Introduction
Diabetes is a chronic metabolic syndrome and is caused by defects in insulin production, insulin secretion, and insulin signaling [1]. Diabetic retinopathy (DR), a major complication of diabetes and the most common cause of blindness in working-age adults, is a progressive disease characterized by microvessel abnormalities, retinal edema, neuronal dysfunction, and breakdown of the blood–retinal barrier (BRB) [2,3]. The classic renin-angiotensin-system (RAS) is involved in the regulation of blood pressure and electrolyte homeostasis, where angiotensin (Ang) II is considered the effector peptide hormone that mediates its function through Ang II Type 1 (AT1R) and type 2 (AT2R) receptors [4]. Recently, a receptor for prorenin and renin named the prorenin receptor (PRR) was identified, which is known to bind renin and prorenin and trigger an intracellular response [5,6]. Prorenin binding to the receptor causes RAS-independent signal transduction via phosphorylation of extracellular signal–regulated kinase (ERK)1/2 [7]. In addition to PRR’s important role in controlling blood pressure, fluid homeostasis, and salt balance [8], PRR has been reported to contribute in the pathogenesis of DR [9] and diabetic nephropathy [10,11]. PRR is upregulated in the kidneys [12] and retinas [9] of diabetic rats and human retinal endothelial cells (hRECs) [9] exposed to high glucose concentrations.
In the ocular tissues, vascular endothelial growth factor (VEGF) is mainly expressed in Müller cells, the RPE, endothelial cells, and ganglion cells [13,14]. Levels of VEGF and VEGF receptors are increased in DR [15,16]. High glucose has been reported to increase the level of the VEGF protein in the RPE [17] and RECs [18], and VEGF is a key factor in the etiology of proliferative DR (PDR) [19]. Thus, VEGF has become a therapeutic target for DR.
MicroRNAs (miRs/miRNAs), a class of small non-coding RNA molecules [20] that negatively regulate gene expression by base-pairing to partially complementary sites in the 3′-untranslated regions (UTRs) of specific target mRNAs, are involved in a large number of biological functions and diseases, such as cancer, cardiovascular diseases, and diabetes. Emerging evidence suggests that miRNAs play significant roles in insulin production, action, and secretion and in diverse aspects of glucose and lipid metabolism [21]. A few recent studies have demonstrated a role for miRNA in DR [22-25]. Therefore, miRNAs have become an intriguing target for therapeutic intervention.
Recent studies have addressed the role of microRNAs in diabetes and its complications. MiRNA-21 is one of the most upregulated miRNAs in response to high glucose as observed in pancreatic beta cells [26], endothelial cells [27], cardiac fibroblasts [28], and the kidney of db/db mice [29]. The involvement of miR-21 in diabetes-associated endothelial dysfunction has been recently reported [27]. MiRNA-21 targets the transcription factor Mothers against decapentaplegic homolog 7 (SMAD7; OMIM 602932) [29], phosphatase and tensin homolog (PTEN; 601728) [30], a negative regulator of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, and Sprouty homolog 1 (SPRY1; 602465) [31], a potent inhibitor of the Ras/MEK/ERK pathway [32]. SMAD7, PTEN, and SPRY are known to modulate the expression of VEGF [29,33,34]. However, miR-21-mediated regulation of VEGF through SMAD7, PTEN, and SPRY has not been explored in the retina. Here, we hypothesize that high glucose might have angiogenic effects through the PRR receptor, independent of Ang II, that leads to the stimulation of VEGF through miR-21-mediated regulation of SMAD7, PTEN, and SPRY. To examine the role of PRR in our experiments, high glucose-mediated induction of VEGF independently of Ang II was investigated by treating ARPE-19 cells with angiotensin converting enzyme (ACE) inhibitor perindopril.
Methods
Cell culture
ARPE-19 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA). To authenticate the cell line, a short tandem repeat (STR) analysis as shown in Appendix 1 was performed by the Emory Integrated Genomics Core (EIGC) using the GenePrint 10 System (Promega, Madison, WI). ARPE-19 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM):F12 medium (ATCC, catalog # 30-2006, Manassas, VA), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (LONZA), at 37 °C in 5% CO2 and 90% relative humidity, and incubated in normoglycemic (NG, 5.5 mmol/l) or hyperglycemic (high glucose, 33 mmol/l) conditions for 48 h. The cells were treated with ACE inhibitor perindopril (10 µmol/l, Sigma-Aldrich, St. Louis, MO) for 24 h, followed by stimulation with human prorenin (Cayman Chemical, Ann Arbor, MI, Item # 10,007,599) at a final concentration of 10 nmol/l and high glucose for 48 h. To examine the role of miR-21 in the regulation of SPRY1, SMAD7, PTEN, ERK, hypoxia-inducible factor (HIF-1α), and VEGF, cells cultured in the NG and/or high glucose conditions were transfected with the scramble control (Scr: Qiagen Inc., Valencia, CA, cat# 1,027,280), mimics (Qiagen, cat# MSY0000076), or antagomirs (Qiagen, cat# MIN0000076) of miR-21 targets the mature sequence 5′-UAG CUU AUC AGA CUG AUG UUG A-3′. The mRNAs/miRNA and proteins were analyzed with quantitative real-time PCR (qRT-PCR) and western blotting, respectively, as described previously [35,36]. Hypoxanthine phosphoribosyltransferase (HPRT)/snRNA U6 and actin were used as internal controls for the qRT-PCR and western blot analyses, respectively.
RNA interference
RNA interference (RNAi) was performed following our previous protocol [37]. Transfection of siRNAs for targeting endogenous genes was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The knockdown of endogenous Ras-related C3 botulinum toxin substrate 1(RAC1) was performed using the predesigned small interfering RNA (siRNA) from Santa Cruz Biotechnology, Inc. (Dallas, TX, cat# sc-36351). The siRNA sequences used in the knockdown of the human PRR (OMIM 300556) and RAC1 (OMIM 602048) genes were as follows: PRR- sense: 5′-GGU CUG UUG UUU UCC GAA ATT-3′, antisense: 5′-UUU CGG AAA ACA ACA GAC CCT-3′; RAC1-sense: 5′-GUU CUU AAU UUG CUU UUC Ctt-3′, antisense: 5′-GGA AAA GCA AAU UAA GAA Ctt-3′. The scrambled siRNA was used as the negative control. Twelve-well plates of ARPE-19 cells were cultured to 80% confluence and transfected with 20 nM of RAC1 siRNA, or 20 nM of the negative control. Cells were harvested for RNA analyses 48 h after siRNA transfection.
Reverse transcription and qRT-PCR
MicroRNA-enriched total RNA was extracted from the ARPE-19 cells using the QIAzol lysis reagent and the miRNeasy kit (cat# 217,004) following the manufacturer’s protocol (Qiagen). For the detection of the genes, first-strand cDNA (cDNA) synthesis was performed on 200 ng of total RNA using a combination of oligo (dT) and random primers following the protocols of the QuantiTect Reverse Transcription Kit (Qiagen, Cat. No. 205313). Total RNA (10 ng) was reverse transcribed to cDNA with miRNA-specific miScript primer assays (Qiagen). The reverse-transcription (RT) mixture was incubated at 37 °C for 30 min followed by incubation at 95 °C for 5 min to inactivate the miScript reverse transcriptase mix.
The qRT-PCR was performed in the MyiQ Cycler (Bio-Rad Laboratories Inc., Hercules, CA) following our previous descriptions [35]. Briefly, 25 μl PCR master mix was prepared as follows: 2 μl cDNA, 1× SYBR Green PCR Master Mix (Qiagen), and 300 nM gene-specific primers. The PCR amplification protocols were the following: 5 min at 95 °C, 40 cycles at 95 °C for 10 s and 60 °C for 30 s. The qRT-PCR data were normalized to the expression levels of hypoxanthine-guanine phosphoribosyltransferase (HPRT) [38] and the highly conserved and universally expressed small nuclear RNA U6 (snRNA U6) [39] for the mRNA and miRNA analyses, respectively. Based on our qRT-PCR data, we have not seen any significant effect of high glucose or miR-21 mimics/antagomirs on the expression of HPRT or U6 in ARPE-19 cells, compared with NG. Therefore, HPRT/snRNA U6 were chosen as normalization controls. The specificity of all the PCR primers was confirmed with 1% agarose gel electrophoresis (Appendix 2). Melt curves showed a single peak (data not shown). All primer sequences and the amplicon sizes are shown in Table 1. The delta cycle threshold (ΔCt) values were used to analyze the expression levels of the mRNA and miRNAs. Briefly, the average ΔCt of each group was calculated according to the following formula: ΔCt = average mRNA or miRNA Ct – average of housekeeping gene (HPRT or U6 snRNA) Ct. The change in gene expression was calculated according to the ΔΔCt, where ΔΔCt = ΔCt of the treated group – ΔCt of the control group. The fold change for the mRNA and miRNA expression level was calculated using 2−ΔΔCt [40].
Table 1. Primers used for quantitative real-time PCR.
| Gene | Primer sequence (5’-3’) | Amplicon size (bp) |
|---|---|---|
| CTGF |
F: GCAGGCTAGAGAAGCAGAGC |
153 |
| |
R:ATGTCTTCATGCTGGTGCAG |
|
| HPRT |
F: ACAGGACTGAACGTCTTGCTCG |
87 |
| |
R:TATAGCCCCCCTTGAGCACAC |
|
| HIF-1α |
F: CAGTCGACACAGCCTGGATATG |
184 |
| |
R: CTGTCCTGTGGTGACTTGTCCT |
|
| LOX |
F: TTACCCAGCCGACCAAGATA |
122 |
| |
R: CCTTCAGCCACTCTCCTCTG |
|
| PRR |
F: CAGACGTGGCTGCATTGTCC |
144 |
| |
R: CTGGGGGTAGAGCCAGTTTGTT |
|
| PTEN |
F: TTCAGGACCCACACGACGGGAAG |
171 |
| |
R: TCCTCTGGTCCTGGTATGAAGAATG |
|
| SMAD7 |
F: GGCTCTACTGTGTCCAGGAG |
115 |
| |
R: ACCTTCTGCACCAGCTGACTC |
|
| SPRY1 |
F: CACCACCAACCAGACCAGTC |
156 |
| |
R: CTCCACACTTGCACTTCC CACA |
|
| VEGF-A |
F: TGCCATCCAATCGAGACCCTG |
156 |
| |
R: GGTGATGTTGGACTCCTCAGTG |
|
| VEGFR2 |
F: CAGTCTGGGAGTGAGATGAAGA |
146 |
| R: ATGGACCCTGACAAATGTGCTG |
Immunoblotting
Protein samples were isolated from the confluent ARPE-19 cells growing on 12-well plates by washing in ice-cold PBS (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.8) and then lysed in radioimmunoprecipitation assay (RIPA) buffer [50 mmol/l Tris-HCl (pH 8.0), 150 mmol/l NaCl, 100 µg/ml phenylmethylsulfonyl fluoride, 1% NP-40, 50 mmol/l NaF, 2 mmol/l EDTA], supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The lysates were centrifuged for 5 min at 14,000 ×g. Protein content was determined with Lowry assay [41], and 100 μg aliquots were solubilized in XT sample buffer (Bio-Rad). The immunoblotting was performed following our previous descriptions [36]. Briefly, the proteins were resolved on Criterion (Bio-Rad) gels, transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA) using Trans-Blot® semi-dry transfer cells (Bio-Rad), and subjected to immunoblot analysis. After blocking with 5% milk for 2 h, the membrane was treated with a primary antibody (SMAD7, sc-365846/SPRY1, sc-100861/PTEN, ab-154812/ERK1–2, Cell Signaling #4370/VEGF, ab-1316/Actin, Sigma #A5441) overnight at 4 °C, washed three times with PBST (1× PBS, 0.05% Tween-20), and then incubated with a secondary antibody conjugated with horseradish peroxidases for 1 h at room temperature (about 22 °C). After the membrane was washed three times in 15 min intervals with PBST, the blots were visualized with the enhanced chemiluminescence detection system (GE Healthcare, City, UK) on Blue Lite Autorad Film (ISC BioExpresss, Kaysville, UT). For repeated immunoblotting, the membranes were stripped in the Restore Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL).
Results
Hyperglycemia upregulates the expression of miR-21 through PRR signaling
Compared with the NG condition (5.5 mM glucose), high glucose (33mM glucose) significantly upregulated the level of miR-21 (p = 0.024) in ARPE-19 cells (Figure 1A). In addition, a significant (p<0.02) increase in PRR, VEGF, and VEGFR2 in ARPE-19 was observed under hyperglycemic conditions when compared with the NG condition (Figure 1A). To confirm the role of PRR in modulating the expression of miR-21 in ARPE-19 cells under a hyperglycemic condition, perindopril-treated cells were transfected with scramble control or PRR siRNA in the presence of high glucose. Compared with the scrambled control, siRNA-mediated silencing of PRR significantly reduced the expression of miR-21 (p<0.001), as well as that of VEGF and VEGFR2, indicative of the involvement of miR-21 in the regulation of VEGF and VEGFR2 (Figure 1B).
Figure 1.
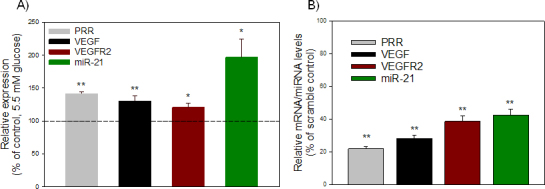
Effect of high glucose on PRR and its downstream targets. A: High glucose increased the transcripts level of PRR, VEGF, VEGFR2, and miR-21. ARPE-19 cells were incubated with perindopril (10 µM) for 24 h and then treated with 33 mM glucose (high glucose) for 48 h. Cells incubated in 5.5 mM glucose (NG) were used as control. B: Knockdown of PRR significantly decreased high glucose–induced upregulation of the expression of VEGF, VEGFR2, and miR-21. Perindopril-treated cells were transfected with 20 nM of control siRNA or PRR siRNA in the presence of high glucose. Cells were harvested for total RNA, and each mRNA/miRNA was measured with quantitative real-time PCR (qRT-PCR). The levels of each transcript were normalized to that of HPRT/snU6. The ratio was then normalized to that of the control and was plotted as a percentage of control. Values are presented as mean ± standard error of the mean (SEM); n = 4; *p<0.05, **p<0.001 versus control (set to 100%).
RAC1 regulates the expression of CTGF, LOX, and miR-21 in a hyperglycemic condition
It has been reported that activation of RAC1 by angiotensin II leads to a connective tissue growth factor (CTGF)- and lysyl oxidase (LOX)-mediated increase of miR-21 expression during atrial fibrillation [42]. To investigate if high glucose–induced PRR signaling activates CTGF, LOX, and miR-21 expression through RAC1, we transfected perindopril-treated cells with 20 nM of control siRNA or RAC1 siRNA in the presence of high glucose. Compared with NG, high glucose significantly increased the expression of CTGF (p = 0.024) and LOX (p = 0.010; Figure 2A). In addition, high glucose induced the expression of miR-21 (p = 0.004), compared with NG (Figure 2B). However, compared with scramble siRNA, silencing of RAC1 significantly reduced the high glucose–induced expression of CTGF (p<0.001), LOX (p = 0.001), and miR-21 (p<0.001) as measured with qRT-PCR, suggesting the critical involvement of CTGF and LOX in the regulation of miR-21 through RAC1 (Figure 2A).
Figure 2.
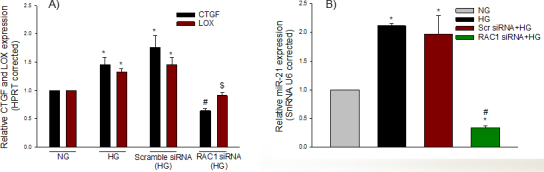
RAC1 regulates CTGF, LOX, and miR-21 expression in hyperglycemic condition. Perindopril-treated cells were transfected with 20 nM of control (Scr) siRNA or RAC1 siRNA in the presence of high glucose and harvested for miRNA-enriched total RNA to measure CTGF and LOX (A) and miR-21 (B) expression as quantified with quantitative real-time PCR (qRT-PCR). Untransfected cells treated with NG were also used as control to high glucose. The level of each transcript was normalized to that of HPRT/snRNA U6. The ratio was then normalized to that of control. Values are presented as mean ± standard error of the mean (SEM); n = 4; *p<0.05, versus NG; #p<0.001 versus scramble siRNA (high glucose) or (2B); $p = 0.001 versus scramble siRNA (high glucose).
MiR-21 in ARPE-19 cells reduces the expression of SMAD7
According to the web-based (Starbase) predictive software developed to explore miRNA-target interaction maps from CLIP-Seq and Degradome-Seq data [43] and data from other groups [44], miR-21 was predicted to be an upstream regulatory miRNA of Smad 7, and based on luciferase reporter assays, SMAD7 appears to be a direct target of miR-21 [29,45]. To examine whether miR-21 affects SMAD7 expression, ARPE-19 cells were transfected with scramble control, miR-21 mimics, or antagomirs of miR-21 in the presence of high glucose. Compared with NG, high glucose decreased the expression of SMAD7 (p = 0.008) and protein (p = 0.003). The miR-21 mimics further suppressed SMAD7 mRNA (Figure 3) and protein (Figure 4), whereas blocking miR-21 by its antagomir significantly increased the expression of SMAD7, compared to the high glucose and miR-21 mimic groups. These data suggest that miR-21 targets SMAD7 in ARPE-19 cells.
Figure 3.
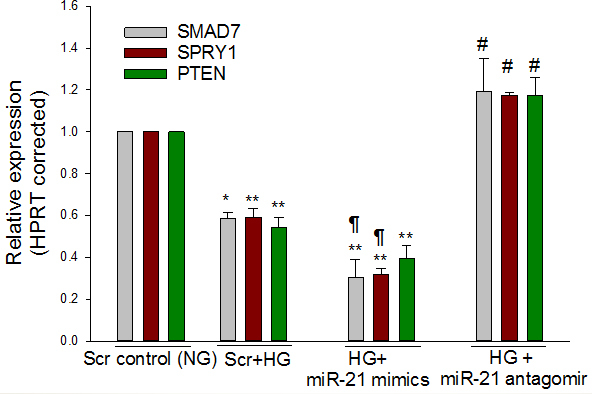
Downregulation of SMAD7, SPRY1 and PTEN by high glucose is mediated by miR-21. Perindopril-treated ARPE-19 cells were transfected with scramble control (NG and high glucose), miR-21 mimic (high glucose), or antagomir of miR-21 (high glucose). Compared to NG, quantitative real-time PCR (qRT-PCR) showed high glucose-mediated suppression of SMAD7, SPRY1, and PTEN. In addition, miR-21 mimics under the high glucose condition further reduced the transcript level of SMAD7 and SPRY1 compared with high glucose alone. However, blocking miR-21 by its antagomir significantly increased the expression of SMAD7, SPRY1, and PTEN. mRNA levels were expressed relative to HPRT, and NG was set to 1. Mean ± standard error of the mean (SEM); n = 4. *p<0.05, **p<0.001 versus NG; #p<0.001 versus high glucose, high glucose and miR-21 mimics; ¶ p<0.05 (SMAD7) or p<0.001 (SPRY1) versus high glucose.
Figure 4.
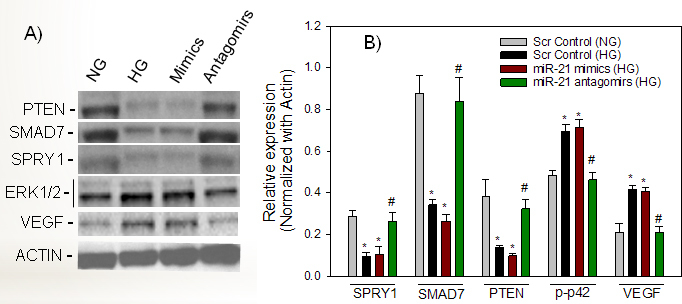
miR-21-regulated SMAD7/SPRY1/PTEN signaling is linked to the activation of pERK and VEGF in ARPE-19 cells under the high glucose condition. Perindopril-treated cells were transfected with scramble control (NG or high glucose), miR-21 mimics (high glucose), or antagomirs of miR-21 (high glucose). Using specific antibodies to SMAD7, SPRY1, PTEN, ERK, and VEGF, the protein/phosphorylation levels were measured with immunoblotting. Compared with scramble NG, a representative western blot (A) and protein analysis (B) showed high glucose-mediated suppression of SPRY1, SMAD7, and PTEN, but increased the activity/expression of ERK and VEGF. Compared with high glucose, miR-21 mimics under high glucose did not show a significant difference at the level of SPRY1, SMAD7, PTEN, and VEGF proteins, as well as the activity of pERK. miR-21 mimics under the high glucose condition did not produce an additional effect compared to high glucose, possibly because the expression of miR-21 is already high in the high glucose condition. The levels of each protein are normalized to that of actin. Values are presented as mean ± standard error of the mean (SEM); n = 3; *p<0.05 versus NG; #p<0.05 versus high glucose control, high glucose and miR-21 mimics.
MiR-21 in ARPE-19 cells reduces the expression of SPRY1
Using bioinformatic algorithms and luciferase reporter assays, Thum et al. [31] identified SPRY1 as a target of miR-21. We also found that miR-21 targets SPRY1 in the ARPE-19 cells. Compared with NG, high glucose significantly decreased the expression of the mRNA (p<0.001) and protein (p = 0.014) levels of SPRY1 (Figure 3, Figure 4) in ARPE-19 cells. In addition, transfection of ARPE-19 cells with the miR-21 mimics resulted in significant suppression of the expression of SPRY1 at the mRNA and protein levels. The high glucose/miR-21 mimics-mediated suppression of SPRY1 at the transcript (Figure 3) and protein (Figure 4) levels was significantly reversed by the antagomir of miR-21 indicating that SPRY1 is a valid target of miR-21.
MiR-21 regulates expression of PTEN and HIF-1α
To assess whether miR-21 regulates PTEN expression in ARPE-19 cells in the high glucose condition, ARPE-19 cells were transiently transfected with scrambled control, miR-21 mimics, or antagomirs of miR-21. We demonstrated that high glucose and miR-21 mimics significantly decreased the expression of PTEN (p<0.01) at the mRNA (Figure 3) and protein (Figure 4) levels, when compared with NG. The miR-21 antagomirs not only significantly (p<0.01) reversed the effect of high glucose on PTEN expression at the transcript and protein levels, but also that of miR-21 mimics in the presence of high glucose, suggesting that high glucose–induced miR-21 downregulates the expression of PTEN. These findings suggest that miR-21 inhibits the expression of PTEN (Figure 3, Figure 4). In addition, it was demonstrated that high glucose significantly increased (p<0.01) the mRNA expression of HIF-1α, compared with NG. However, miR-21 mimics under the high glucose condition did not increase further the expression of HIF-1α at the transcript level, compared to scramble and high glucose (Figure 5). In contrast, the treatment with miR-21 antagomirs significantly decreased (p<0.01) the high glucose–induced expression levels of HIF-1α and restored its expression level to that of the control (Figure 5).
Figure 5.
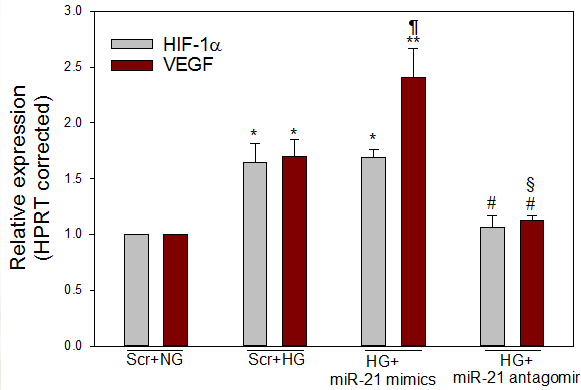
High glucose and miR-21 mimics enhanced the expression of HIF-1α and VEGF. Perindopril-treated ARPE-19 cells were transfected with scramble control (NG and high glucose), miR-21 mimics (high glucose), or antagomirs of miR-21 (high glucose). Compared with NG, quantitative real-time PCR (qRT-PCR) showed high glucose–induced increase of HIF-1α and VEGF. Compared with high glucose, miR-21 mimics under the high glucose condition further increased the transcript level of VEGF, but not HIF-1α. However, blocking miR-21 by its antagomir significantly reduced the expression of VEGF and HIF-1α. mRNA levels were expressed relative to HPRT and NG was set to 1. Mean ± standard error of the mean (SEM); n = 3–4. *p<0.01, **p<0.001 versus NG; ¶p = 0.001 versus high glucose (for VEGF); #p<0.05 versus high glucose, high glucose and miR-21 mimics (for HIF-1α); #p<0.05 versus high glucose or §p<0.001 versus high glucose and miR-21 mimics (for VEGF).
MiR-21 regulates expression of ERK and VEGF
To further evaluate whether high glucose–induced overexpression of miR-21 or the mimics of miR-21 in the high glucose condition modulated the expression of ERK and VEGF, ARPE-19 cells were transfected with scrambled control in the NG and high glucose conditions, the miR-21 mimics (high glucose), or antagomirs of miR-21 (high glucose). Compared with NG, VEGF expression at the mRNA and protein levels was significantly increased in response to high glucose (p<0.05). Compared to scramble and high glucose, miR-21 mimics under the high glucose condition significantly increased the VEGF transcript (p = 0.001) but not the amount of protein (Figure 4, Figure 5). In contrast, transfection with miR-21 antagomirs decreased the level of the mRNA and protein levels of VEGF in high glucose, suggesting that miR-21 in the high glucose condition modulates the expression of VEGF in ARPE-19 cells (Figure 4, Figure 5). To further examine whether miR-21 mimics could induce the phosphorylation level of ERK, we measured p42/p44 expression with western blotting assay using a specific antibody to p44/p42 (ERK1/2). Compared with NG, high glucose increased the phosphorylation level of p42 (p<0.01) and restored its phosphorylation level (p = 0.003) when cells were transfected with miR-21 antagomirs (Figure 4), suggesting that miR-21 modulates the phosphorylation level of ERK under a hyperglycemic condition. However, high glucose and miR-21 mimics when compared to high glucose and scramble did not affect the phosphorylation level of p42.
Discussion
A local RAS with all its components is expressed in the retina, Müller cells, RPE, and RECs [46-48]. We demonstrated that the glucose-induced upregulation of VEGF was associated with the increased expression of miR-21 that led to the suppression of SPRY1, PTEN, and SMAD7, the key regulators of VEGF synthesis. In our recent study [9], we demonstrated that AT1R and PRR mediated the induction of VEGF, VEGFR2, and TGF-β1 production in response to high glucose in human retinal endothelial cells. Compared with control, siRNA-targeted silencing of AT1R and PRR significantly reduced the expression of VEGF, VEGFR2, and TGF-β1. In addition, compared with the separate knockdown of AT1R or PRR, a much larger reduction in the expression of VEGF, VEGFR2, and TGF-β1 was observed upon the siRNA-mediated knockdown of both receptors together, indicating the involvement of both receptors in high glucose-mediated activation of VEGF, VEGFR2, and TGF-β1 [9].
In our present study, high glucose increased the level of miR-21, which targets SMAD7, SPRY1, and PTEN. Increasing evidence suggests that miRNAs play a critical role in the pathogenesis of diabetes and diabetes-related vascular complications [49]. The role of miR-21 in the pathogenesis of diabetes has not been explored previously. Similar to the findings in most cancerous cell types [50-52], a high glucose–induced increase of miR-21 was also observed in our study from ARPE-19 cells. Overexpression of miR-21 has been reported to increase the expression of VEGF and VEGFR2, which promote angiogenesis [53,54].
The role of VEGF/VEGF receptor signaling is well-known for proper vascular development and angiogenesis, processes that have been linked to PI3K signaling [55]. The Ras-MAP kinase pathway has also been reported to signal for VEGF-induced growth [53,56]. It has recently been reported that PI3K not only gets phosphorylated upon VEGF stimulation, but also VEGF induces a striking activation of MAP kinase activity [55]. We showed in the present experiments that high glucose–induced increase of miR-21 suppressed the expression of SMAD7 and the miR-21 antagomir was able to reverse the high glucose–mediated suppression of SMAD7 expression, indicating that SMAD7 is a direct target of miR-21. SMAD7 plays a protective role in diabetic nephropathy because deletion of SMAD7 enhances, whereas overexpression of SMAD7 inhibits, SMAD3-mediated renal fibrosis [57], and suppression of SMAD7 levels may be the mechanism to stimulate activation of TGF-β and nuclear factor (NF)-κB for promoting inflammation in diabetic retinal injury [29].
PTEN, a negative regulator of the PI3K/AKT pathway, is also a potential target of miR-21, which suggests that miR-21 might be associated with insulin resistance or diabetes [58]. In addition, in this study, high glucose–induced increase of miR-21 significantly reduced the expression of PTEN at the transcript and protein levels. PTEN-related angiogenesis might be attributed to PTEN’s upregulation of the expression of VEGF [59], but the mechanism of PTEN-related angiogenesis is not well-known. However, a high concentration of glucose in rat mesangial cells [60] and diabetic mice [61] has been reported to decrease the expression of PTEN and its phosphatase activity, resulting in increased Akt activity. The PTEN/PI3K/Akt pathway has been reported to be involved in miR-21-mediated prevention of mesangial hypertrophy in diabetic db/db mice and mesangial cells [62]. In addition, the inhibition of PTEN activity in cells has been reported to have elevated PIP3 concentrations and higher levels of Akt phosphorylation [63]. Alternatively, high glucose–stimulated expression of TGF-β and VEGF has been reported in ARPE-19 cells [9,64]. It is possible that glucose-mediated stimulation of TGF-β activates Akt through a microRNA-dependent amplifying circuit targeting PTEN [65,66]. It is known that HIF-1α binds to the VEGF gene promoter and induces its expression [67]. Based on experiments with ARPE-19 cells, it was suggested that HIF-1α and VEGF contribute to the pathogenesis of diabetic retinopathy [33]. The increased levels of HIF-1α and VEGF are also reported in the vitreous fluid of patients with proliferative diabetic retinopathy [68]. In our experiments and supported by others [69], it has been shown that high glucose–induced increase of miR-21, possibly through the suppression of PTEN, enhances the expression of HIF-1α and VEGF in ARPE-19 cells, and this process was reversed with the antagomir of miR-21 indicating that the high glucose–induced increase of HIF-1α through suppression of PTEN may be a possible mechanism to enhance the expression of VEGF in ARPE-19 cell. Thus, HIF-1α turns out to be a key downstream target of miR-21 in regulating VEGF.
MiR-21-mediated downregulation of SPRY1 followed by enhancement of ERK activity or phosphorylation has been well demonstrated by several authors [70,71]. It is known that knockdown of SPRY1 expression led to a significant increase in ERK–MAP kinase activity [31]. In ARPE-19 cells, we demonstrated that the downregulation of the expression of the SPRY1 gene mediated by the mimics of miR-21 enhanced the activity of the expression of ERK and VEGF, and this process was reversed by miR-21 antagomirs. Upregulation of SPRY1 in endothelial cells has been reported to decrease VEGF–induced endothelial cell proliferation [34].
It is not known how the RAS is involved in the activation of miR-21 expression under a hyperglycemic condition. However, angiotensin II binding to AT1R is reported to activate RAC1-GTPase that leads to a connective tissue growth factor- and lysyl oxidase-mediated increase in miR-21 expression, contributing to structural remodeling of the atrial myocardium [42]. LOX is known to cross-link the side chain of collagen and elastin and thus contributes to extracellular matrix integrity. In perindopril-treated cells, we have shown for the first time that high glucose–induced PRR stimulates the expression of CTGF, LOX, and miR-21. The small interfering RNA for RAC1 remarkably reduced the high glucose–induced increase in the expression of CTGF, LOX, and miR-21, indicating RAC1, CTGF, and LOX are possible mediators of high glucose–induced regulation of miR-21 in ARPE-19 cells. LOX upregulation is known to increase the expression of Drosha and Dicer, vital endonucleases for miRNA generation [72]. In addition, high glucose–induced expression and activity of LOX were observed in retinal endothelial cells and in diabetic retinas [73]. Additionally, diabetes was found to be associated with a significant increase in the mRNA and protein levels of CTGF, which was attenuated with perindopril treatment [74]. Diabetes-mediated increase in CTGF has been reported to upregulate the expression of VEGF and TGF-β2 and induce apoptosis in the retinas of diabetic rats [75]. RAC1 is also crucial for activating HIF-1α in RPE cells under hypoxia [76]. Similar to the AT1R-mediated activation of RAC1, we hypothesize that prorenin binding to PRR under a hyperglycemic condition activates RAC1, which, in turn, stimulates the expression of CTGF and LOX that leads to high glucose-mediated increase of miR-21.
To our knowledge, this is the first report that high glucose–induced PRR regulates miR-21 through RAC1, which, in turn, modulates the expression of VEGF in ARPE-19 cells through modulation of SMAD7, PTEN, and SPRY1 (Figure 6). Further work is needed to evaluate the role of miR-21 in high glucose–induced stimulation of VEGF, to identify downstream targets, and to develop therapeutic strategies targeting miR-21 in vivo.
Figure 6.
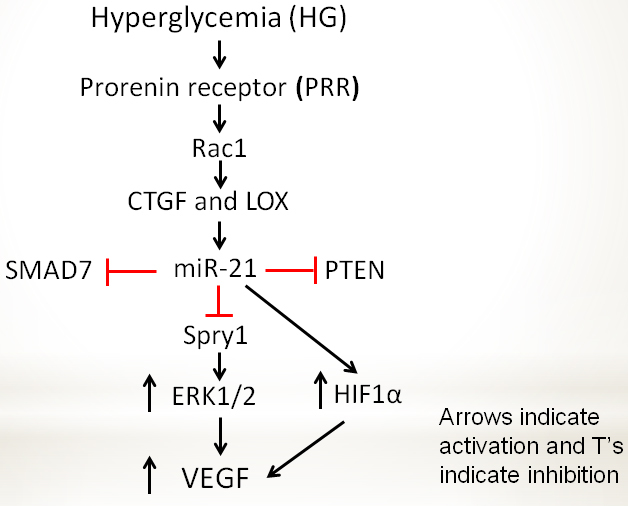
Scheme showing hyperglycemia-induced VEGF expression regulated by PRR, RAC1, and miRNA signaling. Hyperglycemia-induced PRR activates a small GTPase RAC1 that leads to the increased expression of miR-21 via stimulation of CTGF and LOX. Increased miR-21 targeting SMAD7, SPRY1, and PTEN, and using various signaling pathways (see Discussion) regulates VEGF synthesis in ARPE-19 cells. Arrows indicate activation, and T’s indicate inhibition.
Acknowledgments
The research was supported by an unrestricted departmental award from Research to Prevent Blindness (RPB), Inc., and NIH grants R01EY004864, and P30EY006360. The authors thank Jennifer Chung Ah Ro, Curran S. Sidhu, Bhavna Kumar, and Julien Brock for technical assistance. The authors declare no conflict of interest with respect to the research reported herein.
Appendix 1. STR analysis.
To access these data, click or select the words "Appendix 1".
Appendix 2. PCR primers with 1% agarose gel electrophoresis.
To access these data, click or select the words "Appendix 2".
References
- 1.Cohen A, Horton ES. Progress in the treatment of type 2 diabetes: new pharmacologic approaches to improve glycemic control. Curr Med Res Opin. 2007;23:905–17. doi: 10.1185/030079907x182068. [DOI] [PubMed] [Google Scholar]
- 2.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 3.King GL, Kunisaki M, Nishio Y, Inoguchi T, Shiba T, Xia P. Biochemical and molecular mechanisms in the development of diabetic vascular complications. Diabetes. 1996;45:S105–8. doi: 10.2337/diab.45.3.s105. [DOI] [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:26. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int. 1996;50:1897–903. doi: 10.1038/ki.1996.511. [DOI] [PubMed] [Google Scholar]
- 6.Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension. 2005;46:1069–76. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–27. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system–an endocrine and paracrine system. Endocrinology. 2003;144:2179–83. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 9.Haque R, Hur EH, Farrell AN, Iuvone PM, Howell JC. MicroRNA-152 represses VEGF and TGFbeta1 expressions through post-transcriptional inhibition of (Pro)renin receptor in human retinal endothelial cells. Mol Vis. 2015;21:224–35. [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Matavelli LC, Siragy HM. Renal (pro)renin receptor contributes to development of diabetic kidney disease through transforming growth factor-beta1-connective tissue growth factor signalling cascade. Clin Exp Pharmacol Physiol. 2011;38:215–21. doi: 10.1111/j.1440-1681.2011.05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37:277–82. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siragy HM, Huang J. Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol. 2008;93:709–14. doi: 10.1113/expphysiol.2007.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–9. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima DT. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–6. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert RE, Vranes D, Berka JL, Kelly DJ, Cox A, Wu LL, Stacker SA, Cooper ME. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab Invest. 1998;78:1017–27. [PubMed] [Google Scholar]
- 17.Ellis EA, Guberski DL, Somogyi-Mann M, Grant MB. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 2000;28:91–101. doi: 10.1016/s0891-5849(99)00216-6. [DOI] [PubMed] [Google Scholar]
- 18.Tilton RG, Kawamura T, Chang KC, Ido Y, Bjercke RJ, Stephan CC, Brock TA, Williamson JR. Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J Clin Invest. 1997;99:2192–202. doi: 10.1172/JCI119392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues M, Xin X, Jee K, Babapoor-Farrokhran S, Kashiwabuchi F, Ma T, Bhutto I, Hassan SJ, Daoud Y, Baranano D, Solomon S, Lutty G, Semenza GL, Montaner S, Sodhi A. VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62:3863–73. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 21.Pandey AK, Agarwal P, Kaur K, Datta M. MicroRNAs in diabetes: tiny players in big disease. Cell Physiol Biochem. 2009;23:221–32. doi: 10.1159/000218169. [DOI] [PubMed] [Google Scholar]
- 22.McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–23. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:4402–9. doi: 10.1167/iovs.10-6879. [DOI] [PubMed] [Google Scholar]
- 24.Feng B, Chen S, McArthur K, Wu Y, Sen S, Ding Q, Feldman RD, Chakrabarti S. miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–84. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao Y, Mohan R, Zhang S, Tang X. MicroRNAs as pharmacological targets in diabetes. Pharmacol Res. 2013;75:37–47. doi: 10.1016/j.phrs.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roggli E, Britan A, Gattesco S, Lin-Marq N, Abderrahmani A, Meda P, Regazzi R. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes. 2010;59:978–86. doi: 10.2337/db09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng J, Xiong Y, Li G, Liu M, He T, Tang Y, Chen Y, Cai L, Jiang R, Tao J. MiR-21 is overexpressed in response to high glucose and protects endothelial cells from apoptosis. Exp Clin Endocrinol Diabetes. 2013;121:425–30. doi: 10.1055/s-0033-1345169. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Li W, Xu M, Huang H, Wang J, Chen X. Micro-RNA 21Targets dual specific phosphatase 8 to promote collagen synthesis in high glucose-treated primary cardiac fibroblasts. Can J Cardiol. 2014;30:1689–99. doi: 10.1016/j.cjca.2014.07.747. [DOI] [PubMed] [Google Scholar]
- 29.Zhong X, Chung AC, Chen HY, Dong Y, Meng XM, Li R, Yang W, Hou FF, Lan HY. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663–74. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta. 2010;411:846–52. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 31.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 32.Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol. 2002;4:850–8. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 33.Forooghian F, Razavi R, Timms L. Hypoxia-inducible factor expression in human RPE cells. Br J Ophthalmol. 2007;91:1406–10. doi: 10.1136/bjo.2007.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huebert RC, Li Q, Adhikari N, Charles NJ, Han X, Ezzat MK, Grindle S, Park S, Ormaza S, Fermin D, Miller LW, Hall JL. Identification and regulation of Sprouty1, a negative inhibitor of the ERK cascade, in the human heart. Physiol Genomics. 2004;18:284–9. doi: 10.1152/physiolgenomics.00098.2004. [DOI] [PubMed] [Google Scholar]
- 35.Haque R, Chun E, Howell JC, Sengupta T, Chen D, Kim H. MicroRNA-30b-Mediated Regulation of Catalase Expression in Human ARPE-19 Cells. PLoS One. 2012;7:6. doi: 10.1371/journal.pone.0042542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haque R, Ali FG, Biscoglia R, Abey J, Weller J, Klein D, Iuvone PM. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem. 2010;113:1296–306. doi: 10.1111/j.1471-4159.2010.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haque R, Chong NW, Ali F, Chaurasia SS, Sengupta T, Chun E, Howell JC, Klein DC, Iuvone PM. Melatonin synthesis in retina: cAMP-dependent transcriptional regulation of chicken arylalkylamine N-acetyltransferase by a CRE-like sequence and a TTATT repeat motif in the proximal promoter. J Neurochem. 2011;119:6–17. doi: 10.1111/j.1471-4159.2011.07397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin PM, Ananth S, Cresci G, Roon P, Smith S, Ganapathy V. Expression and localization of GPR109A (PUMA-G/HM74A) mRNA and protein in mammalian retinal pigment epithelium. Mol Vis. 2009;15:362–72. [PMC free article] [PubMed] [Google Scholar]
- 39.Hou Q, Tang J, Wang Z, Wang C, Chen X, Hou L, Dong XD, Tu L. Inhibitory effect of microRNA-34a on retinal pigment epithelial cell proliferation and migration. Invest Ophthalmol Vis Sci. 2013;54:6481–8. doi: 10.1167/iovs.13-11873. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 42.Adam O, Löhfelm B, Thum T, Gupta SK, Puhl SL, Schäfers HJ, Böhm M, Laufs U. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. 2012;107:278. doi: 10.1007/s00395-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 43.Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011;39:30. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–97. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Zhang D, Wang Y, Sun P, Hou X, Larner J, Xiong W, Mi J. MiR-21/Smad 7 signaling determines TGF-beta1-induced CAF formation. Sci Rep. 2013;3 doi: 10.1038/srep02038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alcazar O, Cousins SW, Striker GE, Marin-Castano ME. (Pro)renin receptor is expressed in human retinal pigment epithelium and participates in extracellular matrix remodeling. Exp Eye Res. 2009;89:638–47. doi: 10.1016/j.exer.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, Schalekamp MA, Ganten D. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol. 1996;80:159–63. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson-Berka JL. Prorenin and the (pro)renin receptor in ocular pathology. Am J Pathol. 2008;173:1591–4. doi: 10.2353/ajpath.2008.080757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mastropasqua R, Toto L, Cipollone F, Santovito D, Carpineto P, Mastropasqua L. Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res. 2014;43:92–107. doi: 10.1016/j.preteyeres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 52.Li S, Yang X, Yang J, Zhen J, Zhang D. Serum microRNA-21 as a potential diagnostic biomarker for breast cancer: a systematic review and meta-analysis. Clin Exp Med. 2014;17:17. doi: 10.1007/s10238-014-0332-3. [DOI] [PubMed] [Google Scholar]
- 53.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One. 2011;6:0019139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao D, Tu Y, Wan L, Bu L, Huang T, Sun X, Wang K, Shen B. In vivo monitoring of angiogenesis inhibition via down-regulation of mir-21 in a VEGFR2-luc murine breast cancer model using bioluminescent imaging. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999;274:10002–7. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- 56.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–7. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 57.Lan HY. Transforming growth factor-beta/Smad signalling in diabetic nephropathy. Clin Exp Pharmacol Physiol. 2012;39:731–8. doi: 10.1111/j.1440-1681.2011.05663.x. [DOI] [PubMed] [Google Scholar]
- 58.Ling HY, Hu B, Hu XB, Zhong J, Feng SD, Qin L, Liu G, Wen GB, Liao DF. MiRNA-21 reverses high glucose and high insulin induced insulin resistance in 3T3–L1 adipocytes through targeting phosphatase and tensin homologue. Exp Clin Endocrinol Diabetes. 2012;120:553–9. doi: 10.1055/s-0032-1311644. [DOI] [PubMed] [Google Scholar]
- 59.Ma J, Sawai H, Ochi N, Matsuo Y, Xu D, Yasuda A, Takahashi H, Wakasugi T, Takeyama H. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem. 2009;331:161–71. doi: 10.1007/s11010-009-0154-x. [DOI] [PubMed] [Google Scholar]
- 60.Mahimainathan L, Das F, Venkatesan B, Choudhury GG. Mesangial cell hypertrophy by high glucose is mediated by downregulation of the tumor suppressor PTEN. Diabetes. 2006;55:2115–25. doi: 10.2337/db05-1326. [DOI] [PubMed] [Google Scholar]
- 61.Dey N, Das F, Mariappan MM, Mandal CC, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes. J Biol Chem. 2011;286:25586–603. doi: 10.1074/jbc.M110.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z, Peng H, Chen J, Chen X, Han F, Xu X, He X, Yan N. MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice. FEBS Lett. 2009;583:2009–14. doi: 10.1016/j.febslet.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 63.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 64.Grigsby J, Betts B, Vidro-Kotchan E, Culbert R, Tsin A. A possible role of acrolein in diabetic retinopathy: involvement of a VEGF/TGFbeta signaling pathway of the retinal pigment epithelium in hyperglycemia. Curr Eye Res. 2012;37:1045–53. doi: 10.3109/02713683.2012.713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci. 2015;129:1237–49. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 66.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–9. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor–HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19:90–7. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 68.Wang X, Wang G, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148:883–9. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Richart A, Loyer X, Neri T, Howangyin K, Guerin CL, Ngkelo A, Bakker W, Zlatanova I, Rouanet M, Vilar J, Levy B, Rothenberg M, Mallat Z, Puceat M, Silvestre JS. MicroRNA-21 coordinates human multipotent cardiovascular progenitors therapeutic potential. Stem Cells. 2014;32:2908–22. doi: 10.1002/stem.1789. [DOI] [PubMed] [Google Scholar]
- 70.Ma X, Kumar M, Choudhury SN, Becker Buscaglia LE, Barker JR, Kanakamedala K, Liu MF, Li Y. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc Natl Acad Sci USA. 2011;108:10144–9. doi: 10.1073/pnas.1103735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ling M, Li Y, Xu Y, Pang Y, Shen L, Jiang R, Zhao Y, Yang X, Zhang J, Zhou J, Wang X, Liu Q. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-kappaB in arsenite-induced cell transformation. Free Radic Biol Med. 2012;52:1508–18. doi: 10.1016/j.freeradbiomed.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 72.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 73.Chronopoulos A, Tang A, Beglova E, Trackman PC, Roy S. High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes. 2010;59:3159–66. doi: 10.2337/db10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tikellis C, Cooper ME, Twigg SM, Burns WC, Tolcos M. Connective tissue growth factor is up-regulated in the diabetic retina: amelioration by angiotensin-converting enzyme inhibition. Endocrinology. 2004;145:860–6. doi: 10.1210/en.2003-0967. [DOI] [PubMed] [Google Scholar]
- 75.Yang H, Huang Y, Chen X, Liu J, Lu Y, Bu L, Xia L, Xiao W, Chen M, Nie Q, Liu Z. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-beta(2), elucidated by treatment with CTGFsiRNA. Acta Ophthalmol. 2010;88:652–9. doi: 10.1111/j.1755-3768.2009.01641.x. [DOI] [PubMed] [Google Scholar]
- 76.Zhang P, Zhang X, Hao X, Wang Y, Hui Y, Wang H, Hu D, Zhou J. Rac1 activates HIF-1 in retinal pigment epithelium cells under hypoxia. Graefes Arch Clin Exp Ophthalmol. 2009;247:633–9. doi: 10.1007/s00417-008-1031-0. [DOI] [PubMed] [Google Scholar]


