Abstract
Objective
The objective of this study was to investigate the current situation of vancomycin (VAN)-associated acute kidney injury (VA-AKI) in China and identify the risk factors for VA-AKI, as well as to comprehensively examine the risk related to concurrent drug use. Further, we assessed the outcomes of patients who developed VA-AKI and the risk factors for these outcomes. Finally, we aimed to provide suggestions for improving the prevention and treatment of VA-AKI in China.
Methods
We conducted a retrospective observational study of inpatients who had been treated with VAN between January 2013 and December 2013 at Peking University First Hospital. AKI was defined as an increase in SCr of ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours or an increase to ≥1.5 times the baseline certainly or presumably within the past 7 days. VA-AKI was defined as the development of AKI during VAN therapy or within 7 days following the termination of VAN therapy. In addition, we compared patients with NO-AKI, who did not develop AKI during their hospitalization, with those with VA-AKI.
Results
Of the 934 patients treated with VAN during their hospital stay, 740 were included in this study. Among those excluded, 38.1% (74/194) were excluded because of a lack of data on serum creatinine (SCr). Among the included patients, 120 had confirmed VA-AKI, with an incidence of 16.2% (120/740). Multiple logistic regression analysis revealed that an elevated baseline estimated glomerular filtration rate (eGFR) (odds ratio [OR] = 1.009; p = 0.017) and concomitant vasopressor therapy (OR = 2.942; p = 0.009), nitrate use (OR = 2.869; p = 0.007), imipenem-cilastatin treatment (OR = 4.708; p = 0.000), and contrast medium administration (OR = 6.609 p = 0.005) were independent risk factors for VA-AKI; in addition, the receipt of orthopedic/trauma/burn surgery (OR = 0.3575; p = 0.011) and concomitant compound glycyrrhizin use (OR = 0.290; p = 0.017) were independent protective factors for VA-AKI. Multiple logistic regression analysis also demonstrated that among the patients who developed VA-AKI, coronary heart disease (CHD) (OR = 12.6; p = 0.006) and concomitant vasopressor therapy (OR = 15.4; p = 0.001) were independent risk factors for death. We also evaluated the factors influencing improvement of renal function. Multiple logistic regression analysis demonstrated that CHD (OR = 8.858, p = 0.019) and concomitant contrast medium administration (OR = 9.779, p = 0.005) were independent risk factors and that simultaneous β-blocker treatment (OR = 0.124, p = 0.001) was an independent protective factor for improvement of renal function.
Conclusion
Patients treated with VAN received insufficient monitoring of SCr and inadequate therapeutic drug monitoring. We recommend that hospitals increase their investment in clinical pharmacists. An elevated baseline eGFR and concomitant vasopressor therapy, nitrate use, imipenem-cilastatin treatment, and contrast medium administration were independent risk factors for VA-AKI; in addition, orthopedic/trauma/burn surgery and concomitant compound glycyrrhizin use were independent protective factors for VA-AKI.
Background
Vancomycin (VAN), a glycopeptide antibiotic, was discovered in the 1950s. The prescription of VAN has increased in parallel with the rising prevalence of invasive methicillin-resistant Staphylococcus aureus (MRSA) infection [1–3]. Given the increased resistance of deep-seated infections to VAN, clinical guidelines suggest targeting trough VAN levels of 15–20 mg/l for such cases to ensure clinical efficacy and avoid adverse drug reactions (ADRs) as much as possible. However, acute kidney injury (AKI) is still the main serious ADR experienced by patients receiving VAN treatment, and it can threaten patients’ health and even their survival. Some studies have reported AKI incidences of up to 40% [4,5]. Thus, it is clinically important to confirm the risk factors for VA-AKI to help guide clinicians toward the more rational administration of VAN, with increased renal safety and the avoidance of AKI development. Currently, many factors are known to affect AKI development. Patients who are older, receiving therapy for a long duration and/or receiving therapy concomitantly with nephrotoxic agent treatment, have a high trough level of VAN, are critically ill and have compromised renal function prior to VAN treatment are at particularly high risk of VAN-induced nephrotoxicity [5]. However, relevant findings have not been consistent between among studies [6], and there may be ethnic differences in these risk factors [6]. To date, studies of the Chinese population are very limited, and little is known about the risk factors for VA-AKI among Chinese individuals. A recent national survey on AKI in China has revealed a serious condition issue with drug safety in the AKI population, as up to 70% of AKI patients had been exposed to potentially nephrotoxic drugs before or during their kidney injury. This finding raises a major issue, indicating that the healthcare system should increase the renal safety of drug treatments in China [7]. Drug combinations have complex effects on renal toxicity; however, relevant studies are very limited. Several studies have shown that concomitant administration of amphotericin B, tobramycin or tacrolimus, vasopressor agents or intravenous contrast medium may increase the risk of VA-AKI [5], although few drug combinations were examined in these studies.
The objective of this study was to investigate the current situation of VA-AKI in China and to identify the risk factors for VA-AKI in the Chinese population, as well as to comprehensively analyze the potential impacts of drug combinations, including Chinese patent medicines. Further, we examined the outcomes of patients who developed VA-AKI and the risk factors associated with these outcomes. Finally, we aimed to provide some suggestions for improving the prevention and treatment of VA-AKI in the Chinese population.
Methods
Study design and population
This was a single-center retrospective study performed at Peking University First Hospital. We recruited all patients treated with VAN at our hospital from January 2013 to December 2013. Written informed consent given from the patients whose records were used was not given. Because our study was a retrospective research and data was based on discharged patients medical records. The consent procedure approved by the Ethics Committee that was approved this study. This study was approved by the Peking University First Hospital Clinical Research Ethics Committee on December 15th, 2015. The Approval number was 2015 (998] (see S1 File). Patient data was anonymized prior to analysis. Another pharmacist who was not participated in this study was responsible for anonymizing patient data.
Survey design
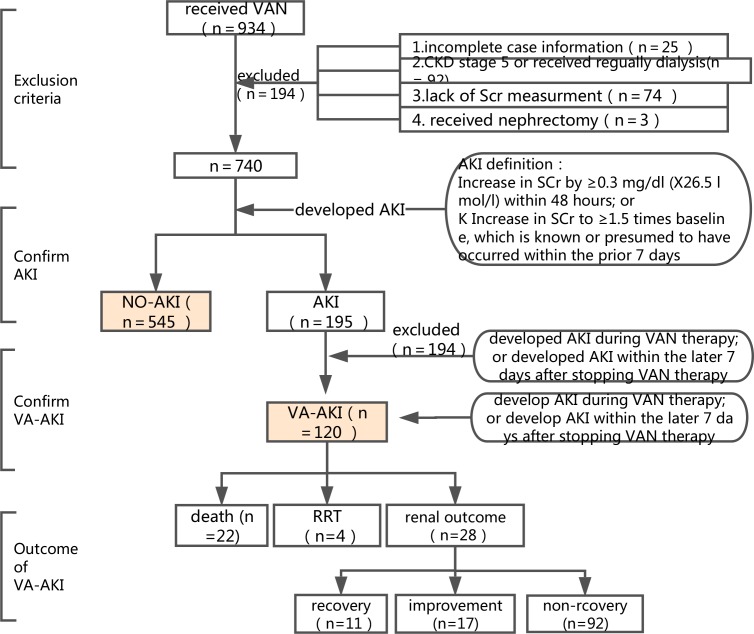
The survey of AKI was designed to include four steps (Fig 1). First, we screened the patients treated with VAN at our hospital; all patients ≥18 years old were included. Patients were excluded if 1) their medical records were incomplete; 2) they had been diagnosed with stage 5 chronic kidney disease (CKD) or were regularly receiving dialysis; 3) their serum creatinine (SCr) were not being adequately monitored to detect the development of AKI; or 4) they had undergone nephrectomy.
Fig 1. Survey design.
Second, we recorded the SCr of the included patients and separated the patients into two groups according to the definition of AKI: the NO-AKI group and the AKI group. We used the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) definition of AKI as the major screening criterion [8]: increase in SCr by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours or increase in SCr to ≥1.5 times baseline which is known or presumed to have occurred within the prior 7 days.
Third, we further excluded patients who developed AKI before receiving VAN treatment or more than 7 days after stopping treatment. The definition of VA-AKI was defined as the development of AKI during VAN treatment or within 7 days following the termination of VAN treatment.
Fourth, for the patients who developed AKI, we further analyzed the development, severity and outcome of AKI. AKI severity was described by the highest stage of AKI (1, 2, or 3) and receipt of renal replacement therapy (RRT), according to the KDIGO criterion. AKI outcome was examined using three variables: all-cause in-hospital death, receipt of RRT and renal outcome at discharge. Renal outcome was categorized into three levels: recovery, improvement and non-recovery. Recovery was defined as restoration of the SCr during hospitalization to the baseline; improvement was defined as a decrease of at least 25% in SCr during hospitalization from beginning of AKI onset; and non-recovery was defined as lack of improvement in SCr at discharge. We combined the recovery and improvement groups into one group for examination of the risk factors related to the renal outcome of VA-AKI.
Data collection
We collected the following variables: demographic characteristics (gender, age, and weight), admission department (medical, surgical, and ICU), concomitant diseases (hypertension, diabetes, coronary heart disease [CHD], CKD, and chronic lung disease [CLD]), laboratory data (baseline SCr), reason for VAN treatment (prophylactic, local infection, and bacteremia), hospitalization factors (length of stay [LOS], ICU admittance, cancer, and surgery), source of VAN, length of therapy (LOT), average daily dosage, therapeutic drug monitoring (TDM), and the use of concomitant drugs (vasopressors, nitrates, β-receptor blockers, phentolamine, amlodipine, sodium nitroprusside, angiotensin II receptor blockers (ARBs), angiotensin II-converting enzyme inhibitors (ACEIs), aminoglycosides, amphotericin B, azole antifungals, micafungin, metronidazole, nystatin, acyclovir, imipenem-cystatins, β-lactam antibiotics, macrolides, quinolones, sulfonamides, tigecycline, diuretics, 20% mannitol, low-molecular-weight dextran, nonsteroidal anti-inflammatory drugs (NSAIDs), immunosuppressants, glucocorticoids, chemotherapy drugs, compound glycyrrhizin, compound fresh bamboo juice, liquorice tablets, Ganmao Qingre granules, Yunnan Baiyao capsules, ursodeoxycholic acid, Simotang, Qingkailing, and senna leaf).
Data analysis
Normally distributed continuous variables were expressed as the mean ±standard deviation (SD), and groups were compared using the independent t test. Non-normally distributed continuous variables were presented as the median (interquartile range [IQR]), and groups were compared using the rank-sum test. In addition, categorical variables were expressed as numbers (percentages) and analyzed using the chi-square test or Fisher’s exact test. Further, logistic regression models were used to assess independent risk factors for VA-AKI incidence and outcome and death. Multiple logistic regression models were used to identify variables with a P value of less than 0.2 in descriptive analysis; these variables were further examined in multivariate analysis to identify independent risk factors. The covariates included in multiple logistic regression analysis of VA-AKI incidence included sex (male vs female), age (change by 10 years), department (medical, surgical and ICU), CHD (yes vs no), baseline SCr (mg/dL), reason for VAN therapy (prophylactic, local infection and bacteremia), LOS (days), ICU admittance (yes vs no), cancer (yes vs no), concomitant low perfusion factors (yes vs no), receipt of orthopedic/trauma/burn surgery and other concomitant situations during hospitalization (yes vs no), LOT (days), mean daily dosage (change by 0.5 g), and receipt of TDM (yes vs no), vasopressors (yes vs no), nitrates (yes vs no), β-receptor blockers (yes vs no), ACEIs (yes vs no), aminoglycosides (yes vs no), azole antifungals (yes vs no), imipenem-cystatins (yes vs no), diuretics (yes vs no), 20% mannitol (yes vs no), contrast medium (yes vs no), compound glycyrrhizin (yes vs no), ursodeoxycholic acid (yes vs no), etc. A backward logistic model was used for the selection of variables. We used the same method to assess the independent risk factors for death and VA-AKI outcome. All p values were two-sided, and a p value of less than 0.05 was deemed significant. Statistical analyses were conducted using Statistical Package for Social Sciences version 20.0 (IBM, Chicago, Ill., USA).
Results
Patients excluded and included
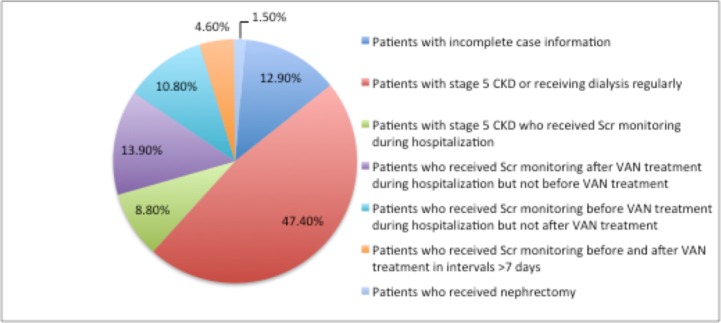
Of the 934 patients who received VAN treatment during their hospital stay, 740 were included in this study. The most common reasons for patient exclusion were the presence of stage 5 CKD or regular receipt of dialysis (47.4%, 92/194) and inadequate SCr monitoring to detect AKI development (38.1%, 74/194)(Fig 2). The total rate of inadequate SCr monitoring among all VAN-treated inpatients was 7.9% (74/934). We further analyzed the departmental distribution of inadequate SCr monitoring, and the results are presented in S1 Table.
Fig 2. Frequency distribution of excluded patients.
A total of 740 patients were included in this study, 50.0% (370/740) of whom were males. The median age was 66 years (IQR = 19). Among the 665 patients included in descriptive analysis, 54.6% (364/665) were females. The ages of 56.2% (374/665) patients were between 60 and 80 years. In addition, 44.4% (291/665) patients concomitant with hypertension, and the rates of concomitant diabetes, CHD, CKD, CLD, cancer and low perfusion factors before or at the time of AKI onset during hospitalization were 22.0% (146/665), 13.7% (91/665), 2.0% (13/665), 2.0% (13/665), and 6.3% (42/665). Further, the median LOS was 25 days (IQR = 14) (Table 1).
Table 1. Comparison of clinical characteristics between VA-AKI and No-AKI patients.
| Total n = 665 |
VA-AKI n = 120 |
No-AKI n = 545 |
P value | |
|---|---|---|---|---|
| Demographic factor | ||||
| Female, n (%) | 364 (54.7) | 52 (43.3) | 312 (57.2) | 0.006 |
| Age (SD), n (%) | 0.000 | |||
| < 50 y | 112 (16.8) | 29 (24.2) | 83 (15.2) | |
| ≥ 50 and < 60 y | 91 (13.7) | 18 (15.0) | 73 (13.4) | |
| ≥ 60 and < 70 y | 175 (26.3) | 20 (16.7) | 155 (28.4) | |
| ≥ 70 and < 80 y | 199 (29.9) | 26 (21.7) | 173 (31.7) | |
| ≥ 80 y | 88 (13.2) | 27 (22.5) | 61 (11.2) | |
| Weight (SD)* | 67.7 ± 12.1 | 64.2 ± 11.4 | 68.4 ± 12.1 | 0.003 |
| Department, n (%) | 0.000 | |||
| Medical | 68 (10.2) | 22 (18.3) | 46 (8.4) | |
| Surgical | 534 (80.4) | 67 (56.3) | 467 (85.7) | |
| ICU | 62 (9.3) | 30 (25.2) | 32 (5.9) | |
| Concomitant diseases | ||||
| Hypertension, n (%) | 0.659 | |||
| No hypertension | 374 (56.2) | 67 (55.8) | 307 (56.3) | |
| Stage 1 | 72 (10.8) | 10 (8.3) | 62 (11.4) | |
| Stage 2 | 83 (12.5) | 18 (15.0) | 65 (11.9) | |
| Stage 3 | 136 (20.5) | 25 (20.8) | 111 (20.4) | |
| Diabetes, n (%) | 146 (22.0) | 27 (22.5) | 119 (21.8) | 0.873 |
| CHD, n (%) | 91 (13.7) | 28 (23.3) | 63 (11.6) | 0.001 |
| CKD, n (%) | 13 (2.0) | 4 (3.3) | 9 (1.7) | 0.401a |
| CLD, n (%) | 13 (2.0) | 3 (2.5) | 10 (1.8) | 0.911a |
| Laboratory variables | ||||
| Baseline SCr, mg/dL | 96.0 ± 37.0 | 120.1 ± 55.3 | 90.2 ± 28.7 | 0.000 |
| Reason for VAN therapy, n (%) #1 | 0.000 | |||
| Prophylactic | 357 (53.7) | 14 (11.7) | 343 (62.9) | |
| Local infection | 277 (41.7) | 87 (72.5) | 190 (34.9) | |
| Bacteremia | 31 (4.7) | 19 (15.8) | 12 (2.2) | |
| Other risk factors | ||||
| Hospitalization-related factors | ||||
| LOS (days), n (n) | 25 (14) | 27 (21) | 17 (12) | 0.000 |
| ICU admittance, n (%) | 109 (16.4) | 48 (44) | 61 (11.2) | 0.000 |
| Cancer, n (%) | 42 (6.3) | 25 (20.8) | 17 (3.1) | 0.000 |
| Concomitant ARDS, shock, or low perfusion factors before or at the time of AKI onset during hospitalization, n (%) | 42 (6.3) | 25 (20.8) | 17 (3.1) | 0.000 |
| Concomitant hemolysis, rhabdomyolysis or crystallization of urine/urine tube during hospitalization, n (%) | 3 (0.5) | 1 (0.8) | 2 (0.4) | 0.450b |
| Concomitant MOF or DIC during hospitalization, n (%) | 12 (1.8) | 7 (5.8) | 5 (0.9) | 0.000 |
| Receipt of endotracheal intubation for ventilator therapy during hospitalization, n (%) | 59 (8.9) | 40 (33.3) | 19 (3.5) | 0.000 |
| Orthopedic/trauma/burn surgery during hospitalization, n (%) | 379 (57.0) | 14 (11.7) | 365 (67.0) | 0.000 |
| Neurosurgery during hospitalization, n (%) | 21 (3.2) | 5 (4.2) | 16 (2.9) | 0.682a |
| Other surgeries during hospitalization, n (%) #2 | 160 (24.1) | 42 (35.0) | 118 (21.7) | 0.002 |
*: n = 592. Only 592 patients’ data on the weight is available. CHD = coronary heart disease, CKD = chronic kidney disease, CLD = chronic liver disease, CEI = angiotensin II-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, NSAID = nonsteroidal anti-inflammatory drug, ARDS = acute respiratory distress syndrome, MOF = multiple organ failure, DIC = disseminated intravascular coagulation.
The VAN used at our hospital was obtained from two sources: Wen kexin and Lai kexin (trade name: Wenkexin, generic name: Vancomycin Hydrochloride for Injection, manufacturer: Eli Lilly KK Seishin Laboratories, specification: 500 mg/bottle; and trade name: Laikexin, generic name: Vancomycin Hydrochloride for Injection, manufacturers: Zhejiang Pharmaceutical Co., Ltd. and Xinchang Pharmaceutical Factory, specification: 500 mg/bottle); 98.6%(655/665) of the patients were treated with VAN from the Wen kexin source. We also surveyed the departmental distribution of the patients receiving VAN treatment, and the results are shown in S2 Table. The reasons for VAN use were mainly prophylaxis and local infection, corresponding to 357 (53.7%, 357/665) and 277 (41.7%, 277/665) patients, respectively. The median LOT was 1 day (IQR = 5), and the median daily dosage was 1.0 g (IQR = 2.0) (Table 2). We also analyzed 38 types of concomitant drugs used by the patients receiving VAN treatment (the details are provided in Table 2).
Table 2. Comparison of concomitant drugs used between VA-AKI and No-AKI patients.
| Total n = 665 |
AKI n = 120 |
NO-AKI n = 545 |
P value | |
|---|---|---|---|---|
| Source of VAN, n (%) | 1.000b | |||
| Wenkexin*1 | 655 (98.6) | 119 (99.2) | 536 (98.5) | |
| Laikexin*2 | 9 (1.4) | 1 (0.8) | 8 (1.5) | |
| LOT, n (n) | 1.0 (5.0) | 6.0 (11) | 1.0 (3.0) | 0.000 |
| Mean daily dosage, n (%) | 0.000 | |||
| 0.5 g | 145 (21.9) | 5 (4.2) | 140 (25.8) | |
| 1.0 g | 273 (41.2) | 34 (28.3) | 239 (44.1) | |
| 1.5 g | 40 (6.0) | 13 (10.8) | 27 (5.0) | |
| 2.0 g | 200 (30.2) | 66 (55.0) | 134 (24.7) | |
| 3.0 g | 4 (0.6) | 2 (1.7) | 2 (0.4) | |
| TDM, n (%) | 36 (5.4) | 19 (15.8) | 17 (3.1) | 0.000 |
| Concomitant drugs | ||||
| Vasopressors, n (%) | 56 (8.4) | 38 (31.7) | 18 (3.3) | 0.000 |
| Nitrates, n (%) | 69 (10.4) | 31 (25.8) | 38 (7.0) | 0.000 |
| β-receptor blockers, n (%) | 156 (23.5) | 58 (48.3) | 98 (18.0) | 0.000 |
| Phentolamine, n (%) | 3 (0.5) | 3 (2.5) | 0 (0.0) | 0.006b |
| Amlodipine, n (%) | 123 (18.5) | 21 (17.5) | 102 (18.7) | 0.756 |
| Sodium nitroprusside, n (%) | 3 (0.5) | 3 (2.5) | 0 (0.0) | 0.006b |
| ARBs, n (%) | 44 (6.6) | 7 (5.8) | 37 (6.8) | 0.703 |
| ACEIs, n (%) | 23 (3.5) | 10 (8.3) | 13 (2.4) | 0.001 |
| Aminoglycosides, n (%) | 19 (2.9) | 9 (7.5) | 10 (1.8) | 0.001 |
| Amphotericin B, n (%) | 3 (0.5) | 1 (0.8) | 2 (0.4) | 0.450b |
| Azole antifungals, n (%) | 63 (9.5) | 30 (25.0) | 33 (6.1) | 0.000 |
| Micafungin, n (%) | 39 (5.9) | 20 (16.7) | 19 (3.5) | 0.000 |
| Metronidazole, n (%) | 26 (3.9) | 16 (13.3) | 10 (1.8) | 0.000 |
| Nystatin, n (%) | 24 (3.6) | 15 (12.5) | 9 (1.7) | 0.000 |
| Acyclovir, n (%) | 24 (3.6) | 15 (12.5) | 9 (1.7) | 0.000 |
| Imipenem-cystatins, n (%) | 61 (9.2) | 20 (16.7) | 41 (7.5) | 0.002 |
| β-lactam antibiotics, n (%) | 593 (89.2) | 103 (85.8) | 490 (89.9) | 0.193 |
| Macrolides, n (%) | 19 (2.9) | 11 (9.2) | 8 (1.5) | 0.000 |
| Quinolones, n (%) | 108 (16.2) | 35 (29.2) | 73 (13.4) | 0.000 |
| Sulfonamides, n (%) | 175 (26.3) | 88 (73.3) | 87 (16.0) | 0.000 |
| Tigecycline, n (%) | 4 (0.6) | 2 (1.7) | 2 (0.4) | 0.310a |
| Diuretics, n (%) | 175 (26.3) | 88 (73.3) | 87 (16.0) | 0.000 |
| 20% mannitol, n (%) | 24 (3.6) | 7 (5.8) | 17 (3.1) | 0.149 |
| Low-molecular-weight dextran, n (%) | 19 (2.9) | 0 (0.0) | 19 (3.5) | 0.033b |
| NSAIDs, n (%) | 222 (33.4) | 35 (29.2) | 187 (34.3) | 0.279 |
| Immunosuppressants, n (%) | 20 (3.0) | 13 (10.8) | 7 (1.3) | 0.000 |
| Glucocorticoids, n (%) | 122 (18.3) | 51 (42.5) | 71 (13.0) | 0.000 |
| Chemotherapy drugs, n (%) | 23 (3.5) | 12 (10.0) | 11 (2.0) | 0.000 |
| Contrast medium, n (%) | 22 (3.3) | 15 (12.5) | 7 (1.3) | 0.000 |
| Compound glycyrrhizin, n (%) | 98 (14.7) | 48 (40.0) | 50 (9.2) | 0.000 |
| Compound fresh bamboo juice, n (%) | 17 (2.6) | 6 (5.0) | 11 (2.0) | 0.061 |
| Liquorice tablets, n (%) | 9 (1.4) | 1 (0.8) | 8 (1.5) | 0.914a |
| Ganmao Qingre granules, n (%) | 41 (6.2) | 11 (9.2) | 30 (5.5) | 0.131 |
| Yunnan Baiyao capsules, n (%) | 19 (2.9) | 11 (9.2) | 8 (1.5) | 0.000 |
| Ursodeoxycholic acid, n (%) | 20 (3.0) | 13 (10.8) | 7 (1.3) | 0.000 |
| Simotang, n (%) | 2 (0.3) | 1 (0.8) | 1 (0.2) | 0.329b |
| Qingkailing, n (%) | 2 (0.3) | 1 (0.8) | 1 (0.2) | 0.329b |
| Senna leaf, n (%) | 267 (39.8) | 7 (5.8) | 258 (47.3) | 0.000 |
*1: Trade name: Wenkexin, generic name: Vancomycin Hydrochloride for Injection, manufacturer: Eli Lilly KK Seishin Laboratories, specification: 500 mg/bottle.
*2: Trade name: Laikexin, generic name: Vancomycin Hydrochloride for Injection, manufacturers: Zhejiang Pharmaceutical Co., Ltd. and Xinchang Pharmaceutical Factory, Specification: 500 mg/bottle.
TDM of included patients
A total of 5.4% (36/665) of the patients received TDM during VAN therapy. The judgment of whether a patient required TDM was based on《Therapeutic monitoring of vancomycin in adult patients》 [9]. One hundred thirty-seven patients met the requirements for TDM. The total inadequate TDM rate was 73.7% (101/137). We further analyzed the TDM rates for all departments (Table 3). Inadequate TDM was more prevalent in surgical departments, particularly the general surgery department, for which the rate reach the standard was 2.9% (1/35). A total of 35 of 37 patients in the general surgery department required monitoring, only 1 of whom was actually monitored; thus, the actual TDM rate was 2.7% (1/37), which is far less than the recommended rate (94.6%, 35/37). Further, the TDM rate for the patients with a normal baseline SCr was 5.1% (33/641), whereas that for the patients with a baseline SCr of higher than 133 mg/dL was 14.3% (3/21).
Table 3. TDM of patients treated with VAN.
| Department | Patients who received TDM (n) | Total patients in department (n) | Patients who required TDM (n) | Actual TDM rate | Recommended TDM rate according to guidelines | Rate reach the guidelines |
|---|---|---|---|---|---|---|
| General surgery | 1 | 37 | 35 | 2.7% | 94.6% | 2.9% |
| Orthopedics | 2 | 387 | 35 | 0.5% | 9.0% | 5.7% |
| SICU | 1 | 17 | 14 | 5.9% | 82.4% | 7.1% |
| Neurology | 1 | 6 | 6 | 16.7% | 100.0% | 16.7% |
| Rheumatism and immunology | 1 | 3 | 3 | 33.3% | 100.0% | 33.3% |
| Respiratory medicine | 8 | 19 | 16 | 42.1% | 84.2% | 50.0% |
| Thoracic surgery | 1 | 2 | 2 | 50.0% | 100.0% | 50.0% |
| CCU | 1 | 2 | 2 | 50.0% | 100.0% | 50.0% |
| Infectious | 1 | 2 | 2 | 50.0% | 100.0% | 50.0% |
CCU = Cardiology Care Unit
Actual TDM rate—patients who received TDM/total no. of patients in department; Supposed TDM rate according to standard—patients who required TDM/total no. of patients in department; Rate reach the standard—actual TDM rate/supposed TDM rate according to standard.
Clinical characteristics of VA-AKI
A total of 198 patients developed AKI, corresponding to an incidence of 26.8% (198/740). Moreover, 120 patients had confirmed VA-AKI, with an incidence of 16.2% (120/740).
Further, we compared the VA-AKI group with the NO-AKI group (Table 1). The majority of the VA-AKI patients were males (56.7% males vs 43.5% females, p = 0.008); in addition, the median age was 65 years (IQR = 29), and the median weight was 64.2 kg (IQR = 18). The VA-AKI patients were more likely have concomitant CHD (23.3% vs 11.6%, p = 0.000), cancer (20.8% vs 3.1%, p = 0.000), ICU admittance (44% vs 11.2%, p = 0.000) and low perfusion factors before or at the time of AKI onset during hospitalization (20.8% vs 3.1%, p = 0.000). Further, the VA-AKI patients had a longer LOS (27 vs 12 days, p = 0.000) and an elevated baseline estimated glomerular filtration rate (eGFR; 120.1 vs 90.2 mg/dL, p = 0.000). With regard to the reason for VAN therapy, most of the VA-AKI patients were treated for local infection (72.5%, 87/120), whereas the treatment was prophylactic for most of the NO-AKI patients (62.9%, 343/545).
Concomitant drugs
To further examine the risk factors for the development of VA-AKI, we first further analyzed the concomitant drugs used with VAN. The average daily dosage was 2.0 g for 55.0% (66/120) of the VA-AKI patients, and the median daily dosage was also 2.0 g (IQR = 1). TDM was significantly more commonly performed for the VA-AKI patients (15.8% vs 3.1%, p = 0.000). With regard to the concomitant drugs, compared with the NO-AKI patients, significantly more of the VA-AKI patients received concomitant vasopressors, nitrates, β-receptor blockers, ACEIs, and the following antimicrobial drugs: aminoglycosides, imipenem-cystatins, quinolones, sulfonamides, etc. In addition, the VA-AKI patients were significantly more likely to receive concomitant diuretics, immunosuppressants, glucocorticoids, and contrast medium. We also evaluated the influence of the concomitant use of Chinese patent drugs and found that the VA-AKI patients were more likely to receive concomitant compound glycyrrhizin, Yunnan Baiyao capsules, and ursodeoxycholic acid but were less likely to receive concomitant senna leaf (Table 2).
Risk factors for VA-AKI
Multiple logistic regression analysis revealed that an elevated baseline eGFR (odds ratio [OR] = 1.009; p = 0.017) and concomitant vasopressor therapy (OR = 2.942; p = 0.009), nitrate treatment (OR = 2.869; p = 0.007), imipenem-cilastatin treatment (OR = 4.708; p = 0.000), and contrast medium administration (OR = 6.609 p = 0.005) were independent risk factors for VA-AKI; in addition, the receipt of orthopedic/trauma/burn surgery (OR = 0.3575; p = 0.011) and concomitant compound glycyrrhizin administration (OR = 0.290; p = 0.017) were determined to be independent protective factors for VA-AKI (See Table 4). The good of fit was evaluated by the analysis of Hosmer and Lemeshow and the result is 0.652.
Table 4. Risk factors for VA-AKI.
| Risk factor | P value | OR | 95% CI |
|---|---|---|---|
| Concomitant renal hypoperfusion during hospitalization | 0.078 | 2.193 | 0.917–5.244 |
| Receipt of endotracheal intubation for ventilation during hospitalization | 0.070 | 2.102 | 0.942–4.691 |
| Receipt of orthopedic/trauma/burn surgery | 0.011 | 0.357 | 0.162–0.786 |
| Baseline eGFR | 0.017 | 1.009 | 1.002–1.016 |
| Vasopressors | 0.009 | 2.942 | 1.302–6.646 |
| Nitrates | 0.007 | 2.869 | 1.331–6.185 |
| Imipenem-cilastatin | 0.000 | 4.708 | 2.611–8.489 |
| Contrast medium | 0.005 | 6.609 | 1.732–21.267 |
| Compound glycyrrhizin | 0.017 | 0.290 | 0.105–0.800 |
| Ganmao Qingre granules | 0.061 | 2.670 | 0.955–6.463 |
VA-AKI severity and treatment
VA-AKI severity was classified according to the highest stage of AKI observed in the patient and receipt of RRT. The highest disease stage was stage 1 for 69.2% (83/120) of the patients, and 2.5% (3/120) of them received RRT (S3 Table). The median time of peak disease severity was 5 days (IQR = 6) after initiation of VAN therapy. Next, we analyzed the departmental distribution of the VA-AKI patients. We excluded the patients with VA-AKI caused by preventive administration of VAN from this analysis because this group of patients consistently exhibited a better baseline condition and lower LOT of VAN treatment. For the purpose of therapy, the departments with highest VA-AKI incidences were the cardiac surgery department, orthopedics department and bone marrow transplant ward, with incidence rates of 59.3% (16/27), 50% (20/40) and 48.2% (13/27), respectively. Therapy adjustments, including termination of VAN treatment or a decrease in the dosage, were only made for 40.8% (49/120) of these VA-AKI patients after the onset of AKI (Table 5).
Table 5. VA-AKI treatments characteristics.
| Treatment | n (%) |
|---|---|
| No adjustment | 68 (56.7) |
| Dose adjustment or other protective measures* | 29 (24.1) |
| Stop/replace VAN | 20 (16.7) |
| Renal replacement therapy | 3 (2.5) |
*protective measures include: addition of renal protective drugs, TDM for VAN, RRT, etc.
Outcomes and risk factors for VA-AKI
The all-cause in-hospital-mortality rate for the VA-AKI patients was 18.33% (22/120), and 76.2% (17/22) had an eGFR that was outside of the normal range at the time of death, suggestive of some degree of kidney injury (Table 6). Multiple logistic regression analysis revealed that the independent risk factors for death for the total VA-AKI patients were CHD (OR = 12.6; p = 0.006) and concomitant vasopressor therapy (OR = 15.4; p = 0.001) (Table 7). We separated the renal outcomes of the VA-AKI patients into three categories: recovery, improvement and non-recovery. Renal function recovered in 11 patients, with a median recovery time of 4 days (IQR = 6) after receipt of VAN therapy and renal function improved in 17 patients. Thus, 28 patients showed either recovery or improvement, representing 23.3% (28/120) of the total VA-AKI patients. The remaining 73.3% (92/120) of the VA-AKI patients had renal insufficiency at the time of hospital discharge. Multiple logistic regression analysis revealed that concomitant CHD (OR = 8.858, p = 0.019) and contrast medium administration (OR = 9.779, p = 0.005) were independent risk factors for VA-AKI and that β-receptor blocker treatment (OR = 0.124, p = 0.001) was an independent protective factor (see Table 8). We further performed an additional post-estimation analysis with Akaike score and a table of the classification of the model (Table 9).
Table 6. VA-AKI patients’ renal function at the time of death.
| Renal function (eGFR) ml/(min·1.73 m2) | n (%) |
|---|---|
| 1. ≥90 | 5 (23.8) |
| 2. ≥60且<90 | 0 (0) |
| 3. ≥30且<60 | 5 (23.8) |
| 4. ≥15且<30 | 8 (38.1) |
| 5. <15 or dialysis | 3 (14.3) |
eGFR = 175 × [SCr (mg/dl)]-1.234 ×[age(y)]-0.179 ×sex(male = 1, female = 0.79).
Table 7. Risk factors for death in VA-AKI patients.
| Factors | P Value | OR | 95% CI |
|---|---|---|---|
| CHD | 0.006 | 12.6 | 2.1–77.1 |
| Vasopressor use | 0.001 | 15.4 | 3.2–75.7 |
| Contrast medium administration | 0.998 | 0.000 | 0.000 |
Table 8. Risk factors for renal outcome in VA-AKI patients.
| Factor | P Value | OR | 95% CI |
|---|---|---|---|
| CHD | 0.019 | 8.858 | 1.442–54.406 |
| LOS | 0.076 | 1.015 | 0.998–1.032 |
| Reception of other surgeries during hospitalization | 0.082 | 3.171 | 0.865–11.623 |
| β-receptor blocker | 0.001 | 0.124 | 0.062–0.881 |
| 20% mannitol | 0.998 | 0.000 | 0.000 |
| Contrast medium | 0.005 | 9.779 | 1.957–48.864 |
| Compound glycyrrhizin | 0.083 | 0.307 | 0.081–1.164 |
| Yunnan Baiyao capsules | 0.998 | 0.000 | 0.000 |
Table 9. Akaike score and a table of the classification of the model.
| Goodness of fit evaluation | Risk Factors For VA-AKI | For Death | For Renal Outcome |
|---|---|---|---|
| Akaike score | 405.233 | 95.222 | 112.233 |
Discussion
VAN is a key antibiotic used for the management of severe Gram-positive infections, particularly MRSA infections. However, VAN treatment failures have occurred in patients infected with MRSA isolates reported to be associated with the development of severe infections. The use of a higher VAN concentration has been recommended, which necessitates evaluations of drug safety, especially nephrotoxic and ototoxic agents. The optimal use of VAN is still a complicated issue [1–3]. This single-center retrospective study conducted at our hospital aimed to investigate the current situation of VA-AKI in China and identify risk factors for VA-AKI to provide some suggestions for improving the prevention and treatment of AKI.
To confirm the occurrence of VA-AKI in this population, we designed a four-step survey abiding by the principles of broad screening and enrollment but utilizing strict identification criteria (Fig 1). During the process of confirming VA-AKI diagnoses, we found that a subset of the VAN-treated patients received insufficient SCr monitoring (7.9%).,Greater attention must be paid to the monitoring of SCr in patients receiving VAN, especially the surgery department, to reduce the misdiagnosis of AKI. The rate of AKI detection was 26.6% and the incidence of VA-AKI was 16.2% at our hospital. Nobutoshi Masuda has reported that intervention by a pharmacist might impact VAN therapy, as it might enable the balancing of a higher trough VAN concentration with the risk of nephrotoxicity. In that study, pharmacists monitored renal function by measuring patients’ SCr levels, and intervention by a pharmacist was determined to be associated with a 45% decrease in the incidence of nephrotoxicity[10]. Kathleen A. Marquis’ study reached similar conclusions, showing that a pharmacist-directed VAN pilot program significantly increased the percentage of patients who were optimally dosed. Patients in the pilot program had a shorter LOT and lower incidence of nephrotoxicity [11]. We recommend that hospitals increase their investment in clinical pharmacists because pharmacists not only enable clinicians, especially surgeons, to focus on their work but also more professionally manage VAN treatment.
In further analyses comparing NO-AKI patients with VA-AKI patients, we found out that the VA-AKI patients were significantly more likely to have CHD as an underlying disease; however, no significant differences in the incidences of other underlying diseases were observed between these groups. Further, the VA-AKI patients were significantly more likely to have an elevated baseline eGFR, longer LOS, higher rate of ICU care and higher rate of concomitant disease. Moreover, the VA-AKI patients had more serious conditions than the NO-AKI patients, and more severe comorbidity is often correlated with a higher risk of nephrotoxicity. This study also revealed that an elevated baseline eGFR was an independent risk factor for the development of VA-AKI, consistent with the findings of a previous study by Brady S. Moffett but in contrast with the results of studies by Lindsey Pritchard and Stephen W. Davies [12–14]. Our explanation for these findings is that clinicians may pay more attention to patients with insufficient renal function. For example, the TDM rate for the patients with a normal baseline SCr was (5.1%) lower than that for the patients with a baseline SCr of higher than 133 mg/dL (14.3%).
We more extensively and more elaborately studied concomitant drugs used during or before VAN treatment compared with previous studies. In addition, this was the first such study to include the concomitant use of Chinese patent drugs. The VA-AKI patients in this study were significantly more likely to receive concomitant compound glycyrrhizin, Yunnan Baiyao capsules, and ursodeoxycholic acid and were less likely to receive concomitant senna leaf. Studies conducted in China have demonstrated that compound glycyrrhizin, Yunnan Baiyao capsules, and senna leaf provide a certain degree of improvement in renal function, but their specific underlying mechanisms require further investigation [15–17]. The sub-classification of drugs in this study was also more detailed compared with previous studies. Many studies had shown that the use of vasoactive drugs is an independent risk factor for the development of VA-AKI [12,18]. The term vasoactive drug covers a wide range of drugs, and in our study, we subdivided vasoactive drugs into 8 specific classes (vasopressors, nitrates, β-blockers, phentolamine, calcium channel blockers, sodium nitroprusside, ARBs, and ACEIs). Multivariate logistic regression analysis revealed that concomitant vasopressor use was an independent risk factor for VA-AKI, consistent with Kathleen A. Hazlewood’s study [4]. The use of vasopressors often indicates that patients have severe concomitant disease symptoms. This is the first study to confirm that concomitant nitrate use is an independent risk factor for VA-AKI. The reason for this finding might be that previous studies did not subcategorize vasoactive drugs into this specific class. Nitrate drugs themselves do not have kidney toxicity. We found that when a patient was using nitrates, he (she) was more likely to concomitant with CHD (49.3%, 34/69 vs 9.6%, 57/596) and hypertension. CHD was reported to be a close connection with worsening renal function [19,20]. Multiple logistic regression analysis also showed that the concomitant use of imipenem-cystatins was an independent risk factor for VA-AKI, in disagreement with most current studies. Blanca Humanes’ study has demonstrated that cilastatin protects against VAN-induced proximal tubule apoptosis and increases cell viability without compromising the antimicrobial effects of VAN. The beneficial effects of cilastatin could be at least partly attributed to the decreased accumulation of VAN in renal proximal tubular epithelial cells [21]. In our study, we assessed imipenem-cystatins rather than cystatins, as there is no “cystatin” drug available in China. Notably, our results may have been influenced by the fact that patients taking imipenem-cystatins always have a concomitant severe infection. In conclusion, greater attention must be paid to patients receiving VAN together with vasopressors, nitrates and/or contrast medium.
Multiple logistic regression analysis revealed that the concomitant use of compound glycyrrhizin was an independent predictor of VA-AKI; this study is the first to report this finding. Compound glycyrrhizin is a preparation composed of β-glycyrrhizic acid glycosides, glycine, methionine and other compounds [22]. Experiments using rats have confirmed that glycyrrhizic acid inhibits the development of renal interstitial fibrosis [23]. We have also observed that compound glycyrrhizin significantly reduces proteinuria and enhances renal function in CKD patients [16]. Receipt of orthopedic/trauma/burn surgery is a prospective factor in our study, but its clinical significance may not be very important. The reason to this statistics result may be that the majority of the patients who received orthopedic/trauma/burn surgery have a better baseline condition, a younger age, shorter LOS, lower rate of ICU stay, less concomitant drugs and so on. All this factors contributed to the result that this group of patients would be less likely to develop AKI and contributed this statistics result further.
Risk factors for mortality and other outcomes
A total of 120 patients developed VA-AKI, and their mortality rate was 18.3% (22/120). The mortality rate for all patients included in this study was 6.1% (45/740). Thus, the mortality rate for AKI patients was higher than that for NO-AKI patients. In addition, the LOS of the AKI patients were significantly longer than that of the NO-AKI patients (27 vs 17, p = 0.000). Chertow GM’s study has also shown that AKI development is associated with increases in the mortality rate and LOS for these patients [24]. In this study, univariate logistic regression analysis revealed that AKI development was a risk factor for mortality (p = 0.000). Because only a small number of patients died (n = 45), it was difficult to further determine whether the occurrence of VA-AKI was an independent risk factor for death in our study. Thus, we could not establish a causal link between AKI and death. Similarly, P. Hanrahan’s study was not able to demonstrate that AKI was an established independent risk factor for increased mortality [25]. However, Glaucia T. F. Seixas’ study and some other published studies have consistently shown that nephrotoxicity is an independent risk factor for death, even after adjusting for comorbidities and disease severity [26]. Furthermore, our multiple logistic regression analysis demonstrated that concomitant CHD (OR = 12.6; p = 0.006) and vasopressor therapy (OR = 15.4; p = 0.001) were independent risk factors for death. Thus, greater attention must be paid to patients using VAN who have concomitant CHD or are simultaneously receiving vasopressor therapy.
Among the patients treated with VAN who developed AKI, 56.7% (68/120) did not receive any targeted treatment, and 2.5% (3/120) received RRT. The type of treatment may be related to AKI severity. The highest AKI stage was stage 1 in 70.8% (85/120) of the patients and stage 3 in 15.0% (18/120) of the patients. For mild cases of AKI, our first consideration is drug dosage adjustment and other renoprotective measures; RRT is used only in severe cases.
We further analyzed the outcomes of AKI. Renal function improved in 23.3% (28/120) of the AKI patients, but 73.3% (92/120) of the patients left the hospital with renal insufficiency. This is a very promising finding. Following the development of AKI, the failure to correct renal function in time would not only increase patient suffering but also increase the LOS and cost of treatment. More importantly, long-term renal insufficiency might promote the progression of AKI to CKD. The consequences of such an outcome are difficult for us to convey. Multiple logistic regression analysis revealed that CHD (OR = 8.858, p = 0.019) and contrast medium administration (OR = 9.779, p = 0.005) were independent risk factors for VA-AKI. The improvement of renal function is more difficult in patients with concomitant CHD and contrast medium administration. Thus, greater attention must be paid to AKI patients with concomitant CHD, and contrast medium should be more carefully administered to these patients. Multiple logistic regression analysis also showed that β-receptor blocker use (OR = 0.124, p = 0.001) was an independent protective factor for AKI. However, the specific mechanism underlying this effect is unclear and warrants further investigation.
Strengths and limitations
The strengths of this study are as follows. First, this study examined a large Chinese population, and previous studies of risk factors for AKI in the Chinese population are very limited. Second, our survey of AKI was designed to include four steps, enabling not only analysis of the situation of AKI and its associated risk factors but also the treatment and outcomes of this condition. In this study, we identified risk factors for VA-AKI to support the prevention and management of AKI. Third, our study analyzed different types of drugs used concomitantly with VAN treatment; the diversity of drugs examined in this study is greater than that in most previous studies, and this is the first study to examine the concomitant use of Chinese patent drugs. However, this descriptive study has several limitations. First, this was a single-center retrospective study. Second, we lacked VAN therapeutic concentrations due to the low rate of TDM in clinical practice in the real word. Third, our study did not analyze the plasma VAN concentration, SCr concentration, or LOS as risk factors for AKI. Fourth, our study did not analyze the cost of AKI.
Conclusion
A subset of patients treated with VAN received insufficient SCr monitoring and clearly inadequate TDM. We recommend that hospitals increase their investment in clinical pharmacists. An elevated baseline eGFR and concomitant vasopressor therapy, nitrate use, imipenem-cilastatin use, and contrast medium administration were independent risk factors for VA-AKI; in addition, the receipt of orthopedic/trauma/burn surgery and concomitant use of compound glycyrrhizin were independent protective factors for VA-AKI.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(PDF)
Acknowledgments
Kunming Pan, Li Yang and Ying Zhou conceived, designed, and organized the study, interpreted the results, and drafted the manuscript. The data were obtained from Peking University First Hospital. Kunming Pan analyzed the data. Kunming Pan, Lingyun Ma and Qian Xiang contributed to collection of the data on-site. Yimin Cui designed and organized the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funded by Peking University First Hospital Department of Pharmacy scientific research funds 4-31 Yimin Cui.
References
- 1.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr., Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66: 82–98. doi: 10.2146/ajhp080434 [DOI] [PubMed] [Google Scholar]
- 2.Wong-Beringer A, Joo J, Tse E, Beringer P. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents. 2011;37: 95–101. doi: 10.1016/j.ijantimicag.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171: 388–416. doi: 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 4.Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? Am J Med. 2010;123: 182 e181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68: 1243–1255. doi: 10.1007/s00228-012-1259-9 [DOI] [PubMed] [Google Scholar]
- 6.Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, et al. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother. 2011;55: 5475–5479. doi: 10.1128/AAC.00168-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, et al. Acute kidney injury in China: a cross-sectional survey. The Lancet. 2015;386: 1465–1471. [DOI] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138. [Google Scholar]
- 9.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49: 325–327. doi: 10.1086/600877 [DOI] [PubMed] [Google Scholar]
- 10.Masuda N, Maiguma T, Komoto A, Haruki Y, Sugiyama T, Kondo S, et al. Impact of pharmacist intervention on preventing nephrotoxicity from vancomycin. Int J Clin Pharmacol Ther. 2015;53: 284–291. doi: 10.5414/CP202274 [DOI] [PubMed] [Google Scholar]
- 11.Marquis KA, DeGrado JR, Labonville S, Kubiak DW, Szumita PM. Evaluation of a Pharmacist-Directed Vancomycin Dosing and Monitoring Pilot Program at a Tertiary Academic Medical Center. Ann Pharmacother. 2015. September;49(9):1009–14. doi: 10.1177/1060028015587900 [DOI] [PubMed] [Google Scholar]
- 12.Moffett BS, Hilvers PS, Dinh K, Arikan AA, Checchia P, Bronicki R. Vancomycin-associated acute kidney injury in pediatric cardiac intensive care patients. Congenit Heart Dis. 2015;10: E6–E10. doi: 10.1111/chd.12187 [DOI] [PubMed] [Google Scholar]
- 13.Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med. 2010;123: 1143–1149. doi: 10.1016/j.amjmed.2010.07.025 [DOI] [PubMed] [Google Scholar]
- 14.Davies SW, Efird JT, Guidry CA, Dietch ZC, Willis RN, Shah PM, et al. Vancomycin-associated nephrotoxicity: The obesity factor. Surg Infect (Larchmt). 2015;16: 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao G, Li XL, Yu H, Sun LP. Clinical observation of Yunnan Baiyao Capsule treatment of hematuria. Modern Medicine & Health. 2008;14:2169. [Google Scholar]
- 16.Yang WJ, Wang HG. Clinical observation of senna treatment to chronic renal failure. Journal of Traditional Chinese Medicine. 1989;04:49. [Google Scholar]
- 17.Song MA, Yan LZ, Wang XX. Effect of Compound Glycyrrhizin on chronic kidney disease proteinuria and renal function. Journal of Medical Forum. 2009;01:82–3. [Google Scholar]
- 18.Costa e Silva VT, Marcal LJ, Burdmann EA. Risk factors for vancomycin nephrotoxicity: still a matter of debate*. Crit Care Med. 2014;42: 2635–2636. doi: 10.1097/CCM.0000000000000635 [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002. June;8(3):136–41. [DOI] [PubMed] [Google Scholar]
- 20.Smith GL, Vaccarino V, Kosiborod M, Lichtman JH, Cheng S, Watnick SG, et al. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003. February;9(1):13–25. doi: 10.1054/jcaf.2003.3 [DOI] [PubMed] [Google Scholar]
- 21.Humanes B, Jado JC, Camano S, Lopez-Parra V, Torres AM, Alvarez-Sala LA, et al. Protective effects of cilastatin against Vancomycin-Induced Nephrotoxicity. Biomed Res Int. 2015;2015: 704382 doi: 10.1155/2015/704382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang ZY. Clinical application of compound glycyrrhizin and its adverse effects. Chinese Journal of Clinical Rational Drug Use. 2012;17:160. [Google Scholar]
- 23.Li KL, Zhang JG, Wang HM, Chen J. Influence of 18α glycyrrhizic acid to NF-κB expression in obstruction nephropathy interstitial rats. Journal of Cellular and Molecular Immunology. 2004;01:31–3. [PubMed] [Google Scholar]
- 24.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740 [DOI] [PubMed] [Google Scholar]
- 25.Hanrahan TP, Kotapati C, Roberts MJ, Rowland J, Lipman J, Roberts JA, Udy A. Factors associated with vancomycin nephrotoxicity in the critically ill. Anaesth Intensive Care, 2015;43:594–9. [DOI] [PubMed] [Google Scholar]
- 26.Seixas GT, Araujo OR, Silva DC, Arduini RG, Petrilli AS. Vancomycin Therapeutic Targets and Nephrotoxicity in Critically Ill Children With Cancer. J Pediatr Hematol Oncol. 2016. March;38(2):e56–62. doi: 10.1097/MPH.0000000000000470 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.