Supplemental Digital Content is available in the text
Keywords: Infection, mortality, multiple organ dysfunction syndrome, severe injury, trauma
Abbreviations: ACCU, adult critical care unit, ACIT-II, activation of coagulation and inflammation in trauma-II, AIS, abbreviated injury score, BD, base deficit, ERMODS, early resolving multiple organ dysfunction syndrome, GCS, Glasgow coma score, ISS, injury severity score, LOS, length of stay, MODS, multiple organ dysfunction syndrome, POET, prospective outcomes evaluation in trauma, PRBC, packed red blood cells, PRMODS, prolonged multiple organ dysfunction syndrome, SOFA, sequential organ failure assessment, TBI, traumatic brain injury, TTR, time to recovery
ABSTRACT
Background:
Multiple organ dysfunction syndrome (MODS) is associated with poor outcomes for trauma patients. Different forms of MODS may exist and have different consequences. The ability to distinguish them clinically may have implications for prognosis and treatment. We wished to study whether prolonged MODS (PRMODS) could be observed as a distinct clinical entity to early resolving MODS (ERMODS) in critically injured patients.
Methods:
Adult major trauma patients recruited to a prospective observational study at a single major trauma center were eligible for inclusion. MODS was defined as Sequential Organ Failure Assessment (SOFA) score >5; and PRMODS as lasting >7 days. Time to recovery (TTR) was calculated as the number of days before the SOFA fell below the MODS threshold (≤5).
Results:
Five hundred ninety-five patients were enrolled of whom 285 developed ERMODS (48%) and 184 (31%) PRMODS. Organ dysfunction was more severe and protracted in PRMODS, especially in patients without brain injury (mean SOFA 11 vs. 6, Day 2, P < 0.001; TTR 17 vs. 3 days, P < 0.001). PRMODS exhibited higher rates of hepatic and renal dysfunction (84% vs. 56%; and 78% vs. 47%, P≤0.001). Patterns of recovery were distinct in hepatic, renal, and neurological systems (TTR 15 vs. 4; 20 vs. 3; and 28 vs. 7 days, P < 0.01). PRMODS was associated with higher infection and mortality rates (91% vs. 41%; and 22% vs. 7%, P < 0.001).
Conclusion:
PRMODS appears common, a distinct clinical entity, and associated with worse patient outcomes. PRMODS may represent an important endpoint for studies evaluating outcomes following trauma.
INTRODUCTION
Multiple organ dysfunction syndrome (MODS) results in poor outcomes for critically injured patients and continues to place large demands on healthcare resources (1, 2). As more patients survive with modern damage control resuscitation, the incidence of MODS continues to be high, as much as 30% in some series of seriously injured patients (1, 3). MODS is managed as a single clinical entity, primarily through organ support until resolution occurs. To date, there have been no successful clinical trials of specific therapeutic agents for the treatment of MODS (4). However, recent studies suggest there may be different forms of MODS, potentially with different etiologies, that have different consequences for patients (5).
Contemporary forms of MODS broadly divide into two patterns. In both patterns the onset of MODS occurs early following the initial injury, but they differ in the relative time course to recovery, and have different complications and outcomes (3). The first pattern describes patients who require initial critical care support, but subsequently recover over the course of a few days with relatively low overall resource requirement. The second pattern describes patients whose MODS fails to recover, instead developing a prolonged or “persistent” course associated with long critical care unit stays, high rates of infection, and greater mortality (3, 5). This form is described variably in the recent literature as “persistant immunosuppression and protein catabolism syndrome (PICS)” (5), “cumulative” MODS (3), or “complicated” MODS (6, 7), although they all share prolonged critical care stays as a common feature. It remains unclear whether these two forms of MODS, here referred to as early resolving MODS (ERMODS) and prolonged MODS (PRMODS), are different clinical entities or are simply manifestations of different severity of physical and physiological insults.
The overall objective of this descriptive study was to examine the contemporary patterns of MODS after severe injury. First, we wished to describe the severity of MODS in the two entities and determine if ERMODS and PRMODS had different rates of evolution or resolution of organ dysfunction. Second, we wanted to investigate whether individual organ components differed in their underlying patterns of dysfunction and in their relative contribution to overall dysfunction in ERMODS and PRMODS. Third, we wanted to examine differences in etiologies and, finally any differences in outcomes for patients. We analyzed a prospectively recruited cohort of adult trauma patients admitted to the critical care unit of an urban major trauma center.
METHODS
Study setting and participants
The study was conducted at an urban major trauma center (Level One). Trauma patients presenting between August 2010 and May 2015 were eligible for inclusion. Adult trauma patients (16 years or older) who met the local criteria for full trauma team activation were included into either the Activation of Coagulation and Inflammation in Trauma (ACIT-II) or Prospective Evaluation of Outcomes in Trauma (POET) observational studies. Severely injured patients subsequently requiring critical care admission were enrolled into this study. We retrospectively excluded patients found to have an injury severity score (ISS) below 16. Additional exclusion criteria for ACIT-II are described elsewhere (8). Ethical approvals were granted by the regional National Health Service Research Ethics Committee.
Data collection
Admission data were collected on patient demographics, mechanism of injury, and baseline physiology. Admission arterial blood analysis for base deficit (BD) was performed during the trauma team resuscitation as part of normal care processes. BD was utilized to indicate the severity of hypovolaemic shock on admission to the emergency department. Blood product use, namely packed red blood cells, fresh frozen plasma, cryoprecipitate and platelets in the first 24 h following admission were recorded.
The primary outcome was the pattern of MODS, which was determined using the Sequential Organ Failure Assessment (SOFA) score (9). Missing component scores were resolved by allocating the same score as the previous day, consistent with methods used by previous authors (2, 10). Patients who were discharged from the critical care unit were allocated a score of “0” for the remainder of the 28-day period, with the assumption that organ dysfunction had recovered. Secondary outcomes were mortality, the presence of infection, ventilator-free days, critical care, and total hospital length of stay (LOS). Patients were reviewed daily for 28 days, or until discharge or death. Causes of death were verified retrospectively using case review notes from biweekly trauma mortality meetings and follow-up postmortem reports where available.
Definitions
MODS was defined as the occurrence of a total SOFA score greater than 5 during the first 7 days of admission. ERMODS was defined as MODS that resolved within 7 days, and PRMODS was defined as MODS that persisted beyond 7 days. We also assessed duration of MODS using the concept of “Time To Recovery—TTR,” as previously described (11). We defined recovery as the time (days) for a patient's SOFA score to fall and remain below 6 for the remainder of the critical care period. Patients who were still in MODS on day 28 were assigned a score of 29. Patients who died were assigned a maximum score of 30. For individual organ components we defined dysfunction as a score of greater than or equal to 1, and recovery as the time taken for the component score to fall and remain below 1. To assess the impact of neurotrauma on the development of MODS, traumatic brain injury (TBI) was defined as a head abbreviated injury score (AIS) of greater than or equal to 3. Infection was defined as presence of a temperature greater than 38.5°C with a positive culture, or clinical evidence of infection (e.g., observation of purulent exudate, pulmonary in-filtrates on chest radiography) requiring treatment with antibiotics. Ventilator-free days were calculated by subtracting the number of days on ventilation from 28 days for those who survived, and from the day of death in patients who died.
Data analysis
Data were analyzed in IBM SPSS Statistics (Version 21). Normal distribution was assessed with the Shapiro–Wilk test. Time to recovery was analyzed using Kaplan–Meier one-minus-survival curves and ERMODS versus PRMODS groups were compared using the log-rank test. Comparison of all other categorical and continuous variables was by chi-squared and Mann–Whitney U tests for non-parametric data. Summary data are reported as medians unless otherwise specified.
RESULTS
During the study period 1,092 patients were enrolled into ACITII or POET and of these, 595 patients were admitted to critical care unit with an ISS > 15. The study cohort was predominantly male (80.2%), having sustained blunt trauma (85.5%) that resulted in severe injury (ISS 29, interquartile range 21–38). TBI was present in 346 (58.2%) patients. A total of 469 patients (78.8%) developed MODS, of whom 285 (47.9% of total) had ERMODS, and 184 (30.9% of total) had PRMODS. MODS was identifiable in majority of cases (86.6%) on the day of admission (Day 0) and peaked in severity on Day 1 (mean SOFA score 9 ± 3).
We examined whether ERMODS and PRMODS exhibited different patterns of MODS evolution and resolution. We found total organ dysfunction in both groups peaked early and within the first 2 days of admission. This was followed by a general trajectory toward resolution, with no discernable second or “late” peak (Fig. 1A). However, patients with PRMODS suffered significantly greater severity of total organ dysfunction when compared with ERMODS. This difference in severity developed early (mean SOFA score 10 vs. 8, Day 2, P < 0.001) and persisted throughout the monitored recovery period. Patients with PRMODS also exhibited significantly slower rates of organ function recovery than might be expected if following a similar trajectory to those with ERMODS (TTR 16 vs. 5 days, P < 0.001, Fig. 1B). The differences between PRMODS and ERMODS were more apparent in the cohort of patients that excluded severe TBI (Fig. 1, C and D) for both organ dysfunction severity (mean SOFA score 11 vs. 6, Day 2, P < 0.001) and rate of recovery (TTR 17 vs. 3 days, P < 0.001). Therefore, we focused subsequent analyses on this non-TBI cohort.
Fig. 1.
Graphs (A) and (C) show mean SOFA scores by day of hospital admission for patients with no multiple organ dysfunction (No MODS), early resolving MODS (ERMODS), and prolonged MODS (PRMODS) in all patients (A) and in patients without traumatic brain injury (TBI) (C).
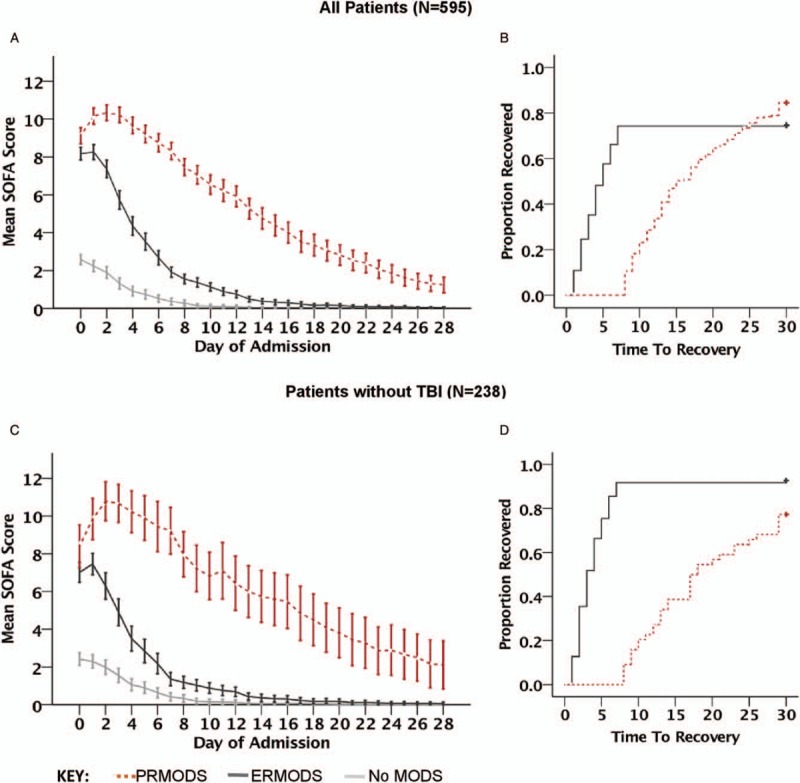
Error bars represent 95% confidence intervals. Graphs (B) and (D) show corresponding Kaplan–Meier 1-Survival Curves illustrating time to recovery (TTR) for ERMODS and PRMODS. Mantel–Cox Log Rank tests comparing the distribution of TTR between these groups showed a statistical difference (P < 0.001) for all patients (B) and those without severe head injury (D) where the effect was greater. SOFA indicates Sequential Organ Failure Assessment.
We found the pattern of dysfunction of individual organ components differed between ERMODS and PRMODS groups (Fig. 2, A–F). First, there were differences regarding the relative contribution of individual organs to the overall MODS burden. Patients with PRMODS (vs. ERMODS) had higher rates of hepatic (84.4% vs. 56.8%, P = 0.01), renal (77.8% vs. 46.8%, P < 0.001), and neurological dysfunction (82.2 vs. 63.1, P = 0.02), and trended toward higher rates of cardiovascular (97.8% vs. 89.2%, P = 0.079) dysfunction. Conversely, there were no differences in the proportion of patients with respiratory (97.8% vs. 97.3%, P = 0.857) or coagulation (88.9% vs. 81.1%, P = 0.236) dysfunction. Second, the groups differed with respect to the evolution of maximal organ dysfunction within individual organs. In PRMODS, maximal dysfunction in respiratory, hepatic, and neurological components evolved gradually and peaked late (Fig. 2, A–C), whereas in the cardiovascular and renal systems, maximal dysfunction evolved rapidly and peaked early (Fig. 2, D and E). In ERMODS, maximal dysfunction in these components occurred on admission or showed only minimal deterioration on the first day. The coagulation system showed similar trajectories of dysfunction in both groups, although PRMODS was more severe throughout (Fig. 2F). Third, the groups differed with respect to the time course of organ dysfunction resolution. The most striking differences (PRMODS vs. ERMODS) were observed in the hepatic, neurological, and renal systems (TTR 15 vs. 4 days; 28 vs. 7 days; and 20 vs. 3 days respectively, P < 0.01), where the recovery trajectories between the two groups appeared to deviate (Fig. 2B, C, and E). PRMODS (vs. ERMODS) also showed prolonged resolution in respiratory, cardiovascular, and coagulation systems (TTR: 27 vs. 8 days; 17 vs. 4 days; 8 vs. 5 days; respectively, P < 0.01); however, the recovery trajectories appeared similar (Fig. 2A, D, and F).
Fig. 2.
Mean component SOFA scores [(A) respiratory, (B) hepatic, (C) neurological, (D) cardiovascular, (E) renal, and (F) coagulation] by day of hospital admission in patients without traumatic brain injury (TBI) for no multiple organ dysfunction (No MODS), early resolving MODS (ERMODS), and prolonged MODS (PRMODS).
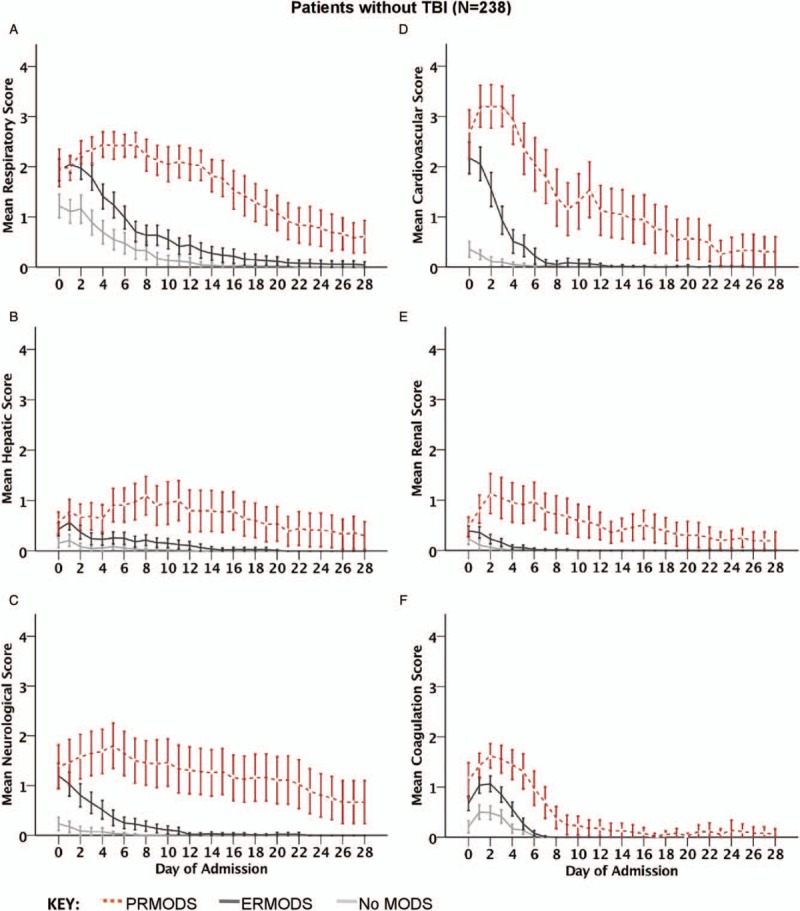
Error bars represent 95% confidence intervals.
Individual organ scores for patients with severe TBI are shown for comparison in Figure 3. While respiratory and cardiovascular systems showed similar patterns to the non-TBI cohort, there were generally low levels of renal and liver dysfunction, and the coagulation system showed no difference between ERMODS and PRMODS in terms of severity or recovery trajectories. Overall, differences in the evolution of organ dysfunction between ERMODS and PRMODS in individual components were less apparent in patients with TBI compared with those without TBI.
Fig. 3.
Mean component SOFA scores [(A) respiratory, (B) hepatic, (C) neurological, (D) cardiovascular, (E) renal, and (F) coagulation] by day of hospital admission in patients with traumatic brain injury (TBI) for no multiple organ dysfunction (No MODS), early resolving MODS (ERMODS), and prolonged MODS (PRMODS).
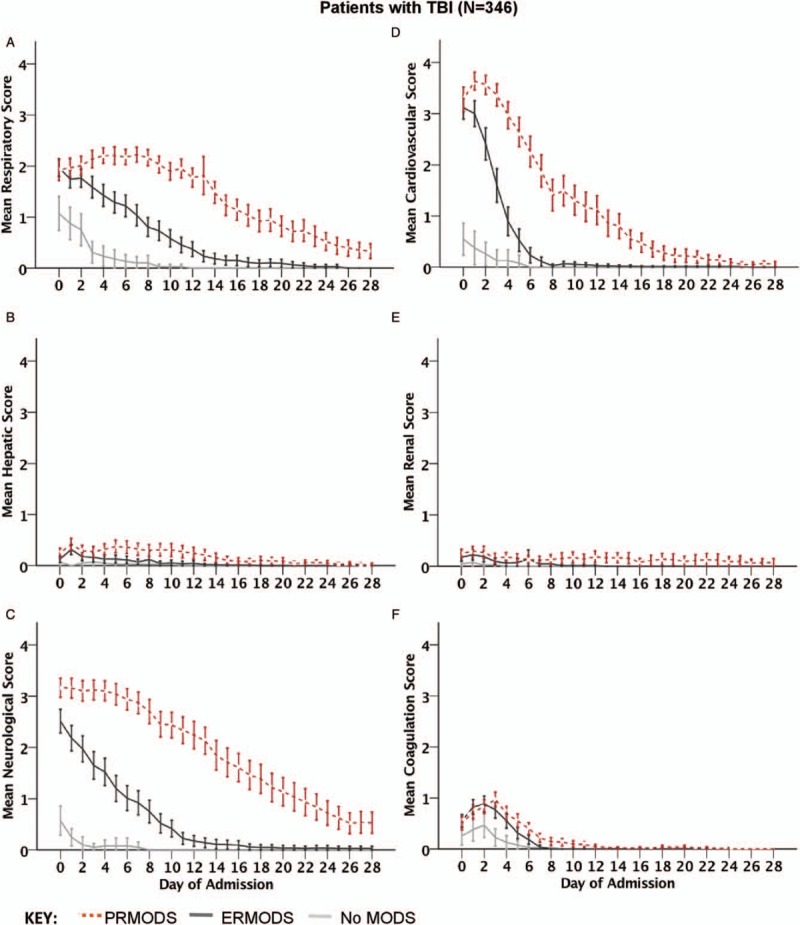
Error bars represent 95% confidence intervals.
Differences in admission variables between ERMODS and PRMODS were examined (Table 1). In the absence of TBI, patients with PRMODS (vs. ERMODS) were older (47 vs. 37 years, P = 0.003), more shocked (base deficit 8.8 vs. 4.3, P < 0.001) and had higher blood product transfusion requirements (red blood cells 8 units vs. 4 units; total blood products 14 units vs. 9 units, P = 0.006). A higher proportion of female patients developed PRMODS. The only admission difference identified in the TBI cohort was a small increase in chest AIS in the PRMODS group (3 vs. 3, P < 0.02).
Table 1.
Admission variables and outcomes
| Patients without TBI | Patients with TBI | |||||||
| No MODS | ERMODS | PRMODS | P value* | No MODS | ERMODS | PRMODS | P value* | |
| N | 82 | 111 | 45 | 43 | 170 | 133 | ||
| Admission variables | ||||||||
| Age | 32 (22–48) | 37 (25–49) | 47 (32–65) | 0.003 | 38 (24–56) | 40 (27–57) | 39 (25–57) | 0.414 |
| Male (%) | 66 (80.5) | 96 (86.5) | 33 (73.3) | 0.049† | 33 (76.7) | 132 (77.6) | 109 (82.0) | 0.356† |
| Blunt (%) | 52 (63.4) | 79 (71.2) | 39 (86.7) | 0.041† | 37 (86.0) | 162 (95.3) | 130 (97.7) | 0.258† |
| First BD | 2.4 (0.4–5.4) | 4.3 (1.4–7.9) | 8.8 (4.6–14.1) | <0.001 | 1.5 (-1.1–3.3) | 3.5 (0.8–6.6) | 4.0 (1.3–7.7) | 0.212 |
| First GCS | 15 (14–15) | 14 (11–15) | 14 (13–15) | 0.777 | 13 (10–14) | 7 (4–12) | 7 (4–9) | 0.327 |
| ISS | 22 (18–28) | 25 (20–33) | 27 (20–36) | 0.552 | 27 (18–34) | 29 (25–41) | 34 (25–43) | 0.205 |
| Head AIS | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.538 | 4 (4–4) | 4 (4–5) | 4 (4–5) | 0.558 |
| Chest AIS | 3 (2–4) | 4 (3–5) | 3 (3–4) | 0.546 | 2 (0–3) | 3 (0–4) | 3 (2–4) | 0.020 |
| Abdominal AIS | 2 (0–3) | 2 (0–3) | 2 (0–4) | 0.283 | 0 (0–0) | 0 (0–2) | 0 (0–2) | 0.335 |
| Extremity AIS | 2 (0–3) | 2 (0–3) | 2 (1–3) | 0.411 | 0.5 (0–2) | 1 (0–3) | 2 (0–3) | 0.243 |
| PRBC units | 1.5 (0–8) | 4 (2–8) | 8 (3–16) | 0.006 | 0 (0–1) | 0 (0–5) | 0 (0–6) | 0.975 |
| Total blood products | 4 (2–6) | 9 (2–18) | 14 (6–36) | 0.006 | 0 (0–1) | 0 (0–9) | 1 (0–12) | 0.963 |
| Outcomes | ||||||||
| Mortality (%) | 0 | 8 (7.2) | 10 (22.2) | 0.007† | 4 (9.3) | 62 (36.5) | 16 (12.2) | <0.001† |
| Infection (%) | 15 (18.8) | 43 (40.6) | 41 (91.1) | <0.001† | 1 (2.4) | 55 (32.4) | 115 (87.8) | <0.001† |
| Day of first infection | 4 (3–5) | 3 (3–4) | 5 (4–6) | 0.007 | 8 (8–8) | 4 (3–6) | 5 (3–7) | 0.115 |
| ACCU LOS | 4 (2–6) | 6 (3–11) | 20 (12–33) | <0.001 | 2 (1–3) | 5 (2–9) | 17 (13–24) | <0.001 |
| Hospital LOS | 13 (9–24) | 18 (9–31) | 35 (21–60) | <0.001 | 8 (4–17) | 14 (3–28) | 41 (26–54) | <0.001 |
| VFD | 28 (26–28) | 25 (21–27) | 7 (0–17) | <0.001 | 27 (26–28) | 19 (0–24) | 12 (5–17) | <0.001 |
Medians (IQR) presented unless expressed otherwise.
*ERMODS versus PRMODS Mann–Whitney U test.
†ERMODS versus PRMODS chi-square test.
ACCU indicates adult critical care unit; AIS, abbreviated injury scale; BD, base deficit; ERMODS, early resolving MODS; GCS, Glasgow coma scale; ISS, injury severity score; LOS, length of stay (days); MODS, multiple organ dysfunction syndrome; PRBC, packed red blood cells; PRMODS, prolonged MODS; TBI, traumatic brain injury; VFD, ventilator-free days.
The presence of PRMODS was associated with worse clinical outcomes (Table 1). All patients with PRMODS developed significantly higher rates of infection (PRMODS vs. ERMODS—without TBI 91.1% vs. 40.6%; with TBI 87.8% vs. 32.4%, P < 0.001), longer critical care and hospital LOS, and fewer ventilator-free days. The day of onset of first infection occurred slightly later in patients with PRMODS (PRMODS vs. ERMODS—without TBI 3 vs. 5 days, P = 0.007; with TBI 4 vs. 5 days, P = 0.199). In patients without TBI, PRMODS was also associated with significantly higher mortality (PRMODS vs. ERMODS 22.2% vs. 7.3%, P = 0.007). In those with TBI, mortality was higher in the ERMODS group (PRMODS vs. ERMODS 12.2% vs. 36.5%, P < 0.001); however when deaths due to unsurvivable TBI were excluded, there was no difference in mortality between PRMODS and ERMODS (7.1% vs. 8.4%, P = 0.698, Supplementary Table 1). In order to ensure that the observed differences were not skewed by early deaths in the ERMODS group (who may have gone on to develop PRMODS if they survived) we performed a subgroup analysis excluding those patients who died within 7 days (Supplementary Table 2) which demonstrated that the patterns of ERMODS and PRMODS were preserved.
DISCUSSION
In this descriptive study of severely injured patients, we have shown that PRMODS has distinct clinical features when compared with ERMODS. PRMODS was more severe than ERMODS and showed differential patterns of evolution and resolution in individual organs. There were also differences in the demographic and injury characteristics between the groups, as well as notable disparities in outcomes. In particular, mortality of PRMODS in the non-TBI group is more than double that of ERMODS, and in all patients PRMODS was associated with exceptionally high rates of infection.
In our study PRMODS exhibited more severe overall organ dysfunction compared with ERMODS with a recovery that was slower than expected relative to the ERMODS group. The difference in severity of dysfunction was evident within the first 2 days and persisted over time, clarifying results of previous studies (3, 6). These two distinct patterns of MODS appear to broadly reflect modern theories of MODS pathogenesis, where prolonged or “complicated” patterns are thought to be the result of an early fulminant dysregulation of the immune system that subsequently fails to normalize (4, 7). However, we also demonstrate differences in the pattern of dysfunction within individual organs. In particular, there were increased contributions from renal and hepatic systems to PRMODS in the non-TBI cohort, which also showed marked prolongation of recovery relative to ERMODS. Models that utilize rises in creatinine or liver enzymes to predict post-trauma MODS (12) could be further developed to determine if they differentiate those at risk of a prolonged MODS course. We found a higher incidence of MODS and PRMODS in patients with severe head injuries, which is consistent with previous work (13). However, patients with TBI appear to show different patterns of organ dysfunction compared with non-TBI patients, with less obvious differences between ERMODS and PRMODS. It is not clear whether this effect occurred because the difference between ERMODS and PRMODS is masked by the effects of TBI, or whether it is a true pattern in these patients.
Differences in the patterns of ERMODS and PRMODS in the non-TBI group in our study occurred independently of both overall and region-specific injury severity scores. This somewhat contrasts previous work in similar patient cohorts, where higher overall injury severity was associated with the clinical presentation of “complicated” MODS (although it was not associated with related changes in gene expression) (6, 7). These studies define complicated MODS using “Time To Recovery,” which incorporates all deaths into the “complicated” MODS category and may explain the propensity toward higher overall injury severity. Other studies identify advanced age as a potential driver of complicated MODS in non-TBI injured patients (14). Our study supports this, and we also show PRMODS to be associated with higher admission base deficit and transfusion requirements, both of which are implicated in immunosuppressive responses after trauma (8). We also observed an association between female gender and PRMODS. Previous studies identify female gender to be protective against MODS in general (predominantly in the reproductive age groups) (15, 16) but a risk factor for MODS-related mortality (2). Although there are some clinical differences in the admission and injury characteristics between ERMODS and PRMODS, they do not fully explain why some patients develop PRMODS and further research is required to provide clarity.
Patients without TBI who developed PRMODS had significantly higher mortality. Previous evidence has associated the cumulative burden of MODS with mortality (3); however, our study reveals that for some patients, MODS-related trauma deaths occur much later than previously described (2, 10). The TBI cohort suffered a much higher mortality in the ERMODS group that appears to be a reflection of early deaths due to non-survivable head injury. The high rates of nosocomial infection in MODS are also described elsewhere (3, 16, 17); however, we observed greater than 100% increase in infections for the PRMODS group compared with ERMODS. Whether PRMODS may be induced by a second hit such as infection remains a hypothesis that requires further investigation. Finally, PRMODS was universally associated with increased ventilator requirements, substantial intensive care unit, and hospital LOS. If PRMODS could be reduced to ERMODS, this could lead to significant outcomes benefits for patients, and reduce the burden this condition places on hospital resources.
There are several limitations to this study. First, in the absence of a universally agreed definition of PRMODS, our definitions are selected somewhat arbitrarily. Previous authors suggest that a “complicated” course of MODS may encompass a critical care stay of 7 to 10 days (6, 10) or a “time to recovery” of greater than 14 days (6, 7, 11). We elected to use an MODS duration of greater than 7 days to represent our PRMODS group as it appeared to capture patients at risk of particularly poor outcomes in terms of infection and mortality. We also chose an MODS threshold of SOFA greater than 5 to capture patients with a high burden of organ dysfunction that manifested as a mild disturbance of several organs. Second, ERMODS and PRMODS are representative measures of injured organs based on the SOFA score. Further precision on early organ injury and recovery may be obtained if more sensitive measures of organ injury were utilized. The neurological component of the SOFA score is considered unreliable due to problems with GCS estimation in sedated and ventilated patients. Nevertheless, when evaluated as a cumulative score, SOFA thresholds greater than 3 are previously shown to have good sensitivity with satisfactory receiver operating curves in predicting LOS and mortality in non-TBI patients (10). Lastly, a proportion of patients who died early may have developed PRMODS if they survived and this may represent a confounding factor. We performed a subgroup analysis of patients who survived for 7 days to provide some clarity on this issue.
PRMODS is common following major trauma, appearing to be clinically distinct from ERMODS and associated with worse outcomes for patients. PRMODS may therefore represent an effective endpoint for studies that examine outcomes following trauma. In order to facilitate treatment improvements for patients, future research is required to understand the immune mechanisms leading to PRMODS, and to develop prediction models that identify patients at risk of PRMODS early in their clinical course.
Supplementary Material
Supplementary Material
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Fröhlich M, Lefering R, Probst C, Paffrath T, Schneider MM, Maegele M, Sakka SG, Bouillon B, Wafaisade A. Committee on Emergency Medicine, Intensive Care and Trauma Management of the German Trauma Society Sektion NIS.. Epidemiology and risk factors of multiple-organ failure after multiple trauma: An analysis of 31,154 patients from the TraumaRegister DGU. J Trauma Acute Care Surg 2014; 76:921–928. [DOI] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, Maier RV, Burlew CC. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg 2014; 76:582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, O’Keefe GE, Cohen MJ, Moldawer LL, Tompkins RG, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med 2012; 40:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, Koenderman L, Kubes P, Lilford RJ. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet 2014; 384:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 2012; 72:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, Ungaro R, Davis R, Cuenca AG, Gentile LF, Nacionales DC, Cuenca AL, Bihorac A, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg 2014; 76:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. A genomic storm in critically injured humans. J Exp Med 2011; 208:2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torrance HD, Brohi K, Pearse RM, Mein CA, Wozniak E, Prowle JR, Hinds CJ, O’Dwyer MJ. Association between gene expression biomarkers of immunosuppression and blood transfusion in severely injured polytrauma patients. Ann Surg 2015; 261:751–759. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710. [DOI] [PubMed] [Google Scholar]
- 10.Dewar DC, White A, Attia J, Tarrant SM, King KL, Balogh ZJ. Comparison of postinjury multiple-organ failure scoring systems: denver versus sequential organ failure assessment. J Trauma Acute Care Surg 2014; 77:624–629. [DOI] [PubMed] [Google Scholar]
- 11.Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, Bankey PE, Nathens AB, Cuenca AG, Efron PA, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg 2012; 255:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewar DC, Tarrant SM, King KL, Balogh ZJ. Changes in the epidemiology and prediction of multiple-organ failure after injury. J Trauma Acute Care Surg 2013; 74:774–779. [DOI] [PubMed] [Google Scholar]
- 13.Maier B, Lefering R, Lehnert M, Laurer HL, Steudel WI, Neugebauer EA, Marzi I. Early versus late onset of multiple organ failure is associated with differing patterns of plasma cytokine biomarker expression and outcome after severe trauma. Shock 2007; 28:668–674. [PubMed] [Google Scholar]
- 14.Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A, et al. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care 2015; 19:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trentzsch H, Lefering R, Nienaber U, Kraft R, Faist E, Piltz S. The role of biological sex in severely traumatized patients on outcomes a matched-pair analysis. Ann Surg 2015; 261:774–780. [DOI] [PubMed] [Google Scholar]
- 16.Frink M, Pape H-C, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on mods and cytokines after multiple injuries. Shock 2007; 27:151–156. [DOI] [PubMed] [Google Scholar]
- 17.Cole E, Davenport R, Willet K, Brohi K. The burden of infection in severely injured trauma patients and the relationship with admission shock severity. J Trauma Acute Care Surg 2014; 76:730–735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


