Abstract
IMPORTANCE
The indications for adjuvant therapy in resected oral tongue cancers are based on both clinical and pathological factors, with clear evidence for adjuvant radiation in patients with pathologically positive neck lymph nodes, positive margins, and extracapsular extension, but the data for patients with no nodal disease are sparse.
OBJECTIVE
To investigate determinants of failure and survival in patients with node-negative oral tongue cancer.
DESIGN, SETTING, AND PARTICIPANTS
Medical records for patients with oral tongue cancer treated with definitive surgery from 2003 to 2013 were reviewed. All patients were cN0 negative and classified as pathologically node-negative (pN0) if a neck dissection was performed. Patients received adjuvant radiotherapy (RT) or chemoradiotherapy (CRT) based on standard clinical and pathological determinants.
MAIN OUTCOMES AND MEASURES
Kaplan-Meier and multivariable (MVA) logistic regression and Cox proportional hazard regression analyses were performed to identify patient, tumor, and treatment characteristics predictive of locoregional control (LRC) and overall survival (OS).
RESULTS
A total of 180 patients met entry criteria, with a median follow-up time of 4.9 years (range, 0.9–12.5 years); 102 patients (56.7%) were female and 42 patients (23.3%) were younger than 45 years at diagnosis. One hundred fifty-three patients (85%) had T1/T2 tumors, and 112 patients (62%) had elective neck dissections with confirmed pN0. Lymphovascular space invasion (LVSI) was present in 36 patients (20%). On MVA, LVSI (OR, 0.06; 95% CI, 0.02–0.19; P < .01) was associated with worse LRC. Elective neck dissection (odds ratio [OR], 2.99; 95% CI, 1.16–7.73; P = .02) and receipt of RT (OR, 7.74; 95% CI, 2.27–26.42; P < .01) were associated with improved LRC. Three-year LRC rates were significantly lower for patients with LVSI (38.8%; 95% CI, 22.8%, 54.6%) than those without LVSI (81.9%; 95% CI, 74.4%, 87.4%). On MVA, only LVSI (hazard ratio, 2.20; 95% CI, 1.19–4.06; P = .01) and age greater than 44 years (hazard ratio, 4.38; 95% CI, 1.34–14.27; P = .01) were associated with worse OS. Three-year OS rates were significantly lower in patients with LVSI (71.3%; 95% CI, 53.2%–83.4%) than those without LVSI (90.3%; 95% CI, 83.8%–94.3%).
CONCLUSIONS AND RELEVANCE
Lymphovascular space invasion in patients with node-negative oral tongue cancer treated with upfront definitive surgery is associated with worse LRC and OS. Node-negative oral cavity cancers with LVSI warrant consideration of further adjuvant therapy, which should be further evaluated in a prospective setting.
There are an estimated 300 000 newly diagnosed oral cavity cancers, of which about a quarter are oral tongue primary lesions, worldwide annually, with about 145000 deaths.1,2 Despite therapeutic advances, cancers of the oral tongue have poorer outcomes when compared with similarly staged cancers of the oropharynx and larynx.3–6 An inferior survival may be due to inherently aggressive histologic characteristics predisposing the cancer to nodal spread to the contralateral neck, “skip metastases” in the ipsilateral neck, or other, unspecified reasons.7–10
Initial surgical resection of oral cavity cancers is the current standard of care.11 Elective neck dissection for clinically node-negative (cN0) patients is recommended in early-stage oral tongue cancers,12 but the need for adjuvant radiotherapy (RT) alone or radiation with combined chemotherapy (CRT) is gleaned from studies incorporating cancers of all head and neck subsites.13–16 There are few studies focusing explicitly on the oral tongue and even fewer addressing the subset of oral tongue cancer patients with negative lymphnodes, but generally the literature supports similar pathological risk factors,9,17,18 in addition to depth of invasion,10 as indications for adjuvant therapy. Because of sparse literature there is controversy about the role of adjuvant therapy in cN0 or pathologically node-negative (pN0) patients.
The objectives of our study were to determine which patient, tumor, or treatment related factors were associated with worse disease control and survival for patients with cN0 and/or pN0 oral tongue cancers.
Methods
Patient Selection
With institutional review board-approval, the medical records of patients from 2003 to 2013 treated with initial total or partial glossectomy for oral tongue squamous cell carcinoma were reviewed. The tumors of all patients were staged prior to surgery with a computerized tomographic (CT) scan of the neck. We excluded patients with node-positive disease (cN positive and/or pN positive) as well as patients with lack of adjuvant treatment records. Staging was based on the 7th edition of the American Joint Committee on Cancer guidelines.19 Chemoradiation was recommended for positive margins. Adjuvant RT alone was generally given for T3 or T4 disease, perineural invasion (PNI), extensive depth of invasion, and close margins of less than 5 mm. All patients were followed with periodic physical examination, including fiberoptic endoscopy when necessary, and surveillance CT imaging.
Statistical Analysis
All statistics were computed using SAS software (version 9.3; SAS Institute Inc). Patient characteristics examined were age, sex, and race. Based on the rising incidence of oral tongue cancers in patients 44 years or younger,20–25 age was divided into those older than 44 years and those 44 years or younger. Tumor characteristics examined included T stage, histological grade, PNI, lym-phovascular space invasion (LVSI), margins (positive, negative, and close, defined as <5 mm), and depth of invasion. Depth of invasion was categorized into less than 3 mm or 3 mm or greater based on previous studies.26 Treatment characteristics analyzed including use of elective neck dissection at time of definitive surgery and type of adjuvant therapy received (none, RT, or CRT). These characteristics were compared across LVSI groups using χ2 tests or Fisher exact tests, where appropriate, for categorical variables, and analysis of variance for numeric variables. Local control (LC) was defined as absence of failure at the primary site. Locoregional control (LRC) was defined as absence of failure at the primary site or bilateral necks. Ultimate locoregional control (ULRC) was defined as absence of failure at the primary site or necks, taking into account salvage therapeutic attempts for failure. Overall survival (OS) was defined as time to death or time to last follow-up from diagnosis. Survival estimates for end points were estimated using the Kaplan-Meier product limit method, and survival distributions were compared using the log-rank test.27 In addition, LC and LRC were defined as time from diagnosis to local failure and locoregional failure, respectively, for the purposes of estimating yearly actuarial rates. Two- and 3-year survival rates for each end point were reported. Univariable (UVA) logistic regression models for LC, LRC, and ULRC and Cox proportional hazard models for OS were fitted to the defined patient, tumor, and treatment characteristics. Multivariable (MVA) logistic regression and proportional hazard models were generated for these end points, using a backward selection with an α-level of removal of .20 based on the local Wald test P values for each variable. Owing to small event sizes in levels of several variables, Firth penalized maximum-likelihood estimation28 was applied for the UVA analysis of ULRC to reduce bias in the 95% CIs and parameter estimates. Separate Kaplan-Meier survival estimates were performed comparing patients with and without LVSI for each end point. The proportional hazards assumption was checked for all survival models. Significance was assessed at the P = .05 level. Graphics were created using R software (version 2.15.1; R Foundation).
Results
Patient Characteristics
After review of 245 patient medical records, 180 patients met study inclusion criteria; 102 patients (56.7%) were female and 42 patients (23.3%) were younger than 45 years at diagnosis. Fifty-five patients were excluded because of clinically (45 patients) or pathologically (10 patients) proven nodal disease found during elective neck dissection. Ten patients were excluded owing to transfer of care to an outside facility after surgery. The patient, tumor, and treatment characteristics of our study population are summarized in Table 1. A total of 153 patients (62%) had preoperative positron emission tomographic-CT staging. Median follow-up time was 4.9 years (range, 0.912.5 years). The median dose of RT was 60 Gy (range, 60–70 Gy). Radiation therapy was delivered for close margins (30 patients), PNI (31 patients), depth of invasion 3 mm or greater (39 patients), and T3/T4 disease (17 patients). Seven patients had positive margins and received adjuvant CRT, 3 with cisplatin, 2 with carboplatin and paclitaxel, and 2 with cetuximab. An additional patient received cetuximab with adjuvant RT as part of RTOG 0920 (NCT00956007).
Table 1.
Summary of the Patient, Tumor, and Treatment Characteristics of the 180 Patients in the Study Population
| Variable | No. (%) |
|---|---|
| Patient Characteristics | |
| Age at diagnosis, y | |
| ≤44 | 42 (23.3) |
| >44 | 138 (76.7) |
| Sex | |
| Female | 102 (56.7) |
| Male | 78 (43.3) |
| Race | |
| White | 144 (80.0) |
| African American | 15 (8.3) |
| Other | 21 (11.7) |
| Tumor Characteristics | |
| T stage | |
| T1 | 126 (70.0) |
| T2 | 37 (20.6) |
| T3 | 10 (5.6) |
| T4a | 7 (3.8) |
| Tumor differentiation | |
| Well differentiated | 64 (35.6) |
| Moderately differentiated | 101 (56.1) |
| Poorly differentiated | 15 (8.3) |
| Perineural invasion | |
| Negative | 125 (69.4) |
| Positive | 55 (30.6) |
| Margins | |
| Negative | 123 (68.3) |
| Close, <5 mm | 50 (27.8) |
| Positive | 7 (3.8) |
| Depth of invasion | |
| <3 mm | 46 (16.0) |
| ≥3 mm | 134 (74.0) |
| Lymphovascular space invasion | |
| Positive | 36 (20.0) |
| Negative | 141 (78.3) |
| Missing | 3 (1.7) |
| Treatment Characteristics | |
| Elective neck dissection | |
| No | 68 (37.8) |
| Yes | 112 (62.2) |
| Adjuvant therapy | |
| None | 131 (72.8) |
| Concurrent chemotherapy and radiation | 8 (4.4) |
| Radiation alone | 41 (22.8) |
Local Control
There were 53 total local failures. On UVA only LVSI was associated with worse LC (odds ratio [OR], 0.26; 95% CI, 0.120.56; P < .01) (eTable 1 in the Supplement), indicating the odds of LC for those with LVSI were 74% lower than the odds of LC for those without LVSI. On MVA, LVSI (OR, 0.24; 95% CI, 0.100.59; P < .01) was associated with worse LC, while receipt of adjuvant radiation (OR, 4.30; 95% CI, 1.40–13.22; P = .01) was associated with improved LC (eTable 2 in the Supplement). There was a nonsignificant finding for PNI (OR, 0.41; 95% CI, 0.16–1.05; P = .06) and African American race (OR, 0.19; 95% CI, 0.03–1.16; P = .09) to be associated with worse LC. The 2- and 3-year LC rates were 81.3% (95% CI, 74.8%–86.4%) and 80.1% (95% CI, 73.3%–85.3%), respectively.
Locoregional Control
There were 71 total locoregional failures. On UVA, LVSI (OR, 0.12; 95% CI, 0.05–0.28; P < .01) was associated with worse LRC, while well-differentiated tumors (vs poorly differentiated referent) (OR, 4.74; 95% CI, 1.43–15.72; P = .01) and receipt of adjuvant radiation (OR, 2.46; 95% CI, 1.12–5.44; P = .03) were associated with higher LRC (eTable 3 in the Supplement). On MVA, LVSI (OR, 0.06; 95% CI, 0.02–0.19; P < .01) was associated with worse LRC. Elective neck dissection (OR, 2.99; 95% CI, 1.16–7.73; P = .02) and receipt of adjuvant radiation (OR, 7.74; 95% CI, 2.27–26.42; P < .01) were associated with improved LRC (Table 2). There was a nonsignificant finding for depth of invasion of at least 3 mm (OR, 0.41; 95% CI, 0.14v1.18; P = .10) and PNI (OR, 0.41; 95% CI, 0.15–1.15; P = .10) for worse LRC. The 2- and 3-year LRC rates were 75.2% (95% CI, 68.2%–80.9%) and 72.7% (95% CI, 65.5%–78.7%), respectively. The sites of regional failure were as follows: 41 in the ipsilateral neck, 3 in the contralateral neck, and 3 in other oral cavity subsites.
Table 2.
Multivariable Analysisa of Patient, Tumor, and Treatment Characteristics and Their Association With Locoregional Control
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Race | ||
| White | 1.65 (0.46–5.94) | .44 |
| African American | 0.50 (0.09–2.80) | .43 |
| Other | 1 [Reference] | |
| Perineural invasion | ||
| Positive | 0.41 (0.15–1.15) | .09 |
| Negative | 1 [Reference] | |
| Lymphovascular space invasion | ||
| Positive | 0.06 (0.02–0.19) | <.01 |
| Negative | 1 [Reference] | |
| Depth of invasion | ||
| ≥3 mm | 0.41 (0.14–1.18) | .10 |
| <3 mm | 1 [Reference] | |
| Use of elective neck dissection | ||
| Yes | 2.99 (1.16–7.73) | .02 |
| No | 1 [Reference] | |
| Adjuvant therapy | ||
| Radiation alone | 7.74 (2.27–26.42) | <.01 |
| Chemotherapy and radiation | 1.83 (0.21–16.15) | .60 |
| None | 1 [Reference] |
Backward selection with an α level of removal of .20 was used. The following variables were removed from the model: age at diagnosis, histological grade, margins, sex, and T-stage. Having locoregional control was the reference group.
Salvage Therapy and Ultimate Locoregional Control
Salvage surgery was attempted in 42 patients for failure at the primary site, with durable control achieved in 30 patients. Adjuvant RT was added in 8 patients, and adjuvant CRT was added in 11 patients. Salvage surgery was attempted in 33 patients who had neck failures, with durable control achieved in 22 patients. Adjuvant RT was added in 9 patients and adjuvant CRT in 15 patients. Of these cases, 12 were reirradiation. Overall, at the time of last follow-up the ULRC rate was 83.3%.
On UVA, African American race (OR, 0.04; 95% CI, 0.01–0.95; P = .04), LVSI (OR, 0.28; 95% CI, 0.12–0.65; P < .01), and close margins (OR, 0.30; 95% CI, 0.13–0.69; P < .01) were associated with worse ULRC (eTable 4 in the Supplement). On MVA, LVSI (OR, 0.22; 95% CI, 0.08–0.59; P < .01) and close margins (OR, 0.24; 95% CI, 0.09–0.62; P < .01) were associated with worse ULRC, with lack of upfront neck dissection showing a nonsignificant finding of worse ULRC (OR, 2.25; 95% CI, 0.895.68; P = .09) (Table 3).
Table 3.
Multivariable Analysisa of Patient, Tumor, and Treatment Variables and Their Association With Ultimate Locoregional Control
| Covariate | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Lymphovascular space invasion | ||
| Positive | 0.22 (0.08–0.59) | <.01 |
| Negative | 1 [Reference] | |
| Margins | ||
| Positive | 0.69 (0.06–7.72) | .76 |
| Close, <5 mm | 0.24 (0.09–0.62) | <.01 |
| Negative | 1 [Reference] | |
| Use of elective neck dissection | ||
| Yes | 1 [Reference] | |
| No | 2.25 (0.89–5.68) | .09 |
Backward selection with an α level of removal of .20 was used. The following variables were removed from the model: age, histological grade, depth of invasion, perineural invasion, race, sex, adjuvant therapy, and T-stage. Having ultimate locoregional control was the reference group.
Overall Survival
On UVA, age greater than 44 years (hazard ratio [HR], 6.15; 95% CI, 1.92–19.68; P < .01), depth of invasion greater than 3 mm (HR, 1.98; 95% CI, 1.01–3.87; P = .04), LVSI (HR, 1.96; 95% CI, 1.10–3.47; P = .02), and close margins (HR, 1.78; 95% CI, 1.03–3.06; P = .04) were associated with worse OS (eTable 5 in the Supplement). On MVA, age older than 44 years (HR, 4.38; 95% CI, 1.34–14.27; P = .01) and LVSI (HR, 2.20; 95% CI, 1.19–4.06; P = .01) were associated with worse OS (Table 4). There was a nonsignificant finding for depth of invasion of at least 3 mm (HR, 2.11; 95% CI, 0.99–4.51; P = .054) to be associated with worse OS. The 2- and 3-year overall survival rates were 94.4% (95% CI, 89.9%–97.0%) and 86.1% (95% CI, 79.9%–90.4%), respectively.
Table 4.
Multivariable Analysisa of Patient, Tumor, and Treatment Characteristics and Their Association With Overall Survival
| Covariate | Overall Survivalb | |
|---|---|---|
| Hazard Ratio (95% CI) | P Value | |
| Age at diagnosis, y | ||
| >44 | 4.38 (1.34–14.27) | .01 |
| ≤44 | 1 [Reference] | |
| Lymphovascular space invasion | ||
| Positive | 2.20 (1.19–4.06) | .01 |
| Negative | 1 [Reference] | |
| Depth of invasion | ||
| ≥3 mm | 2.11 (0.99–4.51) | .054 |
| <3 mm | 1 [Reference] | |
| Use of elective neck dissection | ||
| Yes | 0.64 (0.34–1.19) | .16 |
| No | 1 [Reference] | |
Backward selection with an a level of removal of .20 was used. The following variables were removed from the model: histological grade, margins, perineural invasion, race, sex, adjuvant therapy, and T-stage.
The reference group indicates no survival.
Impact of Lymphovascular Space Invasion
Given that LVSI was associated with worse outcomes as detailed herein, we wanted to more closely examine this cohort compared with the rest of the population. Twenty percent of the population (n = 36) had LVSI. No patients received adjuvant therapy for LVSI alone. Patients with and without LVSI were compared with a trend for more moderately differentiated patients (P = .05) and patients with depth of invasion of at least 3 mm (P = .07) having LVSI (eTable 6 in the Supplement). At 3 years, the LC was 87.0% (95% CI, 80.2%–91.6%) in patients without LVSI vs 54.9% (95% CI, 36.5%–70.0%) in patients with LVSI (log-rank P < .01) (Figure, A). At 3 years, the LRC was 81.9% (95% CI, 74.4%–87.4%) in patients without LVSI vs 38.8% (95% CI, 22.8%–54.6%) in patients with LVSI (log-rank P < .01) (Figure, B). At 3 years the OS rates were 90.3% (95% CI, 83.8%–94.3%) in patients without LVSI vs 71.3% (95% CI, 53.2%–83.4%) in patients with LVSI (log-rank P = .01) (Figure, C). Overall, 5 patients developed distant metastasis, with only 1 patient with LVSI developing distant disease.
Figure. Local Control (LC), Locoregional Control (LRC), and Overall Survival (OS) Among 36 Patients Positive for and 141 Patients Negative for Lymphovascular Space Invasion (LVSI).
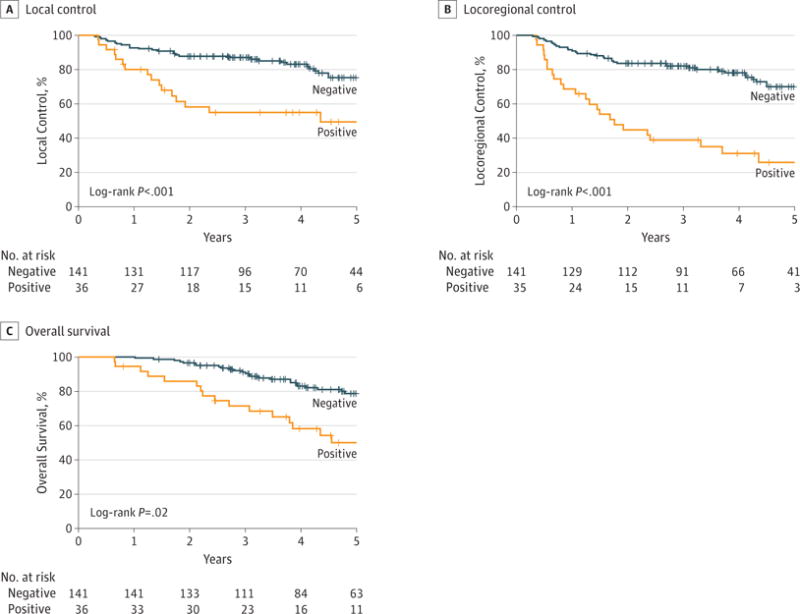
A, Local control, defined as the time from diagnosis to failure at the primary site or time to last follow-up, was analyzed among the patients who had pathological presence of LVSI (36 patients) and compared with those who did not (141 patients). Kaplan-Meier estimates for LC among these patients showed that 3-year LC is significantly worse with LVSI (54.9%) vs no LVSI (87.0%) (P < .01). B, Locoregional control, defined as the time from diagnosist to failure at primary site or in the neck or time to last follow-up, was analyzed among the patients who had pathological presence of LVSI (36 patients) and compared with those who did not (141 patients). Kaplan-Meier estimates for LRC among these patients showed that 3-year LRC is significantly worse with LVSI (38.8%) vs no LVSI (81.9%) (P < .01). C, Overall survival, defined as the time to death or time to last follow-up from diagnosis, was analyzed among the patients who had pathological presence of LVSI (36 patients) and compared with those who did not (141 patients). Kaplan-Meier estimates for OS among these patients showed that 3-yearOS is significantly worse with LVSI (53.2%) vs no LVSI (83.8%) (P = .02). Plus sign indicates censored data.
Toxic Effects
No patient died of treatment-related toxic effects. All patients were able to receive their full course of prescribed RT. Two patients treated with concurrent cisplatin missed 1 cycle; 1 for neutropenia and the other per patient preference. Three patients developed osteoradionecrosis of the mandible. Six patients had long-term percutaneous endoscopic gastrostomy tube dependence.
Discussion
Our series serves as one of the largest in the literature specifically examining patients with node-negative oral tongue cancer treated with initial surgery. We demonstrated that the presence of pathological LVSI is associated with worse LC, LRC, and OS. To our knowledge, this study contains the largest number of patients with node-negative oral tongue cancer with LVSI. The LC, LRC, and OS rates of our series are in line with those of previously published reports.3,5,9,17
The surgical treatment of oral tongue cancer is evolving. A recent randomized clinical trial12 found a survival benefit to elective neck dissection in node-negative oral cavity cancers, but surveillance was poor, which threatened the validity of the conclusions.29 In this series,12 500 patients were randomized to upfront elective neck dissection vs neck dissection for nodal recurrence. At 3 years, those who underwent upfront neck dissection showed improvement in disease-free survival and OS. While this trial included other subsites of the oral cavity, over 85% were oral tongue primary tumors. Adjuvant radiation was delivered for positive nodes, depth of invasion greater than 1 cm, positive margin, or presence of certain risk factors (PNI, LVSI, high grade). On MVA, only depth of invasion was found to be predictive of node positivity. However, only 13% of these patients had PNI or LVSI, and the authors12 did not report them separately, so specific conclusions about LVSI are difficult to make. In our series, we similarly found that use of elective neck dissection was associated with improved LRC (OR, 2.99; 95% CI, 1.16–7.73) but not OS. This suggests that even with appropriately delivered adjuvant therapy, the use of elective neck dissection is beneficial in node-negative oral tongue cancers.
The standard indications for adjuvant therapy in oral tongue cancers have been derived from 2 large randomized trials (EORTC and RTOG).13–16 In both trials, cancers from several subsites were combined together, including the oral cavity, oropharynx, larynx, and hypopharynx. In the EORTC trial, patients with oral cavity or oropharynx primaries with nodal level IV or V disease, advanced T stage, PNI, and LVSI were included. In the RTOG trial, patients with 2 or more positive neck nodes were included. Both trials included pathologically positive margins and extracapsular extension (ECE) in the nodes. While these trials have helped define “high-risk” groups that require adjuvant therapy, only 25% of the patients on these trials had oral cavity cancer, of which only a subset were oral tongue primary tumors. The EORTC trial15 included any patient with LVSI, and given the survival benefit of 13% at 5 years seen, this would suggest that these patients should be getting adjuvant CRT, which was not the case in our series and other reported series of oral cavity cancers.3,5,15,17 Our series helps further delineate risk factors for local and regional control specifically in node-negative oral tongue cancers. We demonstrated that lack of adjuvant radiation therapy is associated with lower LC (OR, 4.30; 95% CI, 1.40–13.22). Because adjuvant therapy in our series was delivered based on guidelines from these randomized trials,16 this would argue that oral tongue cancers may require more aggressive local adjuvant therapy compared with other head and neck subsites, even without standard risk factors. This is in contrast to a recently published abstract30 of a phase 3 randomized clinical trial in locally advanced postoperative oral cavity cancers. In this study, patients were randomized to standard adjuvant RT, accelerated RT given 6 days a week, or concurrent CRT. This was a population with more advanced disease than patients in our series, with 90% having T3/T4 tumors and 48% having N2/N3 disease. Their abstract30 results indicate no benefit to adjuvant therapy escalation, and we await their final published report.
Despite underrepresentation on randomized clinical trials, few studies have specifically examined pathological markers of recurrence and survival in oral tongue cancers. One large series examined all oral cavity subsites, of which 58 patients (25%) were oral tongue primary tumors.17 This study included node-positive patients and examined only patients who were treated with postoperative RT. They determined that 5-year LRC rates were significantly worse in patients with PNI, ECE, and T3 or T4 disease. Diseasespecific survival was worse in patients with ECE or PNI. They did not find LVSI to be a significant predictor of control or survival. This study suggests that adjuvant RT may be sufficient for LVSI, but the inclusion of all oral cavity subsites, node-positive disease, as well as lack of depth of invasion information, makes the study difficult to directly apply to node-negative oral tongue cancers. Similarly, in a series of 88 patients with pathologically node-negative oral tongue cancers, PNI was found to be an independent predictor of nodal disease and extracapsular spread.18 The largest series examining node-negative oral tongue cancers was a multi-institutional review of 164 patients, none of whom received adjuvant therapy.9 The authors9 determined that only tumor thickness of 4 mm or greater was predictive of regional recurrence. While this series suggests that depth of invasion is an independent risk factor for recurrence, it is difficult to make conclusions about LVSI because only 5 patients in their series had this pathological feature.
Limitations
Our series has several strengths and limitations. The major strength is the large number of patients, focusing on a specific clinical scenario: node-negative oral tongue cancers treated with modern therapy. We also have detailed information in regard to treatment type, outcome, and information on toxic effects. The major limitation of our series is the inherent bias of retrospective reviews, namely, the reliance on medical record for accurate information on follow-up. In addition, there is a selection bias with medical record review because there is no randomization of patients.
Conclusions
This series summarizes a large, single-institution experience in the management of clinically and/or pathologically node-negative oral tongue cancer. LVSI seems to be associated with local and regional recurrence, worse survival, without an increase in distant metastasis. Given the locoregional recurrence risk in patients with node-negative oral tongue cancers treated with definitive surgery, the presence of LVSI warrants consideration of adjuvant radiation to the primary site and draining lymphatics, which ideally should be investigated in a prospective setting.
Supplementary Material
Key Points.
Question
What is the impact on disease control and overall survival of lymphovascular space invasion (LVSI) on pathologic findings for patients with node-negative oral tongue cancers treated with upfront definitive surgical resection?
Findings
In this study of 180 consecutive patients treated with standard of care therapy, LVSI was associated with worse local control, locoregional control, and overall survival.
Meaning
In node-negative oral tongue cancers, LVSI on pathologic findings should warrant consideration of more aggressive adjuvant therapy, which should be further investigated in a prospective setting.
Acknowledgments
Funding/Support: Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University and the National Institutes of Health/National Cancer Institute under award No. P30CA138292.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Cassidy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Cassidy, Jegadeesh, Beitler. Acquisition, analysis, or interpretation of data: Cassidy, Switchenko, Sayan, Ferris, Eaton, Higgins, Wadsworth, Magliocca, Saba.
Drafting of the manuscript: Cassidy, Switchenko, Jegadeesh, Sayan, Saba, Beitler.
Critical revision of the manuscript for important intellectual content: Cassidy, Switchenko, Jegadeesh, Ferris, Eaton, Higgins, Wadsworth, Magliocca, Saba, Beitler.
Statistical analysis: Cassidy, Switchenko. Administrative, technical, or material support: Jegadeesh, Sayan, Magliocca, Beitler.
Supervision: Cassidy, Eaton, Higgins, Wadsworth, Saba, Beitler.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No disclosures were reported.
References
- 1.GLOBOCAN 2012: estimated cancer incidence, mortality, and prevalence worldwide in 2012. 2016 http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed December 15, 2016.
- 2.Krolls SO, Hoffman S. Squamous cell carcinoma of the oral soft tissues: a statistical analysis of 14,253 cases by age, sex, and race of patients. J Am Dent Assoc. 1976;92(3):571–574. doi: 10.14219/jada.archive.1976.0556. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DP, Bachar GY, Lea J, et al. Outcomes of squamous cell cancer of the oral tongue managed at the Princess Margaret Hospital. Head Neck. 2013;35(5):632–641. doi: 10.1002/hed.23001. [DOI] [PubMed] [Google Scholar]
- 4.Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer. 2008;112(2):345–351. doi: 10.1002/cncr.23183. [DOI] [PubMed] [Google Scholar]
- 5.Sessions DG, Spector GJ, Lenox J, Haughey B, Chao C, Marks J. Analysis of treatment results for oral tongue cancer. Laryngoscope. 2002;112(4):616–625. doi: 10.1097/00005537-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hicks WL, Jr, North JH, Jr, Loree TR, et al. Surgery as a single modality therapy for squamous cell carcinoma of the oral tongue. Am J Otolaryngol. 1998;19(1):24–28. doi: 10.1016/s0196-0709(98)90061-8. [DOI] [PubMed] [Google Scholar]
- 7.Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10(3):160–167. doi: 10.1002/hed.2890100304. [DOI] [PubMed] [Google Scholar]
- 8.Byers RM, Weber RS, Andrews T, McGill D, Kare R, Wolf P. Frequency and therapeutic implications of “skip metastases” in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19(1):14–19. doi: 10.1002/(sici)1097-0347(199701)19:1<14::aid-hed3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Ganly I, Goldstein D, Carlson DL, et al. Long-term regional control and survival in patients with “low-risk,” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013;119(6):1168–1176. doi: 10.1002/cncr.27872. [DOI] [PubMed] [Google Scholar]
- 10.Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009;115(7):1489–1497. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- 11.Omura K. Current status of oral cancer treatment strategies: surgical treatments for oral squamous cell carcinoma. Int J Clin Oncol. 2014;19(3):423–430. doi: 10.1007/s10147-014-0689-z. [DOI] [PubMed] [Google Scholar]
- 12.D’Cruz AK, Vaish R, Kapre N, et al. Head and Neck Disease Management Group Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373(6):521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JS, Pajak TF, Forastiere AA, et al. Radiation Therapy Oncology Group 9501/Intergroup Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84(5):1198–1205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernier J, Domenge C, Ozsahin M, et al. European Organization for Research and Treatment of Cancer Trial 22931 Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 16.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 17.Hinerman RW, Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head Neck. 2004;26(11):984–994. doi: 10.1002/hed.20091. [DOI] [PubMed] [Google Scholar]
- 18.Chinn SB, Spector ME, Bellile EL, et al. Impact of perineural invasion in the pathologically NO neck in oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013;149(6):893–899. doi: 10.1177/0194599813506867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edge SB, American Joint Committee on Cancer . AJCC Cancer Staging Manual. 7th. New York, NY: Springer; 2010. p. xiv. [DOI] [PubMed] [Google Scholar]
- 20.Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488–1494. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 21.Pitman KT, Johnson JT, Wagner RL, Myers EN. Cancer of the tongue in patients less than forty. Head Neck. 2000;22(3):297–302. doi: 10.1002/(sici)1097-0347(200005)22:3<297::aid-hed14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44years. Cancer. 2005;103(9):1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 23.Myers JN, Elkins T, Roberts D, Byers RM. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg. 2000;122(1):44–51. doi: 10.1016/S0194-5998(00)70142-2. [DOI] [PubMed] [Google Scholar]
- 24.Udeabor SE, Rana M, Wegener G, Gellrich NC, Eckardt AM. Squamous cell carcinoma of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysis. Head Neck Oncol. 2012;4:28. doi: 10.1186/1758-3284-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos HB, dos Santos TK, Paz AR, et al. Clinical findings and risk factors to oral squamous cell carcinoma in young patients: a 12-year retrospective analysis. Med Oral Patol Oral Cir Bucal. 2016;21(2):e151–e156. doi: 10.4317/medoral.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandler K, Vance C, Budnick S, Muller S. Muscle invasion in oral tongue squamous cell carcinoma as a predictor of nodal status and local recurrence: just as effective as depth of invasion? Head Neck Pathol. 2011;5(4):359–363. doi: 10.1007/s12105-011-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu XJ, Lagakos SW. Nonparametric estimation of the mean function of a stochastic process with missing observations. Lifetime Data Anal. 2007;13(1):51–73. doi: 10.1007/s10985-006-9030-0. [DOI] [PubMed] [Google Scholar]
- 28.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57(1):114–119. doi: 10.1111/j.0006-341x.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 29.Beitler JJ, Cassidy RJ, III, Jagadeesh NK. Elective versus therapeutic neck dissection in oral cancer. N Engl J Med. 2015;373(25):2476–247. doi: 10.1056/NEJMc1511351. [DOI] [PubMed] [Google Scholar]
- 30.Laskar SG, Chaukar D, Deshpande M, et al. Phase III randomized trial of surgery followed by conventional radiotherapy (5 fr/Wk) (arm A) vs concurrent chemoradiotherapy (arm B) vs accelerated radiotherapy (6fr/Wk) (arm C) in locally advanced, stage III and IV, resectable, squamous cell carcinoma of oral cavity-oral cavity adjuvant therapy (OCAT): final results ( NCT00193843) J Clin Oncol. 2016;34 Abstr 6004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


