Abstract
With age, stroke prevalence is higher, and stroke outcome, worse, in women. Thus there is an urgent need to identify stroke neuroprotectants for this population. Using a preclinical stroke model, our studies focused on microRNAs (miRNAs), a class of translational repressors, as neuroprotectants. Analysis of circulating miRNA in the acute phase of stroke indicated potential neuroprotective capacity for miR363. Specifically, mir363 is elevated in serum of adult female rats that typically have small infarct volumes, but is deficient in age-matched males or middle-aged males and females, groups that have greater stroke-associated impairment. To directly test the effect of mir363 on stroke outcomes, first, adult females were treated with antagomirs to mir363 post stroke and next, middle-aged females were treated with mimic to mir363-3p post stroke. Antagomir treatment to adult females significantly increased infarct volume and impaired sensory motor performance. Reciprocally, mir363 mimic to middle-aged females reduced infarct volume, preserved forebrain microvessels and improved sensory motor performance. In the early acute stroke phase, mir363-3p mimic reduced the expression and functional activity of caspase-3, a critical component of the apoptotic cell cascade. In contrast, mir363-3p mimic treatment had no effect on stroke outcomes or caspase regulation in young males. Collectively, these studies show that mir363 is neuroprotective for stroke in females and implicates caspase-3 as a sex-specific miRNA-sensitive node for recovery from ischemic stroke.
Keywords: microRNA, cerebral ischemia, caspase-3, aging
Introduction
In view of the growing incidence of ischemic stroke in the US and the paucity of effective treatment options, neuroprotective factors for this disease are urgently needed. In preclinical models, adult females have better stroke outcomes as compared to middle-aged females and adult and middle-aged males in terms of infarct volume and sensory motor behaviors. This has been attributed to the neuroprotective effects of estrogen, and is supported by data showing that bilateral ovariectomy worsens stroke outcomes (Alkayed et al., 1998, Park et al., 2006), while estrogen treatment to ovariectomized females improves stroke-related impairment (Simpkins et al., 1997, Dubal et al., 1998, Rusa et al., 1999, Sawada et al., 2000, Jover et al., 2002). Estrogen treatment to middle-aged, reproductively senescent females, however, is not uniformly neuroprotective for stroke. In some cases, estrogen replacement reduces infarct volume (Alkayed et al., 2000, Wise and Dubal, 2000, Dubal et al., 2001, Toung et al., 2004), while in other cases, hormone treatment is either ineffective or increases infarct volume (Viscoli et al., 2001, Selvamani and Sohrabji, 2010a, Selvamani and Sohrabji, 2010b, Leon et al., 2012)(. Concomitantly, the Women’s Health Initiative study reported that hormone therapy increased the risk for stroke in postmenopausal women (Wassertheil-Smoller et al., 2003). Collectively, the experimental and clinical data underscore the need for alternative effective neuroprotectants for stroke-prone demographic groups such as middle-aged and aging women.
A class of small non-coding RNAs, microRNAs (miRNAs), has emerged as novel therapeutic targets in several diseases including cancer, infectious, immune-mediated, cardiovascular and neurodegenerative diseases (Iorio et al., 2005, Randall et al., 2007, Keller et al., 2009, Martins et al., 2011). MicroRNAs are typically ~25 nucleotides long and regulate gene expression post-transcriptionally by binding to complementary sequences in the 3′ UTR of multiple target mRNAs (Ambros, 2001). Pharmacological and genetic manipulations of individual miRNA or miRNA family in cardiovascular diseases show that they are key regulators of cell survival. Thus stroke outcomes are reportedly improved with infusion or transfection with premir-29c (Pandi et al., 2013) or mir424 mimics (Zhao et al., 2013). Occluding the middle cerebral artery (MCAo) to the mir-223 knock out mouse increases infarct volume as compared to the wildtype control, and rAAV-mediated transfer of miR-223 reduces infarct volume (Harraz et al., 2012). Similarly, antagomirs to Let7f infused after stroke (Selvamani et al., 2012), mir181a infused before stroke (Moon et al., 2013), and mir103-1 delivered before or after stroke (Vinciguerra et al., 2014) also decreased infarct volume. A recent study also shows that the route of miRNA administration may also affect outcomes, such that intravenous but not intracerebroventricular, mir122 improved stroke outcome in adult rats (Liu da et al., 2016). Hence, no studies have identified a miRNA that is neuroprotective in older female animals.
In an expression profiling study comparing 168 serum miRNA in adult and middle age males and females after stroke, we identified mir363-3p, whose expression was inversely correlated with infarct volume. Specifically, in the early acute phase of stroke, adult females (who typically have small infarcts) had high levels of mir363-3p, while groups that typically have large infarct volumes such as middle aged females and adult and middle-aged males, had low levels of this microRNA. Based on this pattern, we hypothesized that elevated expression of miR363 in the early hours after stroke may be functionally associated with reduced infarction in adult females, and could potentially be a therapeutic target for middle-aged females and males of both ages. Using mimic or antagomir to mir363-3p, the present studies show that mir363-3p mediates neuroprotection in stroke-affected females, but has no effect on males. These data underscore the value of miRNA profiling as a strategy to identify new therapeutic targets for stroke, as well as the potential for sexually dimorphic regulation of critical cell death pathways.
Methods
Animals
All animals were purchased from Harlan Laboratories (IN). Females were purchased as adults (6–7 months, 230–320 g) and middle aged (10–12 months, 280–360g) while adult males were age- matched to adult females and middle aged females. All animals were maintained in a 12-h dark:12-h light cycle in AAALAC-accredited vivarium facilities. Food and water were available ad libitum. A week after arrival, females were smeared daily for 14–21 days to determine estrous status (Jezierski and Sohrabji, 2001). Vaginal cells were collected using cotton swabs, placed on slides and cell cytology was examined at a low magnification. Adult females with a normal estrous cycle of 4–6 days were included in the study. Middle aged females were included if cell cytology indicated they were in constant diestrus for at least 7 consecutive days. Adult animals and middle aged animals were at an average of 7 and 11.5 months, respectively, at the time of middle cerebral artery occlusion (MCAo). Within each age and sex, animals were assigned randomly to the treatment groups. A total of 129 animals were used in these studies with group sizes of 5–7. Animals were randomly assigned to treatment groups.
Middle cerebral artery occlusion
All animals were subjected to stereotaxic surgery to occlude the left middle cerebral artery as reported in (Selvamani and Sohrabji, 2010a, Selvamani and Sohrabji, 2010b, Selvamani et al., 2012, Selvamani et al., 2014). Briefly, MCA occlusion was induced by microinjecting 3 ul of Endothelin-1 (American Peptide Company INC, CA; 0.5 ml in 1 ml PBS). Rats were maintained at 37°C throughout surgery and O2 saturation was measured by pulse oximetry. For miR-363-3p experiments: four hours post-stroke, animals were administered tail vein injections, 300 μl (7mg/Kg) of either miRNA mimic negative control or miR-363-3p mimic (AAUUGCACGGUAUCCAUCUGU) oligonucleotide sequences (Thermo Fisher, Grand Island, NY) in In-vivo RNA-LANCEr II (Bioo Scientific, Austin, TX). Animals were terminated at 48h or on day 5 post-MCAo. At termination, the brain was rapidly removed and processed for TTC (Triphenyl Tetrazolium Chloride) staining to assess infarct volume. For molecular analyses, brain tissue was dissected and stored in −80°C. For histological analyses, animals were terminated at 48h post-MCAo and perfused transcardially with saline followed by 4% paraformaldehyde. Brains were then sucrose-loaded and embedded in cryoprotectant sectioning media and stored in −80 °C.
Infarct volume
Infarct volume estimation was performed on animals terminated on day 5 post stroke using our previously described procedures (Selvamani and Sohrabji, 2010b). Briefly, brain slices (2 mm thick) between −2.00 mm and +4.00 mm from Bregma were incubated in a 2% TTC solution at 37°C for 20 min and later photographed using a Nikon E950 digital camera attached to a dissecting microscope. Infarct volume was determined from digitized images using the Quantity One software package (Bio-Rad CA). The volume of the infarct was expressed as a percentage of the contralateral (non-occluded) hemisphere. To ensure reliable, consistent and unbiased estimation of the infarct zone, all images were first coded and all volumetric traces were performed by an investigator blind to the codes (AS).
Behavioral assays
Sensory-motor performance was assessed using procedures described previously for the vibrissae-evoked forelimb placement task (Selvamani and Sohrabji, 2010a, Selvamani and Sohrabji, 2010b) and the adhesive-removal test (Balden et al., 2012). Both tests were performed before and after MCAo surgery. Vibrissae-evoked forelimb placement task: Animals were subject to same-side placing trials and cross-midline placing trials elicited by stimulating the ipsi and contra-lesional vibrissae. During the same-side forelimb placing trials, animal’s ipsi-lesional vibrissae were brushed against the edge of a table to elicit a forelimb placing response, which typically consisted of the forelimb ipsi-lateral to the stimulated vibrissae. In the cross-midline placing trials the ipsi-lesional vibrissae lie perpendicular to the tabletop and the forelimb on that side is gently restrained as the vibrissae was brushed on the top of the table to evoke a response from the contralateral limb and vice versa. Ten trials were performed before the same procedure was repeated for the contra-lesional vibrissae. Adhesive removal test: Two pieces of adhesive-backed foam tape (1 × ½″) were used as bilateral tactile stimuli attached to the palmar surface of the paw of each forelimb. For each forelimb, the time it took to remove each stimulus (tape) from the forelimbs was recorded during three trials per day for each forepaw. Animals were allowed to rest for 5 min between sessions, and each test session had a maximum time limit of 120 sec.
MicroRNA analyses
Circulating microRNA
Circulating miRNA was assessed in blood samples. Blood was collected by saphenous draw at 2d post stroke and trunk blood was collected at termination (5d post stroke). Blood samples were centrifuged at 1300×g for 30 mins to obtain serum.
Mir363-3p was analyzed in serum (n=6 in each experimental group). RNA extraction: RNA was extracted from serum and astrocytes using the miRNeasy Kit (Qiagen, CA) and manufacturer’s instructions, as described in our previous publication (Selvamani et al., 2014). Sample purity was assessed by Nanodrop technology and a ratio of 1.8 was considered acceptable. Samples were stored at −20°C until use. PCR Amplification: Template RNA (25ng total RNA per sample) was incubated with reverse transcriptase for 60 min at 42°C, followed by heat-inactivation of the enzyme (5 min at 95°C) and used immediately. cDNA was diluted 80-fold and then incubated with SYBR® Green master mix. Ten μl was dispensed to each tube. An activation/denaturation step (95°C, 10 min) precedes 40 amplification cycles each at 95°C, 10 s, 60°C, 1 min, ramp-rate 1.6°C/s. Mir363-3p primers were LNA modified (Exiqon, Woburn, MA) which allows for uniform Tm, and confers greater specificity. Samples were then subject to PCR amplification of U6. Delta CT values of miR363-3p were obtained by subtracting the U6 value and ddCT values were obtained by subtracting the mean dCT of the adult female group from each value in all groups. The fold change was expressed as the inverse log of ddCT(1/2^ddCT).
Lectin histochemistry
Lectin staining was performed on 25mm coronal brain sections using Flourescein Lectin (1:200, Vector, Burlingame, CA). Sections were then washed in PBS and counterstained with Hoechst (Polyscience, PA) and coverslipped with ProLong (Invitrogen, CA). Flourescence images were captured using an inverted scope and microvessel density analysed using Image J software (ImageJ 1.48v).
Cellular uptake of mir363-3p in the brain
Immunofluorescence was used to evaluate colocalization of FAM-labelled miR-363-3p in neurons, astrocytes, microglia and endothelial cells. Coronal brain sections (25 mm thickness) were incubated in either NeuN (1:100), anti-GFAP (1:200, Sigma, St. Louis, MO), anti-CD11b (1:100, Serotec, Raleigh, NC), CD31 (1:50, BD pharmagen, San Jose, CA) or tomato lectin (1:200, Vector, Burlingame, CA). Sections were then washed in PBS and incubated with a secondary antibody -goat anti-rabbit (1:2000, Molecular probes, Carlsbad, CA) or goat anti-mouse (1:2000; Grand Island, NY). Finally, the sections were rinsed, counterstained with Hoechst (Polyscience, PA) and coverslipped with ProLong (Invitrogen, CA). Flourescence images were captured using the fully motorized FSX100 inverted microscope.
Cellular localization of Mir363-3p
A combination of in-situ hybridization and immunofluorescence was used to confirm astrocytic localization of mir363-3p. Female rats were euthanized 5d post stroke and the brains were removed following intracardial paraformaldehyde perfusion. Coronal brain sections (25 mm thickness) were incubated in prehybridization solution (100% Formamide, 5 M Nacl, 50X Denhart, 1 M DTT, 10 mg/ml tRNA and 100% Triton) at 45uC for 2 h. The sections were incubated overnight at 45°C in hybridization solution containing 2.5 mM LNA-modified mir363-3p oligonucleotide probe, (ACAGATGGATACCGTGCAATT, Exiqon (Vedbaek, Denmark). The sections were washed with 5% SSC at room temperature for 5 min followed by incubation in formamide wash solution (50% Formamide, 0.1% Tween-20, 1% SSC and DEPC) at 45°C for 30 min. Sections were washed in 0.2%SSC at RT for 30 min and then subject to three 5 min washes with PBS followed by incubation with Rhodamine Avidin (1:500, Vector, Burlingame, CA), for 1h at RT. Following three PBS washes the sections were incubated in the blocking solution (NGS, triton, RNasin and 4 drops/ml of block of avidin blocking solution) at RT for 1 h and then incubated ON with anti-GFAP (Sigma, St. Louis, MO). Sections were then washed in PBS and incubated with a secondary antibody -goat anti-mouse (1:500, Molecular probes, Carlsbad, CA). Finally, the sections were rinsed, counterstained with Hoechst (Polyscience, PA) and coverslipped with ProLong (Invitrogen, CA). Confocal images of the double in situ/immunofluorescence were obtained using Olympus Fluoview 1200, with a BX61WI microscope (Waltham, MA).
Astrocyte microRNA
Astrocytes were harvested from the ischemic hemisphere 2d post stroke, using procedures published in (Chisholm et al., 2015). Briefly, tissues were dissociated using a neural dissociation kit (trypsin) and cells were passed through a 30 mm filter to obtain a single cell suspension. Following myelin removal, cells were collected by positive selection using anti-GLAST antibody (1:5) for 10 min. GLAST was selected as a marker because it is an astrocyte-specific, membrane associated protein, and previous work has established that astrocytes harvested 48 h after ischemia express glutamate transporters (GLT-1 and GLAST) and display no age differences in their expression. Cells were then processed for mir363-3p expression as described above.
Analysis of cell death pathways
Protein extraction
Cell proteins from ischemic hemisphere (cortex and striatum) from animals terminated at 48h were harvested in lysis buffer [50 mm Tris (pH 7.4), 150 mm NaCl, 10% glycerol, 1 mM EGTA, 1 mM Naorthovanadate (pH 10), 5 μM ZnCl2, 100 mM NaF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride in dimethylsulfoxide, 1% Triton X-100] and centrifuged at 20,000 rpm for 30 min. Supernatant was collected and stored at −20°C until further analysis. Protein concentrations were determined using the BCA protein assay kit (Pierce, Rockford, IL).
Western blot analysis of Caspase-3 expression
Samples were size-fractionated on 10% PAGE and transferred to Hybond-C membranes (Amersham Pharmacia Biotech, NJ). Blots were blocked with 1× Tris-buffered saline containing 0.05% Tween 20 and 5% nonfat dry milk. Subsequently blots were probed for Caspase-3 and stripped and reprobed for β-actin. Blots were incubated with primary (1:200, Caspase-3 (7148); Santa Cruz, CA; b-actin (47778) and secondary antibodies (1:2000, goat anti-rabbit; Santa Cruz, CA; Goat anti-mouse 1:2000, BD Pharmagen) subjected to three washes in 1× Tween Tris-buffered saline buffer between the two incubations. The immunosignal was detected by AlphaImager™ Gel Imaging System from Alpha Innotech using chemiluminescence reagents (Fisher Scientific, Pittsburgh, PA). Immunosignal was quantified using computer-assisted densitometric analysis (Quantity One; Bio-Rad). Caspase-3 immunosignal was normalized to b-actin.
Caspase activity
Caspase 3/7 activity was measured using the Caspase-Glo® 3/7 assays (Promega Corp., USA) according to manufacturer’s instructions. Protein lysates from ischemic cortex and striatum were subjected to Caspase 3/7 activity measurement with Caspase-Glo assay kit (Promega, Madison USA). 100ul of cell lysates were aliquoted into 1.5ml tubes followed by 100 μl of Caspase-Glo reagent to each well, the content of well was gently mixed with a plate shaker at 300–500 rpm for 30 seconds. The plate was then incubated at room temperature for 3 hours. The luminescence of each sample was measured in a plate-reading luminometer (TECAN, Switzerland) with parameters of 1minute lag time and 0.5 second/well reading time. The experiments were performed in duplicate.
Statistical analysis
Power analysis for group sizes was computed based on effect sizes seen in previous data and pilot studies. In order to achieve power of 0.9 (1-β) and Type 1 error rate α=0.05, the minimum sample size is 5. For these studies, group sizes ranged from 5–7. For comparisons between adult and middle-aged males and females on infarct volume and mir363-3p expression, a two-way ANOVA with post-hoc comparisons were performed. For behavioral tests, a paired t-test was used for each group, comparing the values obtained pre and post-stroke. For all other tests, an unpaired t-test was performed. Group differences were considered significant at p<0.05 in each case. The statistical package SPSS (v. 21, IBM) was used for these analyses.
RESULTS
Effect of age and sex on post-stroke mir363-3p expression
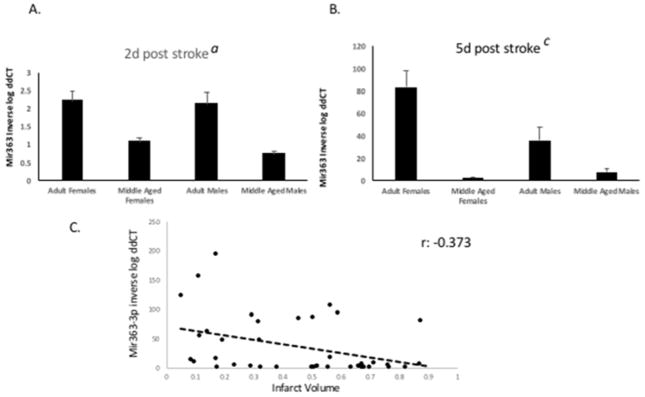
Serum samples were obtained from adult and middle-aged female and male Sprague Dawley rats at 48h and 5d after middle cerebral artery occlusion and analyzed for mir363-3p expression. Two sets of serum samples were used for these analyses; one from a previously published study (Selvamani et al., 2014) and a second set that was separately prepared to confirm these findings. Data from both sets were pooled and shown here. All data were normalized (ddCT) to the middle aged females, since this group showed little variation between the 2d and 5d time point. QRT-PCR analysis of miR-363-3p in serum showed a distinct sex- and age-dependent expression at each time point. At 2d post stroke (Figure 1A), circulating miR-363-3p expression was similar in adult males and females, and both adult groups were more than 2 fold higher than middle aged males and females (main effect of age; F(1,36): 25.146, p<0.001). At 5d post stroke (Figure 1B), miR363-3p levels continued to rise in adult females, and was now significantly higher than all other groups (sex X age interaction; F(1,36): 4.46, p<0.05). Normalized mir363-3p values at 5d post stroke were plotted against infarct volume at 5d post stroke. The relationship between mir363-3p and infarct volume indicates an inverse association, with a correlation of r: −0.37, p<0.05, Pearsons r 2-tailed test). This pattern confirmed that circulating mir363-3p expression at day 5 was inversely related to infarct volume (Figure 1C).
Figure 1. Age and sex differences in circulating mir363-3p expression after stroke.
Mir-363 expression was measured in serum at (A) 2d and (B) 5d post stroke by qRT-PCR. Histogram depicts mean (±SEM) of ddCT values. In all cases, ddCT is normalized to middle-aged females at 2d post stroke. C. Scatterplot representing the relationship between infarct volume and mir363-3p expression at 5d post stroke. Pearsons’s correlation: −.373, p<0.019. a: main effect of age, b: main effect of sex, c: interaction effect. Histograms depict mean±SEM, from 2 separate sets of animals with n=5–6 in each group/set.
Effect of intravenous miR-363-3p antagonists on stroke outcomes in adult females
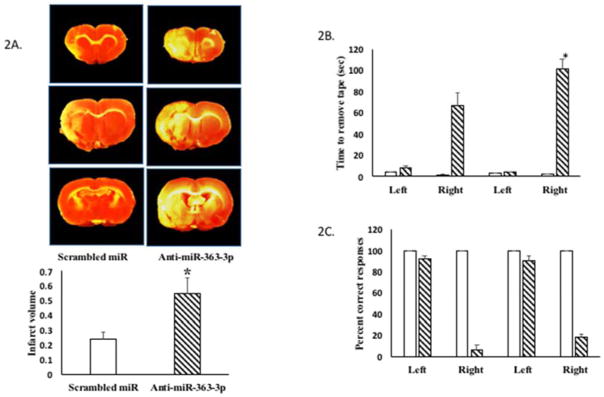
To determine whether elevated levels of mir363-3p in the adult female is associated with better stroke outcomes, adult female rats (5–7 month) were subject to stroke and then injected iv with antagomirs to mir363-3p (a sequence complementary to mir363-3p). As shown in Figure 2A, TTC-stained images indicate a cortico-striatal infarct that is much larger in adult females that received antagomirs to mir363-3p as compared to adult female rats that received injections of a scrambled oligo control. Adult females typically sustain small infarcts after MCAo, while injections of antagomirs post-stroke increased infarct volume by 2.5 fold (Figure 2A; p<0.01).
Figure 2. Effect of intravenous mir363-3p antagomirs on stroke outcomes in adult females.
Adult female rats were injected iv with antagomirs to mir363-3p or scrambled oligos 4h after stroke. A. TTC-stained coronal sections from control oligo and anti-mir-363-3p injected. Histogram depicts average infarct volume (±SEM) normalized to the volume of the non-ischemic hemisphere. B. Sensory motor performance on the adhesive removal test was evaluated before and after stroke. Histograms depict mean (±SEM) latency in seconds to remove the tape. C. Sensory motor performance was also evaluated by vibrissae-evoked forelimb placement task. Histogram depicts percent (+SEM) correct responses over 10 trials. Clear bars represent pre-stroke performance and hatched bars represent post stroke performance. N=5–6 per group. *:p<0.01
The vibrissae-elicited forelimb placing test and the adhesive removal test was performed both before and after MCAo to assess sensory motor deficits commonly seen in this stroke model. Performance on the adhesive removal test is impaired after MCAo (Figure 2B), as shown by the longer latency to tape removal. The latency was significantly increased in the group that received mir363-3p antagomirs, indicating further deterioration on this task. Performance on the vibrissae-evoked forelimb placement task was also worse after stroke although mir363-3p antagomirs did not further impair performance on this task (Figure 2C). Collectively, mir363 antagonists to adult female rats worsened stroke outcomes as measured by infarct volume and sensory motor performance.
Effect of mir363-3p mimic on stroke outcomes in middle-aged females
Middle-aged females display low levels of mir363-3p expression in comparison to adult females, at 2d and 5d post stroke. To determine whether increasing mir363-3p levels would improve stroke outcomes, middle-aged females were subject to stroke and injected iv 4h later with miRNA mimic.
Infarct volume
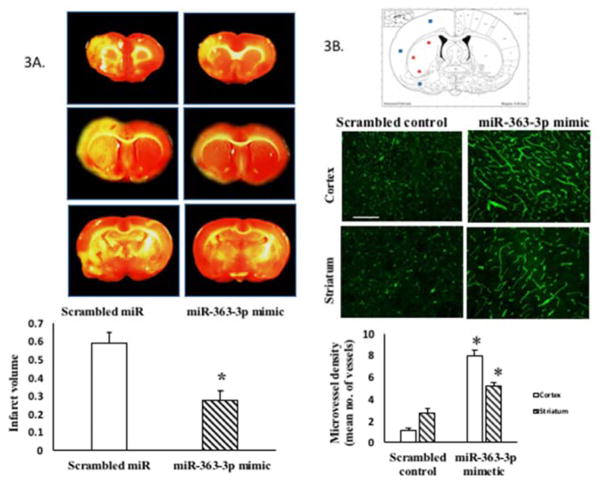
As shown in Figure 3A, TTC-stained sections show that infarct volume in middle-aged females, at 5d post stroke, was significantly reduced in the group that received post stroke iv infusion of miR-363-3p mimic. Specifically, mir363-3p mimic decreased infarct volume by 55% as compared to the group that received the scrambled control (p ≤ 0.001).
Figure 3. Effect of intravenous post stroke miR-363-3p mimic treatment to middle aged females.
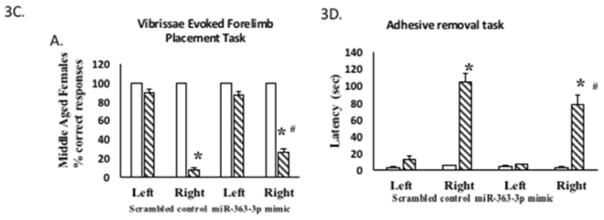
(A) Infarct volume: TTC stained brain sections at 5d post stroke from middle-aged females treated with scrambled oligos (control) or miR363-3p mimic. Histogram depicts average infarct volume (±SEM) normalized to the volume of the non-ischemic hemisphere. B. Microvessel density: Schematic of a brain coronal section (bregma −4.0mm; Paxinos) depicts the three regions of the cortex and striatum that were photographed for microvessel density analysis. Photomicrograph of tomato lectin stained microvessels in the cortex and striatum of mir363-3p or control treated animals after MCAo. Histogram depicts mean (+SEM) density of Tomato lectin labeled microvessels. *: p<0.05. Bar=200 μm. C. Sensory motor performance was evaluated by vibrissae evoked forelimb placement task. Histogram depicts percent (+SEM) correct responses over 10 trials. D. Sensory motor performance on the adhesive removal test was evaluated before and after stroke. Histograms depict mean (±SEM) latency in seconds to remove the tape. (*: p<0.05, comparison of pre versus post stroke performance; #: p<0.05, control versus mir363-3p post stroke performance). In C and D, clear bars represent pre-stroke performance and hatched bars represent post stroke performance
Microvessel density
Microvessel density was also measured in the ischemic hemisphere of control and mir363-3p treated middle aged females, using fluorescein labeled Lycopersicon Esculentum (Tomato) Lectin, a marker for blood vessels and microglia. In each case, stained microvessels were analyzed by fluorescent microscopy in 3 selected regions each of the cortex and in the striatum as indicated in the schematic in Figure 3B. Mean density of tomato lectin-staining confirmed that microvessel density was significantly greater in the cortex and striatum of the miR-363-mimic treated group compared to the scrambled controls (p<0.05).
Behavioral assays
Vibrissae-elicited forelimb placement test: This test revealed a significant deficit in paw-placement due to stroke on the contra-lesional side (p < 0.05), but not on the ipsi-lesional side when compared to pre-stroke performance. Additionally, miR-363 treatment significantly improved performance on the contra-lesional paw post-stroke, compared to the scrambled controls (p<0.05). Histogram depicts ‘percent correct’ responses ±SEM (Figure 3C). Adhesive removal test. Sensory motor function was further evaluated by the latency to adhesive tape removal from the palmar surface of the ipsi-lesional and contra-lesional paw for each group. As expected, there were no significant differences between treatment groups in the average latency for tape removal from the ipsi-lesional paw pre- or post-stroke. In middle aged females, post-stroke performance was significantly impaired on the contra-lesional paw in both groups. However, the group that received the miR-363-3p mimic group had significantly less post-stroke functional loss (77.1 seconds) as compared to the scrambled control group (106.4 seconds) (p<0.05; Figure 3D).
Collectively, mir363-3p mimic to middle-aged females reduced MCAo-induced infarct volume, preserved microvessels and reduced sensory motor impairment
Effect of mir363-3p mimic on stroke outcomes in males
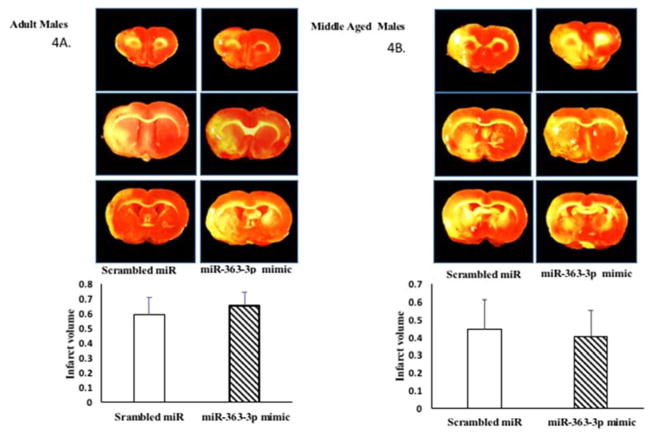
Similar to middle-aged females, adult and middle-aged males also express lower amounts of circulating mir363-3p at 5d post stroke and also display worse stroke outcomes. In this experiment, adult and middle-aged males (age-matched to females) were subject to MCAo and later injected with mir363-3p mimic. As shown in Figure 4A and 4B, infarct volume was not different from between the group that received the scrambled control and mir363-3p treatment in either adult (Fig 4A) or middle-aged (Fig 4B) males.
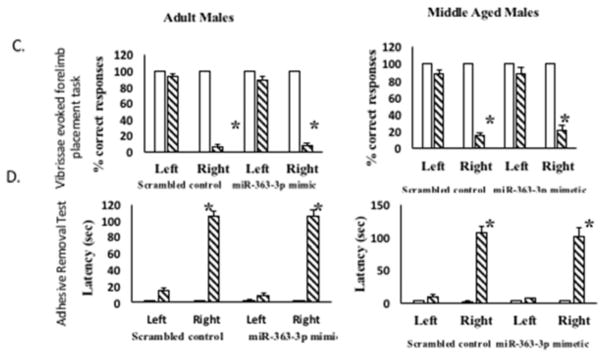
Figure 4. Effect of mir363-3p mimic on post stroke outcome in males.
Infarct volume: TTC stained brain sections at 5d post stroke from (A) adult and (B) middle-aged males treated with scrambled oligos (control) or miR363-3p mimic. Histogram depicts average infarct volume (±SEM) normalized to the volume of the non-ischemic hemisphere. (C) Sensory motor performance was evaluated by vibrissae evoked forelimb placement task. Histograms depict mean (±SEM) latency in seconds to remove the tape. (*: p<0.05, comparison of pre versus post stroke performance. D. Sensory motor performance on the adhesive removal test was also affected by stroke. Histograms depict mean (±SEM) latency in seconds to remove the tape. (*: p<0.05, comparison of pre versus post stroke performance). N = 6–7 per group
Behavioral assays
Vibrissae-elicited forelimb placement test: This test revealed a significant deficit in paw-placement after stroke on the contra-lesional side (p < 0.05), but not on the ipsi-lesional side when compared to pre-stroke performance. Additionally, miR-363 treatment had no effect on post stroke performance on either adult (Fig 4C, left panel) or middle-aged (4C, right panel) males. Adhesive removal test. Sensory motor function was also evaluated by the latency to adhesive tape removal from the palmar surface of the ipsi-lesional and contra-lesional paw. As expected, there were no significant differences between treatment groups in the average latency for tape removal from the ipsi-lesional paw pre- or post-stroke. Post-stroke performance was significantly impaired on the contra-lesional paw in both groups. Furthermore, there were no differences in latency between the groups that received miR-363-3p mimic or the scramnbled control, in either the adult (Fig 4D, left panel) or middle-aged (Fig 4D, right panel) males.
Confirmation of Mir363-3p mimic in plasma and brain
To ensure that the sex difference in the effect of mir363-3p was not due to different availability of the mimic, tissues from middle-aged females and males injected (iv) with the mimic and the scrambled control were subject to qRT-PCR. Tail vein injections of mir363 significantly elevated serum expression of this microRNA when measured 48h post stroke in both males (4.01 fold) and females (3.76 fold), as compared to animals injected with scrambled control oligos (Table 1a). By 5d post stroke, control and mimic injected groups did not differ in their expression of serum mir363-3p. Brain tissue also showed significantly higher expression of miR-363 in the ischemic hemisphere in the group that received the mimic at 48h. MiR363 was elevated to a similar extent in both females (3.76 fold) and males (3.52). At 5d post stroke, mir363 levels were further elevated in the ischemic hemisphere, and to a similar extent in females (8.43 fold) and males (9.02 fold). No difference was observed between the ischemic and non-ischemic hemisphere in scrambled control injected animals, indicating that the mimic was preferentially recruited to the ischemic hemisphere (Table 1b). Overall, in both males and females, mir363-3p injections transiently elevate this microRNA in circulation (2d) and persistently (2d–5d) elevate mir363-3p levels in the brain.
Table 1a.
Fold change in serum mir363 after injection with mimetic
p<0.05 compared to scrambled controls
Table 1b.
Fold change in brain mir363 after injection with mimetic
| Females | Males | |||
|---|---|---|---|---|
| Brain hemisphere | Ischemic | Non-Ischemic | Ischemic | Non-Ischemic |
| 2d post stroke | 3.76* | 1.098 | 3.52* | 1.31 |
| 5d post stroke | 8.43* | 0.82 | 9.02* | 0.82 |
p<0.05 compared to scrambled controls
Cellular localization of mir363-3p mimic in the brain
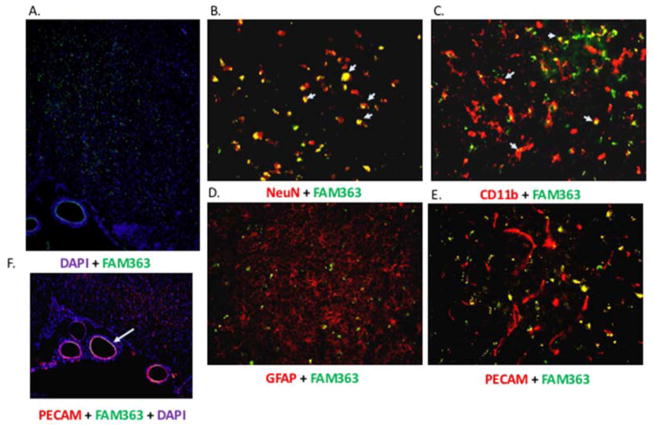
To determine which neural cell types localized exogenous mir363-3p, combined immunofluorescence was performed for cell specific markers and fluorescein (FAM) labeled mir363-3p mimic. Female rats were injected with FAM-mir363-3p 4h post stroke by tail vein injection. Two days post -stroke, brain tissue obtained from mimic-injected animals was processed for immunohistochemistry for neuronal (NeuN), astrocytic (GFAP), microglial (Iba1) and endothelial (PECAM) markers. FAM-labeled mir363-3p was widely detected in the forebrain (Figure 5), as indicated by the fluorescein label (green) in a section counter stained with a nuclear dye (DAPI, Hoescht #33342). The majority of NeuN-positive (red) cells in the cortex and striatum were also labeled with FAM-363-3p, indicating a robust internalization of the mimic in neurons. FAM-mir363-3p was also localized to a few Iba1-positive cells, indicative of microglial uptake of the mimic. Virtually no double-label was seen in GFAP–positive cells or PECAM-positive cells in the cortex or striatum, indicating that the mimic was not internalized by astrocytes and microvascular endothelial cells. Interestingly, FAM-mir363-3p is clearly visible in the endothelial lining of the middle cerebral artery, confirmed by colocalization of the mimic to PECAM positive cells in this major artery.
Figure 5. Cellular uptake of mir363-3p mimic.
A. Coronal section of the forebrain depicting uptake of FAM-labeled mir363-3p mimic (green), injected iv 4h after MCAo,. Section is counterstained with DAPI (Hoescht nuclear dye; blue). FAM-labeled mir363-3p is extensively colocalized with NeuN positive cells (B) in the cortex and striatum (indicated by arrows; shown here are striatal neurons), and localized, although less extensively, to CD11b positive (microglial) cells (C). Little or no colocalization of FAM-363-3p mimic is seen in GFAP positive cells (astrocytes) (D) or microvessel PECAM-positive cells (E). In contrast, FAM-mir363-3p was localized to PECAM positive endothelial cells lining the middle cerebral artery (F).
Local synthesis of mir363-3p
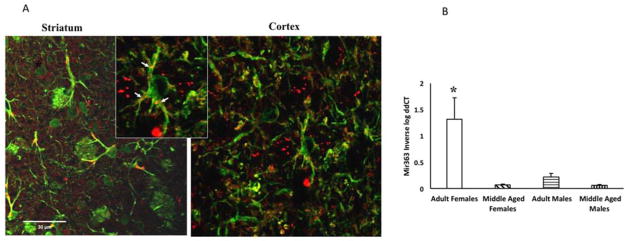
To determine if mir363-3p is locally synthesized in the brain, sections through the forebrain of adult female rats were incubated with a probe complementary to mir363-3p. Punctate staining, indicative of mir363-3p hybridization, was observed in the cortex and striatum. As shown in Figure 6A, hybridization signal was localized to GFAP-positive cells (astrocytes). Mir363-3p hybridization was localized to the distal processes of the astrocyte. To determine age and sex differences in mir363-3p expression, astrocytes were harvested from the ischemic hemisphere and subject to qPCR. Mir363-3p was present in astrocytes from all groups however, the pattern of expression varied by age and sex. Adult females had the highest expression of mir363-3p, as compared to adult males (6-fold higher), middle aged females (18 fold higher) and middle aged males (21-fold higher) (Figure 6B).
Figure 6. Mir363 expression in astrocytes.
A. In situ hybridization for mir363-3p combined with immunohistochemistry for GFAP. Inset shows punctate mir363-3p hybrids in astrocytic processes, indicated by arrows. B. Astrocytes were harvested from the ischemic hemisphere of adult and middle aged male and female rats and subject to qRT-PCR for mir363-3p. Histogram depicts mean (±SEM) mir363-3p analyzed by qRT-PCR. *: interaction effect, p<0.05.
Effect of mir363-3p mimic on the cell death effector protein Caspase-3
Mir363-3p has 1492 putative gene targets and 460 validated gene targets (miRWalk; http://zmf.umm.uni-heidelberg.de/). Only a few gene targets have been rigorously validated by reporter assays and these are shown in Table 2. Overall, mir363-3p appears to targets genes directly or indirectly involved with apoptotic cell death (Li et al., 2016, Wang et al., 2016), and some of these genes display either functional sex differences or are responsive to estrogen (Tsukahara et al., 2006, Tsukahara, 2009, Utge et al., 2010, Sharma et al., 2011, Caliceti et al., 2013, Fan et al., 2014, Zhao et al., 2014, Shannonhouse et al., 2015). Both caspase-3 and -9 play a role in apoptotic cell death, with caspase-9 being an initiator caspase, and caspase-3, an executioner caspase. Both caspases have been implicated in stroke-mediated cell death, and caspase inhibition improves stroke outcomes in a sex-dependent manner (Liu et al., 2009, Liu et al., 2011). The long non-coding RNA (lnc) MALAT1, another mir363-3p target, also regulate caspase-3 and caspase-9 expression and promotes apoptosis when inhibited (Chen et al., 2015). NOTCH1, a cell surface receptor, can be cleaved to release the NOTCH intracellular domain NICD which is implicated in stroke/hypoxia mediated cell death (Arumugam et al., 2006). In view of the convergence of mir363-3p on apoptosis related genes, we next determined mir363-3p mimic regulation of caspase-3, as a marker for the apoptotic cell death pathway.
Table 2.
| Target Gene | Reference | Sex Difference/Hormone regulation | Reference |
|---|---|---|---|
| CASP3 (Caspase-3) | (Floyd et al., 2014) | Neonatal expression in the hypothalamus Functional difference: Females are more sensitive to caspase-mediated cell death |
(Tsukahara et al., 2006, Tsukahara, 2009) (Liu et al., 2009, Liu et al., 2011, Sharma et al., 2011) |
| Caspase-9 | (Floyd et al., 2014) | Functional difference: Females are more sensitive to caspase-mediated cell death | (Liu et al., 2011) |
| BIM Bcl2-like protein 11 |
(Floyd et al., 2014) | Neonatal expression in the hypothalamus | (Tsukahara et al., 2006, Tsukahara, 2009) |
| NOTCH1 | (Song et al., 2015) | Estrogen enhances NOTCH signaling in bone marrow-derived mesenchymal stem cells (BMSCs) Estrogen increases NOTCH1 processing in endothelial cells |
(Fan et al., 2014) (Caliceti et al., 2013) |
| CREB1 | (Li et al., 2016) | Associated with depression in men Elevated CREB1 RNA after high fat diet in females only |
(Utge et al., 2010) (Shannonhouse et al., 2015) |
| MALAT1 | (Wang et al., 2016) | Estradiol decreases MALAT1 RNA in MCF10 cells Increased MALAT1 RNA expression at male-specific temperature-dependent sex determination |
(Zhao et al., 2014) (Chojnowski and Braun, 2012) |
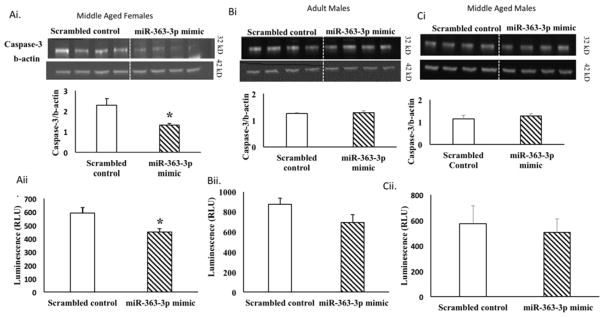
Protein lysates from the ischemic hemisphere of middle-aged females, adult and middle aged males injected with either control or mir363-3p post stroke was subject to Western blot analysis for caspase 3. As shown in Figure 7A, caspase-3 immunopositive signal (32kD) was seen in all groups, however capsase-3 expression was significantly reduced by 50% in mir363-3p-treated middle aged females as compared to scrambled controls (Fig 7Ai). An ELISA-based caspase activity assay further showed that caspase-3/7 activity, as measured by cleavage of the proluminescent tetrapeptide substrate DEVD, was decreased by 25% in protein extracts from miR-363-3p treated middle-aged females as compared to scrambled controls (p <0.05) (Figure 7Aii). Collectively, in middle aged females, mir363-3p treatment reduced both expression and functional activation of the caspase-3. By contrast, in adult males and middle aged males, mir363-3p mimic did not alter the expression of caspase-3 (Figure 7Bi, 7Ci). Similarly, caspase activity was also not altered (Figure 7Bii, 7Cii), when compared to scrambled control treated animals, indicating that caspase-3 expression was insensitive to mir363-3p treatment in males.
Figure 7. Effect of miR363-3p mimic on caspase-3 expression and activity.
Caspase -3 protein expression: Western blot expression of caspase-3 in cortico-striatal lysates from the ischemic hemisphere in control and mir363-3p injected animals. Histogram depicts mean (+SEM) caspase-3 expression (normalized to b-actin) in middle-aged females (Ai), adult males (Bi) and middle aged males (Ci). Caspase-3/7 activity: Protein lysates from the ischemic cortex and striatum were treated with caspase-glo 3/7 substrate in 96-well microplates and luminescence was measured in relative light units (RLU). Histogram depicts mean (+SEM) caspase-3/7 activity in middle aged females (Aii), adult males (Bii) and middle-aged males (Cii). *p ≤ 0.01
DISCUSSION
The present study confirms that circulating mir-363 expression is elevated in the group that displayed the smallest infarct volumes, namely adult females. In females, mir363-3p appears to be closely linked to stroke outcomes, such that iv injections of mir363-3p antagomirs to adult females increased infarct volume and further impaired sensory motor performance, while iv infusion of mir363 mimic to middle aged females significantly reduced neuronal damage, preserved cortico-striatal microvasculature and improved sensory motor function in this group, when compared to scrambled oligonucleotide treated controls. Mir363-3p mimic also reduced the expression and activation of caspase-3 in middle aged females. While it is unlikely that caspase-3 is the sole effective target of the mir363-3p mimic, regulation of this target gene by mir363-3p maps coordinately with its effects on infarct volume, and suggests that it may be a critical target in the acute phase of stroke. Consistent with this hypothesis, mir363-3p mimics were found localized to neurons where its effects on apoptotic processes would likely reduce infarct volume. To our knowledge this is the first instance of a microRNA mediator that improves stroke outcomes in older female animals, a group where MCAo typically results in larger infarction and greater behavioral deficits.
MiRNAs are attractive therapeutic candidates for acute and chronic diseases, due to their ability to regulate large gene networks and several approaches have been pursued to identify stroke-protective miRNA. These include ‘gene-focused’ approaches, where miRNAs are selected because they regulate known neuroprotectants, as in the case of Let7f and mir1 which regulate IGF-1 (Selvamani et al., 2012). In the case of mir181, spatial-segregation of this microRNA (elevated in the ischemic core and low in the penumbra), was exploited to show that pre- (Ouyang et al., 2012) and post-stroke (Xu et al., 2015) infusion of mir181 antagomirs reduced infarct volume. Similarly, miR-223 consensus sites in glutamate receptor subunits, GluR2 and NR2B, were manipulated to reduce cell death in hippocampal neurons following global transient ischemia (Harraz et al., 2012). A recent study further showed that the route of administration of miRNA is also critical, such that intravenous but not intracerebroventricular injection of mir122 improved stroke outcome in adult rats (Liu et al., 2015). Age and sex also modify the impact of microRNA treatment, thus while anti-Let7f was neuroprotective for stroke in young females, we found that it had no effect on young males or ovariectomized females (Selvamani et al., 2012). Furthermore, anti-Let7f paradoxically, increased infarct volume in middle age females (Selvamani and Sohrabji, unpublished observations), indicating that the endocrine environment modifies the effect of miRNA therapies. In contrast to anti-Let7f, a unique feature of the present study is that a miRNA that discriminates stroke outcomes based on age and sex, such as mir363-3p, can be reverse engineered to develop new therapeutic targets, and is effective for both young and middle-aged females.
The present data also confirm an emerging trend in stroke neuroprotectants where a specific treatment is effective in one sex but not the other. Despite its strong neuroprotective effect in middle aged females, miR-363 treatment did not reduce infarct volume in males nor improve sensory motor function. We reported a similar phenomenon with anti-Let-7f treatment, which was neuroprotective in adult females but not in age-matched males (Selvamani et al., 2012). Sex differences in stroke outcome have been attributed to the immediate presence of sex hormones or prenatal hormone exposure or sex chromosomes (Turtzo et al., 2011, Manwani et al., 2015), and estrogen treatment has been shown to be neuroprotective in both young females and males (Simpkins et al., 1997, Dubal et al., 1998, Rusa et al., 1999, Toung et al., 2004). However, sex differences in neuroprotection extends beyond sex steroids, such that nitric oxide inhibitors, PARP inhibitors, and the anti-inflammatory drug minocycline are reported to be effective for stroke in males but not for females (Hagberg et al., 2004, Li and McCullough, 2009).
In addition, functional sex differences are well recognized in ischemia-induced cell death pathways. Cerebral ischemia triggers an intrinsic apoptotic pathway (Dirnagl et al., 1999) and caspase-3 is the major apoptotic executioner protease (Budihardjo et al., 1999, Nicholson, 1999). Caspase inhibition has been shown to delay cell death in different experimental models of neurodegeneration (Knoblach et al., 2004, Renolleau et al., 2007). Caspase activation is the major pathway activated by ischemia in females and caspase inhibition benefits females following neonatal hypoxia (Renolleau et al., 2007) and adult ischemia (Liu et al., 2009) but not males. Not surprisingly, microRNAs that regulate proteins associated with the apoptotic cell death pathway such as the caspases (Rink and Khanna, 2011), intermediary proteins such XIAP and those associated with critical choice points for survival or death such as the BCL-2 family members (Ouyang and Giffard, 2014) are consistently found to modulate ischemic stroke outcomes. Sex specific regulation has also been reported for XIAP, the X-linked inhibitor of apoptosis protein, which is regulated by mir23a. Stroke elevates mir23a and reduces XIAP in females. Furthermore, inhibition of XIAP exacerbates stroke outcomes in females but not males (Siegel et al., 2011). Interestingly, both XIAP and mir363-3p are located on the X chromosome. In the present study, mir363-3p mimic injections improved stroke outcomes in middle-aged females and reduced both protein expression and caspase-3 activity in this group, consistent with evidence that mir-363-3p inhibits caspase-3, caspase-9 and BIM genes in luciferase reporter assays (Floyd et al., 2014). However, mir363-3p mimic injections to males failed to regulate either infarct volume or caspase 3 expression/activity in this group, even though mimic levels were similar in males and females. Since caspase 3 is a predicted target of several miRNA, an important future direction would be to evaluate sex differences in groups of miRNA instead of a single one.
Astrocytes play a key role in pathological processes like stroke, and recent studies show that manipulating astrocyte-enriched miRNA, such as mir181 and mir29 can reduce ischemia-induced cell death (Ouyang et al., 2012, Ouyang and Giffard, 2014). These miRNAs target members of the Bcl-2 family of pro- and anti-apoptotic proteins, and reduce mitochondrial dysfunction and apoptosis induced by glucose deprivation and ischemia. Our data show that miR363-3p is also expressed in astrocytes, and displays similar age and sex dependent expression patterns as circulating (serum) mir363-3p, in that it is elevated in adult females as compared to adult males and middle aged males and females. Collectively the evidence suggests that astrocyte-derived miRNA may play an important role in reducing cell death. With the growing evidence for secreted miRNA, transported by exosomes, microvesicles or chaperone particles (Chen et al., 2012), these data also suggest that neurons may have a readily available pool of anti-apoptotic miRNA from astrocytes under conditions of ischemia.
In conclusion, while most microRNA profiling studies in stroke have focused on microRNA that can distinguish types of brain injuries, or reperfusion times, and prognosis, and stroke induced transcription, the present study utilized a novel strategy of correlating miRNA with stroke outcomes, to identify candidate therapeutic target microRNAs for stroke. The complete mechanism of action for miR-363 needs further study, and the significant increase in its expression during the 2–5d post stroke period suggests that it may affect more or different stroke-associated processes with time. During the acute phase, it likely involves (sex-specific) regulation of cell death pathways, and since predicted targets of mir363-3p include extracellular matrix pathway and cell adhesion molecules, mir363-3p may also be involved in long term repair and recovery. A recent study of male and female stroke and control (with vascular risk factors) patients found that mir363 was among a small group of miRNA that are differentially expressed in blood samples collected in the acute stroke phase (Jickling et al., 2014). While this study did not assess the association of mir363 and stroke impairment or sex differences in its expression, it underscores the relevance of this miRNA to early events in ischemic injury.
Highlights.
Circulating miRNA 363-3p is inversely associated with infarct volume and stroke outcomes
Post stroke mir363-3p antagomirs increase infarct volume in young females, while post-stroke mir363-3p mimetics improve stroke outcomes
Mir363-3p treatment reduces caspase-3 activity and expression in middle-aged females
The effect of mir363-3p treatment is sex-specific, such that it is ineffective in improving stroke outcomes in young or middle aged males
Acknowledgments
We thank Andrea C. Armenta for her assistance with scoring behavioral assays. Supported by NS074895, AG042189 and a Women’s Health in Neuroscience Program pilot grant from TAMHSC VPR to FS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke; a journal of cerebral circulation. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke; a journal of cerebral circulation. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Chan SL, Jo D-G, Yilmaz G, Tang S-C, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–2435. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annual review of cell and developmental biology. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Caliceti C, Aquila G, Pannella M, Morelli MB, Fortini C, Pinton P, Bonora M, Hrelia S, Pannuti A, Miele L, Rizzo P, Ferrari R. 17beta-estradiol enhances signalling mediated by VEGF-A-delta-like ligand 4-notch1 axis in human endothelial cells. PLoS One. 2013;8:e71440. doi: 10.1371/journal.pone.0071440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Meng T, Liu X, Sun M, Tong C, Liu J, Wang H, Du J. Long non-coding RNA MALAT-1 is downregulated in preeclampsia and regulates proliferation, apoptosis, migration and invasion of JEG-3 trophoblast cells. International Journal of Clinical and Experimental Pathology. 2015;8:12718–12727. [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liang H, Zhang J, Zen K, Zhang C-Y. Secreted microRNAs: a new form of intercellular communication. Trends in Cell Biology. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Chisholm NC, Henderson ML, Selvamani A, Park MJ, Dindot S, Miranda RC, Sohrabji F. Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics. 2015;10:142–152. doi: 10.1080/15592294.2014.1001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnowski JL, Braun EL. An unbiased approach to identify genes involved in development in a turtle with temperature-dependent sex determination. BMC genomics. 2012;13:308. doi: 10.1186/1471-2164-13-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JZ, Yang L, Meng GL, Lin YS, Wei BY, Fan J, Hu HM, Liu YW, Chen S, Zhang JK, He QZ, Luo ZJ, Liu J. Estrogen improves the proliferation and differentiation of hBMSCs derived from postmenopausal osteoporosis through notch signaling pathway. Molecular and cellular biochemistry. 2014;392:85–93. doi: 10.1007/s11010-014-2021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DH, Zhang Y, Dey BK, Kefas B, Breit H, Marks K, Dutta A, Herold-Mende C, Synowitz M, Glass R, Abounader R, Purow BW. Novel anti-apoptotic microRNAs 582-5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS One. 2014;9:e96239. doi: 10.1371/journal.pone.0096239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. Journal of neurochemistry. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- Harraz MM, Eacker SM, Wang X, Dawson TM, Dawson VL. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18962–18967. doi: 10.1073/pnas.1121288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer research. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiology of aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Jickling GC, Ander BP, Zhan X, Noblett D, Stamova B, Liu D. microRNA Expression in Peripheral Blood Cells following Acute Ischemic Stroke and Their Predicted Gene Targets. PLoS ONE. 2014;9:e99283. doi: 10.1371/journal.pone.0099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Leidinger P, Lange J, Borries A, Schroers H, Scheffler M, Lenhof HP, Ruprecht K, Meese E. Multiple sclerosis: microRNA expression profiles accurately differentiate patients with relapsing-remitting disease from healthy controls. PLoS One. 2009;4:e7440. doi: 10.1371/journal.pone.0007440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Alroy DA, Nikolaeva M, Cernak I, Stoica BA, Faden AI. Caspase inhibitor z-DEVD-fmk attenuates calpain and necrotic cell death in vitro and after traumatic brain injury. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:1119–1132. doi: 10.1097/01.WCB.0000138664.17682.32. [DOI] [PubMed] [Google Scholar]
- Leon RL, Li X, Huber JD, Rosen CL. Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17beta-estradiol. Endocrinology. 2012;153:3386–3393. doi: 10.1210/en.2011-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen D, Li Y, Jin L, Liu J, Su Z, Qi Z, Shi M, Jiang Z, Ni L, Yang S, Gui Y, Mao X, Chen Y, Lai Y. Oncogenic cAMP responsive element binding protein 1 is overexpressed upon loss of tumor suppressive miR-10b-5p and miR-363-3p in renal cancer. Oncology reports. 2016;35:1967–1978. doi: 10.3892/or.2016.4579. [DOI] [PubMed] [Google Scholar]
- Liu da Z, Jickling GC, Ander BP, Hull H, Zhan X, Cox C, Shroff N, Dykstra-Aiello C, Stamova B, Sharp FR. Elevating microRNA-122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:1374–1383. doi: 10.1177/0271678X15610786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, McCullough LD. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke; a journal of cerebral circulation. 2011;42:1090–1096. doi: 10.1161/STROKEAHA.110.594861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke; a journal of cerebral circulation. 2009;40:1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:221–229. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M, Rosa A, Guedes LC, Fonseca BV, Gotovac K, Violante S, Mestre T, Coelho M, Rosa MM, Martin ER, Vance JM, Outeiro TF, Wang L, Borovecki F, Ferreira JJ, Oliveira SA. Convergence of miRNA expression profiling, alpha-synuclein interacton and GWAS in Parkinson’s disease. PLoS One. 2011;6:e25443. doi: 10.1371/journal.pone.0025443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1976–1982. doi: 10.1038/jcbfm.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell death and differentiation. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Giffard RG. MicroRNAs affect BCL-2 family proteins in the setting of cerebral ischemia. Neurochemistry international. 2014;77:2–8. doi: 10.1016/j.neuint.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiology of disease. 2012;45:555–563. doi: 10.1016/j.nbd.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R. MicroRNA miR-29c down-regulation leading to de-repression of its target DNA methyltransferase 3a promotes ischemic brain damage. PLoS One. 2013;8:e58039. doi: 10.1371/journal.pone.0058039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, Chien M, Weir DB, Russo JJ, Ju J, Brownstein MJ, Sheridan R, Sander C, Zavolan M, Tuschl T, Rice CM. Cellular cofactors affecting hepatitis C virus infection and replication. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. Journal of neurochemistry. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiological genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke; a journal of cerebral circulation. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of IGF-1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010a;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiology of aging. 2010b;31:1618–1628. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Williams MH, Miranda RC, Sohrabji F. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clinical science (London, England: 1979) 2014;127:77–89. doi: 10.1042/CS20130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannonhouse JL, Grater DM, York D, Wellman PJ, Morgan C. Sex differences in motivational responses to dietary fat in Syrian hamsters. Physiology & behavior. 2015;147:102–116. doi: 10.1016/j.physbeh.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J, Nelluru G, Wilson MA, Johnston MV, Hossain MA. Sex-specific activation of cell death signalling pathways in cerebellar granule neurons exposed to oxygen glucose deprivation followed by reoxygenation. ASN neuro. 2011:3. doi: 10.1042/AN20100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Li J, Liu F, Benashski SE, McCullough LD. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11662–11667. doi: 10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. Journal of neurosurgery. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Song B, Yan J, Liu C, Zhou H, Zheng Y. Tumor Suppressor Role of miR-363-3p in Gastric Cancer. Medical science monitor: international medical journal of experimental and clinical research. 2015;21:4074–4080. doi: 10.12659/MSM.896556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- Tsukahara S. Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. Journal of neuroendocrinology. 2009;21:370–376. doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Kakeyama M, Toyofuku Y. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. Journal of neurobiology. 2006;66:1411–1419. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, Siegel C, McCullough LD. X chromosome dosage and the response to cerebral ischemia. J Neurosci. 2011;31:13255–13259. doi: 10.1523/JNEUROSCI.0621-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utge S, Soronen P, Partonen T, Loukola A, Kronholm E, Pirkola S, Nyman E, Porkka-Heiskanen T, Paunio T. A population-based association study of candidate genes for depression and sleep disturbance. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2010;153b:468–476. doi: 10.1002/ajmg.b.31002. [DOI] [PubMed] [Google Scholar]
- Vinciguerra A, Formisano L, Cerullo P, Guida N, Cuomo O, Esposito A, Di Renzo G, Annunziato L, Pignataro G. MicroRNA-103-1 selectively downregulates brain NCX1 and its inhibition by anti-miRNA ameliorates stroke damage and neurological deficits. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22:1829–1838. doi: 10.1038/mt.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. The New England journal of medicine. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Wang S-H, Zhang W-J, Wu X-C, Weng M-Z, Zhang M-D, Cai Q, Zhou D, Wang J-D, Quan Z-W. The lncRNA MALAT1 functions as a competing endogenous RNA to regulate MCL-1 expression by sponging miR-363-3p in gallbladder cancer. Journal of cellular and molecular medicine. 2016:n/a–n/a. doi: 10.1111/jcmm.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. Jama. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB. Estradiol protects against ischemic brain injury in middle-aged rats. Biology of reproduction. 2000;63:982–985. doi: 10.1095/biolreprod63.4.982. [DOI] [PubMed] [Google Scholar]
- Xu LJ, Ouyang YB, Xiong X, Stary CM, Giffard RG. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Experimental neurology. 2015;264:1–7. doi: 10.1016/j.expneurol.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, Tao Z, Xu C, Song J, Ji X, Luo Y. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke; a journal of cerebral circulation. 2013;44:1706–1713. doi: 10.1161/STROKEAHA.111.000504. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Chen C, Liu Y, Wu C. 17beta-Estradiol treatment inhibits breast cell proliferation, migration and invasion by decreasing MALAT-1 RNA level. Biochemical and biophysical research communications. 2014;445:388–393. doi: 10.1016/j.bbrc.2014.02.006. [DOI] [PubMed] [Google Scholar]