Abstract
Several locations in the Elizabeth River, VA, USA are highly contaminated with polycyclic aromatic hydrocarbons (PAHs), due to the release of creosote mixtures from wood treatment facilities. Interestingly, some populations of Atlantic killifish (Fundulus heteroclitus) inhabiting the Elizabeth River (ER) are resistant to PAH-induced teratogenesis. However, evolutionary resistance to PAHs due to chronic PAH exposure is associated with reduced fitness and increased susceptibility to other environmental stressors in at least one PAH-resistant ER killifish population. More specifically, wild-caught and first generation PAH-resistant juvenile killifish have altered metabolic demands when compared to non-resistant fish. Herein, we investigated this association further by examining a previously under-studied population captured from the creosote-contaminated site Republic Creosoting (Rep). We assessed PAH toxicity and effects on energy metabolism in Rep killifish in comparison with killifish from the reference site Kings Creek (KC). Following exposures to simple and complex PAH mixtures, Rep killifish exhibited several phenotypes associated with PAH resistance including decreased incidences of developmental cardiovascular deformities and recalcitrant cytochrome P450 1A (CYP1A) activity. We evaluated bioenergetics in killifish embryos throughout development and found elevated basal oxygen consumption rates in Rep embryos relative to KC embryos. Furthermore, juvenile F1 Rep fish had significantly lower maximal metabolic rates and aerobic scopes than KC juveniles. These results suggest that populations of killifish that have adapted or evolved to withstand the toxicity associated with PAHs consequently have altered energetic metabolism or demands. Such consequences could result in an enhanced vulnerability to other environmental and anthropogenic stressors in PAH-resistant killifish.
Keywords: Atlantic killifish, Bioenergetics, CYP1A, Developmental toxicity, Polycyclic aromatic hydrocarbons
Introduction
Since the 19th century, when one of the first-recognized occurrences of pollution-driven evolution manifested as altered wing coloration of moth populations (Bowater 1914; Cook and Saccheri 2013), there have been many incidences of rapid organismal evolution in response to chronic exposure to anthropogenic pollutants (Fox 1995; Hendry 2013). Evolved resistance to pesticides is now seemingly a common trait in many insect species including the frequently-targeted disease vectors such as mosquitos (e.g. members of the Culex genus) (Hemingway et al. 2004) and widespread, highly adaptable agricultural pests such as the green peach aphid (Myzus persicae) (Silva et al. 2012). Additionally, abiotic stressors stemming from climate change (e.g. increases in temperature, changes in ocean pH, and hypoxia) can also affect organismal fitness and may drive evolution (Lohbeck et al. 2012; Kelly and Hofmann 2013; Pespeni et al. 2013; Stillman and Paganini 2015). Mechanisms and tradeoffs of evolutionary responses to pollutants may include limited organismal plasticity and a reduced capacity to respond to other abiotic stressors. This has become an increasingly important area of research.
At several sites along the Elizabeth River (VA, USA), exposure to decades of creosote pollution from wood treatment facilities has resulted in evolved resistance of a native fish, Fundulus heteroclitus (Atlantic killifish), to the toxic effects associated with its chemical components (Clark et al. 2013). Creosote, a complex mixture often utilized during wood preservation processes, is primarily made up of a class of ubiquitous compounds known as polycyclic aromatic hydrocarbons (PAHs). These compounds come in a variety of forms, many of which display varying degrees and mechanisms of toxicity (National Toxicology Program, 2012). In fish species, including Atlantic killifish, exposures to PAHs during embryonic development results in cardiovascular deformities similar to those seen after exposures to dioxin-like compounds and halogenated aromatic compounds (Billiard et al. 1999; Incardona et al. 2004; Wassenberg and Di Giulio 2004a).
Specific populations of killifish inhabiting highly contaminated sites in the Elizabeth River are genetically distinct from populations that live in uncontaminated regions (Mulvey et al. 2002; Mulvey et al. 2003; Wills et al. 2010). Although killifish are one of the most genetically diverse vertebrate species and can live in a variety of ecological conditions, populations found at PAH-contaminated and other dioxin-like compound-contaminated sites all share genetic mutations localized to genes involved in metabolism of these compounds (Reid et al. 2016). Populations at contaminated sites have evolved to withstand the acute toxicity normally observed after exposures to creosote. Incidences of cardiovascular deformities as well as the characteristic induction of the Phase I metabolic enzyme cytochrome P4501A (CYP1A) are reduced in PAH resistant killifish after PAH exposures during embryonic development (Meyer et al. 2002; Ownby et al. 2002; Wassenberg and Di Giulio 2004a; Clark et al. 2013; Brown et al. 2016a); for example, Brown et al. (2016a) found that a statistically significant induction of cardiovascular deformities was not seen in a PAH-resistant population of killifish until exposure levels reached 100 times higher than what was required to induce a similar magnitude of deformities in a non-resistant population.
Killifish collected from the Atlantic Wood Industries Superfund site (AW) in the Elizabeth River display the phenotypes of PAH resistance as described above (Jung and Di Giulio 2010; Clark et al. 2013). The wood treatment facility located there primarily utilized creosote during operations and although it was closed and placed on the National Priorities List in 1990, the surrounding area is still highly contaminated with high molecular weight PAHs (Di Giulio and Clark 2015). Although resistance similar to that seen in AW killifish is beneficial for acute exposures to PAH mixtures, our previous work has shown that there are fitness-related consequences of the adaptation at both early and later life stages. For example, reduced aerobic scope, behavioral changes, and increased susceptibility to other anthropogenic and environmental stressors are all associated with PAH resistance in these fish (Brown et al. 2016a, 2016b; Meyer and Di Giulio, 2003).
Historically, AW fish have served as a unique model to investigate mechanisms and costs of PAH toxicity. However, with the closing of the Atlantic Wood Industries Superfund Site for remediation, there was a need to identify a new population of killifish that are similarly resistant to the toxicological effects of PAHs and would not be affected, genetically or physically, by remediation activities. Other populations of contaminant-resistant Atlantic killifish have been found along the eastern seaboard and in a related species, Fundulus grandis, in the Gulf of Mexico (Nacci et al. 2010; Oziolor et al. 2014; Bugel et al. 2010). These groups have also shown that contaminant resistance can result in fitness costs including decreased tolerance to additional biotic and abiotic stressors and impaired immune and reproductive function (Nacci et al. 2009; Oziolor et al. 2016; Bugel et al. 2010; Frederick et al. 2006). Further, several other populations of killifish inhabiting differentially contaminated sites along the Elizabeth River exhibit varying degrees of PAH resistance in correlation with the level of pollution at those respective sites (Clark et al. 2013; Di Giulio and Clark 2015). Therefore, our goals for this study were to more thoroughly examine a second population of Elizabeth River killifish from another highly contaminated site for PAH resistance and identify consequences of this type of adaptation in laboratory-reared offspring.
Republic (Rep), currently the most highly PAH-contaminated and accessible study site in the Elizabeth River and located approximately 2 miles upstream of the AW site, is also polluted with creosote due to the activities of a Republic Creosoting Incorporated wood treatment facility (Vogelbein and Unger 2008; Clark et al. 2013). Analyses of sediment samples for a suite of low and high molecular weight PAHs with GCMS demonstrate that the Rep site contains approximately 113,886 ng tPAH/g dry sediment, a value over 200 times higher than the reference site Kings Creek (KC; 526 ± 624 ng tPAH/g dry sediment) (Vogelbein and Unger 2008; Clark et al. 2013); the total PAH content of sediment found at Elizabeth River sites is associated with the PAH resistance seen in local killifish populations, with highly adapted populations found at highly contaminated sites (Clark et al. 2013; Jayasundara et al. in review). Additionally, Clark et al. (2013) partially characterized the Rep population and demonstrated that they show similar levels of developmental resistance to PAHs as displayed by AW fish.
The first objective of this study was to characterize the level of PAH resistance shown by killifish captured at the creosote-contaminated Rep site. This involved exposing wild-caught adults and laboratory-reared first generation killifish embryos to individual PAHs or PAH mixtures and assessing them for the distinctive responses normally seen in non-adapted populations after PAH exposures. As hypothesized, results demonstrate that Rep killifish from two generations and two life stages displayed PAH-resistant phenotypes similar to those of AW killifish.
Energy metabolism is biologically important for all living species and at any life stage. Based on the results from the first objective and previous work that has shown alterations in energy metabolism in PAH resistant fish (Brown et al. 2016b; Oziolor et al. 2016; Jayasundara et al. in review), our second objective was to test the hypothesis that PAH resistant fish have altered bioenergetic phenotypes during multiple life stages. Our results confirm that evolution of PAH resistance and early-life or chronic exposures to PAHs may alter the bioenergetic function of adapted killifish populations during embryonic development as well as juvenile stages. These bioenergetic consequences could potentially result in increased vulnerability of these fish to additional biotic and abiotic stressors in their natural environment and may have population- and ecosystem-level consequences.
Materials and Methods
Fish Collection, Care, and Handling
Wild Fundulus heteroclitus were collected from the Rep (36°47′39.65” N, 76°17′31.94” W) and KC (37°18′16.2” N, 76°24′58.9” W) sites in the summer of 2015 (Commonwealth of Virginia Marine Resources Commission Scientific Collection Permit # 15-003). Fish were maintained in the laboratory in approximately 15ppt artificial seawater (ASW) (Instant Ocean, Foster & Smith, Rhinelander, WI, USA), at 25-28°C with a 14:10 light:dark cycle and were fed Aquamax Fingerling Starter 300 fish feed (PMI Nutritional International, LLC, Brentwood, MO, USA). Adults were kept in clean water for at least one month before handling occurred.
Eggs and sperm from reproductive-aged F0 males and females were collected manually and placed in 400 mL beakers. After mixing with sperm, the eggs were given one hour to complete fertilization after which they were washed with a 0.3% hydrogen peroxide (VWR International, Radnor, PA, USA)-20ppt ASW solution. All embryos were kept in an incubator at 28°C until use.
Chemicals
Benzo[a]pyrene (BaP), fluoranthene (FL), β-naphthoflavone (BNF), ethoxyresorufin, protease inhibitor, magnesium sulfate (MgSO4), potassium chloride (KCl), Tris-HCl, ethylenediaminetetraacetic acid (EDTA), NADPH, NADH, dithiothreitol (DTT), antimycin A, rotenone, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Corn oil and sucrose were purchased from VWR International (Radnor, PA, USA). HEPES was purchased from Acros Organics (Thermo Fisher Scientific, Waltham, MA, USA). Tris-base was purchased from Omni Pur (EMD Millipore, Darmstadt, Germany). BaP (1 mg/mL) and FL (10 mg/mL) stocks were prepared in DMSO. BNF solutions were prepared in corn oil.
Atlantic Wood Industries sediment was previously collected and processed in order to extract and characterize an aqueous solution containing a complex PAH mixture, hereafter known as Atlantic Wood sediment extract (AWSE) which was used during experimentation. For complete collection and extraction methods and analytical characterization, see Clark et al. (2013).
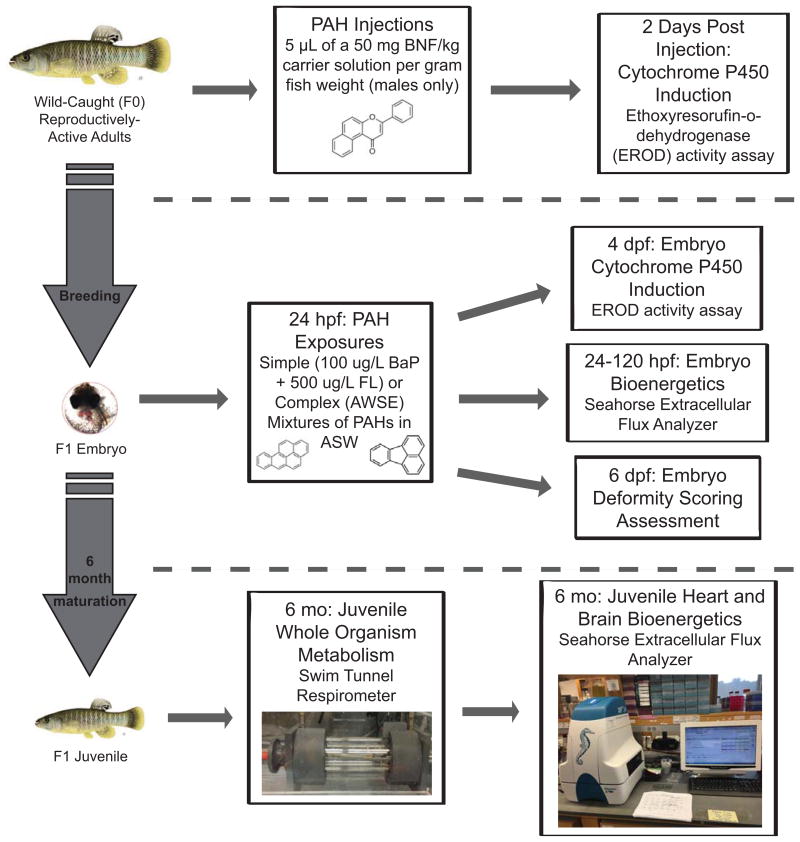
A schematic of all of the methods described below can be found in Figure 1. The Duke University Institutional Animal Care and Use Committee approved all of the F. heteroclitus care, handling, and techniques used during this work (Protocol # A184-13-07).
Fig. 1.
Schematic of the methodology used in this study. Wild-caught males were utilized for the liver microsomal EROD assay and were also bred with wild-caught females to obtain the F1 generation. F1 embryos were either exposed at 24 hpf to PAH mixtures and utilized during the embryo EROD, deformity scoring, and/or bioenergetics assessments or raised under normal conditions for 6 months. These 6 month old juvenile fish were then utilized for whole-organism metabolic testing and then sacrificed for tissue bioenergetics testing
F0 Generation Intraperitoneal Injections, Liver Dissections, and Liver Microsomal EROD Assay
Reproductive age male F0 F. heteroclitus from KC and Rep populations were submerged in 15 ppt ice water until loss of equilibrium. Only male fish were chosen to prevent effects on CYP1A activity due to sex as found in Meyer et al. (2002). Fish were injected intraperitoneally with 5 μL/g wet weight fish of either a corn oil carrier or a 50 mg/kg BNF solution, in accordance with Meyer et al. (2003). Fish were then placed in recovery tanks, allowed to re-equilibrate, and were kept in conditions described above until sacrifice. The livers of each fish were removed 48 hours after injections, flash frozen in liquid nitrogen, and stored at -80°C until analysis. Six fish per population were injected for each treatment.
Microsomal fractions were isolated as described in Jung and Di Giulio (2010) with modification. Specifically, isolated livers were weighed, minced, and then rinsed with ice-cold washing solution (0.15 M KCl, 10 mM DTT, pH 7.4) until the solution remained clear. Each liver was then transferred to a 2 mL microcentrifuge tube in 4X volume of homogenization buffer (0.25 M sucrose, 0.1 M Tris-HCl, 1 mM EDTA, 10 mM DTT, pH 7.4) plus protease inhibitor (1 μL for every 1 mL homogenization buffer) with an approximately equal volume of 1 mm blending beads (SKU: ZROB10). Samples were placed in a bullet blender (Next Advance, Inc., Averill Park, NY, USA) and blended at high speed for three, 1 minute pulses, or until fully homogenized. The homogenate was transferred to an ultracentrifuge tube and centrifuged at 10,000 g for 20 minutes at 4°C. The top fatty layer was discarded and the supernatant was transferred to a new ultracentrifuge tube. The supernatant was spun at 100,000 g for 60 minutes at 4° C. The supernatant was discarded, and the microsomal pellet was washed with 1-3 mL of microsomal pellet washing solution (0.15 M Tris-base, 10 mM DTT, pH 8.0) until the solution remained clear. The pellet was resuspended in microsomal resuspension buffer (0.25 M sucrose, 1 mM EDTA, 0.1 M Tris-HCl in 20% glycerol, pH 7.4), of approximately 1 mL per gram of liver tissue. The microsomal suspension was then aliquoted, flash frozen, and stored at -80°C until thawed for protein quantification and the EROD assay.
For protein quantification, one aliquot of microsomal solution was diluted to 1:10 in DI water and quantified with the Pierce™ BCA Protein Assay Kit as per manufacturer's protocol for microplate procedure (Thermo Fisher Scientific Inc., Waltham, MA, USA). Microsomal CYP1A activity was then quantified via the EROD assay, as described in Jung and Di Giulio (2010). First, 50 μg of the microsomal fraction from each sample were loaded in a 96-well microplate (in duplicate) and freshly prepared, ice cold cofactor buffer (102 μM NADPH, 120 μM NADH, 5 mM MgSO4 into 100 mM HEPES, pH 8.0) was added to bring the volume to 100 μL. Afterwards, 100 uL of 2.5 μM 7-ethoxyresorufin in cofactor buffer was then added to each well and fluorescence was measured at 530/590 nM. The results were calculated against the standard curve of known concentrations of resorufin stock to report activity (pmoles resorufin/mg protein/minute).
F1 Generation Embryo Exposures, EROD Activity Assay, and Cardiac Deformity Screening
At 24 hpf, embryos were screened for deformities and healthy embryos were dosed individually with 10 mL dosing solution in glass scintillation vials (Wheaton, Millville, NJ, USA). Treatments for both KC and Rep embryos included the 20 ppt ASW control, 1%, 3%, and 5% (v/v) AWSE solutions, along with a 10% (v/v) AWSE solution for Rep embryos only. Exposures of 10% AWSE result in mass mortality in KC embryos and therefore were not used for that population. Additionally, embryos were dosed with a simple mixture of 100 μg/mL BaP and 500 μg/mL FL (100/500 BaP+FL), a synergistically toxic combination of an AhR agonist and CYP1A inhibitor, respectively (Wassenberg and Di Giulio 2004b), and a 0.1% (v/v) DMSO control solution. All dosing solutions were supplemented to contain 21 μg/L ethoxyresorufin for the ethoxyresorufin-o-deethylase (EROD) assay. After dosing, embryos were kept at 28°C until assessment. Three experimental replicates of these procedures took place with a total n of 33-35 embryos for each treatment.
At 96 hpf (4 dpf) embryos were removed from vials and EROD activity was measured according to the protocols described in Matson et al. (2008). Although embryos had begun to display deformities depending on treatment group, there did not appear to be any resulting developmental delays. All embryos had small, but visible urinary bladders, as is necessary to conduct the EROD assay and appropriate for stages 28 and 29 of F. heteroclitus embryonic development (Armstrong and Child 1965). All EROD values for KC and Rep treatments were expressed as a percent of the KC 20 ppt ASW control group response. After measurements were recorded, embryos were placed back into their respective vials and stored in 28°C incubators until 144 hpf (6 dpf) when cardiac deformity screenings were performed. Under a light microscope, cardiac abnormalities were recorded in a blind assessment. As described in Matson et al. (2008), deformities were scored either as a 0 (normal), 1 (moderate deformity) or 2 (severe deformity). The primary deformities seen, also described in Clark et al. (2010), were heart elongation, or the “stringy heart” phenotype, misalignment of the atrium and ventricle, slowed heartbeat, and limited blood flow. As stated above, there were visible deformities resulting from treatment at that time, but development did not appear to be affected, although in some cases, embryos treated with higher concentrations of AWSE (i.e. 5% and 10% AWSE treatments) tended to be slightly smaller in size. There was visible circulation and pigmentation in all surviving embryos which indicated that they were approaching stage 31 of embryonic development (Armstrong and Child 1965).
F1 Generation Embryonic Bioenergetic Assessment
KC and Rep embryos were collected and dosed at 24 hpf similarly to the methods described above, but with only two treatments: 20 ppt ASW Control and 1% (v/v) AWSE. Using the Seahorse Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA), basal respiration, reported as oxygen consumption rate (OCR), was measured for individual embryos at time points of 24 hpf, 48 hpf, 72 hpf, 96 hpf, and 120 hpf (methods adapted from Stackley et al. 2011). With the exception of the 24 hpf time point, embryos were removed from the dosing solutions at the designated time and placed into individual wells filled with 750 μL of a 20 ppt ASW solution. Basal respiration measurements were recorded for one minute every five minutes for approximately 1.5 hours. The sample size per plate was 5 embryos per treatment per population and 2-3 plates were run for each time point. At the 24 hpf time point, only KC and Rep embryos from the 20 ppt ASW treatment groups were tested. A total of 2 plates were run with a sample size per plate of 11 embryos per population. Embryos were only used if there were no apparent developmental deformities.
Additionally, beginning at 24 hpf, KC and Rep embryos were placed in individual wells and the OCR was measured for one minute, five times per hour for 12 hours (until 36 hpf). These measurements created a time course to further examine the significant developmental changes in basal OCR that were seen from 24 hpf to 48 hpf. The sample size per plate was 11 embryos per treatment per population and 4 plates were run for each.
F1 Generation Juvenile Respirometry and Tissue-Specific Bioenergetics Assessment
KC and Rep embryos were collected as described above and incubated at 28°C on dampened filter paper until hatching (14 dpf). These embryos were not exposed to PAH mixtures during development. Larvae were raised in groups of 15-20 per 9 L tanks at 28°C and approximately 15 ppt salinity in an Aquatic Habitats™ (Apopka, FL, USA) system and were fed once per day with freshly hatched Artemia franciscana (Brine Shrimp Direct, Ogden, Utah, USA) and once per day with Zeigler Adult Zebrafish Diet (Zeigler Bros., Inc., Gardners, PA, USA). No measurements of hatching or survival were taken during development. Approximately six months post fertilization, the juveniles were collected and tested for whole-organism basal and maximal metabolic rates using a Swim Tunnel Respirometer (Loligo Systems, Denmark) with methods adapted from Jayasundara et al. (2015). Briefly, live fish were placed into the respirometer chamber and allowed to acclimate for a period of 1 hour. Four basal respiratory measurements were taken during which water flow to the chamber was closed for a period of 5 minutes and oxygen concentration of the water was recorded in μmol/L. After the 5 minute period, water flow was restored and the chamber was reoxygenated for a period of 10 minutes. To assess the maximal metabolic rate, fish were removed from the chambers and were chased in a separate tank for 15-20 minutes, until exhaustion. Immediately following the chasing period, fish were placed back in the respiratory chambers, water flow was shut off, and the oxygen concentration of the water was measured once every minute for 5 minutes. The lowest basal measurement recorded and highest maximal measurement recorded were used during calculations of basal and maximal metabolic rates, respectively. The weight of each fish was also recorded to correct for size during calculations. KC killifish had an average weight of 0.47 g and Rep killifish had an average weight of 0.39 g. Afterward, juveniles were given a 1 week recovery period before sacrifice and dissection of hearts and brains. Metabolic rates in those whole, live tissues were measured in accordance with the methods of Jayasundara et al. (2015) using the Seahorse Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA). During tissue-specific metabolic measurements, FCCP at a concentration of 5 μM was utilized as an uncoupling agent to induce maximal respiration. Afterwards, a combination of antimycin A and rotenone at concentrations of 0.044 mM and 0.044 mM, respectively, was utilized to inhibit the activities of mitochondrial Complex III and I, respectively, essentially inhibiting mitochondrial respiration in the tissues. A total of 15 juvenile killifish per population were utilized during these experiments.
Data Analysis
All analyses were performed using GraphPad Prism 6 (GraphPad Prism, Inc., La Jolla, CA), Statview version 5.0.1 (SAS Institute Inc., Cary, NC), and the Fisher's Exact Test online calculator (DanielSloper.com). Individual embryos, livers, hearts, and brains were the units of replication. The embryo EROD activity assay, cardiac deformity screening, embryonic bioenergetic assessments, and juvenile bioenergetics assessments were all replicated and therefore were tested for batch effects prior to combining the replicates into one data set. Although there was a significant difference seen with one replicate of the embryonic cardiac deformity screening, the removal of this group did not alter the results. We considered this to be an adequate rationale for recombining all three experimental groups for further statistical analyses.
The cardiac deformity screening data were analyzed non-parametrically using Fisher's Exact Test (2×3 contingency tables) for effects by population and treatments. Significance was Bonferroni corrected for multiple comparisons. The 24 hpf embryonic bioenergetic assessment and whole-organism respirometry data were analyzed using an unpaired t-test. All other data sets were analyzed using a 2-way analysis of variance (ANOVA) and post hoc Fisher's LSD. Statistical significance was accepted at p<0.05 for all tests that were not corrected for multiple comparisons.
Results
F0 Generation Liver Microsomal EROD Activity
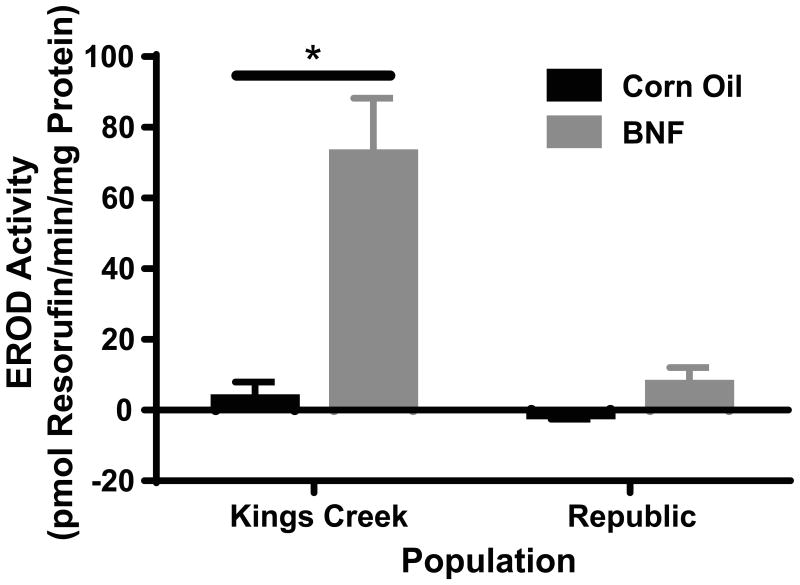
KC adults injected with 50 mg/kg BNF had significantly elevated levels of EROD activity (72.3 ± 15.9 pmol resorufin/min/mg protein; p<0.0001), while Rep adults injected with the same concentrations of BNF did not (7.1 ± 4.9 pmol resorufin/min/mg protein) when compared with the KC corn oil control (3.1 ± 4.8 pmol resorufin/min/mg protein) (Figure 2).
Fig. 2.
F0 liver microsomal EROD activity. KC killifish injected with BNF had significantly induced liver microsomal CYP1A activity. Rep killifish injected with BNF did not display a significantly different change in fluorescence when compared with the KC corn oil-injected controls or Rep corn oil-injected controls. Error bars represent mean ± SEM. Values marked by * are statistically different than the KC corn oil treatment group at p<0.0001 (2-way ANOVA, Fisher's LSD)
F1 Generation Embryo EROD Activity
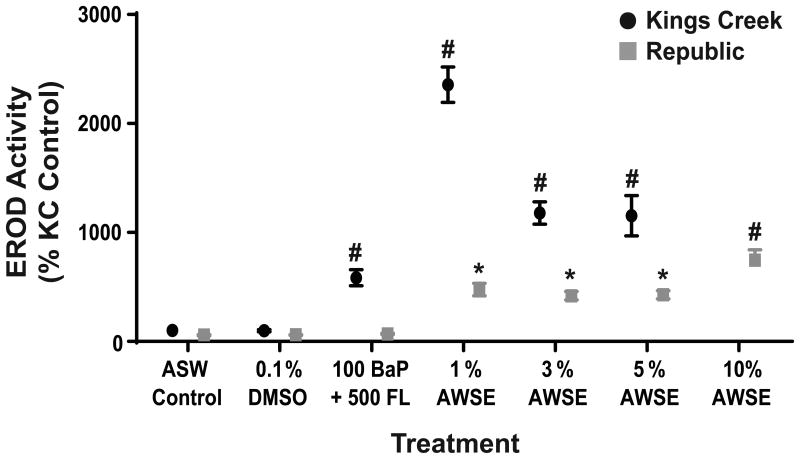
As expected from previous studies (Clark et al. 2013; Brown et al. 2016a), KC embryos were prone to both induction of CYP1A and cardiovascular teratogenesis after exposures to simple and complex PAH mixtures compared to ASW or DMSO controls. EROD activity peaked with the 1% AWSE treatment and declined with increasing treatment concentrations as embryos developed severe deformities. All PAH treatments induced a significant increase in EROD activity (p<0.0001) compared to controls (Figure 3). CYP1A inductions for BaP+FL and 1%, 3%, and 5% AWSE treatment groups were 5.9, 23.6, 11.8, and 11.5 fold higher, respectively, than inductions in the KC ASW control group.
Fig. 3.
In ovo EROD activity of KC and Rep embryos at 4 dpf. Error bars represent mean ± SEM. Statistical significance (2-way ANOVA, Fisher's LSD) of p<0.01 is denoted by * and p<0.0001 is denoted by # when compared against the KC ASW control group
Rep embryos also displayed significant increases in EROD activity for several exposures, however the inductions in activity were 2.5-5 times lower than in KC fish depending on treatment. Additionally, these inductions were not associated with significantly different deformity score distributions in those groups. In Rep embryos, the 10% AWSE treatment induced the highest level of EROD activity (7.5 times higher than the KC ASW control group; p<0.0001) and the 1%, 3%, and 5% AWSE treatments also significantly induced EROD activity, but to a lesser extent (4.8, 4.2, and 4.3 times higher, respectively, than the KC ASW control group; all p<0.008). Treatment with the simple mixture of BaP+FL resulted in no significant increase in EROD activity (Figure 3).
F1 Generation Embryo Cardiovascular Deformity Screening
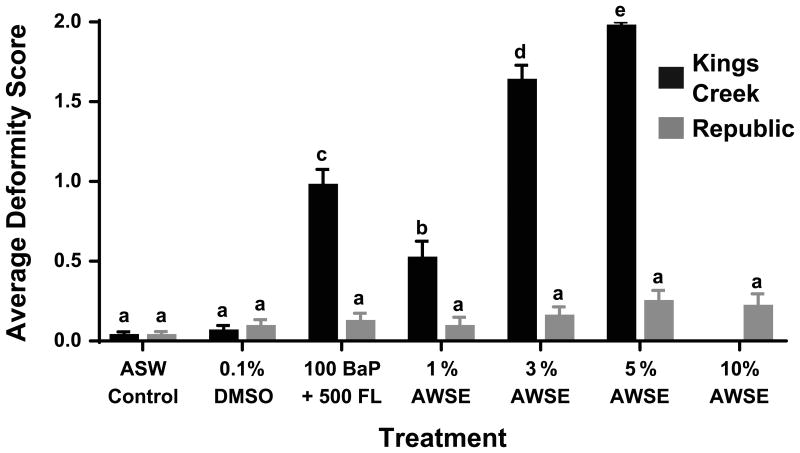
In order to accurately represent these results, the data were analyzed in the context of distribution of score frequencies (i.e. were the incidences of twos, ones, and zeros found after one treatment differently distributed than the incidences of twos, ones, and zeros found after another treatment). Figure 4 concisely displays these data as an average of the scores found after each treatment which can be more easily compared to past data published with similar methods (Clark et al. 2013; Brown et al. 2016a). For an in-depth portrayal of score distributions, reference Figure A1.
Fig. 4.
Cardiovascular deformity score averages at 6 dpf. Error bars represent mean ± SEM. Bars marked with different letters are significantly different from each other after corrections for multiple comparisons (Fisher's Exact Test)
Within the KC population, the distribution of deformity score frequencies differed significantly between the BaP+FL, 3% AWSE, 5% AWSE (all p<0.0001), and 1% AWSE (p<0.0005) and both the control groups (ASW and DMSO). There were no significant differences between the distributions of deformity score frequencies for any of the Rep treatments groups when compared with the Rep control groups. Comparisons were also made for treatment groups between the two populations. The KC distributions of score frequencies significantly differed from the Rep distributions after treatment with BaP+FL, 3% AWSE, 5% AWSE (all p<0.0001), and 1% AWSE (p<0.0005), but not in either of the control groups.
F1 Generation Embryo Bioenergetic Assessment
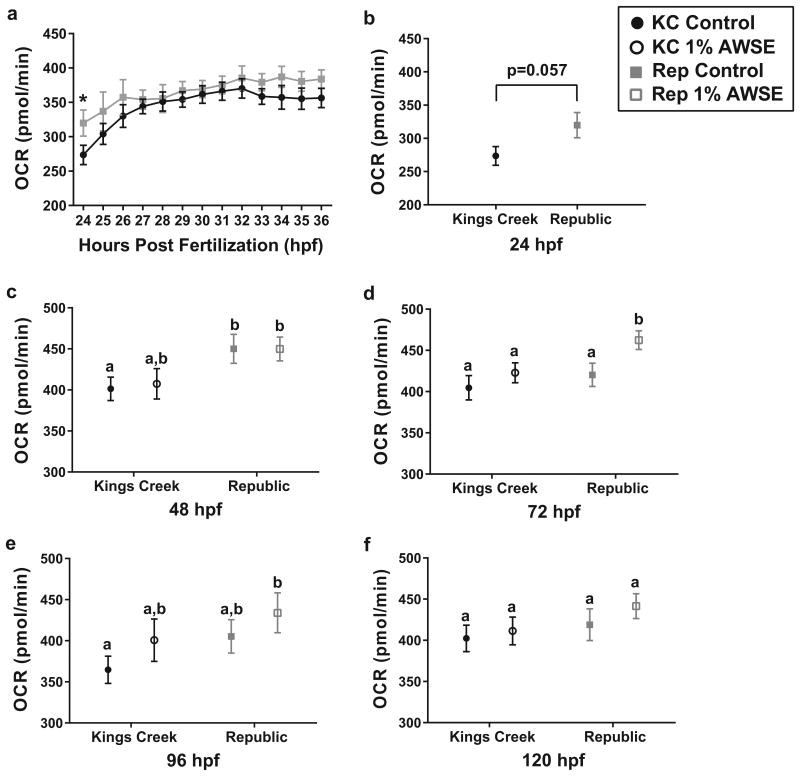
Across all points measured within the 24-36 hpf time course, Rep embryos had increased basal OCR when compared with KC embryos (Figure 5a). Although the only individual time point when Rep embryos had statistically different OCRs than KC embryos was at 24 hpf (p<0.05), completion of a 2-way ANOVA revealed that population and time were both statistically significant factors (p<0.005 and p<0.0001, respectively). This trend was also observed during the 24 hpf-120 hpf series (Figure 5b-e). The only time point when the untreated (ASW control) Rep embryos significantly differed from untreated KC embryos was at 48 hpf (p<0.05), although statistical significance was also almost achieved at 24 hpf (p<0.06).
Fig. 5.
Basal OCR of Rep and KC embryos exposed to control conditions (ASW) or 1% (v/v) AWSE during development. Filled black circles represent untreated (ASW control) KC embryos, open black circles represent treated (1% AWSE) KC embryos, closed gray squares represent untreated Rep embryos, and open gray squares represent treated Rep embryos. Error bars represent mean ± SEM. (a) 12-hour time course of basal OCR values from 24-36 hpf. Both time and population were significant factors (p<0.0001 and p<0.002, respectively). Rep groups marked with * are significantly different than KC groups at p<0.05 (2-way ANOVA). (b-e) Measurements of basal OCR in untreated and treated (1% AWSE) Rep and KC embryos at 24, 48, 72, 96, and 120 hpf, respectively. Values marked by different letters are significantly different than each other at p<0.05 (unpaired t-test or 2-way ANOVA)
Treatment with 1% AWSE did not significantly increase the basal OCR of either population when compared to their respective ASW control groups except in Rep embryos at 72 hpf. However, Rep embryos treated with 1% AWSE had significantly or trending higher basal OCR values at 48 hpf, 72 hpf, 96 hpf, and 120 hpf (p<0.05, p<0.003, and p<0.03, p=0.11 respectively) when compared with the ASW control KC embryos.
F1 Generation Juvenile Respirometry and Bioenergetic Assessment
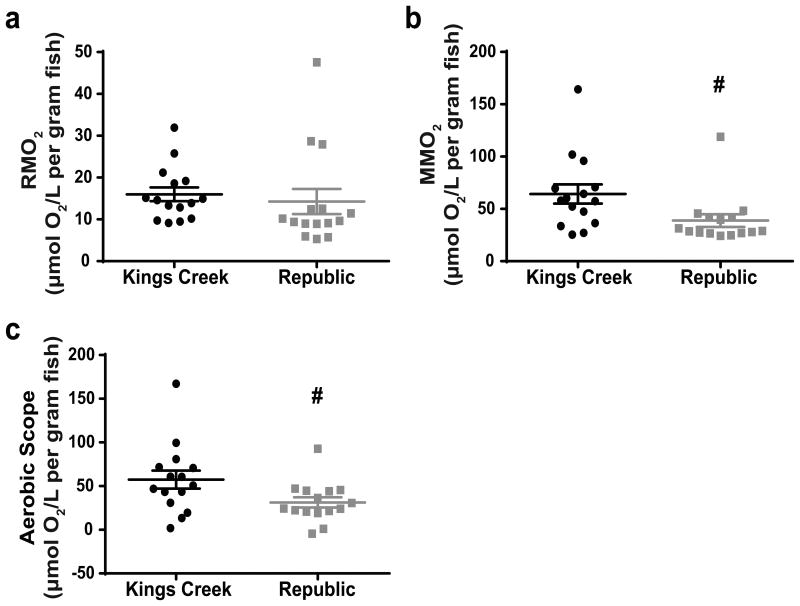
Whole-organism basal metabolic rates of Rep and KC juvenile fish were not significantly different (Figure 6a). However, Rep fish did have a significantly lower maximal metabolic rate (p<0.05) which translated to a significantly lower aerobic scope (p<0.05) (Figure 6b and 6c, respectively). There were no significant differences found in any of the bioenergetic parameters measured between the two populations during ex vivo assessments of heart and brain bioenergetics possibly due to the large variability between plate replicates (data not shown).
Fig. 6.
F1 juvenile respirometry measurements. (a) Resting metabolic rates (RMO2) of KC and Rep killifish normalized to body weight. (b) Maximal metabolic rates (MMO2) of KC and Rep killifish normalized to body weight. (c) Calculated aerobic scope (MMO2-RMO2) of KC and Rep killifish. Error bars represent mean ± SEM. Rep groups marked with # are statistically different than the corresponding KC groups at p<0.05 (unpaired t-test)
Discussion
Characterization of Killifish from Rep Site as PAH-Resistant
PAH resistance in Rep killifish was evident during multiple life stages and across two generations. Adult wild-caught (F0) Rep killifish were recalcitrant to hepatic microsomal CYP1A activity after injections of BNF, a model PAH and AhR agonist (Figure 2). Similarly, F1 Rep embryos had a reduced CYP1A response to simple and complex mixtures of PAHs compared to KC embryos (Figure 3). Rep embryos did not show significant induction of EROD activity after exposures to a simple PAH mixture of BaP and FL, but there was induction after exposures to the complex, creosote-contaminated sediment extract. This could be because the primary PAH constituents of the complex PAH mixture AWSE are high molecular weight AhR agonists whereas the simple mixture contained a higher ratio of CYP1A inhibitor to AhR agonist (Clark et al. 2013); the addition of a CYP1A inhibitor (e.g. FL) into an exposure with an AhR agonist (e.g. BaP) reduces the CYP1A activity of the agonist, potentially to levels that allow PAH-resistant embryos to display recalcitrance (Wassenberg and Di Giulio 2004b).
However, the CYP1A induction seen after exposures of Rep embryos to AWSE was a different response than that of F1 embryos from the AW population of PAH-resistant killifish. Clark et al. (2013) found that there was no significant induction of EROD activity in AW embryos after any treatment, including 10% AWSE. One possible explanation for this difference may be that there are higher migration rates at the Rep site which reintroduces non-resistant killifish into the gene pool, therefore, the killifish found at that site are not as resistant to PAH toxicity. Alternatively, creosoting activities at the Rep site were stopped two decades prior to activity at the AW Industries site which might have altered the composition of PAHs found in the sediment at the Rep site and subsequently altered the response of Rep embryos to the AW sediment-based extract. Current work is underway to analyze sediment and porewater extracts taken from the Republic site for toxicity. A more detailed comparison of results from this study and those that utilized similar methods to assess PAH-resistance in AW killifish can be found in Table A1.
Rep embryos displayed a complete recalcitrance to the cardiovascular deformities normally associated with PAH exposures, even after treatments with 10% AWSE, a level that is lethal for KC embryos (Figure 4, Figure A1). This phenotype was similar to the response of the AW killifish population (Clark et al. 2013). The KC population predictably displayed deformity score frequency distributions significantly different than those of KC control groups and every Rep treatment group (Wassenberg and Di Giulio 2004a; Clark et al. 2010; Wills et al. 2010; Clark et al. 2013). Specifically, moderate to severe cardiac deformities were observed in KC embryos with increasing concentrations of AWSE, in a dose-dependent manner. For example, treatment with 1% AWSE resulted in mostly normal and moderately deformed embryos with only a few severely deformed embryos. However, treatment with 5% AWSE resulted in almost all severely deformed embryos.
Previous studies have noted that EROD activity and physiological deformities are associated, although our results support the observation that the relationship between PAH-induced EROD activity and cardiovascular deformities in ovo is not linearly predictable (Whyte et al. 2000; Wassenberg et al. 2002; Incardona et al. 2005; Incardona et al. 2006). In KC embryos, there was reduced EROD activity after exposures to 3% and 5% AWSE compared to 1% AWSE, but a higher incidence of the most severe cardiovascular deformities in the 3% and 5% treated embryos. This can be explained by a loss of localization of the fluorescence in the urinary bladder due to increased incidence of severe deformities, as described in Wills et al. (2010), which manifested as decreased EROD activity measurements. Additionally, the binary mixture of BaP+FL produced higher incidences of severe cardiovascular deformities than 1% AWSE in KC embryos, but did not induce a higher response in CYP1A activity. This was expected due to CYP1A inhibition by FL, as described earlier (Wassenberg and Di Giulio 2004b). In Rep embryos, cardiovascular deformities did not track with EROD activity either, although for this population EROD activity overestimated the risk of teratogenesis whereas EROD activity underestimated the risk of teratogenesis in KC embryos. More specifically, Rep embryos that appeared normal during deformity analysis had significantly elevated EROD activity.
Based on the results presented above, we concluded that the Rep population of killifish can be characterized as PAH-resistant. Although CYP1A activity was induced in AWSE dosed Rep F1 embryos, this was not the case in the PAH-resistant AW embryos; CYP1A induction was diminished and often significantly lower than that of KC embryos. Further, in both the liver microsomal EROD assay as well as during the cardiovascular deformity screening, Rep embryos displayed complete recalcitrance to the effects normally associated with PAH exposure and toxicity, clearly demonstrating that Rep killifish display PAH-resistant phenotypes across multiple life stages and generations. Therefore, we further tested this population for potential bioenergetic consequences of this evolved adaptation, the outcomes of which are discussed below.
Bioenergetic Consequences of PAH Resistance
Herein, we report the energetic profiling of F1 Atlantic killifish from embryonic and juvenile (approximately 6 months post fertilization) life stages, both of which are critical windows for survival to adulthood. These data include basal respiration rates of embryos exposed to clean conditions or non-teratogenic doses of a complex PAH mixture throughout development. During the 24-36 hpf respirometric time course (Figure 5a), a significant increase in basal respiration was measured over time for both populations of killifish. The spike in OCR from 24-26 hpf coincides with the timing of the transition from blastula to gastrula phases, a point at which the midblastula transition occurs and rapid RNA synthesis and mitochondrial biogenesis begin (Armstrong and Child 1965; Dawid et al. 1985; Stackley et al. 2011). Interestingly, the OCRs for later time points (48, 72, 96, and 120 hpf) remain relatively constant, around 350-400 pmol O2/min for KC embryos and 400-450 pmol O2/min for Rep embryos (Figure 5b-f). This plateau is surprising because these developmental stages encompass organodifferentiation, angiogenesis, the onset of cardiovascular function, brain region differentiation, increased mitochondrial content of cells, and increased frequency of body movements (Armstrong and Child 1965; Stackley et al. 2011). This is in contrast to the work conducted by Stackley et al. (2011) who found that basal respiration in zebrafish embryos increased linearly with time and progressing development. Further studies are needed to assess respiration later in embryonic development (up to 14 dpf) and into larval phases in order to characterize the increased energy demand and dependence on oxidative phosphorylation expected to result from later developmental stages and hatching (Stackley et al. 2011)..
In both the 12-hour time course and the measurements taken every 24 hours, there was a trend of increased respiration in Rep embryos compared to KC embryos (Figure 5). As seen clearly from 24-36 hpf, the difference varied with time, but this trend persisted at every time point tested. This is consistent with results found in Oziolor et al. (2016) which showed that larvae from one population of non-resistant Gulf killifish had a significantly lower basal OCR than two contaminant-resistant populations and one other non-resistant population. This difference in basal respiration could be a result of several changes within Rep embryos including, but not limited to a) an increased demand for ATP production and/or b) inefficient oxidative phosphorylation due to damaged mitochondria or perturbed mitochondrial processes associated with chronic PAH exposure (Jung and Di Giulio 2010; Meyer et al. 2013; Du et al. 2015; Du et al. 2016). Here, we assume that an increased OCR is a negative consequence because this increase means that the embryos are respiring closer to their upper or maximal limit of respiration. Once that limit is reached, the organism can no longer aerobically respond to alterations in its environment. However, we cannot exclude potential benefits from this raise in OCR or that Rep embryos have higher maximal respiratory limits than KC embryos, which was not measured here, although data discussed later from our juvenile studies does not support that claim. Optimization of the killifish embryo Seahorse XF Analyzer protocol for the use of pharmacological agents such as those already used in zebrafish embryos (Stackley et al. 2011; Massarsky et al. 2015) would allow for further evaluation of the various parameters of energy metabolism (e.g. proton leak, spare capacity, ATP turnover, etc.) that could play a role in the differences observed between PAH-resistant and non-resistant populations.
Treatment with 1% AWSE did not have a statistically significant effect on respiration rates of killifish embryos from either population when compared to their own controls, but potentially could still have a biologically significant effect. It is likely that major differences in oxygen consumption rate in embryos are only seen after PAH exposures that also cause teratogenic effects and therefore were not seen in this study; we purposefully chose embryos that were not visibly deformed during these assays. However, it is clear that treatment with 1% AWSE did increase the OCRs of the Rep embryos because of the increased differences seen between those embryos and the KC control embryos. At 48, 72, and 96 hpf, Rep embryos treated with 1% AWSE had significantly higher basal OCR values than KC control embryos. This is in contrast to Rep control embryos, which were only significantly different than KC control embryos at 48 hpf. This is supported by work conducted in zebrafish which found that exposures to individual PAHs and PAH mixtures resulted in increased resting metabolic rates in adults (Gerger et al. 2014; Gerger and Weber 2015). We hypothesize that the significant increase in basal OCR of Rep embryos treated with 1% AWSE was due in part because there is a difference in OCR between the populations and in part to an increase in OCR after PAH exposures, although the differences are not statistically significant at p<0.05.
Previous results have shown that AWSE treatments did not affect AW killifish during larval mobility, habituation, and novel tank/anxiety behavior assays, or swimming performance tests that measured critical swimming speeds compared to KC killifish (Brown et al. 2016a; Brown et al. 2016b in press). Du et al. (2015, 2016) also showed that AW killifish, but not KC killifish, were resistant to the effects of BaP exposure on oxidative phosphorylation. However, current data suggest that embryonic OCR of KC and Rep embryos were affected almost equivalently by exposures to AWSE. Although OCR in AW embryos have never been tested previously, our embryonic data are in direct contrast with existing juvenile AW killifish data- 5 month old AW killifish basal oxygen consumption rate was not significantly altered by acute exposures to AWSE (Brown et al. 2016b in press)- suggesting an ontogenetic shift in mitochondrial response to PAHs in these fish.
In addition to alterations in embryonic OCR, we also observed differences in whole-organismal respiration in Rep F1 juvenile killifish (Figure 6), which indicates that changes in metabolic function is potentially a lifelong consequence of PAH resistance and/or chronic PAH exposure, not a phenotype seen only at certain phases of development. The resting metabolic rate for Rep killifish was not significantly different than that of KC killifish, suggesting that PAH resistance does not impact the organism's ability to meet minimal requirements for metabolic demands (Davoodi and Claireaux 2007), which contrasts our embryonic data. The embryos used during our measures of basal OCR were not deformed and likely had a better chance of survival to larval and juvenile stages than deformed embryos. Our results assert that in Rep killifish the increase in basal OCR we see during embryonic development shifts during another phase of development, but there is the possibility that embryos that had the highest basal OCRs did not survive to juvenile stages and therefore the Rep juvenile resting metabolic rate was not significantly different from KC.
However, the maximal metabolic rate for Rep killifish was significantly lower than that of KC fish which resulted in a significantly decreased aerobic scope in Rep killifish. As hypothesized by Davoodi and Claireaux (2007), this suggests that Rep killifish have an altered ability to utilize energy resources when faced with high energy demands which could result in reduced metabolic flexibility in stressful situations. This could have important implications for PAH-resistant killifish if they are faced with taxing scenarios and need to mobilize energy resources in order to respond to altered environmental conditions or other stressors. If this decrease in maximal metabolic rate is also present in Rep embryos, which already have an increased basal OCR and potentially a limited aerobic scope, their respiratory capacity would be even further restricted by this upper bound. Additionally, several physiological functions, including reproduction, growth, and immune responses, could be limited by energetic restraints that appear to result from evolved PAH resistance (Jayasundara et al. in review).
Further optimization of methodology will be needed to assess whether altered mitochondrial function of heart or brain tissues contribute to the whole-organism-level metabolic changes observed in Rep killifish. During ex vivo bioenergetic experimentation, there was variability within population, both KC and Rep, and tissue type in all metabolic parameters (i.e. mitochondrial respiration, reserve capacity, non-mitochondrial respiration) measured, to the extent that in some cases trends seen in initial experimentation reversed in subsequent experimental groups. This could be due to inter-individual variability in organ size which affected dosing of pharmacological agents used during testing (Jayasundara et al. 2015) or there is simply no significant difference in either tissue type between the two populations. As of now, we cannot claim that the significant differences in whole-organism aerobic metabolism should be attributed to metabolic shifts in heart or brain tissue. However, future work will be dedicated to reexamining bioenergetic differences between killifish populations in heart, brain, and gonadal tissues.
The goal of this study was to examine two populations of killifish directly removed from their natural environments for differences in tolerance to contaminant exposure and energy metabolism. However, one of the lasting questions that arises from this work and other work with contaminant-resistant killifish populations is whether the alterations we see in PAH-resistant populations are due to chronic PAH exposures, genetic evolution, or both. From these results and others in Rep, AW, and other ER populations (Brown et al. 2016b; Jayasundara et al. in review), we hypothesize that the bioenergetic consequences we found are inherited traits that did not arise from direct or chronic PAH exposures. On the other hand, it is possible that removal of these fish from constant chronic exposure (i.e. their natural environmental conditions) or other unknown environmental pressures was the primary cause of the observed changes. All current work points to these consequences as an adaptive response to PAHs, but chronic exposure could potentially exacerbate or placate the effects of evolutionary resistance.
Summary
Several assays spanning two generations support our conclusion that killifish residing at the creosote-contaminated Republic Creosoting Inc. site constitute a PAH-resistant population. These fish demonstrated recalcitrance of CYP1A induction and absence of the usual suite of developmental deformities after PAH exposures, although the adaptive response of Rep killifish was slightly less striking than the population of killifish previously found at the Atlantic Wood Industries Superfund site. The Rep population of killifish also had markedly different measurements of aerobic metabolism, observed in two life stages, than a non-resistant population of killifish. Rep F1 killifish had higher oxygen consumption rates throughout embryonic development as well as lowered aerobic scopes, or reduced metabolic plasticity, at 6 months of age (Davoodi and Claireaux 2007). These results suggest that populations of killifish that have evolved to withstand the toxicity associated with PAHs and inhabit PAH-contaminated ecosystems may consequently have altered energy demands or metabolic abilities. Such consequences could result in an enhanced vulnerability to other environmental and anthropogenic stressors in PAH-resistant killifish. The rapid evolution of certain traits that suppress physiological PAH toxicity appear to be beneficial in closed-system scenarios and laboratory settings. However, with increasing frequency and severity of environmental stressors such as climate change, these adaptations could result in the increased susceptibility and vulnerability of populations of this ecologically important fish species.
Supplementary Material
Fig. A1 Cardiovascular deformity score frequency distributions of KC and Rep embryos at 6 dpf. Black symbols denote significance within a population compared to ASW controls. Gray symbols denote significance within a treatment between populations. Bars marked with * are significantly different at p<0.0005. Bars marked with # are significantly different at p<0.0001 (Fisher's Exact Test)
Acknowledgments
We would like to thank members of the Di Giulio Laboratory for their help and support during preparation of this manuscript, as well as the reviewers for their insightful comments. This work was supported by Duke University's Program in Environmental Health Training Grant (NIEHS T32-ES021432) and the Duke University Superfund Research Center (NIEHS P42-ES010356).
Footnotes
Compliance with Ethical Standards: Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: This article does not contain any studies with human participants. The Duke University Institutional Animal Care and Use Committee approved all of the F. heteroclitus care, handling, and techniques used during this work (Protocol # A184-13-07).
References
- Armstrong PB, Child JS. Stages in the Normal Development of Fundulus heteroclitus. Biol Bull. 1965;128:143–168. [Google Scholar]
- Billiard SM, Querbach K, Hodson PV. Toxicity of retene to early life stages of two freshwater fish species. Environ Toxicol Chem. 1999;18:2070–2077. [Google Scholar]
- Bowater W. Heredity of melanism in Lepidoptera. W Journ of Gen. 1914;3:299–315. doi: 10.1007/BF02981796. [DOI] [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, et al. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol Teratol. 2016;53:55–63. doi: 10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, et al. Later Life Swimming Performance and Persistent Heart Damage Following Subteratogenic PAH Mixture Exposure in the Atlantic Killifish (Fundulus heteroclitus) Environ Toxicol Chem. 2016 doi: 10.1002/etc.3877. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel SM, White LA, Cooper KR. Impaired reproductive health of killifish (Fundulus heteroclitus) inhabiting Newark Bay, NJ, a chronically contaminated estuary. Aquat Toxicol. 2010;96:182–193. doi: 10.1016/j.aquatox.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Clark BW, Cooper EM, Stapleton HM, Di Giulio RT. Compound- and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus subpopulations throughout the Elizabeth River estuary (Virginia, USA) Environ Sci Technol. 2013;47:10556–10566. doi: 10.1021/es401604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat Toxicol. 2010;99:232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LM, Saccheri IJ. The peppered moth and industrial melanism: evolution of a natural selection case study. Heredity (Edinb) 2013;110:207–12. doi: 10.1038/hdy.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi F, Claireaux G. Effects of exposure to petroleum hydrocarbons upon the metabolism of the common sole Solea solea. Mar Pollut Bull. 2007;54:928–934. doi: 10.1016/j.marpolbul.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Dawid IB, Haynes SR, Jamrich M, et al. Gene expression in Xenopus embryogenesis. J Embryol Exp Morphol. 1985;89(Suppl):113–124. [PubMed] [Google Scholar]
- Di Giulio RT, Clark BW. The Elizabeth River Story: A case study in evolutionary toxicology. J Toxicol Environ Health Part B. 2015;18:2159–258. doi: 10.1080/15320383.2015.1074841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Crawford DL, Nacci DE, Oleksiak MF. Heritable oxidative phosphorylation differences in a pollutant resistant Fundulus heteroclitus population. Aquat Toxicol. 2016;177:44–50. doi: 10.1016/j.aquatox.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Du X, Crawford DL, Oleksiak MF. Effects of Anthropogenic Pollution on the Oxidative Phosphorylation Pathway of Hepatocytes from Natural Populations of Fundulus heteroclitus. Aquat Toxicol. 2015;165:231–240. doi: 10.1016/j.aquatox.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Frederick LA, van Veld PA, Rice CD. Bioindicators of immune function in creosote-adapted estuarine killifish, Fundulus heteroclitus. J Toxicol and Env Heal A. 2007;70(17):1433–1442. doi: 10.1080/15287390701382910. https://doi.org/10.1080/15287390701382910. [DOI] [PubMed] [Google Scholar]
- Fox GA. Tinkering with the tinkerer: Pollution versus evolution. Environmental Health Perspectives. 1995:93–100. doi: 10.1289/ehp.103-1519277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerger CJ, Thomas JK, Janz DM, Weber LP. Acute effects of β-naphthoflavone on cardiorespiratory function and metabolism in adult zebrafish (Danio rerio) Fish Physiol Biochem. 2014;41:289–298. doi: 10.1007/s10695-014-9982-z. [DOI] [PubMed] [Google Scholar]
- Gerger CJ, Weber LP. Comparison of the acute effects of benzo-a-pyrene on adult zebrafish (Danio rerio) cardiorespiratory function following intraperitoneal injection versus aqueous exposure. Aquat Toxicol. 2015;165:19–30. doi: 10.1016/j.aquatox.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochemistry and Molecular Biology. 2004:653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Hendry AP. Key questions in the genetics and genomics of eco-evolutionary dynamics. Heredity (Edinb) 2013;111:456–466. doi: 10.1038/hdy.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, et al. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Jayasundara N, Kozal JS, Arnold MC, et al. High-throughput tissue bioenergetics analysis reveals identical metabolic allometric scaling for teleost hearts and whole organisms. PLoS One. 2015 doi: 10.1371/journal.pone.0137710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Di Giulio RT. Identification of mitochondrial cytochrome P450 induced in response to polycyclic aromatic hydrocarbons in the mummichog (Fundulus heteroclitus) Comp Biochem Physiol - C Toxicol Pharmacol. 2010;151:107–112. doi: 10.1016/j.cbpc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MW, Hofmann GE. Adaptation and the physiology of ocean acidification. Funct Ecol. 2013;27:980–990. doi: 10.1111/j.1365-2435.2012.02061.x. [DOI] [Google Scholar]
- Lohbeck KT, Riebesell U, Reusch TBH. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat Geosci. 2012;5:346–351. doi: 10.1038/ngeo1441. [DOI] [Google Scholar]
- Massarsky A, Jayasundara N, Bailey JM, et al. Teratogenic, bioenergetic, and behavioral effects of exposure to total particulate matter on early development of zebrafish (Danio rerio) are not mimicked by nicotine. Neurotoxicol Teratol. 2015;51:77–88. doi: 10.1016/j.ntt.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CW, Clark BW, Jenny MJ, et al. Development of the morpholino gene knockdown technique in Fundulus heteroclitus: A tool for studying molecular mechanisms in an established environmental model. Aquat Toxicol. 2008;87:289–295. doi: 10.1016/j.aquatox.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Di Giulio RT. Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol Appl. 2003;13:490–503. doi: 10.1890/1051-0761(2003)013[0490:HAAFCI]2.0.CO;2. [DOI] [Google Scholar]
- Meyer JN, Leung MCK, Rooney JP, et al. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Nacci DE, Di Giulio RT. Cytochrome P4501A (CYP1A) in killifish (Fundulus heteroclitus): Heritability of altered expression and relationship to survival in contaminated sediments. Toxicol Sci. 2002;68:69–81. doi: 10.1093/toxsci/68.1.69. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Wassenberg DM, Karchner SI, et al. Expression and inducibility of aryl hydrocarbon receptor pathway genes in wild-caught killifish (Fundulus heteroclitus) with different contaminant-exposure histories. Environ Toxicol Chem. 2003;22:2337–2343. doi: 10.1897/02-495. [DOI] [PubMed] [Google Scholar]
- Mulvey M, Newman MC, Vogelbein W, Unger MA. Genetic structure of Fundulus heteroclitus from PAH-contaminated and neighboring sites in the Elizabeth and York Rivers. Aquat Toxicol. 2002;61:195–209. doi: 10.1016/S0166-445X(02)00055-3. [DOI] [PubMed] [Google Scholar]
- Mulvey M, Newman MC, Vogelbein WK, et al. Genetic structure and mtDNA diversity of Fundulus heteroclitus populations from polycyclic aromatic hydrocarbon-contaminated sites. Environ Toxicol Chem. 2003;22:671–677. doi: 10.1897/1551-5028(2003)022<0671:GSAMDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nacci DE, Champlin D, Jayaraman S. Adaptation of the estuarine fish Fundulus heteroclitus (Atlantic Killifish) to polychlorinated biphenyls (PCBs) Estuaries and Coasts. 2010;33:853–864. doi: 10.1007/s12237-009-9257-6. [DOI] [Google Scholar]
- Nacci D, Huber M, Champlin D, Jayaraman, et al. Evolution of tolerance to PCBs and susceptibility to a bacterial pathogen (Vibrio harveyi) in Atlantic killifish (Fundulus heteroclitus) from New Bedford (MA, USA) harbor. Environ Pollut. 2009;157(3):857–864. doi: 10.1016/j.envpol.2008.11.016. https://doi.org/10.1016/j.envpol.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program. NTP Research Concept : Polycyclic Aromatic Hydrocarbons (PAHs) NTP Board Sci Couns Meet. 2012:1–14. [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, et al. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ Toxicol Chem. 2002;21:1897–1902. doi: 10.1002/etc.5620210917. [DOI] [PubMed] [Google Scholar]
- Oziolor EM, Dubansky B, Burggren WW, et al. Cross-resistance in Gulf killifish (Fundulus grandis) populations resistant to dioxin-like compounds. Aquat Toxicol. 2016;175:222–231. doi: 10.1016/j.aquatox.2016.03.019. https://doi.org/10.1016/j.aquatox.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Oziolor EM, Bigorgne E, Aguilar L, et al. Evolved resistance to PCB- and PAH-induced cardiac teratogenesis, and reduced CYP1A activity in Gulf killifish (Fundulus grandis) populations from the Houston Ship Channel, Texas. Aquat Toxicol. 2014;150:210–219. doi: 10.1016/j.aquatox.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Pespeni MH, Sanford E, Gaylord B, et al. Evolutionary change during experimental ocean acidification. Proc Natl Acad Sci. 2013;110:6937–6942. doi: 10.1073/pnas.1220673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid NM, Proestou DA, Clark BW, et al. The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wild fish. Science. 2016;354(6317):1305. doi: 10.1126/science.aah4993. LP-1308. https://doi.org/10.1126/science.aah4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AX, Jander G, Samaniego H, et al. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS One. 2012 doi: 10.1371/journal.pone.0036366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackley KD, Beeson CC, Rahn JJ, Chan SSL. Bioenergetic profiling of zebrafish embryonic development. PLoS One. 2011 doi: 10.1371/journal.pone.0025652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman JH, Paganini a W. Biochemical adaptation to ocean acidification. J Exp Biol. 2015;218:1946–1955. doi: 10.1242/jeb.115584. [DOI] [PubMed] [Google Scholar]
- Vogelbein WK, Unger MA. The Elizabeth River Monitoring Program 2006-2007: Assocation between mummichog liver histopathology and sediment chemical contamination 2008 [Google Scholar]
- Wassenberg DM, Di Giulio RT. Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. In: Marine Environmental Research. 2004a:163–168. doi: 10.1016/j.marenvres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004b;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Swails EE, Di Giulio RT. Effects of single and combined exposures to benzo(a)pyrene and 3,3′4,4′5-pentachlorobiphenyl on EROD activity and development in Fundulus heteroclitus. Marine Environmental Research. 2002:279–283. doi: 10.1016/s0141-1136(02)00182-4. [DOI] [PubMed] [Google Scholar]
- Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit Rev Toxicol. 2000;30(4):347–570. doi: 10.1080/10408440091159239. https://doi.org/10.1080/10408440091159239. [DOI] [PubMed] [Google Scholar]
- Wills LP, Matson CW, Landon CD, Di Giulio RT. Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquat Toxicol. 2010;99:33–41. doi: 10.1016/j.aquatox.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. A1 Cardiovascular deformity score frequency distributions of KC and Rep embryos at 6 dpf. Black symbols denote significance within a population compared to ASW controls. Gray symbols denote significance within a treatment between populations. Bars marked with * are significantly different at p<0.0005. Bars marked with # are significantly different at p<0.0001 (Fisher's Exact Test)