Abstract
We previously reported that the kinase AKT is activated in the nucleus accumbens (NAc) of rodents in response to excessive consumption of alcohol. One of the important downstream targets of AKT is the mammalian Target Of Rapamycin in Complex 1 (mTORC1), which was also activated by alcohol intake. mTORC1 controls dendritic protein translation, and we showed that the mTORC1-dependent translational machinery is activated in the NAc in response to alcohol intake. Importantly, systemic or intra-NAc inhibition of the AKT/mTORC1 pathway attenuated alcohol-drinking behaviors. Here, we mapped the activation patterns of AKT and mTORC1 in corticostriatal regions of rodents consuming large amounts of alcohol. We found that the activation of AKT and mTORC1 in response to cycles of binge drinking of 20% alcohol was centered in the NAc shell. Both kinases were not activated in the dorsolateral striatum (DLS), however, AKT but not mTORC1, was activated in the dorsomedial striatum (DMS) of mice but not rats. Interestingly, excessive intake of alcohol produced a selective activation of the AKT/mTORC1 pathway in the orbitofrontal cortex (OFC), which was not observed in medial prefrontal cortex (mPFC). Furthermore, this signaling pathway was not activated in the NAc shell or OFC of rats consuming moderate amounts of alcohol nor was it activated in rats consuming sucrose. Together, our results suggest that excessive alcohol intake produces a brain region selective activation of the AKT/mTORC1 pathway, which is likely to contribute to NAc shell and OFC-dependent mechanisms that underlie the development and maintenance of alcohol drinking behavior.
Introduction
Signal transduction cascades transduce extracellular signal to intracellular compartments, and as such molecular signaling plays crucial role in biological responses in all cell-types including neurons. A prominent signaling cascade consists of the phospholipid kinase phosphoinositide 3-kinase (PI3K), which activates the kinase Phosphoinositide-dependent kinase-1 (PDK-1) that phosphorylates and activates the serine and threonine kinase, AKT (also known as protein kinase B (PKB)) (Manning and Cantley, 2007).
In the adult central nervous system (CNS), the PI3K/AKT axis plays a role in neuronal outgrowth and morphology (Read and Gorman, 2009; Sui et al., 2008). In addition, AKT has been implicated in synaptic plasticity and learning and memory (Sui et al., 2008). Interestingly, the PI3K/AKT pathway in the nucleus accumbens (NAc) of rodents plays a role in the molecular and behavioral adaptations that are induced by alcohol, and that in turn drives alcohol-drinking behaviors. Specifically, using the intermittent access to 20% alcohol 2-bottle choice (IA20%-2BC) paradigm, we observed that repeated cycles of binge drinking and withdrawal increase the PI3K-dependent activation of AKT in the NAc of mice and rats which was maintained even after 24-hr withdrawal (Neasta et al., 2011). We further showed that one of the consequences of AKT activation by alcohol is the phosphorylation of GSK3β in the NAc of rodents, a molecular outcome that contributes to alcohol-dependent microtubule assembly (Liu et al., 2016). Importantly, blockade of the activity of either PI3K or AKT in the NAc decreased rat voluntary consumption of alcohol in the IA20%-2BC paradigm as well as in an operant 20% alcohol self-administration paradigm (Neasta et al., 2011).
AKT, through intermediate steps, also activates the mammalian Target Of Rapamycin in Complex 1 (mTORC1) (Dibble and Cantley, 2015). Active mTORC1 initiates protein synthesis through phosphorylation of eIF4E-binding protein (4E-BP) and the ribosomal protein S6 kinase (S6K), which in turn phosphorylates its substrate, S6 (Takei and Nawa, 2014). These phosphorylation steps initiate the assembly of the translation initiation machinery of a subset of mRNAs (Takei and Nawa, 2014). mTORC1 is present in dendrites where it induces the local dendritic translation of synaptic mRNAs (Liu-Yesucevitz et al., 2011). Because of its role in activating the dendritic translational machinery, mTORC1 has been shown to play an important role in synaptic morphology, synaptic plasticity, and memory processes (Buffington et al., 2014; Hoeffer and Klann, 2010).
Previously, we reported that like AKT, mTORC1 was robustly activated in the NAc of mice and rats that were consuming high levels of alcohol as indicated by increased phosphorylation of S6K and 4E-BP (Neasta et al., 2010). Interestingly, we also observed that a single session of excessive alcohol intake was sufficient to activate mTORC1 signaling in the NAc of mice, which was localized to dopamine D1 receptor expressing neurons (Beckley et al., 2016). Furthermore, we and others showed that the consequence of alcohol-mediated activation of mTORC1 was an increased translation of Homer, postsynaptic density protein 95 (PSD95), the GluA1 subunit of AMPAR, and CRMP-2 in the NAc of rodents (Beckley et al., 2016; Cozzoli et al., 2009; Liu et al., 2016; Neasta et al., 2010). Finally, we showed that the cellular consequences of alcohol-dependent mTORC1-mediated translation in the NAc is the enhancement of synaptic plasticity (Beckley et al., 2016), and enhancement of microtubule assembly (Liu et al., 2016). Finally, systemic and intra-NAc administration of the mTORC1 inhibitor, rapamycin, attenuates alcohol seeking and drinking (Beckley et al., 2016; Cozzoli et al., 2016; Neasta et al., 2010). Taken together, these findings indicate that the PI3K/AKT/mTORC1 axis plays a central role in neuroadaptations underlying phenotypes such as alcohol drinking, seeking, and relapse.
To gain further insight on the interaction between alcohol intake and the AKT/mTORC1 axis, we surveyed the activity of this signaling pathway in corticostriatal regions of both mice and rats in response to chronic intermittent consumption of high levels of alcohol. Interestingly, we found that AKT and mTORC1 are active in response to alcohol in a brain-region restricted fashion.
Material and Methods
Animals
Male Long-Evans rats (Harlan, Indianapolis, IN) and male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were 8–9 weeks old at the beginning of the experiment and individually housed in separate temperature- and humidity- controlled rooms under a 12-hour light/dark cycle (lights on at 07:00 AM) or a reversed 12 hour light/dark cycle (lights on at 10:00 PM), respectively, with food and water available ad libitum. All animal procedures in this report were approved by the University of California San Francisco (UCSF) Institutional Animal Care and Use Committee and conducted in agreement with the Guide for the Care and Use of Laboratory Animals (National Research Council, Gallo Center) and the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC, UCSF).
Two-bottle choice drinking paradigm
Intermittent access to 20% alcohol
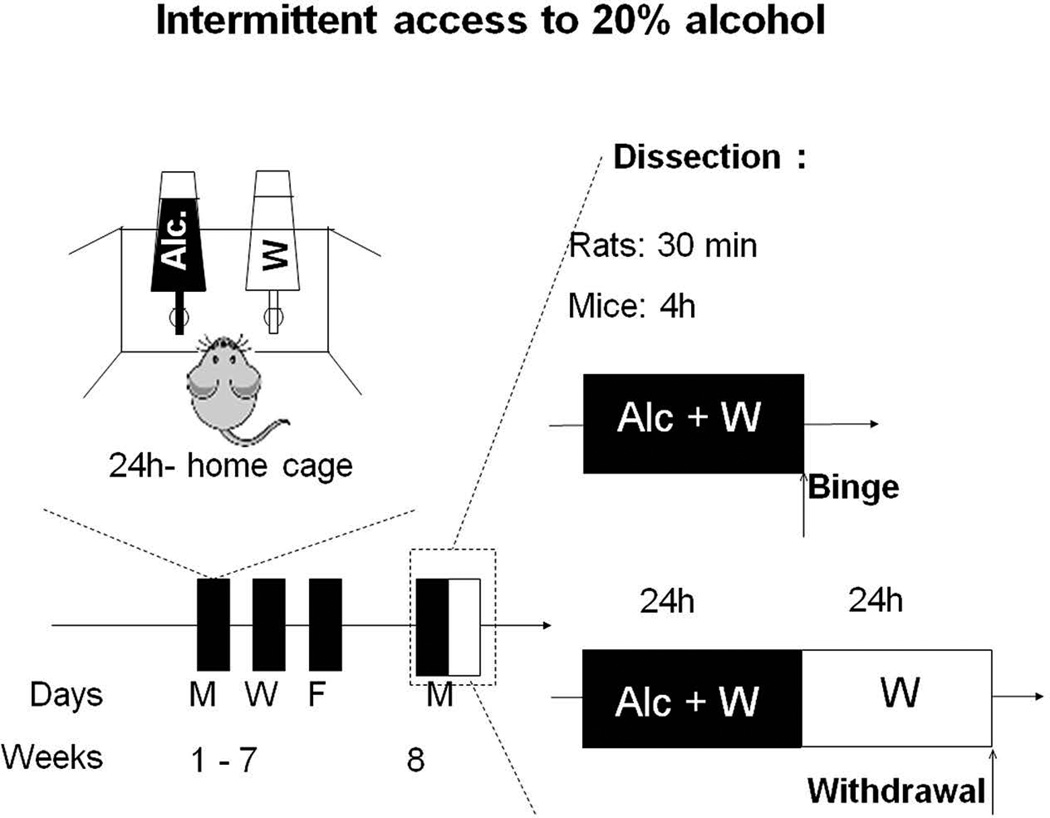
The IA20%-2BC procedure was conducted as previously described in rats (Carnicella et al., 2014) and mice (Warnault et al., 2013), and is depicted in Fig. 1. Rodents were given 24 hrs of concurrent access to one bottle of 20% alcohol (v/v) in water and one bottle of water on Monday, Wednesday and Friday, with 24- or 48 hrs (weekend) alcohol-deprivation periods between the alcohol drinking sessions for 7–8 weeks (i.e. 21 drinking sessions). Rats and mice that drink more than 3.5 g/kg/24h and 14 g/kg/24h, respectively, were considered excessive alcohol drinkers and included in the study (65–70% of the animals). Further details can be found in the online supporting information.
Figure 1. Excessive alcohol-drinking model.
Rats and mice experienced 7–8 weeks of intermittent access to 20% alcohol two-bottle choice drinking paradigm (IA20%-2BC). Control animals had access to water (W) only. Brain regions were removed after a short period of access to alcohol (Binge, 30 min after the last drinking session in rats, 4 hrs in mice) or after a withdrawal period of 24 hrs after the end of the last drinking session (Withdrawal).
Continuous access to 10% alcohol
Rats underwent a 3 weeks (21 drinking sessions) continuous access to two bottles (CA10%-2BC) one containing a 10% alcohol solution (v/v) and the other containing tap water, as depicted in Fig. 3b. Rats consumed on average 1.5 g/kg/24h and were considered moderate alcohol drinkers. Further details can be found in the online supporting information.
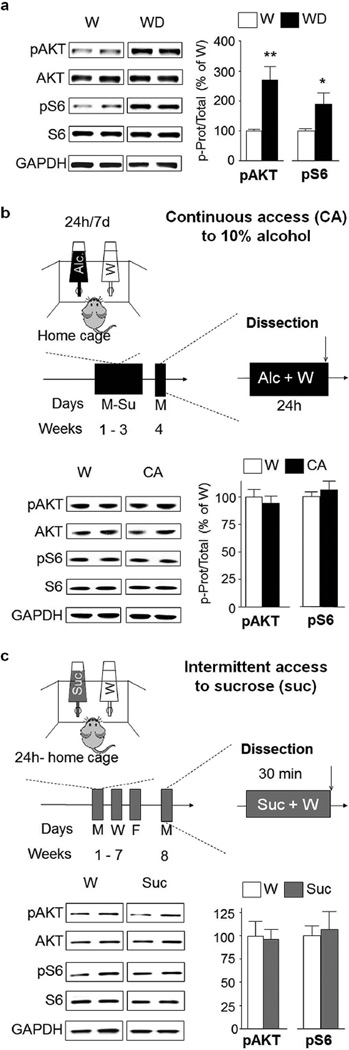
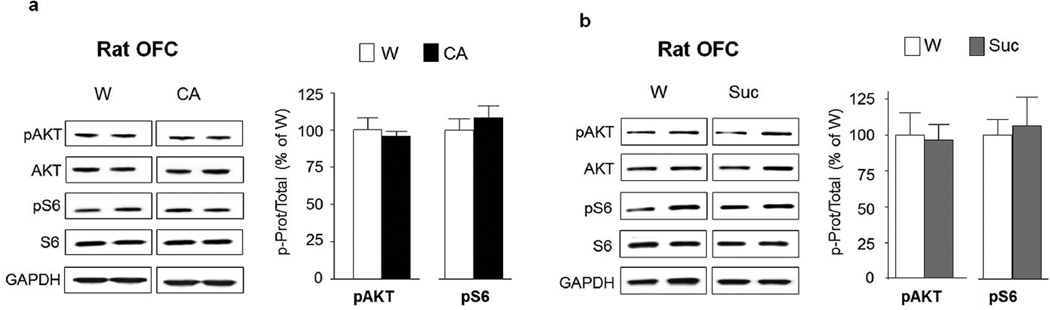
Figure 3. AKT/mTORC1 activation by alcohol in nucleus accumbens shell is long lasting and specific.
(a) After 7–8 weeks of IA20%-2BC, the NAc shell of rats was removed after 24 hrs of withdrawal following the end of the last drinking session (Withdrawal, WD, see Fig. 1), and the phosphorylation of AKT and S6 in the NAc shell was determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the average ratio +/− S.E.M of phospho-AKT to AKT and phospho-S6 to S6 and are expressed as percentage of water (W) controls. Significance was determined using two-tailed unpaired t-test. pAKT p<0.01; pS6 p<0.05. n=6 for alcohol group, n=7 for water group. (b) A diagram depicting the 10% continuous access 2 bottle choice (CA10%-2BC) alcohol drinking paradigm. Rats experienced 3 weeks (21 sessions) of CA10%-2BC. Controls animals had access to water (W) only. Brain regions were removed at the end of the last drinking session. Phosphorylation of AKT and S6 in the NAc shell after CA10%-2BC (CA) was determined by western blot analysis. pAKT p>0.05; pS6 p>0.05 n=4 per group. (c) Intermittent access to a natural reward. Rats experienced 7–8 weeks of IA to 1% sucrose in the two-bottle choice drinking paradigm (IA1%-2BC). Control animals had access to water (W) only. Brain regions were removed 30 min after the beginning of the last drinking session. Phosphorylation of AKT and S6 in the NAc shell after IA1%-2BC (Suc) was determined by western blot analysis. pAKT p>0.05; pS6 p>0.05 n=5 for sucrose group, n=4 for water group. *p<0.05; **p<0.01.
Intermittent access to 1% sucrose
Rats underwent an IA-2BC to 1% (IA1%-2BC) sucrose as depicted in Fig. 3c. Further details can be found in the online supporting information.
Immunochemistry (IHC)
The procedure was conducted as previously described (Barak, 2013) and details can be found in the online supporting information.
Collection of brain samples for biochemical analyses
Rats and mice were euthanized using CO2, 30 min or 4 hrs, respectively, after the beginning of the last drinking session (binge) and 24 hrs after the end of the last drinking session (withdrawal) (Timeline, Figure 1). Brains were then quickly removed and placed on an ice-cold platform prior to dissection. Tissue was homogenized in ice-cold radio immunoprecipitation assay (RIPA) buffer (in mM: 50 Tris-HCL, 5 EDTA, 120 NaCl, 1%NP-40, 0.1% deoxycholate, 0.5% SDS, protease and phosphatase inhibitors cocktails). Samples were homogenized using a sonic dismembrator. Protein content was determined using BCA kit.
Western blot analysis
Procedure was conducted as previously described (Gibb et al., 2011; Neasta et al., 2010). Details can be found in the online supporting information.
Data analysis
Data are expressed as the mean ± SEM. Dual comparisons were analyzed using unpaired two-tailed Student’s t-tests, by graphPad Prism 5.0 Software (GaphPad software Inc, CA, USA). Statistical significance was set at p<0.05.
Results
To survey the activation patterns of the AKT/mTORC1 signaling pathway in corticostriatal regions of rodents that voluntary consume excessive amounts of alcohol, we utilized the IA20%-2BC paradigm (Carnicella et al., 2014) in which rats and mice have a free access to a bottle of water and access to a bottle containing 20% alcohol every other day for a period of 7–8 weeks (Carnicella et al., 2014) (Fig. 1). This paradigm results in escalation of alcohol drinking in 65–70% of the rats that reach a stable and high level of alcohol consumption (2.4±0.7 and 6.0±0.6 g/kg/24 hrs on the first and last drinking session, respectively). Rats also display a binge-drinking phenotype with about one-third of the total amount of consumed alcohol occurring within the first 30 min period, an amount that generates a blood alcohol concentration (BAC) of >80 mg%, which meets the criteria of the NIAAA for binge drinking in humans (Carnicella et al., 2014). Mice on the other hand, drink 5.5±0.30g/kg alcohol in a 4 hrs session, an amount that is equivalent to a BAC >80 mg% (Neasta et al., 2010). Furthermore, unlike rats, mice consume high amounts of alcohol early on (Beckley et al., 2016), and as a result the escalation alcohol consumption during the 7 weeks of exposure is less pronounced (14.6±1.7 and 15.1±0.9 g/kg/24 hrs on first and last drinking session, respectively). Brain regions were harvested 30 min (rat) and 4-hrs (mice) after the initiation of the last drinking session (Binge) or after a period of 24-hr of withdrawal following the last 24-hr drinking session (Withdrawal) (Fig. 1).
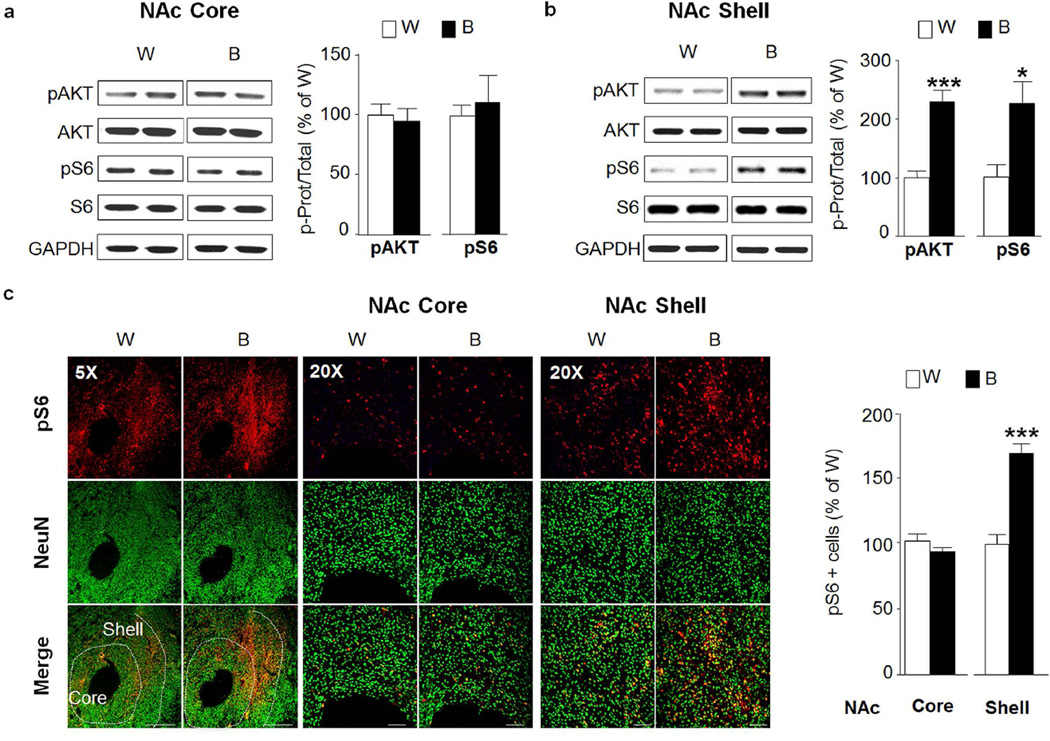
We previously observed that IA20%-2BC results in a robust and sustained activation of the AKT/mTORC1 pathway in the NAc of mice (Neasta et al., 2014; Neasta et al., 2011), results which were replicated herein (Fig. S1). The NAc is divided into two subregions: the core and shell, which receive distinct cortical inputs and may have dissociative roles in guiding motivated behaviors (Corbit et al., 2016). We were therefore interested to determine whether the activation of the AKT/mTORC1 pathway is localized in one of the two subregions of the NAc. As the NAc of mice is too small to definitely dissect the two subregions, we measured the level of AKT and mTORC1 activation in response to repeated cycles of binge drinking and withdrawal in the NAc core and shell of rats. Activation of AKT and mTORC1 was measured by using specific antibodies that recognize the phosphorylation site ser473 on AKT, as an indicator of AKT activation (Manning and Cantley, 2007) as well as the phosphorylation sites ser235/236 on S6, a downstream target of mTORC1, which is a commonly used marker for mTORC1 activation (Tsai et al., 2014; Zeng et al., 2009). We found that binge drinking of alcohol did not activate AKT/mTORC1 pathway in the NAc core of rats (Fig. 2a, pAKT t(12)=0.36, p=0.728.; pS6 t(12)=0.43, p=0.675), but produced a robust activation in the shell subregion of the NAc (Fig. 2b, pAKT t(12)=5.69, p<0.001; pS6 t(12)=2.95, p=0.012) as compared to control rats that consumed only water for the same duration of time. Next, we set to confirm the biochemical data using immunohistochemistry (IHC) as a second independent method. Phospho-AKT cannot be reliably used for IHC studies, therefore to test for the activation of this pathway, we used anti-phospho-S6 antibodies that specifically detect mTORC1 activation by IHC (Barak et al., 2013). As shown in Fig. 2c, binge drinking of alcohol led to a significant increase in the immunoreactivity of phospho-S6 in the NAc shell (pS6 t(12)=6.55, p<0.001) but not core (pS6 t(12)=1.27 p=0.226) as compared to water only consuming rats.
Figure 2. Excessive alcohol intake activates AKT/mTORC1 signaling in the rat nucleus accumbens shell.
After 7–8 weeks of IA20%-2BC, the core and shell subregions of rats NAc were removed 30 min after the beginning of the last drinking session (Binge, B, see Fig. 1). (a,b) Phosphorylation of AKT and S6 was determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the average ratio +/− S.E.M of phospho-AKT to AKT and phospho-S6 to S6 and are expressed as percentage of water (W) control. Significance was determined using two-tailed unpaired t-test. (a) Phosphorylation level of AKT and S6 in the NAc core of binge drinking rats (B) pAKT p>0.05; pS6 p>0.05. n= 7 per group. (b) Phosphorylation level of AKT and S6 in the NAc shell of binge drinking rats (B) pAKT p<0.001; pS6 p<0.05. n=7 per group. (c) IHC analysis of phospho-S6 levels in the NAc shell and core of binge-drinking rats (B) compared to water (W) controls. Representative images of rat NAc labeled with phospho-S6 in red and NeuN in green. Bar scale 5×, 500µm; 20×, 80µm. Phospho-S6 labeled cells are expressed as percentage of water controls. Phospho-S6 in NAc core p>0.05; phospho-S6 in NAc shell p<0.001. n=7 per group. *p<0.05; ***p<0.001.
Next, we tested whether the alcohol-dependent activation of AKT and mTORC1 in the rat NAc shell is long-lasting, and is maintained after a period of 24-hr of withdrawal. We found that the phosphorylation levels of both AKT and mTORC1 were still elevated in the NAc shell of rats 24-hr after the last drinking session (Fig 3a, pAKT t(11)=4.02, p=0.002; pS6 t(11)=2.38, p=0.036).
Next, we examined whether the activation of the pathway in the NAc shell is determined by the concentration of alcohol consumed and/or by the regimen of intake. To address this question, we used a paradigm of moderate consumption of alcohol in which rats had continuous access to a solution of 10% alcohol concurrently with a bottle of water for 21 drinking sessions (i.e. 21 days), matching the number of drinking sessions of the IA20%-2BC (Fig. 3b). In contrast to IA20%-2BC, rats with CA10%-2BC do not display escalation of alcohol intake across sessions. Instead, the level of consumption is constant and relatively low (1.2±0.2 g/kg/24 hrs). As shown in Fig. 3b, CA10%-2BC did not alter the activation state of AKT and S6 in the NAc shell (pAKT t(6)=0.62 p=0.558; pS6 t(6)=0.69 p=0.511), suggesting that a chronic intermittent drinking of high levels of alcohol (i.e. a model of excessive drinking) is necessary for the activation of the AKT/mTORC1 pathway in the NAc shell of rats.
We further examined whether the activation of the AKT/mTORC1 pathway in the rat NAc shell is specific for alcohol or is shared with other rewarding substances. To examine this possibility, we measured the phosphorylation levels of AKT and S6 in the NAc shell of rats consuming the natural and rewarding substance, sucrose. We used an IA-2BC paradigm to mimic the alcohol drinking regimen and a concentration of sucrose (1%) that is highly rewarding as the rats drink a considerably high amount of the solution in 30 min (10.6±1.7 ml/kg/30 min) (Fig. 3c). We found that 7–8 weeks of access to sucrose did not alter the phosphorylation levels of AKT and S6 in the NAc shell (Fig. 3c, pAKT t(7)=0.16 p=0.876; pS6 t(7)=0.27 p=0.793), suggesting that the AKT/mTORC1 pathway in the NAc shell is activated specifically in response to excessive alcohol intake.
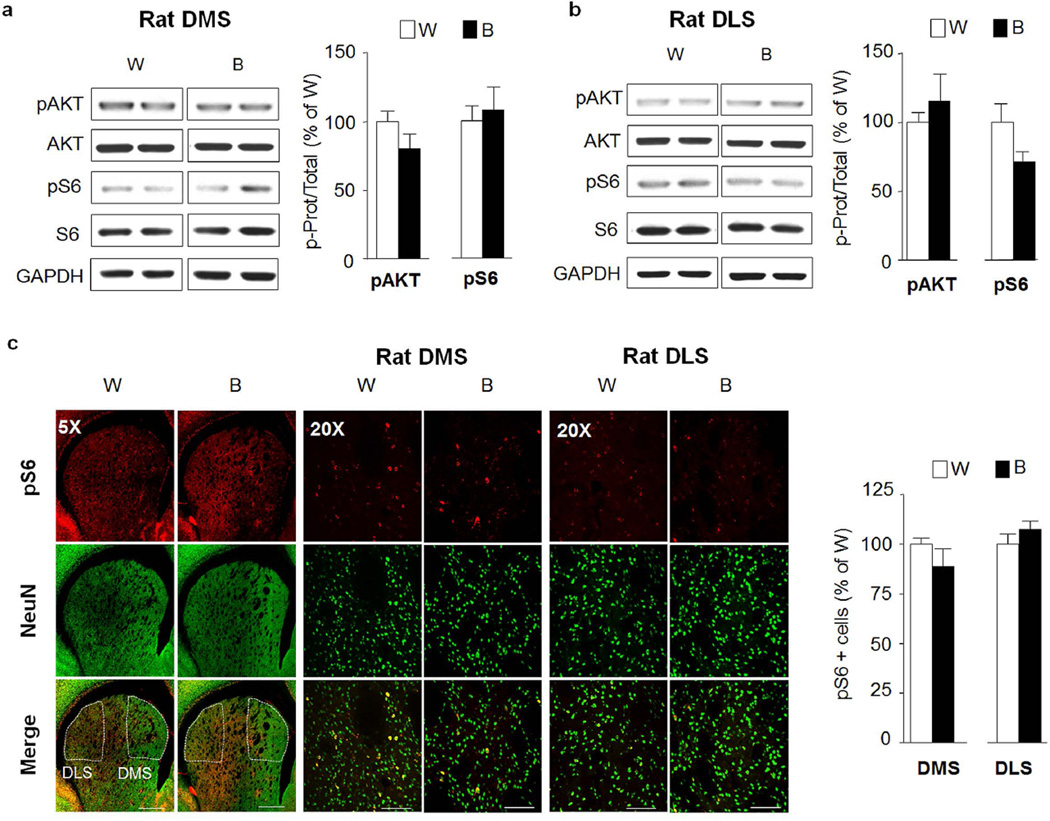
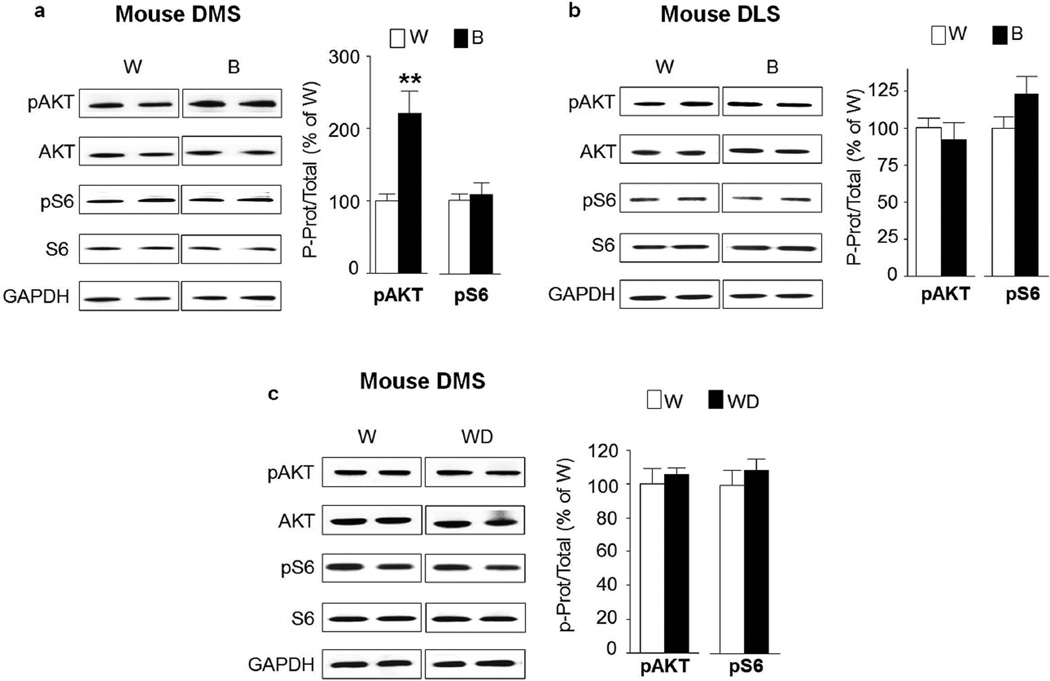
The dorsal striatum plays a critical role in the development of alcohol addiction (Everitt and Robbins, 2013). The dorsomedial striatum (DMS) contributes to the expression of goal-directed seeking of alcohol, whereas the dorsolateral striatum (DLS) is crucial for the development and expression of habitual alcohol seeking (Corbit et al., 2012). We therefore tested whether the AKT/mTORC1 pathway is activated in the dorsostriatal subregions in response to repeated cycles of excessive binge drinking and withdrawal. As shown in Figure 4, no change in AKT or S6 phosphorylation was observed in the DMS (Fig. 4a, pAKT t(12)=1.52 p=0.154 ; pS6 t(12)=0.39 p=0.699) or the DLS (Fig. 4b, pAKT t(12)=0.74 p=0.472; pS6 t(12)=1.94 p=0.077) of rats after binge drinking of alcohol compared to water only drinking controls. These biochemical data were confirmed using IHC as we observed no change in the immunoreactivity of pS6 in binge-drinking rats (Fig. 4c, DMS pS6 t(8)=1.16 p=0.279; DLS pS6 t(12)=1.20 p=0.252). Surprisingly however, the phosphorylation levels of AKT (pAKT t(20)=3.76 p=0.0012) but not S6 (pS6 t(20)=0.41 p=0.688) were increased in the DMS of mice following a binge drinking session (Fig. 5a), although this change was not observed in the DLS (Fig. 5b, pAKT t(20)=0.65 p=0.526; pS6 t(20)=1.64 p=0.116). Alcohol-mediated increase in the activation of AKT in the DMS of mice was short-lasting and was not detected after a 24-hr of withdrawal (Fig. 5c, pAKT t(6)=0.5426 p=0.607; pS6 t(6)=0.7899 p=0.459).
Figure 4. Excessive alcohol intake does not activate AKT/mTORC1 signaling in the rat dorsal striatum.
After 7–8 weeks of IA20%-2BC, the DMS and DLS of rats were removed 30 min after the beginning of the last drinking session (Binge, B, see Fig. 1). (a,b) Phosphorylation of AKT and S6 was determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the average ratio +/− S.E.M of phospho-AKT to AKT and phospho-S6 to S6 and are expressed as percentage of water (W) controls. Significance was determined using two-tailed unpaired t-test. (a) Phosphorylation level of AKT and S6 in the DMS of binge drinking rats (B) pAKT p>0.05; pS6 p>0.05. n=7 per group (b) Phosphorylation level of AKT and S6 in the DLS of binge drinking rats (B) pAKT p>0.05; pS6 p>0.05. n=7 per group. (c) IHC analysis of phospho-S6 levels in the DMS and DLS of alcohol binge-drinking rats (B) compared to water (W) controls. Representative images of rat dorsal striatum labeled with phospho-S6 in red and NeuN in green. Bar scale 5×, 500µm; 20×, 80µm. Phospho-S6 labeled cells are expressed as percentage of the controls. Phospho-S6 in DMS p>0.05; phospho-S6 in DLS p>0.05. n=5 per group for DMS, n=7 per group for DLS.
Figure 5. Excessive alcohol intake activates AKT in the mouse dorsomedial but not in the lateral striatum and mTORC1 signaling is not activated in either striatal regions.
After 7–8 weeks of IA20%-2BC, the DMS and DLS of mice were removed 4 hrs after the beginning of the last drinking session (Binge, B, see Fig. 1). Phosphorylation of AKT and S6 was determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the average ratio +/− S.E.M of phospho-AKT to AKT and phospho-S6 to S6 and are expressed as percentage of water (W) controls. Significance was determined using two-tailed unpaired t-test. (a) Phosphorylation level of AKT and S6 in the DMS of alcohol binge drinking mice (B) pAKT p<0.01; pS6 p>0.05. n=11 per group. (b) Phosphorylation level of AKT and S6 in the DLS of binge drinking mice (B) pAKT p>0.05; pS6 p>0.05. n=11 per group. (c) Phosphorylation level of AKT and S6 in the DMS of mice after a 24-hr period of withdrawal (WD) pAKT p>0.05; pS6 p>0.05. n=4 per group.**p<0.01
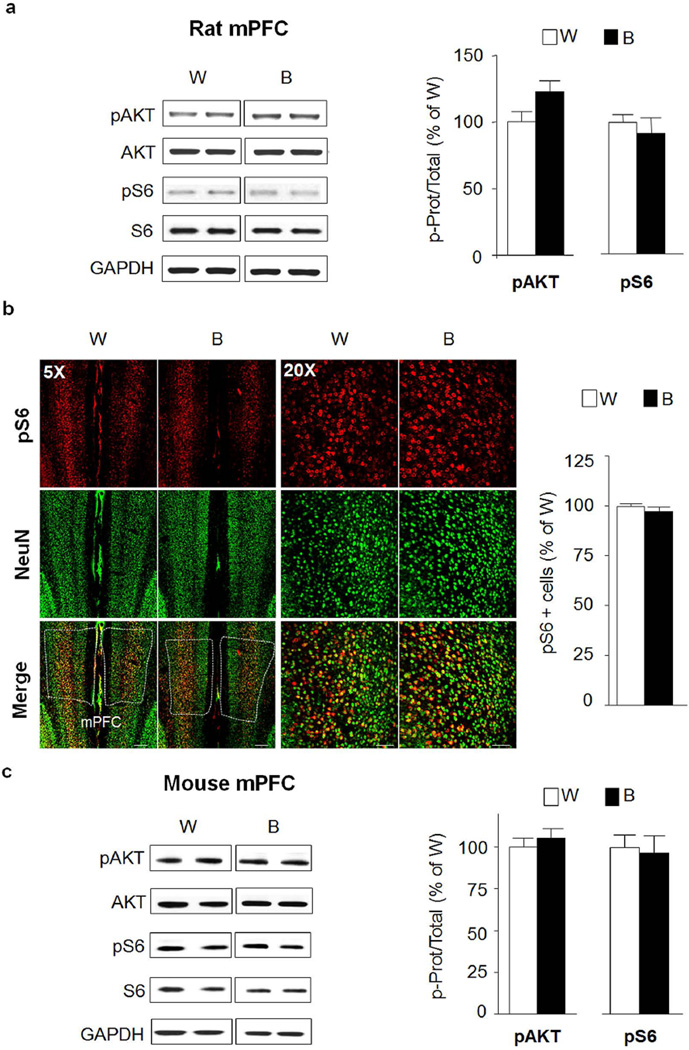
Next, we tested the levels of AKT/mTORC1 activation in cortical regions that play a role in mechanisms associated with alcohol drinking phenotypes. First, we surveyed the phosphorylation levels of AKT and S6 in the medial prefrontal cortex (mPFC) which plays a central role in executive functions that are thought to be disrupted by substance of abuse, including alcohol (Everitt and Robbins, 2016). As shown in Fig. 6, AKT an mTORC1 were not activated in the mPFC of rats (Fig. 6a, pAKT t(12)=1.99 p=0.07; pS6 t(12)=0.62 p=0.546; Fig. 6b, pS6 t(12)=1.00 p=0.335) or mice (Fig. 6c, pAKT t(20)=0.72 p=0.48; pS6 t(20)=0.24 p=0.814) in response to IA20%-2BC. We also observed no change in the activation state of this signaling pathway in the cingulate cortex (data not shown).
Figure 6. Excessive alcohol intake does not activate AKT/mTORC1 signaling in the rat medial prefrontal cortex.
(a,b) After 7–8 weeks of IA20%-2BC, the mPFC of rats was removed 30 min after the beginning of the last drinking session (Binge, B, see Fig. 1). (a) Phosphorylation of AKT and S6 was determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the average ratio +/− S.E.M of phospho-AKT to AKT and phospho-S6 to S6 and are expressed as percentage of water (W) controls. Significance was determined using two-tailed unpaired t-test. Phosphorylation level of AKT and S6 in the mPFC of alcohol binge drinking rats (B) pAKT p>0.05; pS6 p>0.05. n= 7 per group (b) IHC analysis of phospho-S6 levels in the mPFC of alcohol binge-drinking rats (B) compared to water (W) controls. Representative images of rat mPFC labeled with phospho-S6 in red and NeuN in green. Bar scale 5×, 500µm; 20×, 80µm. Phospho-S6 labeled cells are expressed as percentage of the control. Phospho-S6 in mPFC p>0.05. n=7 per group. (c) After 7–8 weeks of IA20%-2BC, the mPFC of mice was removed 4 hrs after the beginning of the last drinking session (Binge, B, see Fig. 1). Phosphorylation level of AKT and S6 in the mPFC of alcohol binge drinking mice (B) pAKT p>0.05; pS6 p>0.05. n=12 for alcohol group, n=10 for water group.
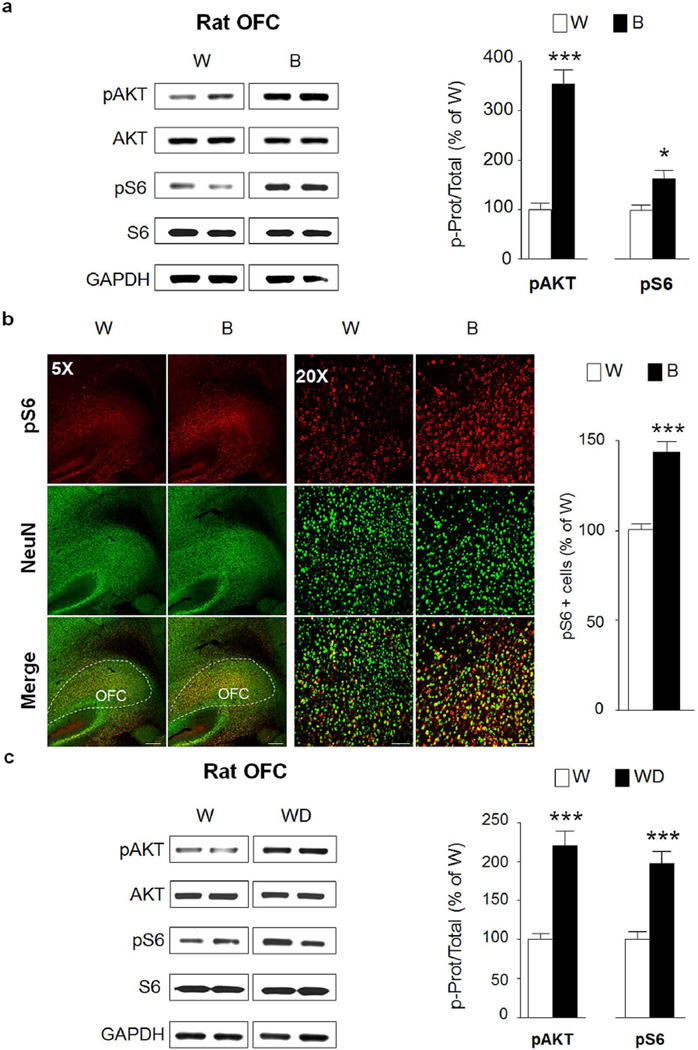
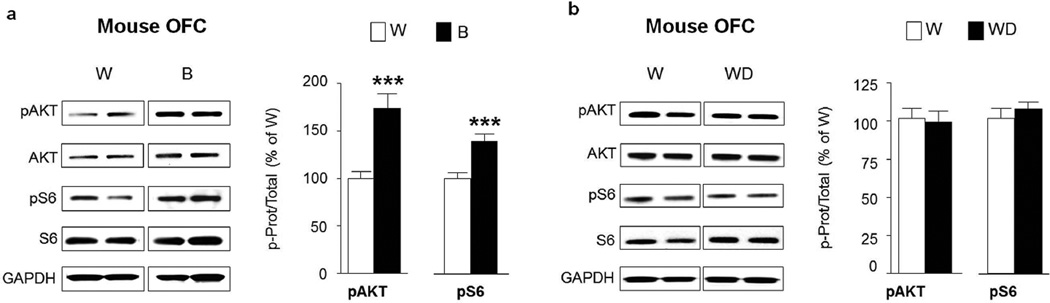
Finally, we examined whether AKT and/or mTORC1 are activated in the orbitofrontal cortex (OFC), an important region for adaptive behavior and flexible decision-making (Schoenbaum et al., 2009). Interestingly, both AKT and S6 were phosphorylated in response to a binge alcohol drinking in rats consuming alcohol in a IA20%-2BC (Fig. 7a, pAKT t(12)=8.12 p<0.001; pS6 t(12)=2.96 p=0.012) suggesting that the pathway is robustly activated in the OFC in response to repeated cycles of binge drinking and withdrawal. The activation of the pathway was confirmed using IHC, indicating that mTORC1 activation is localized at the boundary between the lateral and ventral OFC subregions (Fig. 7b, pS6 t(12)=6.41 p<0.001). Finally, similar to the pattern of activation observed in the NAc shell, AKT and mTORC1 were still activated in the OFC of rats that underwent a 24-hr withdrawal period after the last 24-hr drinking session (Fig. 7c, pAKT t(12)=6.084 p<0.001; pS6 t(12)=5.212 p=0.0002). Furthermore, AKT and mTORC1 were also activated in the OFC of mice in response to a binge drinking session (Fig. 8a; pAKT t(18)=4.39 p=0.0004; pS6 t(18)=4.00 p=0.0008), although the activation of the pathway was not maintained after a 24-hr of withdrawal (Fig. 8b, pAKT t(14)=0.27 p=0.792; pS6 t(14)=0.83 p=0.42).
Figure 7. Excessive alcohol intake activates AKT/mTORC1 signaling in the rat orbitofrontal cortex.
After 7–8 weeks IA20%-2BC, the OFC of rats were removed 30 min after the beginning of the last drinking session (Binge, B, see Fig. 1) (a,b) or 24 hrs after the end of the last drinking session (Withdrawal, WD) (c). Phosphorylation of AKT and S6 was determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the average ratio +/− S.E.M of phospho-AKT to AKT and phospho-S6 to S6 and are expressed as percentage of water (W) controls. Significance was determined using two-tailed unpaired t-test. (a) Phosphorylation level of AKT and S6 in the OFC of alcohol binge drinking rats (B) pAKT p<0.001; pS6 p<0.05. n=7 per group (b) IHC analysis of phospho-S6 levels in the OFC of alcohol binge-drinking rats (B) compared to water (W) controls. Representative images of rat OFC labeled with phospho-S6 in red and NeuN in green. Bar scale 5×, 500µm; 20×, 80µm. Phospho-S6 labeled cells are expressed as percentage of the control. Phospho-S6 in OFC p<0.001. n=7 per group. (c) Phosphorylation level of AKT and S6 in the OFC of rats after 24 hrs of withdrawal (WD) pAKT p<0.001; pS6 p<0.001. n=7 per group. *p<0.05; ***p<0.001.
Figure 8. Excessive alcohol intake activates AKT/mTORC1 signaling in the mouse orbitofrontal cortex.
After 7–8 weeks of IA20%-2BC, the OFC of mice were removed 4 hrs after the beginning of the last drinking session (Binge, B, see Fig. 1) (a) or 24 hrs after the end of the last drinking session (Withdrawal, WD) (b). Phosphorylation of AKT and S6 was determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the average ratio +/− S.E.M of phospho-AKT to AKT and phospho-S6 to S6 and are expressed as percentage of water (W) controls. Significance was determined using two-tailed unpaired t-test. (a) Phosphorylation level of AKT and S6 in the OFC of alcohol binge drinking mice (B) pAKT p<0.001; pS6 p<0.001 n=10 per group. (b) Phosphorylation level of AKT and S6 in the OFC of mice after 24 hrs of withdrawal (WD) pAKT p>0.05; pS6 p>0.05. n=8 per group.***p<0.001.
To determine the specificity of alcohol-mediated activation of the AKT/mTORC1 pathway in the OFC, rats underwent CA10%2BC (Fig. 3b) or 1% sucrose IA1%-2BC (Fig. 3c), and the phosphorylation levels of AKT and S6 were measured. We found that neither 10% alcohol nor 1% sucrose intake alter the activation of AKT and mTORC1 in the OFC (Fig. 9a; pAKT t(6)=0.58 p=0.585; pS6 t(6)=0.76 p=0.479 and Fig. 9b; pAKT t(7)=1.00 p=0.348; pS6 t(7)=0.78 p=0.459). Together these data suggest that the AKT/ mTORC1 pathway is activated in the OFC of both rats and mice when exposed to repeated cycles of excessive alcohol drinking and withdrawal but not after moderate consumption of alcohol or intake of natural reward.
Figure 9. Moderate consumption of alcohol as well as sucrose consumption do not activate AKT/mTORC1 signaling in the orbitofrontal cortex.
(a) Phosphorylation of AKT and S6 in the OFC of rats after 3 weeks of CA10%-2BC was determined by western blot analysis. Phosphorylation of AKT and S6 was determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the average ratio +/− S.E.M of phospho-AKT to AKT and phospho-S6 to S6 and are expressed as percentage of water (W) controls. Significance was determined using two-tailed unpaired t-test. pAKT p>0.05; pS6 p>0.05. n=4 per group. (b) Phosphorylation of AKT and S6 in the OFC after 7–8 weeks of IA1%-2BC of sucrose was determined by western blot analysis. pAKT p>0.05; pS6 p>0.05. n=5 for sucrose group, n=4 for water group.
Discussion
Here we used physiologically relevant drinking paradigms in rodent and described a detailed biochemical and IHC analysis of alcohol-mediated activation of the AKT/mTORC1 signaling pathway in striatal and cortical regions of rodents. The major and rather surprising finding is that the activation of this signaling pathway by alcohol is very discrete and is localized to the shell of the NAc and the OFC. The results also imply that this pathway is activated only in response to an excessive alcohol-drinking regimen.
We found that repeated cycles of binge drinking and withdrawal activate AKT/mTORC1 signaling in the shell but not in the core of the NAc of rats. The NAc has been shown to control alcohol seeking and relapse with a complex dichotomy of function between the NAc shell and core depending on the cues and context associated with alcohol intake (Corbit et al., 2016). It is possible that alcohol-dependent activation of AKT/mTORC1 pathway co-opts the function of the NAc shell to preferentially promote action toward obtaining alcohol. Consistently with this hypothesis, we previously found that inhibition of AKT in the NAc “shore” (i.e. the boundary between the core and the shell) reduces alcohol intake in the IA20%-2BC, as well as in an operant self-administration of 20% alcohol paradigm (Neasta et al., 2011). Accordingly, intra-NAc infusion of rapamycin reduces alcohol intake in the IA20%-2BC model and alcohol responding during self-administration as well as extinction sessions in the operant paradigm, indicating that mTORC1 in the NAc promotes actions of taking and seeking alcohol (Neasta et al., 2014; Neasta et al., 2010). Given that excessive alcohol intake activates the AKT/mTORC1 pathway in the NAc shell but not core, the effect of local AKT and mTORC1 inhibition on alcohol-related behaviors is most likely attributed to the NAc shell.
A previous study by Cozzoli et al. found that PI3K, an upstream kinase of AKT, is activated in response to alcohol drinking in male mice (Cozzoli et al., 2009), data which are in line with our previous findings showing that inhibition of PI3K in the NAc reduces excessive alcohol drinking (Neasta et al., 2011). Furthermore, as described above, excessive alcohol intake activates mTORC1 in the NAc of male mice and rats in numerous studies including the data herein (Beckley et al., 2016; Liu et al., 2016; Neasta et al., 2010) and Fig. S1 (mice) and Fig. 1 (rats). Curiously Cozzoli et al. recently reported that binge drinking of alcohol reduces the phosphorylation levels of PI3K (and thus AKT activity), as well as the phosphorylation levels of the mTORC1 substrates S6K and 4E-BP in the NAc of male mice (Cozzoli et al., 2016). Thus, it is difficult to reconcile the opposite profile of activation of the same signaling pathway in the Cozzoli et al. 2016 study with Cozzoli et al. 2009 and our studies. A major difference between the studies is the alcohol drinking paradigms. We utilized the IA20%-2BC paradigm, a well-validated model of excessive drinking in which mice have unlimited access to water. In contrast, Cozzoli et al used a scheduled fluid access in which mice undergo fluid restriction prior to short alcohol access sessions (Cozzoli et al., 2016). It is important to note that fluid restriction is an environmental stressor promoting physiological stress response (i.e. elevated plasma corticosterone level), that can potentially explain the high levels of substrate phosphorylation in the water control group in Cozzoli’s study (Cozzoli et al., 2016), as compared to our studies ((Beckley et al., 2016; Liu et al., 2016; Neasta et al., 2010) and data herein). One of the consequences of mTORC1 activation is the translation of the scaffolding protein Homer, and we showed that mTORC1 activation in the NAc by alcohol increases the translation of Homer (Beckley et al., 2016; Liu et al., 2016; Neasta et al., 2010). These findings are in line with the study by Cozzoli and Szmulinski showing that the protein levels of Homer are increased in response to alcohol intake (Cozzoli et al., 2009). Thus, it is rather perplexing that the recent Cozzoli et al. reports a reduction in Homer mRNA levels in response to alcohol consumption without showing a consequent decrease in the level of the protein (Cozzoli et al., 2016). Finally, similar to our previous study (Neasta et al., 2010), Cozzoli (2016) reported that rapamycin reduces alcohol binge drinking in male mice (Cozzoli et al., 2016). Since rapamycin is a highly selective mTORC1 inhibitor, it is difficult to envision how an inhibitor can reduce a behavior without prior activation of the pathway.
Importantly, we observed that excessive consumption of alcohol also produces a robust activation of AKT as well as mTORC1 in the OFC. To our knowledge this is the first report of an activation of this signaling pathway in the OFC in response to a behavioral input. The OFC controls the shift between goal-directed and habitual responses (Gremel et al., 2016; Gremel and Costa, 2013), which is a hallmark of substance use disorders (Everitt and Robbins, 2016). In addition, alcohol-dependent patients perform poorly in neurocognitive tasks dependent on the OFC compared to healthy subjects suggesting that the disease is associated with OFC dysfunction (Beck et al., 2012). Consistent with human studies, chronic alcohol exposure using vapor chambers impairs OFC-dependent behavioral performance in rodents (Badanich et al., 2011). Alcohol vapor exposure also induces an increase in excitability, synaptic plasticity and spine density in lateral OFC neurons that may contribute to the OFC-dependent behavioral-deficits (McGuier et al., 2015). A recent study showed that inactivation of the lateral OFC by excitotoxic lesion or in response to the activation of inhibitory DREADD (designer receptor exclusively activated by designer drug) reduced alcohol drinking in mice previously exposed to vapor, revealing a role for the OFC in alcohol-related behaviors (Hartog et al., 2016). In addition, we previously found that a short period of reconsolidation of alcohol memories resulted in mTORC1 activation in the OFC (but not in the NAc) of rats, and that systemic administration of rapamycin following reactivation of alcohol memories disrupted reconsolidation leading to reduction in alcohol seeking on the following day (Barak et al., 2013). Thus, the normal function of mTORC1 in the OFC and the potential role of the AKT/mTORC1 signaling pathway in the OFC in alcohol-drinking behaviors are of great interest and currently under active investigation. The contribution of mTORC1 in the OFC to reconsolidation of alcohol-associated memories has yet to be confirmed by local manipulation, however, it is possible that mTORC1 in the OFC contributes to the strengthening of memories for alcohol, promoting seeking, craving and relapse.
The Nac is composed of D1 and D2 medium spiny neurons (MSNs) (Gerfen and Surmeier, 2011). We previously found that mTORC1 is activated only in D1 receptor-expressing MSNs (Beckley et al., 2016). The OFC is composed of glutamatergic and GABAergic neurons (Kolb and Gibb, 2015), and whether mTORC1 activation by alcohol is segregated into one type of neurons or is shared by both types of neurons is an open question that merits further investigation. Furthermore, as the OFC and the NAc are different in their cellular composition, another open question is whether the mechanism(s) leading to mTORC1 activation in the two brain region is similar or different.
It is intriguing that the AKT/mTORC1 pathway is activated by alcohol in such discrete brain regions, and one possibility is that specific yet unidentified neurotransmitter, neuropeptide or growth factors are mediating activation of the pathway only in these two brain regions. We recently generated data showing that the activation of mTORC1 in the NAc after the first drink of alcohol is mediated by the activation of dopamine D1 receptor and restricted to D1-expressing MSNs of the shell (Beckley et al., 2016). Heavy alcohol intake is thought to be due in part to dysregulation of the mesolimbic reward circuitry and to a hypodopaminergic state during withdrawal, an observation which was confirmed in animal studies (Barak et al., 2011). Thus, it is plausible that neurotransmitter other than dopamine contribute to the activation of the AKT/mTORC1 in the NAc shell in response to prolonged excessive drinking of alcohol. Moreover, we found that AKT and mTORC1 are activated during a withdrawal period. As increased glutamatergic excitability is observed during withdrawal periods (Wang et al., 2010), thus it is plausible that glutamatergic signaling contributes to the activation of this pathway in the NAc shell and/or OFC. Interestingly, Hirth et al recently demonstrated a hyperdopaminergic state during protracted abstinence, showing that opposite dynamical changes take place in the mesolimbic dopaminergic system in alcohol acute withdrawal and protracted abstinence (Hirth et al., 2016). It would be of interest to test the activation of AKT/mTORC1 signaling pathway after a longer period of abstinence. Furthermore, one of the main upstream kinases activated by alcohol is protein kinase A (PKA) (Ron and Barak, 2016) and we previously provided findings that link the AKT/mTORC1 to PKA signaling in the NAc (Beckley et al., 2016; Ben Hamida et al., 2012), and thus it is plausible that the recruitment of PKA signaling in the NAc shell and OFC in response to repeated cycles of binge alcohol intake initiates the activation of the AKT/mTORC1 pathway.
Interestingly, we observed an increased AKT phosphorylation in the DMS of mice but not rats after binge drinking of alcohol. These molecular differences may be due to the distinct pattern of alcohol consumption in the IA20%-2BC paradigm between species, with rats displaying a noticeable escalation in alcohol consumption and preference across drinking sessions, and mice showing a rapid onset of excessive consumption and preference. As a result of the different profile of escalation, mice consume a high level of alcohol for 21–24 sessions, whereas rats consume excessive amounts of alcohol for only 10 drinking sessions (starting when the alcohol intake curve reaches a maximal plateau). An increased phosphorylation of AKT but not S6 in the DMS suggests that AKT-dependent signaling pathways other than mTORC1 are activated by binge drinking of alcohol. For examples, AKT phosphorylates and inactivates GSK3β (Manning and Cantley, 2007), and we recently showed that AKT-mediated inactivation of GSK3β in the NAc participates in mechanisms underlying excessive alcohol drinking (Liu et al., 2016) and Cheng et al. recently reported that excessive alcohol drinking increases GSK3β phosphorylation in the DMS of mice (Cheng et al., 2016), raising the possibility that GSK3β phosphorylation by AKT may be a transducer of alcohol’s actions in the DMS. Another interesting downstream target of AKT signaling is the forkhead transcription factor FOXO3a, which has been shown to regulate cocaine-elicited behavioral responses in the NAc (Ferguson et al., 2015). Moreover, AKT regulates the activity of the transcription factor NFkB by inducing the phosphorylation and subsequent degradation of inhibitor of kB (IkB) and allowing NFkB to be transported into the nucleus to regulates gene transcription (Bai et al., 2009). AKT is also able to phosphorylate and activate cAMP response element binding protein (CREB) (Lv et al., 2013), which is an important transcription factor in the brain’s reward pathways after exposure to drugs of abuse (Robison and Nestler, 2011). Thus, it is plausible that a rapid onset of alcohol consumption triggers the transcriptional machinery downstream of AKT in the DMS.
Another difference in the profile of activation of AKT/mTORC1 between rats and mice is observed in the OFC, in which the activation of the kinases is sustained and detected after a 24-hr withdrawal period in rats but not in mice. It is plausible that the short duration of AKT/mTORC1 activation in the OFC of mice is due to the fact that phosphatases that dephosphorylate AKT (Qiao et al., 2008) are more rapidly activated in the OFC of mice compared to rats, and/or that the Phosphatase and Tensin homolog (PTEN), which directly antagonizes the activity of PI3K (Worby and Dixon, 2014) is activated more rapidly in the OFC of mice than rats.
A growing number of studies in recent years have started to elucidate specific neuroadaptations that underlie the development and maintenance of excessive alcohol seeking and drinking in brain region specific manner (Ron and Barak, 2016), our findings that the AKT/mTORC1 pathway is activated by alcohol in very restricted brain regions paves the way for future studies on the role of the pathway in these corticostriatal brain regions.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Alcohol Abuse and Alcoholism, P50AA017072 (D.R.).
References
- Badanich KA, Becker HC, Woodward JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci. 2011;125:879–891. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer. 2009;125:2863–2870. doi: 10.1002/ijc.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Carnicella S, Yowell QV, Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 2011;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, Janak PH, Ron D. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci. 2013;16:1111–1117. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Beckley JT, Laguesse S, Phamluong K, Morisot N, Wegner SA, Ron D. The First Alcohol Drink Triggers mTORC1-Dependent Synaptic Plasticity in Nucleus Accumbens Dopamine D1 Receptor Neurons. J Neurosci. 2016;36:701–713. doi: 10.1523/JNEUROSCI.2254-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamida S, Neasta J, Lasek AW, Kharazia V, Zou M, Carnicella S, Janak PH, Ron D. The small G protein H-Ras in the mesolimbic system is a molecular gateway to alcohol-seeking and excessive drinking behaviors. J Neurosci. 2012;32:15849–15858. doi: 10.1523/JNEUROSCI.2846-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Huang W, Costa-Mattioli M. Translational Control in Synaptic Plasticity and Cognitive Dysfunction. Annu Rev Neurosci. 2014;37:17–38. doi: 10.1146/annurev-neuro-071013-014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Huang CC, Ma T, Wei X, Wang X, Lu J, Wang J. Distinct Synaptic Strengthening of the Striatal Direct and Indirect Pathways Drives Alcohol Consumption. Biological psychiatry. 2016 doi: 10.1016/j.biopsych.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Fischbach SC, Janak PH. Nucleus accumbens core and shell are differentially involved in general and outcome-specific forms of Pavlovian-instrumental transfer with alcohol and sucrose rewards. Eur J Neurosci. 2016;43:1229–1236. doi: 10.1111/ejn.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Kaufman MN, Nipper MA, Hashimoto JG, Wiren KM, Finn DA. Functional regulation of PI3K-associated signaling in the accumbens by binge alcohol drinking in male but not female mice. Neuropharmacology. 2016;105:164–174. doi: 10.1016/j.neuropharm.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25:545–555. doi: 10.1016/j.tcb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Ferguson D, Shao N, Heller E, Feng J, Neve R, Kim HD, Call T, Magazu S, Shen L, Nestler EJ. SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J Neurosci. 2015;35:3100–3111. doi: 10.1523/JNEUROSCI.4012-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb SL, Hamida SB, Lanfranco MF, Ron D. Ethanol-induced increase in Fyn kinase activity in the dorsomedial striatum is associated with subcellular redistribution of protein tyrosine phosphatase alpha. J Neurochem. 2011;119:879–889. doi: 10.1111/j.1471-4159.2011.07485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, Costa RM. Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron. 2016 doi: 10.1016/j.neuron.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog CD, Zamudio-Bulcock P, Nimitvilai S, Gilstrap M, Fedarovich H, Motts A, Woodward JJ. Inactivation of the lateral orbitofrontal cortex increases drinking in ethanol-dependent but not non-dependent mice. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Broccoli L, Vengeliene V, Rossmanith M, Perreau-Lenz S, Kohr G, Sommer WH, Spanagel R, Hansson AC. Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:3024–3029. doi: 10.1073/pnas.1506012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Plasticity in the prefrontal cortex of adult rats. Front Cell Neurosci. 2015;9:15. doi: 10.3389/fncel.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bassell GJ, Gitler AD, Hart AC, Klann E, Richter JD, Warren ST, Wolozin B. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–16093. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Laguesse S, Legastelois R, Morisot N, Ben Hamida S, Ron D. mTORC1-dependent translation of collapsin response mediator protein-2 drives neuroadaptations underlying excessive alcohol-drinking behaviors. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv T, Wang SD, Bai J. Thioredoxin-1 was required for CREB activity by methamphetamine in rat pheochromocytoma cells. Cell Mol Neurobiol. 2013;33:319–325. doi: 10.1007/s10571-012-9897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuier NS, Padula AE, Lopez MF, Woodward JJ, Mulholland PJ. Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol. 2015;49:21–27. doi: 10.1016/j.alcohol.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Barak S, Hamida SB, Ron D. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem. 2014;130:172–184. doi: 10.1111/jnc.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell QV, Carnicella S, Ron D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry. 2011;70:575–582. doi: 10.1016/j.biopsych.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M, Sheng S, Pardee AB. Metastasis and AKT activation. Cell Cycle. 2008;7:2991–2996. doi: 10.4161/cc.7.19.6784. [DOI] [PubMed] [Google Scholar]
- Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci. 2009;66:2975–2984. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci. 2016;17:576–591. doi: 10.1038/nrn.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L, Wang J, Li BM. Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn Mem. 2008;15:762–776. doi: 10.1101/lm.1067808. [DOI] [PubMed] [Google Scholar]
- Takei N, Nawa H. mTOR signaling and its roles in normal and abnormal brain development. Front Mol Neurosci. 2014;7:28. doi: 10.3389/fnmol.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai V, Parker WE, Orlova KA, Baybis M, Chi AW, Berg BD, Birnbaum JF, Estevez J, Okochi K, Sarnat HB, Flores-Sarnat L, Aronica E, Crino PB. Fetal brain mTOR signaling activation in tuberous sclerosis complex. Cereb Cortex. 2014;24:315–327. doi: 10.1093/cercor/bhs310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine A, Barak S, Ron D. Chromatin remodeling--a novel strategy to control excessive alcohol drinking. Transl Psychiatry. 2013;3:e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. Pten. Annu Rev Biochem. 2014;83:641–669. doi: 10.1146/annurev-biochem-082411-113907. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.