Introduction
Interleukin-17 (IL-17) is a multifaceted cytokine with diverse roles both in immune-protection and also immunopathology. IL-17 has a well-recognized role in immune surveillance at mucosal and barrier surfaces (Miossec & Kolls, 2012, Song et al., 2016) but also has been increasingly implicated as a driver of immunopathology in settings of autoimmunity and chronic inflammation (Gaffen et al., 2014). The current review introduces basic aspects of IL-17 biology and examines the protective and pathogenic roles of IL-17 with a focus on oral mucosal immunity and inflammation. Specific emphasis is given to the role of the IL-17 response as a catalyst in “shaping the microbiome at the oral barrier”.
IL-17 cytokine and its signaling
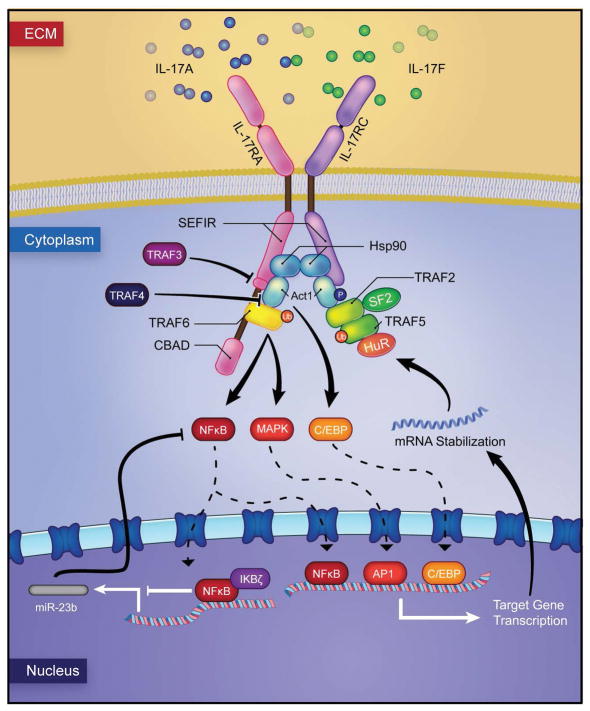
IL-17 is formally called ‘IL-17A’, but is usually referred to as IL-17 since it was the first described member of the IL-17 family (Rouvier et al., 1993). To date, the IL-17 family includes six members (from IL-17A to IL-17F) that share sequence homology (Patel & Kuchroo, 2015). IL-17A and IL-17F exhibit high sequence similarity and can form homodimers and heterodimers to signal (Miossec & Kolls, 2012). In fact, IL-17A and IL-17F signal through the same receptor complex (known as ‘IL-17R’, an heterodimer of IL-17RA and IL-17RC subunits) (Toy et al., 2006) and largely share biological functions (Miossec & Kolls, 2012). The IL-17 receptor family has additional members namely IL-17 RB, IL-17RC, IL-17RD and IL-17RE. All IL-17 receptor subunits are structurally similar, consisting of an outer membrane fibronectin III-like domain, a conserved cytoplasmic SEF/IL-17R (SEFIR) domain and a distal activation domain (CBAD) (Gaffen et al., 2014). The SEFIR domain exhibits sequence homology with the Toll/IL-1R domain (Novatchkova et al., 2003), suggesting commonalities between IL-17 and TLR signaling cascades. Engagement of IL-17 (IL-17A, IL-17F and IL-17A/F) to the IL-17R heterodimeric complex, leads to the association of the adaptor protein Act1 (previously named CIKS) through the SEFIR domain (Qian et al., 2007). Act1 recruits the TNFR-associated factor (TRAF) 6 that is poly-ubiquitinated by Act1 via its E3 ligase activity (Liu et al., 2009), triggering the activation of the canonical nuclear factor κB (NF-κB) pathway and some components of the mitogen-activated protein kinase (MAPK) pathways, namely JUN N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 (Sonder et al., 2011, Patel et al., 2007). Additionally, IL-17 signaling can activate members of the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors that are critical for the expression of certain target genes (Patel et al., 2007). mRNA stabilization events are also involved in the regulation of the IL-17 signaling pathway. Important for mRNA stabilization is the formation of the TRAF2/TRAF5 complex and recruitment of HuR and ASF/SF2, which stabilize mRNA transcript targets (Datta et al., 2010, Sun et al., 2011, Herjan et al., 2013) (Figure 1). Moreover, there are other regulators of IL-17 signaling pathway that act at different levels. The adaptor protein TRAF3 has the ability to inhibit the association of the IL-17R with Act1 and TRAF4 interferes with the Act1 and TRAF6 interaction, inhibiting downstream signal transduction (Zhu et al., 2010, Zepp et al., 2012). Act1 is stabilized by the chaperone Hsp90 that prevents its targeting for proteasomal degradation by the negative regulator βTrCP E3 ligase complex (Wang et al., 2013, Wu et al., 2014). Deubiquitinating enzymes (DUB) are also important negative regulators of this pathway. The DUB A20 removes ubiquitination residues on TRAF6 suppressing activation of NF-κB and MAPK signaling; likewise, the DUB USP25 negatively regulates IL-17 signaling by targeting both TRAF5 and TRAF6 (Song & Qian, 2013). MicroRNAs (miRNAs) have also been implicated as negative regulators of IL-17, miR-23b targets the kinases TAB2, TAB3 and IKKα, all of which are integral to the NF-κB pathway (Zhu et al., 2012). Additionally, the distal activator domain (CBAD) of the IL-17RA subunit participates on inhibitory events for IL-17 signaling, such as coordinating phosphorylation of C/EBPβ and associating with the DUB A20 (Garg et al., 2013). Lastly, it has been recently shown that the signaling molecule MCPIP1 (also known as Regnase-1), acts as a negative regulator of IL-17-induced genes, degrading mRNA of cytokines (Il6) and promoters needed for other gene targets (Garg et al., 2015).
Figure 1. The IL-17 signaling.
IL-17 (IL-17A, IL-17F, IL-17A/F) engages the heteromeric IL-17R complex and recruits the adaptor protein Act1. Act1 triggers the ubiquitination of TRAF6. TRAF6 activates three major pathways namely NF-κB, MAPK and C/EBP, triggering the transcription of IL-17 target genes. This signaling cascade is regulated at multiple steps. Hsp90 acts as a positive regulator of this cascade by stabilizing Act1. TRAF2 and TRAF5 are also positive regulators that form a complex with SF2 and recruit HuR, to mediate mRNA stabilization. TRAF3 inhibits the association of the IL-17R with Act1 and TRAF4 inhibits the recruitment of TRAF6 by Act1. miR-23b negatively regulates NF-κB activation.
In sum, the IL-17 signaling cascade is complex, involving multiple pathways and levels of regulation that continue to be interrogated expanding knowledge in the IL-17 field.
Cellular sources of IL-17
IL-17 is primarily secreted by a distinct CD4+ T cell subset, known as T helper 17 cells (Th17), named after their signature cytokine (Harrington et al., 2005). Th17 cells in different contexts can also produce other cytokines such as IL-17F, IL-21, IL-22 and granulocyte-macrophage colony-stimulating factor (GM-CSF), at varying expression levels (Liang et al., 2006, Korn et al., 2007, Codarri et al., 2011). Typically, the differentiation of naïve CD4+ T cells towards an effector subset necessitates antigen engagement through the T cell receptor (TCR) and depends on the surrounding cytokine milieu. Differentiation of murine Th17 cells has been shown to be dependent on transforming growth factor β (TGF-β) and IL-6 (Veldhoen et al., 2006, Bettelli et al., 2006, Mangan et al., 2006), whereas human Th17 cells can be induced in the presence of TGF-β/IL-1β, IL-6 and IL-23 (Acosta-Rodriguez et al., 2007a, Manel et al., 2008, Volpe et al., 2008). However, human and murine Th17 cells have also been shown to be generated independent of TGF-β (Ghoreschi et al., 2010), suggesting that presence or absence of TGF-β during differentiation may determine distinct functional profiles of Th17 cells (Acosta-Rodriguez et al., 2007a, Volpe et al., 2008). IL-21 also has the ability to drive Th17 differentiation in conjunction with TGF-β (Korn et al., 2007), and is proposed as an amplification signal for differentiation since IL-21 production from Th17 cells leads to increased IL-23R expression. This is important because IL-23 is critical for stabilization and maintenance of the Th17 phenotype and IL-23R is not typically expressed on naïve T cells. (Bettelli et al., 2008) (Burkett et al., 2015).
A critical step in the differentiation of Th17 cells is the activation of the signal transducer and activator of transcription 3 (STAT3) (Yang et al., 2007). Upon binding of the Type I cytokines IL-6, IL-21 and IL-23 to their receptors, Janus kinases (Jaks) phosphorylate the receptors, leading to the recruitment and phosphorylation of STAT3, which dimerizes and then translocates to the nucleus to enhance the expression of target genes (O’Shea et al., 2009). Then, STAT3 induces the expression of the transcription factor orphan nuclear receptor ROR-γt, which is recognized as the Th17 lineage-specific master regulator (Ivanov et al., 2006). Transcription factors BAFT and IRF4 cooperate with STAT3 to initiate the Th17 differentiation program (Ciofani et al., 2012). High throughput transcriptional network analyses have revealed that Th17 cell development is tightly controlled by 22 genes that act as positive regulators and a module of 5 genes that are negative regulators of differentiation, illustrating the complexity of the Th17 cell developmental program (Yosef et al., 2013).
After differentiation, Th17 cells are able to produce type 17 cytokines and express the CC chemokine receptor 6 (CCR-6), which allows for their preferential migration into mucosal and barrier sites (Hirota et al., 2007, Acosta-Rodriguez et al., 2007b).
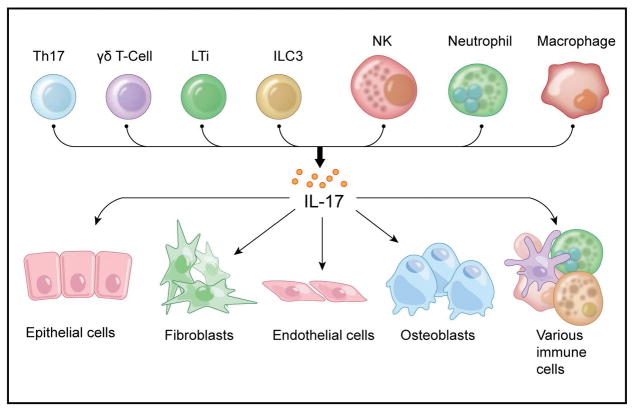
Apart from Th17 cells, IL-17 is produced by other cellular sources that include γδ T cells, lymphoid tissue inducer cells (LTi), innate lymphoid cells type 3 (ILC3s) and natural killer cells (NK) (Patel & Kuchroo, 2015, Artis & Spits, 2015) (Figure 2). Particular disease settings appear to provide favorable conditions for other immune cells to produce IL-17, such as neutrophils during fungal infections and breast cancer metastasis (Taylor et al., 2014, Coffelt et al., 2015) and alveolar macrophages during allergic lung inflammation related to asthma (Song et al., 2008). In addition, mast cells have also been involved as relevant IL-17 producers in psoriatic skin (Lin et al., 2011, Mashiko et al., 2015) and in rheumatoid arthritis affected joints (Hueber et al., 2010), however, a recent report suggests that human mast cells rather than making IL-17 have the ability to capture and store exogenous IL-17A using a receptor-mediated exocytosis mechanism (Noordenbos et al., 2016).
Figure 2. Cellular sources and targets of IL-17.
(Upper panel) Main cellular sources of IL-17 are Th17 cells and other immune cells such as γδ T cells, lymphoid tissue inducer cells (LTi), innate lymphoid cells type 3 (ILC3s) and natural killer cells (NK). During inflammation IL-17 can also be produced by neutrophils and macrophages. (Lower panel) Cellular targets of IL-17 are primarily non-hematopoietic cells, including keratinocytes, fibroblasts, endothelial and osteoblasts cells. Immune cells such as T, B and NK cells can also be IL-17 targets.
Cellular targets of IL-17
The IL-17 receptor complex has a ubiquitous expression and is present in a wide variety of tissues and cell types (Yao et al., 1995a, Haudenschild et al., 2002). The IL-17RA subunit of the receptor shows higher expression in cells of hematopoietic origin (Kuestner et al., 2007, Ishigame et al., 2009). In contrast, the IL-17RC subunit is primarily found in cells of non-hematopoietic lineage, such as mesenchymal, epithelial and endothelial cells, which constitute the main targets of IL-17 (Kuestner et al., 2007, Ge & You, 2008, Ishigame et al., 2009). Interestingly, macrophages seem to constitute an exception, bearing both IL-17RA and IL-17RC (Ishigame et al., 2009).
IL-17 signaling on epithelial cells is critical for physiologic regulation of mucosal immunity and barrier defenses (discussed in detail below). Additionally, in settings of inflammation IL-17 has been shown to exert its activity on a variety of cell types including keratinocytes, fibroblasts, osteoblasts, endothelial and immune cells (Figure 2). For instance, IL-17 stimulates the production of pro-inflammatory mediators such as IL-6, IL-8, Prostaglandin E2 (PGE2) and GM-CSF from epithelial, endothelial and fibroblastic cells (Yao et al., 1995b, Fossiez et al., 1996). In the context of rheumatoid arthritis (RA) IL-17 has been shown to mediate tissue pathology by acting on a variety of cell targets. IL-17 can induce the release of connective tissue destructive enzymes matrix metalloproteinase 1 (MMP-1) and MMP-3 (van Hamburg et al., 2011) from synovial fibroblasts. IL-17 is also shown to directly act on osteoblasts through different mechanisms. It can stimulate the release of PGE2 followed by osteoclast differentiation factor (ODF), which induces osteoclast maturation and further bone destruction in RA (Kotake et al., 1999). In addition, IL-17 can enhance RANKL expression on osteoblasts and activate RANK signaling on osteoclasts, promoting osteoclastogenesis (Miossec & Kolls, 2012). Lastly, macrophages are reported to increase their production of the pro-inflammatory cytokines IL-1β and TNF-α upon IL-17 stimulation, further amplifying inflammatory responses (Jovanovic et al., 1998, Mosser & Edwards, 2008).
In the context of fungal infection, it has been shown that endogenous and/or exogenous IL-17 can directly enhance reactive oxygen species (ROS) production in neutrophils (Taylor et al., 2014). However, this is controversial since it has been reported previously that neutrophils do not exhibit the IL-17RC on their surface (Pelletier et al., 2010). IL-17 is also shown to play a role in the development of NK cells, which during fungal infections increase GM-CSF expression in an IL-17RA dependent manner (Bar et al., 2014).
Interestingly, IL-17 signaling can also affect T and B cell differentiation and functions. IL-17 modulates T helper cell differentiation, by inhibiting the transcription factors T-bet and STAT-1 (O’Connor et al., 2009). On autoreactive B cells, IL-17 has been documented to modulate chemotaxis and positively impacts their survival and proliferation (Xie et al., 2010).
IL-17 as a key Mediator of Mucosal Surveillance and Barrier Integrity
IL-17 producing cells are considered by many the sentinels of mucosal barrier immunity (Cua & Tato, 2010). In fact, IL-17 exerts its function as a protective mediator in barrier immunity by multiple mechanisms. To date it is recognized that: 1) IL-17 has key roles in maintaining barrier integrity, 2) IL-17 promotes the production of antimicrobial factors which are key for the containment of pathogens and commensals at barrier sites, 3) innate cells producing IL-17 are a first line of defense strategically positioned at barrier sites to regulate the recruitment and generation of neutrophils (Figure 3).
Figure 3. IL-17 is critical for oral mucosal integrity and immunosurveillance.
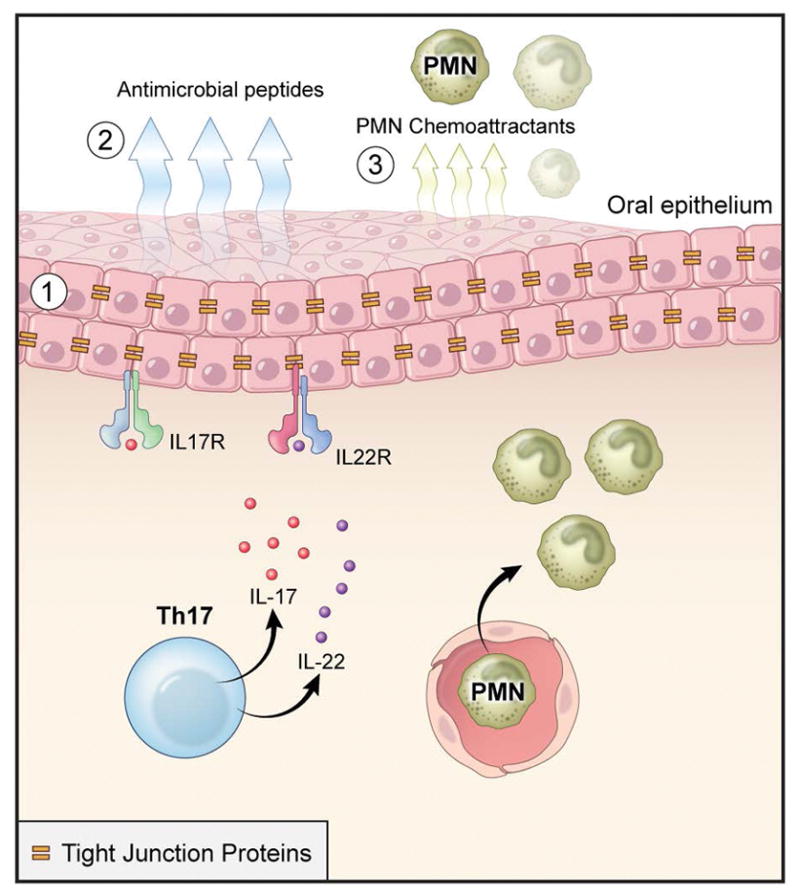
Th17 cells, largely through its effector cytokine IL-17 (but also through IL-22), have an important role in maintaining mucosal barrier integrity. Key functions in IL-17-mediated mucosal surveillance are (1) regulation of epithelial tight junction protein expression (2), induction of antimicrobial peptide production (3) and release of neutrophil chemo-attractants.
IL-17 promotes epithelial integrity by regulating tight junction proteins that connect and stabilize epithelial cell connections with the purpose of maintaining the barrier and keeping out gut luminal contents and commensal organisms. To date it’s been shown that IL-17 regulates the production of the tight junction protein claudin (Karp et al., 2010) and that IL-17A-dependent regulation of the tight junction protein occludin during epithelial injury is key in limiting excessive permeability and maintaining barrier integrity (Lee et al., 2015). Accordingly, IL-17A or IL-17RA inhibition has been associated with severe weakening of the intestinal epithelial barrier (Maxwell et al., 2015).
Another mechanism by which IL-17 contributes to mucosal immune surveillance is the induction of antimicrobial mediators. IL-17 alone and in coordination with IL-22 induces the production of β-defensins (HBD), regenerating (ReG) proteins, S100 proteins, cathelicidins, lipocalins and lactoferrins (Liang et al., 2006, Kolls et al., 2008, Peric et al., 2008). These microbicidal agents are predominantly produced by epithelial cells. IL-17 also promotes epithelial cell secretion of chemokines such as CC-chemokine ligand 20 (CCL-20) for recruitment of neutrophils when the mucosal barrier is breached.
In fact, neutrophil recruitment is a major IL-17 function. For this, innate IL-17-producing cells are strategically positioned at barrier sites to sense injury and infection and rapidly recruit neutrophils for initial containment of any insult. This early IL-17 production is required for optimal neutrophil recruitment and resistance to infection. It is important to note that innate cells IL-17 populations not only interact with pathogens during infection, but also are critical under physiologic conditions for the containment of commensal flora and the maintenance of mucosal homeostasis (Cua & Tato, 2010). IL-17 exerts its neutrophil stimulatory functions by inducing epithelial cell secretion of granulopoietic factors such as G-CSF, GM-CSF and chemokines such as CXCL-1, 2, 5, which promote neutrophil chemotaxis (Mantovani et al., 2011)
These immune-protective functions of IL-17 have been shown to be particularly important for the clearance of specific extracellular pathogens and fungi at barrier sites. Specifically, disruptions in IL-17 signaling or production have been linked to susceptibility to Staphylococcus aureus, Citrobacter rodentium and Klebsiella pneumoniae, which infect the skin, colon and lung, respectively (Ishigame et al., 2009, Cho et al., 2010, Aujla et al., 2008). IL-17 immunity undoubtedly has emerged as a critical component in mucosal fungal surveillance (Lionakis et al., 2014). In both humans and animal models disruptions in the IL-17 pathway have been linked to increased susceptibility to oral and mucocutaneous candida infections (CMC) (Cypowyj et al., 2012). CMC is a disease characterized by recurrent symptomatic infections of the nails, skin, genital and oral mucosa caused by members of the genus Candida, mostly due to the commensal Candida albicans (Puel et al., 2012). Candida albicans does not cause chronic infections in healthy subjects; nonetheless, in immunocompromised patients might trigger a variety of distinct disease forms, with systemic and/or mucosal involvement (Cypowyj et al., 2012).
Humans with disruptions in the development of Th17 cells do exhibit susceptibility to select bacterial and fungal infections. Specifically, patients with hyper-IgE syndrome have a defect in the differentiation of Th17 cells due to a mutation in the STAT3 gene (Milner et al., 2008) and are susceptible to skin S. aureus infections and recurrent pneumonias, most commonly due to S. aureus, but also Streptococcus pneumoniae and Haemophilus influenza infections. Lung infections with Aspergillus are also seen secondary to bronchiectasis. Mucocutaneous candidiasis is detected in the majority of patients (Freeman & Holland, 2009). Patients with other genetic disruptions in the IL-17 cascade or response all also present with mucocutaneous and oral candidiasis (see below).
IL-17 as a driver of Inflammation/Immunopathology/Autoimmunity
Evidence from human disease and disease models
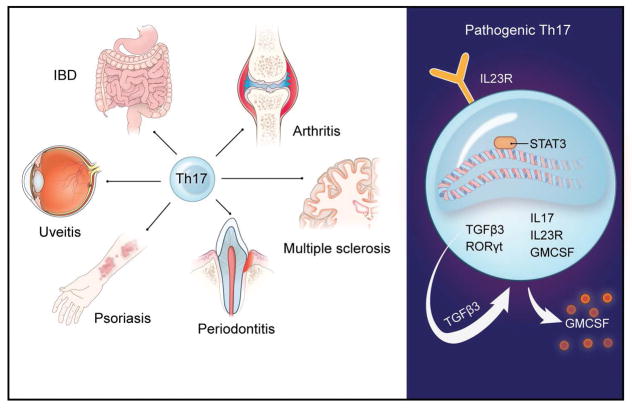
IL-17 secreting cells have been documented in inflammatory lesions of patients with a variety of human inflammatory and autoimmune diseases including psoriasis, inflammatory bowel disease, rheumatoid arthritis, type 1 diabetes, multiple sclerosis and periodontitis (Gaffen et al., 2014) (Zenobia & Hajishengallis, 2015) and speculated and/or shown to be involved in the pathogenesis of the respective diseases. The link between over-activated IL-17 related responses and various inflammatory and autoimmune diseases in humans is also supported by results from genome wide association studies (GWAS) studies revealing IL-23R polymorphisms being linked with susceptibility to autoimmune diseases such as psoriasis, psoriatic arthritis, ankylosing spondylitis, multiple sclerosis and Crohn’s disease (Duerr et al., 2006, Wellcome Trust Case Control et al., 2007, Liu et al., 2008) (Figure 4).
Figure 4. Th17 in inflammatory disease and their pathogenic Th17 signature.
(Left) Th17 cells have been implicated in the pathogenesis of various inflammatory and autoimmune diseases. (Right) Cellular and molecular signature of pathogenic Th17 cells.
Consistent with human observations, mice deficient in IL-17A/F and related cytokines and mediators (including IL-22-, IL-23A- or IL-23RA-deficient mice) or treated with antibodies against IL-17/23 have increased susceptibility to EAE, collagen antibody-induced arthritis, and CIA, and models of inflammatory bowel disease (IBD), ankylosing spondylitis and psoriasis (Murphy et al., 2003, Langrish et al., 2005, Hue et al., 2006, van der Fits et al., 2009, Sherlock et al., 2012). On the basis of these human observations and genetic linkage studies as well as preclinical models, IL-17-specific and IL-23-specific antibody treatments have emerged as candidate therapeutic targets for Crohn’s disease, psoriasis and psoriatic arthritis. Specifically, to date Secukinumab the human IL-17 antagonist has been FDA approved for the treatment of psoriatic arthritis, ankylosing spondylitis and plaque psoriasis (Langley et al., 2014). Ustekinumab inhibitor of the p40 subunit that is shared by IL-23 and IL-12 is currently FDA approved for the treatment of psoriasis and psoriatic arthritis and has shown efficacy in the treatment of Crohn’s disease (Sandborn et al., 2012).
Pathogenic Th17 cell subsets and cooperative IL-17 signaling in inflammation
IL-17’s reason for being (teleology) is however not to cause disease, it is a protective mechanism geared towards the establishment of mucosal immunity. The pathologic role of IL-17 in disease appears to be context dependent and related to the development of a subset of pathogenic IL-17 secreting cell subsets. In fact, it has been recently established that there is a pathogenic subset of Th17 cells that are increasingly capable of mediating inflammatory pathology (Figure 4). Pathogenic murine Th17 cells express a unique transcriptional signature compared to non-pathogenic Th17, which includes the elevated expression of the IL-23R (Lee et al., 2012). Importantly, IL-23R deficient cells cannot induce autoimmunity irrespective of how they are differentiated in vitro (Lee et al., 2012). Accumulating data support a central role for IL-23 in promoting the pathogenicity of Th17 cells by several mechanisms, including through the maintenance and stabilization of the Th17 signature gene expression program (Rorc and Il17), the induction of effector genes as well as upregulation of the Il23r expression (Gaffen et al., 2014). Amongst the currently appreciated roles of IL-23 in mediating Th17 pathogenicity is its ability to stimulate the production of endogenous TGF-β3, which will thereafter drive development of a pathogenic Th17 phenotype (Lee et al., 2012). It is also well recognized that GM-CSF, which is produced by pathogenic Th17 is dependent on IL-1 and IL-23 and is required for pathogenicity (Codarri et al., 2011, El-Behi et al., 2011). Additionally, exposure to IL-23 diminishes the concentration of the anti-inflammatory cytokine IL-10 in developing Th17 cells, which renders these cells pathogenic (McGeachy et al., 2007). Pathogenic signaling of IL-23 through the IL-23R is thought to be STAT3 mediated with STAT3 representing a major downstream mediator of IL-23R signaling in mice and humans and implicated in the pathogenicity of Th17 cells (Burkett et al., 2015). Consistent with a critical role for STAT3 in pathogenic Th17 responses, gain of function mutation in STAT3 in humans display early onset multi-organ autoimmunity (Flanagan et al., 2014, Milner et al., 2015). However, IL-23R/STAT3 activation alone cannot explain the unique requirement for IL-23 in pathogenic Th17 cell commitment, as IL-6 is an even more potent activator of STAT3. Recently IL-6 activation of STAT3 has been shown to contribute to the pathogenicity of Th17 cells via induction of the microRNA miR-183C, which is shown to inhibit Foxo1 (a negative regulator of Th17 pathogenicity)(Ichiyama et al., 2016). However, additional transcriptional regulators or signaling pathways may be operating to promote inflammatory Th17 cell effector function in different settings.
The ability of IL-17 to signal cooperatively with other cytokines is probably one of the most important aspects of its biology as it relates to disease. Importantly, on their own, IL-17A (and IL-17F) are modest activators of signaling, but they function cooperatively with other pro-inflammatory molecules, particularly TNF, but also IFNγ, IL-22, lymphotoxin, IL-1β and lipopolysaccharide (Gaffen, 2009). The molecular basis for this synergy is not completely understood and probably involves multiple mechanisms (Gaffen, 2009). In synovial tissue, IL-17 upregulates TNFR2 expression, and thereby enhances responsiveness to TNF (Zrioual et al., 2009). For some genes, cooperation between IL-17 and TNF occurs at the level of the promoter (for example, Il6 and Lcn2) and/or mRNA stability (for example, mRNA encoding chemokines such as CXCL1) (Shen & Gaffen, 2008). IL-17 also upregulates the expression of NF-κB inhibitor-ζ (IκBζ) — which promotes the expression of select target genes (Karlsen et al., 2010).
IL-17 in oral mucosal immunity and inflammatory disease (periodontititis)
Importantly the IL-17 cytokine has been shown to play a major role both in mucosal surveillance and immunopathology at the oral mucosal barrier. Consistent with its immunoprotective role towards fungi, IL-17 is critical for oral mucosal protection against Candida albicans.
The Th17 lineage, acting largely through IL-17, has been elegantly shown in animal model systems to confer a dominant protective response to oral candidiasis through neutrophil recruitment and induction of antimicrobial factors (Conti et al., 2009). Consistent with experimental data HIES patients (bearing a STAT3 mutation and defects in Th17 differentiation) exhibit great susceptibility to oral candidiasis (thrush) which has been attributed to low levels of salivary AMPs, including HBD-2 and various histatins and reduced candidacidal activity of patient saliva (Conti et al., 2011).
Similarly, patients harboring mutations in molecules required for IL-17 signaling, such as IL-17RA, IL-17RC, IL-17F and the adaptor protein ACT1 also exhibit increased susceptibility to mucocutaneous, including oral, candidiasis (Puel et al., 2011, Boisson et al., 2013, Ling et al., 2015). Additional primary immunodeficiencies (PIDs) that may affect Th17 differentiation, such as gain-of-function mutations in STAT1, also manifest with CMC, reinforcing the importance of Th17 cells in coordinating fungal mucosal immunity (Zhang et al., 2009, Liu et al., 2011). Of the PIDs that affect fungal recognition molecules, mutations on DECTIN-1 and its adaptor CARD9 have been linked to defects in mounting effective IL-17 responses and CMC susceptibility (Glocker et al., 2009, Ferwerda et al., 2009). Additionally, individuals harboring genetic mutations in AIRE (and high titers of neutralizing autoantibodies to IL-17A, IL-17F and IL-22), almost invariably present with CMC and oral thrush (Meloni et al., 2012, Ferre et al., 2016), and show diminished Candida killing activity in saliva and decreased levels of the salivary AMP cystatin SA1 (Lindh et al., 2013). Finally, some of the patients with severe combined immunodeficiency (SCID) may also present with CMC, depending on their mutation and whether it affects the T lymphocytic lineage (Lionakis et al., 2014). Importantly, this continuously growing knowledge gained from the study of PIDs is helping define the role of IL-17 in human immunity (Table).
Table 1.
Primary Immunodeficiencies Affecting the Th17 Cell Pathway Linked to Susceptibility to Oral Mucosal Infection.
| Oral manifestation | Gene affected by mutation | Clinical Syndrome (If described) | Reference |
|---|---|---|---|
| Chronic Mucocutaneous Candidiasis (CMC) |
IL-17RA, IL-17RC IL-17F, ACT1 |
(Puel et al., 2011) (Boisson et al., 2013) (Ling et al., 2015) |
|
| Loss-of -function STAT3 | Autosomal dominant hyper IgE syndrome (AD-HIES) | (Freeman & Holland, 2009) | |
| Gain-of-function STAT1 | (Liu et al., 2011) | ||
| AIRE | Autoimmune polyendocrinopathy- candidiasis-ectodermal dystrophy (APECED) | (Meloni et al., 2012) | |
| DOCK8 | Autosomal recessive hyper IgE syndrome (AR-HIES) | (Zhang et al., 2009) | |
| DECTIN-1 | (Ferwerda et al., 2009) | ||
| CARD-9 | (Glocker et al., 2009) | ||
| IRF8 | (Hambleton et al., 2011) | ||
| STK4 | (Abdollahpour et al., 2012) | ||
|
CD45, IL7RA, RAG1, RAG2, ADA, AK2 JAK3, ARTEMIS |
Severe combined immunodeficiency (SCID) | (Buckley, 2004) |
Conversely, exaggerated IL-17 responses have been linked to immunopathology, particularly periodontitis in the oral cavity. Several studies have found high levels of IL-17 in chronic periodontitis (reviewed in (Zenobia & Hajishengallis, 2015)), and indicate a correlation of increased IL-17 expression with disease severity and with clinical parameters of periodontal destruction (Johnson et al., 2004, Lester et al., 2007, Dutzan et al., 2012). In fact, IL-17 expression is higher in periodontitis than in gingivitis and is almost undetectable in healthy control tissues (Honda et al., 2008, Okui et al., 2012, Moutsopoulos et al., 2012). IL-17 is also shown to be increased in aggressive periodontitis compared to chronic periodontitis and controls (Shaker & Ghallab, 2012). Finally, numerous studies have found an increase in Th17-related cytokines in periodontal lesions, such as IL-23, IL-21 and other pro-inflammatory and osteoclastogenic mediators such as IL-6 and RANKL, respectively (Lester et al., 2007, Cardoso et al., 2009, Dutzan et al., 2009, Ohyama et al., 2009, Allam et al., 2011). In humans, the presence of Th17 cells has been previously shown in periodontal lesions (Cardoso et al., 2009) and recently Th17 cells have been identified as the main source of IL-17 in periodontitis (Dutzan et al., 2016).
Critically, tissue neutrophils are now recognized as key cellular regulators of Th17 responses in periodontitis. Studies from our group and collaborators have demonstrated that the lack or severe reduction in tissue neutrophils in patients with Leukocyte Adhesion Deficiency Type 1 (LAD-1) and relevant animal models is linked to exaggerated IL-17 responses (Moutsopoulos et al., 2014). Conversely, over-abundance of tissue neutrophils in patients with chronic and aggressive periodontitis is also linked to excessive IL-17 responses (as discussed above). Consistent with these observations, mechanistic evidence from animal models also supports that excessive neutrophil recruitment can lead to exaggerated IL-17 responses. In fact, unregulated neutrophil recruitment in the absence of Del-1 (an endogenous inhibitor of neutrophil extravasation) leads to a deregulation of the IL-17 response (Eskan et al., 2012). Collectively, all of this evidence suggests that a balance of tissue neutrophils is key in IL-17 regulation.
Dysregulated IL-17 production could be playing a pathogenic role in periodontitis though distinct but possibly overlapping mechanisms. IL-17 has the ability to amplify inflammation through excessive neutrophil recruitment, by enhancing pro-inflammatory cytokine production and by activating osteoclasts, all of which could contribute to immunopathology and bone destruction (Moutsopoulos et al., 2015b). Experimental studies in animal models strongly support a pathogenic role for IL-17 in periodontitis. IL-17 blockade has been shown to reverse immunopathology linked to excessive neutrophil recruitment in Del-1-deficient mice (Eskan et al., 2012). Likewise, in mouse models of LADI periodontitis (LFA-1KO) inhibition of IL-17 or IL-23p19 was able to arrest inflammatory bone loss (Moutsopoulos et al., 2014). However, human studies targeting the IL-17 cytokine pathway are necessary to conclusively define the role of IL-17 in the pathogenesis and progression of periodontal diseases.
What is the role of IL-17surveillance in the establishment of the oral microbiome
IL-17 has emerged as a critical cellular regulator of mucosal immunity and inflammation at the oral barrier. Given the well-recognized role of the commensal microbiome in health and disease it becomes important to understand what the role of IL-17 immunity is in shaping microbiome colonization in the oral cavity. To date it is well appreciated that IL-17 responses are critical for fungal surveillance in the oral cavity, particularly for surveillance of oral Candida. Yet, little is known of the role of IL-17 responses in microbiome/bacterial surveillance. Interestingly, despite the fact that IL-17 signaling has been shown to participate in immunity against bacterial pathogens at various tissue sites (Isailovic et al., 2015), data from various PID patient cohorts with disruptions in Th17 immunity do not indicate susceptibility to any other oral infections in patients with blunted Th17 responses. Yet one cannot disregard a possible role for the microbiome as a contributor in the susceptibility to oral candidiasis. The interplay between microbiome and mycobiome is a well-recognized factor in the control of fungal infections as evidenced by the increase in fungal mucosal infections in patients following antibiotics (Sobel, 2007, Diaz et al., 2014). Therefore, evaluating microbiome/mycobiome interactions in the setting of defective IL-17 immunity becomes of greater interest.
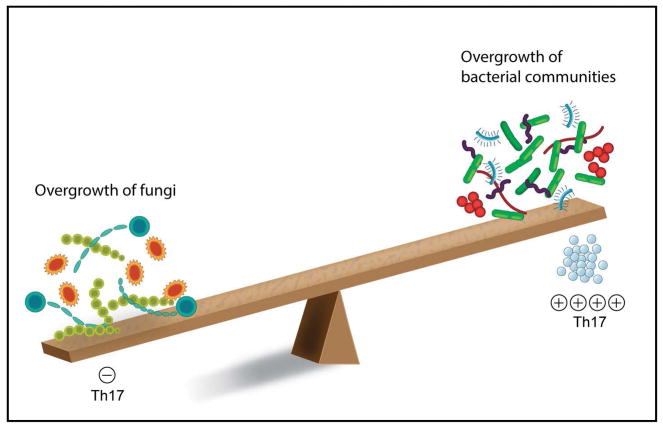
Interestingly, while the oral microbiome in the setting of reduced IL-17 immunity is only recently beginning to be evaluated (Smeekens et al., 2014), it is well documented that exaggerated IL-17 oral responses are linked to microbial overgrown and dysbiosis (Figure 5). Exaggerated IL-17 production in the context of chronic and LAD-1 periodontitis is accompanied by an increase in microbial load and presence of dysbiotic bacterial communities (Eskan et al., 2012, Moutsopoulos et al., 2015a, Abusleme et al., 2013). However, strong evidence supporting a role for exaggerated IL-17 inflammation in facilitating the formation of dysbiotic microbial communities comes from animal models of LAD-1 periodontitis. Murine LAD-1 (LFA-1KO) is associated with an increase in oral microbial load and anaerobic counts. Importantly, inhibition of IL-17 or IL-23 alone (without restoration of the genetic defect) reverses microbial dysbiosis suggesting that exaggerated IL-17 inflammation is a driving force for microbial imbalance in periodontitis (Moutsopoulos et al., 2014). It has been theorized previously that the microbial communities associated with periodontitis have an ‘inflammophilic’ character and thrive in the presence of inflammation and its nutrients (Hajishengallis, 2014). In accordance with this hypothesis and data from experimental models, in humans it has been shown that periodontal inflammation drives an increase in microbial load in periodontitis (Abusleme et al., 2013). It is possible (based on data from the LAD model) that amplification of the IL-17 axis is a critical component of periodontal inflammation which facilitates a shift towards a “pathogenic” microbial community. However, further studies in humans targeting the IL-17 response can only conclusively define the role of IL-17 in the pathogenesis of periodontitis and its contribution to microbial dysbiosis.
Figure 5. Concepts of how IL-17 immunity may participate in the establishment of the oral microbiome/mycobiome.
Defects in the Th17 pathway are associated with an overgrowth of fungi leading to oral and mucocutaneous candidiasis. Conversely, exaggerated IL-17 responses in the context of periodontitis have been linked to bacterial overgrowth and dysbiosis.
Acknowledgments
This work was funded by the Intramural Program of the National Institute of Dental and Craniofacial Research. The authors would like to acknowledge Erina He at the NIH Medical Arts department and Ms. Teresa Wild for critically reviewing the manuscript.
References
- Abdollahpour H, Appaswamy G, Kotlarz D, Diestelhorst J, Beier R, Schaffer AA, Gertz EM, Schambach A, Kreipe HH, Pfeifer D, Engelhardt KR, Rezaei N, Grimbacher B, Lohrmann S, Sherkat R, Klein C. The phenotype of human STK4 deficiency. Blood. 2012;119:3450–7. doi: 10.1182/blood-2011-09-378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–25. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007a;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007b;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Allam JP, Duan Y, Heinemann F, Winter J, Gotz W, Deschner J, Wenghoefer M, Bieber T, Jepsen S, Novak N. IL-23-producing CD68(+) macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J Clin Periodontol. 2011;38:879–86. doi: 10.1111/j.1600-051X.2011.01752.x. [DOI] [PubMed] [Google Scholar]
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014;40:117–27. doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, Puel A, Li X, Casanova JL. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39:676–86. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- Burkett PR, Meyer zu Horste G, Kuchroo VK. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest. 2015;125:2211–9. doi: 10.1172/JCI78085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–73. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, Newberry KM, Meadows S, Greenfield A, Yang Y, Jain P, Kirigin FK, Birchmeier C, Wagner EF, Murphy KM, Myers RM, Bonneau R, Littman DR. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–7. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, de Visser KE. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–8. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4:448–55. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Cypowyj S, Picard C, Marodi L, Casanova JL, Puel A. Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol. 2012;42:2246–54. doi: 10.1002/eji.201242605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Novotny M, Pavicic PG, Jr, Zhao C, Herjan T, Hartupee J, Hamilton T. IL-17 regulates CXCL1 mRNA stability via an AUUUA/tristetraprolin-independent sequence. J Immunol. 2010;184:1484–91. doi: 10.4049/jimmunol.0902423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz PI, Strausbaugh LD, Dongari-Bagtzoglou A. Fungal-bacterial interactions and their relevance to oral health: linking the clinic and the bench. Front Cell Infect Microbiol. 2014;4:101. doi: 10.3389/fcimb.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL) -17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis. J Clin Periodontol. 2009;36:396–403. doi: 10.1111/j.1600-051X.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016 doi: 10.1038/mi.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Vernal R, Vaque JP, Garcia-Sesnich J, Hernandez M, Abusleme L, Dezerega A, Gutkind JS, Gamonal J. Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients. J Periodontol. 2012;83:948–54. doi: 10.1902/jop.2011.110482. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–73. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre EM, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, Rosen LB, Break TJ, Gu W, Hunsberger S, Browne SK, Hsu AP, Rampertaap S, Swamydas M, Collar AL, Kong HH, Lee CR, Chascsa D, Simcox T, Pham A, Bondici A, Natarajan M, Monsale J, Kleiner DE, Quezado M, Alevizos I, Moutsopoulos NM, Yockey L, Frein C, Soldatos A, Calvo KR, Adjemian J, Similuk MN, Lang DM, Stone KD, Uzel G, Kopp JB, Bishop RJ, Holland SM, Olivier KN, Fleisher TA, Heller T, Winer KK, Lionakis MS. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight. 2016:1. doi: 10.1172/jci.insight.88782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morre SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Lango Allen H, De Franco E, McDonald TJ, Rajala H, Ramelius A, Barton J, Heiskanen K, Heiskanen-Kosma T, Kajosaari M, Murphy NP, Milenkovic T, Seppanen M, Lernmark A, Mustjoki S, Otonkoski T, Kere J, Morgan NG, Ellard S, Hattersley AT. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46:812–4. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AF, Holland SM. Clinical manifestations, etiology, and pathogenesis of the hyper-IgE syndromes. Pediatr Res. 2009;65:32R–37R. doi: 10.1203/PDR.0b013e31819dc8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AV, Ahmed M, Vallejo AN, Ma A, Gaffen SL. The deubiquitinase A20 mediates feedback inhibition of interleukin-17 receptor signaling. Sci Signal. 2013;6:ra44. doi: 10.1126/scisignal.2003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AV, Amatya N, Chen K, Cruz JA, Grover P, Whibley N, Conti HR, Hernandez Mir G, Sirakova T, Childs EC, Smithgall TE, Biswas PS, Kolls JK, McGeachy MJ, Kolattukudy PE, Gaffen SL. MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation. Immunity. 2015;43:475–87. doi: 10.1016/j.immuni.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge D, You Z. Expression of interleukin-17RC protein in normal human tissues. Int Arch Med. 2008;1:19. doi: 10.1186/1755-7682-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschlager N, Gross O, Ruland J, Grimbacher B. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–57. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, McDonald D, Carey P, Ginhoux F, Alsina L, Zumwalt TJ, Kong XF, Kumararatne D, Butler K, Hubeau M, Feinberg J, Al-Muhsen S, Cant A, Abel L, Chaussabel D, Doffinger R, Talesnik E, Grumach A, Duarte A, Abarca K, Moraes-Vasconcelos D, Burk D, Berghuis A, Geissmann F, Collin M, Casanova JL, Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–38. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Haudenschild D, Moseley T, Rose L, Reddi AH. Soluble and transmembrane isoforms of novel interleukin-17 receptor-like protein by RNA splicing and expression in prostate cancer. J Biol Chem. 2002;277:4309–16. doi: 10.1074/jbc.M109372200. [DOI] [PubMed] [Google Scholar]
- Herjan T, Yao P, Qian W, Li X, Liu C, Bulek K, Sun D, Yang WP, Zhu J, He A, Carman JA, Erzurum SC, Lipshitz HD, Fox PL, Hamilton TA, Li X. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J Immunol. 2013;191:640–9. doi: 10.4049/jimmunol.1203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–12. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Aoki Y, Takahashi N, Maekawa T, Nakajima T, Ito H, Tabeta K, Okui T, Kajita K, Domon H, Yamazaki K. Elevated expression of IL-17 and IL-12 genes in chronic inflammatory periodontal disease. Clin Chim Acta. 2008;395:137–41. doi: 10.1016/j.cca.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–40. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- Ichiyama K, Gonzalez-Martin A, Kim BS, Jin HY, Jin W, Xu W, Sabouri-Ghomi M, Xu S, Zheng P, Xiao C, Dong C. The MicroRNA-183-96-182 Cluster Promotes T Helper 17 Cell Pathogenicity by Negatively Regulating Transcription Factor Foxo1 Expression. Immunity. 2016;44:1284–98. doi: 10.1016/j.immuni.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isailovic N, Daigo K, Mantovani A, Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–21. [PubMed] [Google Scholar]
- Karlsen JR, Borregaard N, Cowland JB. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. J Biol Chem. 2010;285:14088–100. doi: 10.1074/jbc.M109.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp DR, Marthandan N, Marsh SG, Ahn C, Arnett FC, Deluca DS, Diehl AD, Dunivin R, Eilbeck K, Feolo M, Guidry PA, Helmberg W, Lewis S, Mayes MD, Mungall C, Natale DA, Peters B, Petersdorf E, Reveille JD, Smith B, Thomson G, Waller MJ, Scheuermann RH. Novel sequence feature variant type analysis of the HLA genetic association in systemic sclerosis. Hum Mol Genet. 2010;19:707–19. doi: 10.1093/hmg/ddp521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–35. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–73. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C, Group ES, Group FS. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–38. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, Cua DJ. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727–38. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–9. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester SR, Bain JL, Johnson RB, Serio FG. Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J Periodontol. 2007;78:1545–50. doi: 10.1902/jop.2007.060458. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh E, Brannstrom J, Jones P, Wermeling F, Hassler S, Betterle C, Garty BZ, Stridsberg M, Herrmann B, Karlsson MC, Winqvist O. Autoimmunity and cystatin SA1 deficiency behind chronic mucocutaneous candidiasis in autoimmune polyendocrine syndrome type 1. J Autoimmun. 2013;42:1–6. doi: 10.1016/j.jaut.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, Ikinciogullari A, Dogu F, Belkadi A, Levy R, Migaud M, Boisson B, Bolze A, Itan Y, Goudin N, Cottineau J, Picard C, Abel L, Bustamante J, Casanova JL, Puel A. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212:619–31. doi: 10.1084/jem.20141065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Netea MG, Holland SM. Mendelian genetics of human susceptibility to fungal infection. Cold Spring Harb Perspect Med. 2014:4. doi: 10.1101/cshperspect.a019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, Masson C, Toth B, Flatot J, Migaud M, Chrabieh M, Kochetkov T, Bolze A, Borghesi A, Toulon A, Hiller J, Eyerich S, Eyerich K, Gulacsy V, Chernyshova L, Chernyshov V, Bondarenko A, Grimaldo RM, Blancas-Galicia L, Beas IM, Roesler J, Magdorf K, Engelhard D, Thumerelle C, Burgel PR, Hoernes M, Drexel B, Seger R, Kusuma T, Jansson AF, Sawalle-Belohradsky J, Belohradsky B, Jouanguy E, Bustamante J, Bue M, Karin N, Wildbaum G, Bodemer C, Lortholary O, Fischer A, Blanche S, Al-Muhsen S, Reichenbach J, Kobayashi M, Rosales FE, Lozano CT, Kilic SS, Oleastro M, Etzioni A, Traidl-Hoffmann C, Renner ED, Abel L, Picard C, Marodi L, Boisson-Dupuis S, Puel A, Casanova JL. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–48. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Helms C, Liao W, Zaba LC, Duan S, Gardner J, Wise C, Miner A, Malloy MJ, Pullinger CR, Kane JP, Saccone S, Worthington J, Bruce I, Kwok PY, Menter A, Krueger J, Barton A, Saccone NL, Bowcock AM. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4:e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2015;136:351–9. e1. doi: 10.1016/j.jaci.2015.01.033. [DOI] [PubMed] [Google Scholar]
- Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M, Stevens E, Bigler J, Davis JA, Rottman JB, Budelsky AL, Symons A, Towne JE. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity. 2015;43:739–50. doi: 10.1016/j.immuni.2015.08.019. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Meloni A, Willcox N, Meager A, Atzeni M, Wolff AS, Husebye ES, Furcas M, Rosatelli MC, Cao A, Congia M. Autoimmune polyendocrine syndrome type 1: an extensive longitudinal study in Sardinian patients. J Clin Endocrinol Metab. 2012;97:1114–24. doi: 10.1210/jc.2011-2461. [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, Lyons JJ, Engelhardt KR, Zhang Y, Topcagic N, Roberson ED, Matthews H, Verbsky JW, Dasu T, Vargas-Hernandez A, Varghese N, McClain KL, Karam LB, Nahmod K, Makedonas G, Mace EM, Sorte HS, Perminow G, Rao VK, O’Connell MP, Price S, Su HC, Butrick M, McElwee J, Hughes JD, Willet J, Swan D, Xu Y, Santibanez-Koref M, Slowik V, Dinwiddie DL, Ciaccio CE, Saunders CJ, Septer S, Kingsmore SF, White AJ, Cant AJ, Hambleton S, Cooper MA. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125:591–9. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Chalmers NI, Barb JJ, Abusleme L, Greenwell-Wild T, Dutzan N, Paster BJ, Munson PJ, Fine DH, Uzel G, Holland SM. Subgingival microbial communities in Leukocyte Adhesion Deficiency and their relationship with local immunopathology. PLoS Pathog. 2015a;11:e1004698. doi: 10.1371/journal.ppat.1004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Kling HM, Angelov N, Jin W, Palmer RJ, Nares S, Osorio M, Wahl SM. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 2012;39:294–303. doi: 10.1016/j.jaut.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, Uzel G, Chen W, Chavakis T, Holland SM, Hajishengallis G. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med. 2014;6:229ra40. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Lionakis MS, Hajishengallis G. Inborn errors in immunity: unique natural models to dissect oral immunity. J Dent Res. 2015b;94:753–8. doi: 10.1177/0022034515583533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordenbos T, Blijdorp I, Chen S, Stap J, Mul E, Canete JD, Lubberts E, Yeremenko N, Baeten D. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J Leukoc Biol. 2016;100:453–62. doi: 10.1189/jlb.3HI1215-542R. [DOI] [PubMed] [Google Scholar]
- Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–9. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–9. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Steward-Tharp SM, Laurence A, Watford WT, Wei L, Adamson AS, Fan S. Signal transduction and Th17 cell differentiation. Microbes Infect. 2009;11:599–611. doi: 10.1016/j.micinf.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, Yamada N, Hata M, Yamane J, Terada N. The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res. 2009;88:633–8. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- Okui T, Aoki Y, Ito H, Honda T, Yamazaki K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res. 2012;91:574–9. doi: 10.1177/0022034512446341. [DOI] [PubMed] [Google Scholar]
- Patel DD, Kuchroo VK. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity. 2015;43:1040–51. doi: 10.1016/j.immuni.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem. 2007;282:27229–38. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–43. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, Ruzicka T, Gallo RL, Schauber J. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504–12. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12:616–22. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–56. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–56. [PubMed] [Google Scholar]
- Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG, Group CS. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–28. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- Shaker OG, Ghallab NA. IL-17 and IL-11 GCF levels in aggressive and chronic periodontitis patients: relation to PCR bacterial detection. Mediators Inflamm. 2012;2012:174764. doi: 10.1155/2012/174764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, Eberl G, Buckley CD, Kastelein RA, Pierce RH, Laface DM, Cua DJ. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- Smeekens SP, Huttenhower C, Riza A, van de Veerdonk FL, Zeeuwen PL, Schalkwijk J, van der Meer JW, Xavier RJ, Netea MG, Gevers D. Skin microbiome imbalance in patients with STAT1/STAT3 defects impairs innate host defense responses. J Innate Immun. 2014;6:253–62. doi: 10.1159/000351912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961–71. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- Sonder SU, Saret S, Tang W, Sturdevant DE, Porcella SF, Siebenlist U. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J Biol Chem. 2011;286:12881–90. doi: 10.1074/jbc.M110.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng ZH. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–24. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- Song X, He X, Li X, Qian Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol Immunol. 2016;13:418–31. doi: 10.1038/cmi.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175–82. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat Immunol. 2011;12:853–60. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15:143–51. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–9. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–45. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, Dolhain RJ, Lubberts E. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–7. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Wang C, Wu L, Bulek K, Martin BN, Zepp JA, Kang Z, Liu C, Herjan T, Misra S, Carman JA, Gao J, Dongre A, Han S, Bunting KD, Ko JS, Xiao H, Kuchroo VK, Ouyang W, Li X. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat Immunol. 2013;14:72–81. doi: 10.1038/ni.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust Case Control C, Australo-Anglo-American Spondylitis C. Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Davison D, Easton D, Evans DM, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskivina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop TD, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Matthew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–37. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Wang C, Boisson B, Misra S, Rayman P, Finke JH, Puel A, Casanova JL, Li X. The differential regulation of human ACT1 isoforms by Hsp90 in IL-17 signaling. J Immunol. 2014;193:1590–9. doi: 10.4049/jimmunol.1400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Li J, Wang JH, Wu Q, Yang P, Hsu HC, Smythies LE, Mountz JD. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol. 2010;184:2289–96. doi: 10.4049/jimmunol.0903133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995a;3:811–21. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995b;155:5483–6. [PubMed] [Google Scholar]
- Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, Gennert D, Satija R, Shakya A, Lu DY, Trombetta JJ, Pillai MR, Ratcliffe PJ, Coleman ML, Bix M, Tantin D, Park H, Kuchroo VK, Regev A. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496:461–8. doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000. 2015;69:142–59. doi: 10.1111/prd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp JA, Liu C, Qian W, Wu L, Gulen MF, Kang Z, Li X. Cutting edge: TNF receptor-associated factor 4 restricts IL-17-mediated pathology and signaling processes. J Immunol. 2012;189:33–7. doi: 10.4049/jimmunol.1200470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, Davis J, Turner ML, Uzel G, Holland SM, Su HC. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–55. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, Liu Y, Zhao L, Li X, Shi Y, Qian Y. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J Exp Med. 2010;207:2647–62. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, Shi Y, Harley JB, Shen N, Qian Y. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18:1077–86. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- Zrioual S, Ecochard R, Tournadre A, Lenief V, Cazalis MA, Miossec P. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112–20. doi: 10.4049/jimmunol.0801967. [DOI] [PubMed] [Google Scholar]