Abstract
During development, neurons establish inappropriate connections as they seek out their synaptic partners, resulting in supernumerary synapses that must be pruned away. The removal of miswired synapses usually involves electrical activity, often through a Hebbian spike-timing mechanism. A novel form of activity-dependent refinement is used by Drosophila that may be non-Hebbian, and is critical for generating the precise connectivity observed in that system. In Drosophila, motoneurons use both glutamate and the biogenic amine octopamine for neurotransmission, and the muscle fibers receive multiple synaptic inputs. Motoneuron growth cones respond in a time-regulated fashion to multiple chemotropic signals arising from their postsynaptic partners. Central to this mechanism is a very low frequency (<0.03 Hz) oscillation of presynaptic cytoplasmic calcium, that regulates and coordinates the action of multiple downstream effectors involved in the withdrawal from off-target contacts. Low frequency calcium oscillations are widely observed in developing neural circuits in mammals, and have been shown to be critical for normal connectivity in a variety of neural systems. In Drosophila these mechanisms allow the growth cone to sample widely among possible synaptic partners, evaluate opponent chemotropic signals, and withdraw from off-target contacts. It is possible that the underlying molecular mechanisms are conserved widely among invertebrates and vertebrates.
Keywords: oscillation, chemorepulsion, neuromuscular junction, non-Hebbian, second messengers
It is estimated that the nearly 1011 neurons of the human nervous system establish over 1014 synaptic connections (Azevedo et al., 2009; Kasthuri et al., 2015). To wire up a system of such astonishing complexity requires mechanisms that are highly efficient and flexible. Rather than uniquely specifying each synaptic connection, the developing nervous system can initially establish connections that are characterized by supernumerary synaptic contacts, as widely observed in neural networks. Inappropriate off-target synapses are subsequently pruned away through activity-dependent mechanisms to yield a more precise and functional connectome (reviewed in Katz and Shatz, 1996; Yamamoto and López-Bendito, 2012; Doll and Broadie, 2014; Koropouli and Kolodkin, 2014; Arroyo and Feller, 2016). Errors in synaptic pruning are associated with several neurological disorders, including autism and schizophrenia (Berridge, 2012; Tang et al., 2014; Sekar et al., 2016).
In this review article, we examine synaptic refinement with a focus on the embryonic and larval neuromuscular system of Drosophila, where some of the underlying molecular mechanisms have been resolved (Carrillo et al., 2010; Vonhoff and Keshishian, 2017). This simple array of synapses is established by two distinct classes of motoneurons that use as neurotransmitters either glutamate (Johansen et al., 1989) or the biogenic amine octopamine (Monastirioti, 1999).
Activity Dependent Refinement
The refinement of neural connections occurs in vertebrates and invertebrates, and has been extensively studied in the developing visual system (reviewed in D’Orazi et al., 2014; Pratt et al., 2016). Although activity-independent synapse elimination has been observed in mouse retinal cells (Morgan et al., 2011; Wei et al., 2011; Yonehara et al., 2011), activity-dependent mechanisms play a crucial role in establishing precise network connectivity (reviewed in Huberman et al., 2008; Cang and Feldheim, 2013). Pioneering work by Hubel and Wiesel showed that visual experience was required for the formation of ocular dominance columns between axons of the lateral geniculate nucleus (LGN) of the thalamus, and layer 4 neurons in primary visual cortex (Wiesel and Hubel, 1963). The requirement for neural activity in the segregation of visual projections was subsequently tested using TTX eye injections in both cold blooded vertebrates (Meyer, 1982), and in mammals (Shatz and Stryker, 1988; Sretavan et al., 1988). Patterned neural activity was also found to be essential for refining retinotopic map projections at other visual centers, such as the superior colliculus (McLaughlin et al., 2003). Activity-dependent refinement is also involved in controlling the balance between excitatory and inhibitory synapses, as found for the Xenopus optic tectum (Akerman and Cline, 2007). Elsewhere activity is involved in the elimination of supernumerary contacts at the vertebrate neuromuscular junction (reviewed by Sanes and Lichtman, 2001), and for synapse elimination of climbing fiber inputs to cerebellar Purkinje cells (reviewed by Purves and Lichtman, 1980; Kano and Hashimoto, 2009).
The remodeling that occurs during synaptic refinement suggests that electrical activity influences neurite growth or retraction. The link between activity and growth is a general feature of neural systems. For example, in Drosophila altered levels of neural activity in embryonic olfactory projection neurons (Prieto-Godino et al., 2012) and in larval and adult motoneurons (Duch et al., 2008; Hartwig et al., 2008) affects dendrite size and complexity, and thus directly influences synaptic connections. Similarly, in larval motoneurons manipulation of neural activity alters presynaptic NMJ size and arbor complexity, and affects presynaptic bouton morphology (Budnik et al., 1990; Zhong et al., 1992; Lnenicka et al., 2003; Mosca et al., 2005; Berke et al., 2013).
Molecular Mechanisms Underlying Refinement
How is neural activity linked to the cell biology of neuronal growth and retraction? Depolarization elevates intracellular free calcium (Ca2+) levels through voltage-gated calcium channels (VGCCs). As a result, the mechanisms regulating synaptic connectivity generally involve Ca2+-dependent effectors. Ca2+-dependent signaling can influence early growth events, such as the motility and exploration of the growth cone (Kater and Shibata, 1994; Zheng and Poo, 2007; Rosenberg and Spitzer, 2011). In some cases this is due to the modulation of the growth cone’s response to various exogenous chemotropic factors, such as netrin-1-induced attraction, myelin-associated glycoprotein (MAG)-induced repulsion (Ming et al., 2001), or Ephrin-A induced repulsion of mouse retinal ganglion cells (Nicol et al., 2007).
Within the cytoplasm, Ca2+ regulates the activity of various GTPases (Jin et al., 2005), that in turn affect cytoskeletal dynamics within the growing contact. GTPases serve as a key molecular link between changes in free Ca2+ levels in the growth cone due to activity, and subsequent responses to chemotropic factors (Lowery and Van Vactor, 2009). One potential mechanism linking neural activity and cytoskeletal dynamics would involve the regulation of actin by the activity of Rho GTPases. Rho is known to regulate ROCK function, which in turn activates LIM Kinase (LIMK; Amano et al., 2010). LIMK inhibits cofilin, an actin severing protein that promotes actin recycling. Consistent with this hypothesis, LIMK is known to regulate synaptic function in mice (Meng et al., 2002) as well as NMJ growth in Drosophila (Ang et al., 2006).
A second molecular mechanism regulating activity-dependent refinement involves interactions between Ca2+ and cyclic nucleotides such as cAMP and cGMP. Intracellular cyclic nucleotide levels regulate chemotropic growth cone turning (Lohof et al., 1992; Song et al., 1997; Nishiyama et al., 2003), synaptic plasticity (Zhong et al., 1992), and the refinement of axon branches in both retinal cells (Nicol et al., 2006) and Drosophila motoneurons (Vonhoff and Keshishian, 2017). Whether cAMP levels are positioned upstream or downstream of Ca2+ signaling remains incompletely resolved, as there is evidence in the literature for both scenarios. cAMP levels may act downstream of Ca2+ as connectivity defects arise following misregulation of Ca2+-dependent adenylyl cyclases, such as AC1 in mouse retinal neurons (Nicol et al., 2006), ADCY8 in zebrafish retinal neurons (Xu et al., 2010), and Rutabaga in Drosophila motoneurons (Vonhoff and Keshishian, 2017). By contrast, cAMP also regulates Ca2+-signaling as it promotes Ca2+-induced Ca2+-release (CICR) from internal stores (Gomez and Zheng, 2006; Zheng and Poo, 2007), modulates the amplitude of growth cone Ca2+-transients (Nicol et al., 2011), and cyclic nucleotide-gated (CNG) ion channels, to allow for Ca2+-influx in growth cones (Togashi et al., 2008).
Intracellular Ca2+ activates several pathways that converge on transcription factors that control the expression of activity-regulated genes that may be involved in guidance mechanisms. This was first revealed for the immediate early gene c-fos, downstream of Ca2+ influx (Greenberg et al., 1986). Fos protein together with Jun family members comprises the AP-1 transcription factor (Curran and Franza, 1988). AP-1 has been involved in synaptic plasticity in mouse hippocampal neurons (Fleischmann et al., 2003) as well as in activity-dependent dendritic growth of Drosophila motoneurons (Hartwig et al., 2008; Vonhoff et al., 2013) and synaptic development at the Drosophila NMJ (Sanyal et al., 2002).
Finally, there is good evidence from both vertebrates and invertebrates that synaptic refinement requires temporally patterned changes or oscillations in the levels of second messengers. This dynamism has been particularly evident for Ca2+, where spontaneous retinal waves are critical for the refinement of visual maps in the mouse brain (Wong, 1999; Arroyo and Feller, 2016), as well as for the refinement of neuromuscular junctions in Drosophila embryos (Carrillo et al., 2010; Vonhoff and Keshishian, 2017). It is intriguing that cAMP levels are also required to oscillate for the refinement of mouse retinal axons (Nicol et al., 2007), or to be dynamically maintained within an optimal level for the refinement of Drosophila motoneuron axon branches (Vonhoff and Keshishian, 2017).
Drosophila NMJ as a Genetic Model to Study Synaptic Refinement
The Drosophila larval bodywall offers an anatomically stereotypic genetic model system for studying many aspects of neuronal connectivity (for reviews see Ruiz-Cañada and Budnik, 2006; Menon et al., 2013). Among its features are singly identifiable glutamatergic motoneurons with very narrow connectivity, innervating only one or two muscle fibers each, and a subset of efferent neuromodulatory neurons that express the biogenic amine octopamine (Monastirioti et al., 1995, 1996; Monastirioti, 1999) that project widely and innervate multiple muscle fibers.
The stereotypic connectivity of the embryonic and larval Drosophila NMJ crucially relies on the expression of molecular recognition cues (reviewed in Nose, 2012). Whereas some molecules are expressed by all muscles, the expression pattern of other cues is restricted to individual muscles (Winberg et al., 1998). Examples of muscle-specific cues include Fasciclin III (Halpern et al., 1991), Capricious (Shishido et al., 1998), Connectin (Nose et al., 1992), and NetrinB (Harris et al., 1996). By contrast, other molecules are expressed by numerous muscle fibers, as for example Fasciclin II (Lin and Goodman, 1994), Teneurin-m (Mosca et al., 2012), Dpr11 (Carrillo et al., 2015), and Semaphorin2a (Matthes et al., 1995).
During embryonic development Drosophila motoneuron growth cones sample widely among muscle fibers, and inevitably make inappropriate contacts, as shown schematically in Figure 1A (Halpern et al., 1991; Sink and Whitington, 1991; Chiba et al., 1993). The off-target contacts are removed during an early critical period (late embryo to early 1st instar; Figure 1B), otherwise they mature into functional ectopic synapses (Jarecki and Keshishian, 1995; Carrillo et al., 2010). Ultimately, neural activity refines the motoneuron contacts, so that their connectivity is limited only to their appropriate muscle fiber targets. Silencing electrical activity in the motoneurons during the critical period increases the frequency of ectopic motoneuron contacts throughout the bodywall (Figure 1C; Jarecki and Keshishian, 1995; White et al., 2001; Carrillo et al., 2010).
Figure 1.

The events associated with synaptic targeting at the Drosophila NMJ. (A) Initial motoneuron projections make filopodial contacts (green) onto both the target muscle as well as to multiple off-target muscle fibers (Halpern et al., 1991; Sink and Whitington, 1991). (B) During normal development, off-target contacts are withdrawn, leading to the final specific connectivity (green). The refinement must occur during an early critical period and depends on presynaptic electrical activity (Jarecki and Keshishian, 1995; White et al., 2001; Carrillo et al., 2010). (C) When neural activity is suppressed, the off-target contacts are retained (red), leading to ectopic synapses. The transition from a growth cone filopodium to a synapse is rapid and the refinement of ectopic contacts occurs while growth cones are still motile, consistent with the critical period for refinement at the Drosophila NMJ. Ectopic contacts that fail to withdraw develop into functional synapses. This is in contrast to scenarios observed in other systems where synaptic contacts have to be stabilized and then refined by mechanisms that rely on prolonged period of synaptic competition.
In vivo electrical activity in the embryo is highly patterned, with brief (~15 s) bursts of action potentials spaced every 2–3 min (Pereanu et al., 2007; Crisp et al., 2008; Vonhoff and Keshishian, 2017). Normal synaptic refinement depends on the presence of two voltage-gated Ca2+ channels, Cacophony (Cac), the Ca(v)2.1 channel (Carrillo et al., 2010), and Dmca1G, the Ca(v)3 channel (Vonhoff and Keshishian, in preparation). The experimental rescue of the cac mutation to restore normal synaptic connectivity requires oscillatory presynaptic Ca2+ entry, timed to resemble the native electrical oscillations (Carrillo et al., 2010). This indicates that Ca2+-oscillations at a specific frequency and pattern (in the range of 0.01–0.03 Hz) are required for proper synaptic refinement.
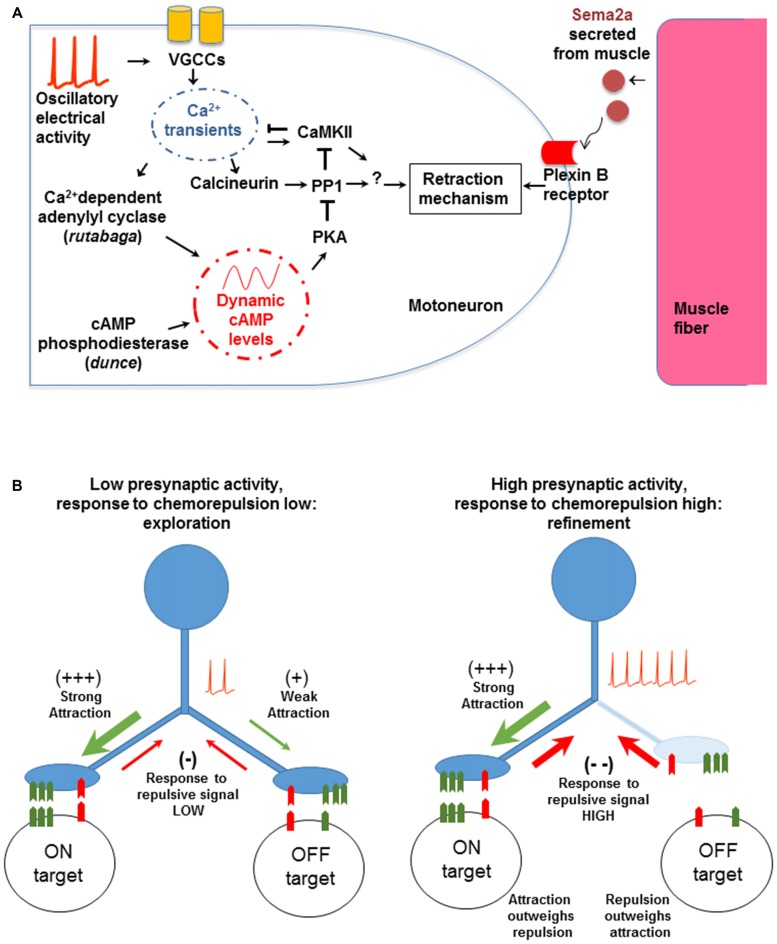
In addition to the activity-dependent entry of Ca2+ through Ca2+ channels (Figure 2A), refinement also depends on the activity of at least three downstream Ca2+-dependent signaling systems in the presynaptic terminal: the Ca2+/calmodulin-dependent serine/threonine kinase II (CaMKII; Carrillo et al., 2010), the Ca2+/calmodulin-dependent serine/threonine protein phosphatase Calcineurin (CaN; Vonhoff and Keshishian, in preparation), and the Ca2+-dependent adenylyl cyclase Rutabaga (Vonhoff and Keshishian, 2017). Rutabaga elevates intracellular cAMP-levels, which are degraded by the activity of the cAMP-phosphodiesterase Dunce. Similarly, molecules whose activity is typically downstream of cAMP such as PKA and PP1 are also required for synaptic refinement (Vonhoff and Keshishian, 2017). Notably, PKA and CaN are known to interact with PP1 (Blitzer et al., 1998; Oliver and Shenolikar, 1998), which in turn can regulate CaMKII. Collectively, these interactions suggest a complex signaling network to govern synaptic refinement in this system (Figure 2A).
Figure 2.
The molecular and cellular mechanisms involved in synaptic refinement. (A) The interactions were identified by genetic tests and transgenic manipulations. A low frequency voltage oscillation activates voltage gated Ca2+ channels (VGCCs). The resulting Ca2+ entry regulates Ca2+-dependent effectors including Ca2+/calmodulin-dependent serine/threonine kinase II (CaMKII), Calcineurin (CaN), and Rutabaga. The latter increases cAMP levels, which in turn regulate PKA and PP1. The chemorepellant Sema2a is secreted by the muscle and activates the presynaptic PlexinB receptor. The response to Sema2a is gated by the level of presynaptic Ca2+ activity (see text for details). Arrows and T-shape lines indicate positive and negative regulation, respectively. The subcellular physical location and region of action of the molecular components have not been determined yet. (B) A model for non-Hebbian refinement at the Drosophila NMJ. The left panel shows an initial contact made by a motoneuron onto on-target and off-target muscle fibers. The molecular match is stronger with the on-target fiber. When Ca2+ levels are low, the response to the retrograde chemorepulsive signal from the muscle is muted, allowing the off-target contact to be retained. With neural activity and elevated presynaptic Ca2+ (right panel), the repulsive response is elevated, leading to the withdrawal of the off-target contact. Note that the model does not depend on correlated activity between the synaptic partners, as would be expected in a Hebbian mechanism.
How are off-target contacts withdrawn? There is strong evidence that synaptic pruning depends on an active response by the presynaptic growth cone to Sema2a, a chemorepulsive molecule secreted by muscle fibers that acts via the PlexinB receptor in motoneurons (Winberg et al., 1998; Ayoob et al., 2006; Carrillo et al., 2010). We hypothesize that Ca2+ entry into the developing motoneuron terminal modulates the cell’s chemorepulsive response to Sema2a. A similar role for neural activity and Ca2+ waves in modulating chemotropic and guidance responses of growth cones has been proposed for vertebrate neurons (Spitzer et al., 2000; Ming et al., 2001; Nicol et al., 2006, 2011; Rosenberg and Spitzer, 2011).
We therefore propose a model where the response of the motoneuron growth cone to muscle-derived Sema2a is episodically modulated in an oscillatory fashion (Figure 2B). When Ca2+-levels in growth cones are low, exploratory filopodia are favored to contact and extend on membrane surfaces. By contrast, during activity bouts, Ca2+- and cAMP levels transiently increase, raising the responsiveness of the neuron to the Sema2a-chemorepellant and withdrawing the less firmly-associated filopodial contacts from off-target surfaces. Thus, presynaptic electrical activity regulates complex molecular interactions in a time-dependent fashion, to modulate the neuron’s responsiveness to chemorepulsion exerted by the muscle fibers. These results provide a coherent picture of the links between neural activity, chemorepulsion, and the refinement of synaptic connectivity.
Molecular Candidates that may be Involved in Activity-Dependent Refinement
Although a crucial role for Ca2+-influx via VGCCs in the withdrawal of off-target neuromuscular contacts has been observed, a role for CICR in synaptic refinement in Drosophila remains untested. CICR is influenced by cAMP (Gomez and Zheng, 2006; Zheng and Poo, 2007) and is required for netrin-1 induced growth cone turning (Hong et al., 2000). Furthermore, filopodial Ca2+ transients have been shown to activate the protease calpain to promote growth cone repulsive turning (Robles et al., 2003). Several calpain genes with neural expression have been identified in Drosophila (Friedrich et al., 2004), and have been associated with Ca2+-dependent dendrite pruning (Kanamori et al., 2013), offering a potential regulatory mechanism for future examination.
Alternative links between neural activity and CaN for synaptic refinement also remain untested, as for example molecular pathways involving the activity-dependent transcription factor AP1. In murine T-cells, CaN dephosphorylates NFAT, a DNA-binding phosphoprotein that forms a complex with Fos and Jun to activate gene transcription (Jain et al., 1993). In cultured mouse primary neurons, the CaN-NFAT signaling is required to promote the netrin-1 dependent axonal outgrowth (Graef et al., 2003). In Drosophila motoneurons, AP1 promotes activity-dependent dendritic growth (Hartwig et al., 2008; Vonhoff et al., 2013) and synaptic plasticity (Sanyal et al., 2002) together with NFAT at the larval NMJ (Freeman et al., 2011). Furthermore, CaN and the GSK-3β kinase homolog Shaggy have been recently described to regulate bouton stabilization at the larval NMJ by activating or inhibiting the microtubule associated protein-1b fly ortholog futsch/MAP-1b, respectively (Wong et al., 2014). Shaggy activates the CaN-regulator Sra in Drosophila eggs (Takeo et al., 2012), and also negatively regulates neuronal AP1 function by inhibiting the JNK pathway, as described in an in vivo genetic screen in Drosophila (Franciscovich et al., 2008). Interestingly, the genes sema2a and fkbp13 (a protein predicted to bind the pharmacological agent FK506, a known inhibitor of CaN) were identified in the same screen among the molecules that regulate AP1 function (Franciscovich et al., 2008). Whether these genes play a role in the activity-dependent withdrawal of ectopic contacts or in the modulation of chemorepulsion remains to be tested.
Biogenic Amines and Refinement
Synaptic connectivity in Drosophila can range from precise targeting, as seen for the glutamatergic motoneurons that limit their connections to just one or two bodywall muscle fibers, to efferents that establish broad projections across the musculature, such as those expressing the biogenic amine octopamine. To what extent are the molecular mechanisms governing guidance and synaptic refinement conserved between these two distinct patterns of synaptic connectivity?
The octopaminergic motoneurons are highly plastic and respond to elevated electrical activity by expanding their peripheral arbors on the musculature (Zhong et al., 1992; Budnik, 1996; Koon et al., 2011). Although the octopaminergic projections are made over a broad expanse of the musculature, the wiring is nevertheless subject to activity-dependent refinement. Over half of the activity-dependent ectopic contacts found on muscle fibers are made by the octopaminergic motoneurons and those ectopic contacts are largely eliminated when neuromuscular activity is normal (Jarecki and Keshishian, 1995; Carrillo et al., 2010; Vonhoff and Keshishian, 2017). Thus similar mechanisms are likely at play to refine the connections made by the glutamatergic motoneurons that project to only one or two muscle fibers and the octopaminergic neurons that project to large regions of the musculature.
Octopamine regulates the activity-dependent plasticity of glutamatergic motoneurons in a paracrine fashion, acting through Octβ2R receptors that regulate cAMP levels at the NMJs (Koon et al., 2011; Koon and Budnik, 2012). It is therefore possible that the octopaminergic efferents are themselves involved in regulating synaptic refinement. Drosophila expresses four distinct octopamine receptors (El-Kholy et al., 2015), including multiple forms that are found in neurons and muscles. As the Drosophila octopamine GPCRs modulate cAMP levels as well as Ca2+ signaling (Balfanz et al., 2005; Evans and Maqueira, 2005; Maqueira et al., 2005; Maiellaro et al., 2016), this raises the possibility that octopamine influences the refinement process by modulating the levels of these second messengers.
Concluding Thoughts
The refinement of synaptic connections often involves Hebbian, spike-timing correlation between synaptic partners, with asynchronous inputs removed (an idea first elaborated by Stent, 1973). This ubiquitous mechanism is involved in topographic map development and synaptic refinement throughout the vertebrate CNS. By contrast, the Drosophila NMJ apparently does not require postsynaptic depolarization for the removal of off-target contacts (Jarecki and Keshishian, 1995; White et al., 2001; Carrillo et al., 2010), suggesting a fundamentally different mechanism for synaptic refinement. Moreover, there is no evidence for competition based on correlated synaptic activity at the Drosophila NMJ, as is the case for refinement in other systems.
At the Drosophila NMJ connectivity is governed by a combinatorial system of recognition molecules expressed by motoneurons and muscles. A correct molecular “match” is needed to stabilize the motoneuronal contact leading to a functional synapse (Furrer and Chiba, 2004; Menon et al., 2013; Carrillo et al., 2015). As noted above, the motoneurons sample among possible synaptic partners, with off-target contacts withdrawn in an activity-dependent fashion. The challenge is to make guidance decisions based on opponent signals that are presented simultaneously: a global chemorepellant signal from all muscles, and a local chemoattractive signal from the target cell. Assuming that the response to the chemorepellant is governed by Ca2+ levels, then the growth cone sampling and withdrawal phases would be coordinated by the Ca2+ oscillations (Figure 2B). We view this mode of error correction as a form of time-dependent signal multiplexing, where the neuron can respond to distinct chemotropic signals depending on the phase of the Ca2+ oscillation. Vital imaging experiments currently underway (Vonhoff and Keshishian, in preparation), are testing whether there is a direct correlation between growth cone motility and the underlying low frequency Ca2+ oscillation.
Author Contributions
FV and HK wrote the manuscript and designed the figures.
Funding
This study was supported by the National Institutes of Health (grant no. 1R21NS053807, 5R01NS031651).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Robert Carrillo, University of Chicago, for helpful comments on the manuscript.
References
- Akerman C. J., Cline H. T. (2007). Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 30, 382–389. 10.1016/j.tins.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Amano M., Nakayama M., Kaibuchi K. (2010). Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67, 545–554. 10.1002/cm.20472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L.-H., Chen W., Yao Y., Ozawa R., Tao E., Yonekura J., et al. (2006). Lim kinase regulates the development of olfactory and neuromuscular synapses. Dev. Biol. 293, 178–190. 10.1016/j.ydbio.2006.01.030 [DOI] [PubMed] [Google Scholar]
- Arroyo D. A., Feller M. B. (2016). Spatiotemporal features of retinal waves instruct the wiring of the visual circuitry. Front. Neural Circuits 10:54. 10.3389/fncir.2016.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoob J. C., Terman J. R., Kolodkin A. L. (2006). Drosophila Plexin B is a Sema-2a receptor required for axon guidance. Development 133, 2125–2135. 10.1242/dev.02380 [DOI] [PubMed] [Google Scholar]
- Azevedo F. A. C., Carvalho L. R. B., Grinberg L. T., Farfel J. M., Ferretti R. E. L., Leite R. E. P., et al. (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532–541. 10.1002/cne.21974 [DOI] [PubMed] [Google Scholar]
- Balfanz S., Strünker T., Frings S., Baumann A. (2005). A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 93, 440–451. 10.1111/j.1471-4159.2005.03034.x [DOI] [PubMed] [Google Scholar]
- Berke B., Wittnam J., McNeill E., Van Vactor D. L., Keshishian H. (2013). Retrograde BMP signaling at the synapse: a permissive signal for synapse maturation and activity-dependent plasticity. J. Neurosci. 33, 17937–17950. 10.1523/JNEUROSCI.6075-11.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. (2012). Calcium signalling remodelling and disease. Biochem. Soc. Trans. 40, 297–309. 10.1042/BST20110766 [DOI] [PubMed] [Google Scholar]
- Blitzer R. D., Connor J. H., Brown G. P., Wong T., Shenolikar S., Iyengar R., et al. (1998). Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 280, 1940–1943. 10.1126/science.280.5371.1940 [DOI] [PubMed] [Google Scholar]
- Budnik V. (1996). Synapse maturation and structural plasticity at Drosophila neuromuscular junctions. Curr. Opin. Neurobiol. 6, 858–867. 10.1016/s0959-4388(96)80038-9 [DOI] [PubMed] [Google Scholar]
- Budnik V., Zhong Y., Wu C. F. (1990). Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J. Neurosci. 10, 3754–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J., Feldheim D. A. (2013). Developmental mechanisms of topographic map formation and alignment. Annu. Rev. Neurosci. 36, 51–77. 10.1146/annurev-neuro-062012-170341 [DOI] [PubMed] [Google Scholar]
- Carrillo R. A., Olsen D. P., Yoon K. S., Keshishian H. (2010). Presynaptic activity and CaMKII modulate retrograde semaphorin signaling and synaptic refinement. Neuron 68, 32–44. 10.1016/j.neuron.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo R. A., Özkan E., Menon K. P., Nagarkar-Jaiswal S., Lee P. T., Jeon M., et al. (2015). Control of synaptic connectivity by a network of Drosophila IgSF cell surface proteins. Cell 163, 1770–1782. 10.1016/j.cell.2015.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba A., Hing H., Cash S., Keshishian H. (1993). Growth cone choices of Drosophila motoneurons in response to muscle fiber mismatch. J. Neurosci. 13, 714–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp S., Evers J. F., Fiala A., Bate M. (2008). The development of motor coordination in Drosophila embryos. Development 135, 3707–3717. 10.1242/dev.026773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr. (1988). Fos and Jun: the AP-1 connection. Cell 55, 395–397. 10.1016/0092-8674(88)90024-4 [DOI] [PubMed] [Google Scholar]
- Doll C. A., Broadie K. (2014). Impaired activity-dependent neural circuit assembly and refinement in autism spectrum disorder genetic models. Front. Cell. Neurosci. 8:30. 10.3389/fncel.2014.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazi F. D., Suzuki S. C., Wong R. O. (2014). Neuronal remodeling in retinal circuit assembly, disassembly, and reassembly. Trends Neurosci. 37, 594–603. 10.1016/j.tins.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch C., Vonhoff F., Ryglewski S. (2008). Dendrite elongation and dendritic branching are affected separately by different forms of intrinsic motoneuron excitability. J. Neurophysiol. 100, 2525–2536. 10.1152/jn.90758.2008 [DOI] [PubMed] [Google Scholar]
- El-Kholy S., Stephano F., Li Y., Bhandari A., Fink C., Roeder T. (2015). Expression analysis of octopamine and tyramine receptors in Drosophila. Cell Tissue Res. 361, 669–684. 10.1007/s00441-015-2137-4 [DOI] [PubMed] [Google Scholar]
- Evans P. D., Maqueira B. (2005). Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert. Neurosci. 5, 111–118. 10.1007/s10158-005-0001-z [DOI] [PubMed] [Google Scholar]
- Fleischmann A., Hvalby O., Jensen V., Strekalova T., Zacher C., Layer L. E., et al. (2003). Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J. Neurosci. 23, 9116–9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciscovich A. L., Mortimer A. D., Freeman A. A., Gu J., Sanyal S. (2008). Overexpression screen in Drosophila identifies neuronal roles of GSK-3β/shaggy as a regulator of AP-1-dependent developmental plasticity. Genetics 180, 2057–2071. 10.1534/genetics.107.085555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A., Franciscovich A., Bowers M., Sandstrom D. J., Sanyal S. (2011). NFAT regulates pre-synaptic development and activity-dependent plasticity in Drosophila. Mol. Cell. Neurosci. 46, 535–547. 10.1016/j.mcn.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich P., Tompa P., Farkas A. (2004). The calpain-system of Drosophila melanogaster: coming of age. Bioessays 26, 1088–1096. 10.1002/bies.20106 [DOI] [PubMed] [Google Scholar]
- Furrer M.-P., Chiba A. (2004). Molecular mechanisms for Drosophila neuronetwork formation. Neurosignals 13, 37–49. 10.1159/000076157 [DOI] [PubMed] [Google Scholar]
- Gomez T. M., Zheng J. Q. (2006). The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 7, 115–125. 10.1038/nrn1844 [DOI] [PubMed] [Google Scholar]
- Graef I. A., Wang F., Charron F., Chen L., Neilson J., Tessier-Lavigne M., et al. (2003). Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell 113, 657–670. 10.1016/s0092-8674(03)00390-8 [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B., Greene L. A. (1986). Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science 234, 80–83. 10.1126/science.3749894 [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Chiba A., Johansen J., Keshishian H. (1991). Growth cone behavior underlying the development of stereotypic synaptic connections in Drosophila embryos. J. Neurosci. 11, 3227–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R., Sabatelli L. M., Seeger M. A. (1996). Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 17, 217–228. 10.1016/s0896-6273(00)80154-3 [DOI] [PubMed] [Google Scholar]
- Hartwig C. L., Worrell J., Levine R. B., Ramaswami M., Sanyal S. (2008). Normal dendrite growth in Drosophila motor neurons requires the AP-1 transcription factor. Dev. Neurobiol. 68, 1225–1242. 10.1002/dneu.20655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Nishiyama M., Henley J., Tessier-Lavigne M., Poo M. (2000). Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403, 93–98. 10.1038/47507 [DOI] [PubMed] [Google Scholar]
- Huberman A. D., Feller M. B., Chapman B. (2008). Mechanisms underlying development of visual maps and receptive fields. Annu. Rev. Neurosci. 31, 479–509. 10.1146/annurev.neuro.31.060407.125533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Miner Z., Kerppola T. K., Lambert J. N., Verdine G. L., et al. (1993). The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 365, 352–355. 10.1038/365352a0 [DOI] [PubMed] [Google Scholar]
- Jarecki J., Keshishian H. (1995). Role of neural activity during synaptogenesis in Drosophila. J. Neurosci. 15, 8177–8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Guan C.-B., Jiang Y.-A., Chen G., Zhao C.-T., Cui K., et al. (2005). Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J. Neurosci. 25, 2338–2347. 10.1523/JNEUROSCI.4889-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J., Halpern M. E., Johansen K. M., Keshishian H. (1989). Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J. Neurosci. 9, 710–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori T., Kanai M. I., Dairyo Y., Yasunaga K.-I., Morikawa R. K., Emoto K. (2013). Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science 340, 1475–1478. 10.1126/science.1234879 [DOI] [PubMed] [Google Scholar]
- Kano M., Hashimoto K. (2009). Synapse elimination in the central nervous system. Curr. Opin. Neurobiol. 19, 154–161. 10.1016/j.conb.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Kasthuri N., Hayworth K. J., Berger D. R., Schalek R. L., Conchello J. A., Knowles-Barley S., et al. (2015). Saturated reconstruction of a volume of neocortex. Cell 162, 648–661. 10.1016/j.cell.2015.06.054 [DOI] [PubMed] [Google Scholar]
- Kater S. B., Shibata A. (1994). The unique and shared properties of neuronal growth cones that enable navigation and specific pathfinding. J. Physiol. Paris 88, 155–163. 10.1016/0928-4257(94)90001-9 [DOI] [PubMed] [Google Scholar]
- Katz L. C., Shatz C. J. (1996). Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138. 10.1126/science.274.5290.1133 [DOI] [PubMed] [Google Scholar]
- Koon A. C., Ashley J., Barria R., DasGupta S., Brain R., Waddell S., et al. (2011). Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat. Neurosci. 14, 190–199. 10.1038/nn.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon A. C., Budnik V. (2012). Inhibitory control of synaptic and behavioral plasticity by octopaminergic signaling. J. Neurosci. 32, 6312–6322. 10.1523/JNEUROSCI.6517-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropouli E., Kolodkin A. L. (2014). Semaphorins and the dynamic regulation of synapse assembly, refinement and function. Curr. Opin. Neurobiol. 27, 1–7. 10.1016/j.conb.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. M., Goodman C. S. (1994). Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron 13, 507–523. 10.1016/0896-6273(94)90022-1 [DOI] [PubMed] [Google Scholar]
- Lnenicka G. A., Spencer G. M., Keshishian H. (2003). Effect of reduced impulse activity on the development of identified motor terminals in Drosophila larvae. J. Neurobiol. 54, 337–345. 10.1002/neu.10133 [DOI] [PubMed] [Google Scholar]
- Lohof A. M., Quillan M., Dan Y., Poo M. M. (1992). Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 12, 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery L. A., Van Vactor D. (2009). The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 10, 332–343. 10.1038/nrm2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiellaro I., Lohse M. J., Kittel R. J., Calebiro D. (2016). cAMP signals in Drosophila motor neurons are confined to single synaptic boutons. Cell Rep. 17, 1238–1246. 10.1016/j.celrep.2016.09.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueira B., Chatwin H., Evans P. D. (2005). Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 94, 547–560. 10.1111/j.1471-4159.2005.03251.x [DOI] [PubMed] [Google Scholar]
- Matthes D. J., Sink H., Kolodkin A. L., Goodman C. S. (1995). Semaphorin II can function as a selective inhibitor of specific synaptic arborizations. Cell 81, 631–639. 10.1016/0092-8674(95)90084-5 [DOI] [PubMed] [Google Scholar]
- McLaughlin T., Torborg C. L., Feller M. B., O’Leary D. D. M. (2003). Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160. 10.1016/s0896-6273(03)00790-6 [DOI] [PubMed] [Google Scholar]
- Meng Y., Zhang Y., Tregoubov V., Janus C., Cruz L., Jackson M., et al. (2002). Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron 35, 121–133. 10.1016/s0896-6273(02)00758-4 [DOI] [PubMed] [Google Scholar]
- Menon K. P., Carrillo R. A., Zinn K. (2013). Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdiscip. Rev. Dev. Biol. 2, 647–670. 10.1002/wdev.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. L. (1982). Tetrodotoxin blocks the formation of ocular dominance columns in goldfish. Science 218, 589–591. 10.1126/science.7123262 [DOI] [PubMed] [Google Scholar]
- Ming G.-L., Henley J., Tessier-Lavigne M., Song H.-J., Poo M.-M. (2001). Electrical activity modulates growth cone guidance by diffusible factors. Neuron 29, 441–452. 10.1016/s0896-6273(01)00217-3 [DOI] [PubMed] [Google Scholar]
- Monastirioti M. (1999). Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc. Res. Tech. 45, 106–121. [DOI] [PubMed] [Google Scholar]
- Monastirioti M., Gorczyca M., Rapus J., Eckert M., White K., Budnik V. (1995). Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J. Comp. Neurol. 356, 275–287. 10.1002/cne.903560210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M., Linn C. E., Jr., White K. (1996). Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 16, 3900–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. L., Soto F., Wong R. O., Kerschensteiner D. (2011). Development of cell type-specific connectivity patterns of converging excitatory axons in the retina. Neuron 71, 1014–1021. 10.1016/j.neuron.2011.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca T. J., Carrillo R. A., White B. H., Keshishian H. (2005). Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc. Natl. Acad. Sci. U S A 102, 3477–3482. 10.1073/pnas.0406164102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca T. J., Hong W., Dani V. S., Favaloro V., Luo L. (2012). Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature 484, 237–241. 10.1038/nature10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X., Hong K. P., Spitzer N. C. (2011). Spatial and temporal second messenger codes for growth cone turning. Proc. Natl. Acad. Sci. U S A 108, 13776–13781. 10.1073/pnas.1100247108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X., Muzerelle A., Rio J. P., Métin C., Gaspar P. (2006). Requirement of adenylate cyclase 1 for the ephrin-A5-dependent retraction of exuberant retinal axons. J. Neurosci. 26, 862–872. 10.1523/JNEUROSCI.3385-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X., Voyatzis S., Muzerelle A., Narboux-Nême N., Südhof T. C., Miles R., et al. (2007). cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat. Neurosci. 10, 340–347. 10.1038/nn1842 [DOI] [PubMed] [Google Scholar]
- Nishiyama M., Hoshino A., Tsai L., Henley J. R., Goshima Y., Tessier-Lavigne M., et al. (2003). Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 423, 990–995. 10.1038/nature01751 [DOI] [PubMed] [Google Scholar]
- Nose A. (2012). Generation of neuromuscular specificity in Drosophila: novel mechanisms revealed by new technologies. Front. Mol. Neurosci. 5:62. 10.3389/fnmol.2012.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Mahajan V. B., Goodman C. S. (1992). Connectin: a homophilic cell adhesion molecule expressed on a subset of muscles and the motoneurons that innervate them in Drosophila. Cell 70, 553–567. 10.1016/0092-8674(92)90426-d [DOI] [PubMed] [Google Scholar]
- Oliver C. J., Shenolikar S. (1998). Physiologic importance of protein phosphatase inhibitors. Front. Biosci. 3, D961–D972. 10.2741/a336 [DOI] [PubMed] [Google Scholar]
- Pereanu W., Spindler S., Im E., Buu N., Hartenstein V. (2007). The emergence of patterned movement during late embryogenesis of Drosophila. Dev. Neurobiol. 67, 1669–1685. 10.1002/dneu.20538 [DOI] [PubMed] [Google Scholar]
- Pratt K. G., Hiramoto M., Cline H. T. (2016). An evolutionarily conserved mechanism for activity-dependent visual circuit development. Front. Neural Circuits 10:79. 10.3389/fncir.2016.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Godino L. L., Diegelmann S., Bate M. (2012). Embryonic origin of olfactory circuitry in Drosophila: contact and activity-mediated interactions pattern connectivity in the antennal lobe. PLoS Biol. 10:e1001400. 10.1371/journal.pbio.1001400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. (1980). Elimination of synapses in the developing nervous system. Science 210, 153–157. 10.1126/science.7414326 [DOI] [PubMed] [Google Scholar]
- Robles E., Huttenlocher A., Gomez T. M. (2003). Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron 38, 597–609. 10.1016/s0896-6273(03)00260-5 [DOI] [PubMed] [Google Scholar]
- Rosenberg S. S., Spitzer N. C. (2011). Calcium signaling in neuronal development. Cold Spring Harb. Perspect. Biol. 3:a004259. 10.1101/cshperspect.a004259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Cañada C., Budnik V. (2006). Introduction on the use of the Drosophila embryonic/larval neuromuscular junction as a model system to study synapse development and function and a brief summary of pathfinding and target recognition. Int. Rev. Neurobiol. 75, 1–31. 10.1016/s0074-7742(06)75001-2 [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Lichtman J. W. (2001). Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805. 10.1038/35097557 [DOI] [PubMed] [Google Scholar]
- Sanyal S., Sandstrom D. J., Hoeffer C. A., Ramaswami M. (2002). AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature 416, 870–874. 10.1038/416870a [DOI] [PubMed] [Google Scholar]
- Sekar A., Bialas A. R., de Rivera H., Davis A., Hammond T. R., Kamitaki N., et al. (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. 10.1038/nature16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. (1988). Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science 242, 87–89. 10.1126/science.3175636 [DOI] [PubMed] [Google Scholar]
- Shishido E., Takeichi M., Nose A. (1998). Drosophila synapse formation: regulation by transmembrane protein with Leu-rich repeats, CAPRICIOUS. Science 280, 2118–2121. 10.1126/science.280.5372.2118 [DOI] [PubMed] [Google Scholar]
- Sink H., Whitington P. M. (1991). Pathfinding in the central nervous system and periphery by identified embryonic Drosophila motor axons. Development 112, 307–316. [DOI] [PubMed] [Google Scholar]
- Song H. J., Ming G. L., Poo M. M. (1997). cAMP-induced switching in turning direction of nerve growth cones. Nature 388, 275–279. 10.1038/40864 [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Lautermilch N. J., Smith R. D., Gomez T. M. (2000). Coding of neuronal differentiation by calcium transients. Bioessays 22, 811–817. [DOI] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J., Stryker M. P. (1988). Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature 336, 468–471. 10.1038/336468a0 [DOI] [PubMed] [Google Scholar]
- Stent G. S. (1973). A physiological mechanism for Hebb’s postulate of learning. Proc. Natl. Acad. Sci. U S A 70, 997–1001. 10.1073/pnas.70.4.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S., Swanson S. K., Nandanan K., Nakai Y., Aigaki T., Washburn M. P., et al. (2012). Shaggy/glycogen synthase kinase 3β and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis. Proc. Natl. Acad. Sci. U S A 109, 6382–6389. 10.1073/pnas.1120367109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Gudsnuk K., Kuo S. H., Cotrina M. L., Rosoklija G., Sosunov A., et al. (2014). Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83, 1131–1143. 10.1016/j.neuron.2014.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi K., von Schimmelmann M. J., Nishiyama M., Lim C. S., Yoshida N., Yun B., et al. (2008). Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron 58, 694–707. 10.1016/j.neuron.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Vonhoff F., Keshishian H. (2017). Cyclic nucleotide signaling is required during synaptic refinement at the Drosophila neuromuscular junction. Dev. Neurobiol. 77, 39–60. 10.1002/dneu.22407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonhoff F., Kuehn C., Blumenstock S., Sanyal S., Duch C. (2013). Temporal coherency between receptor expression, neural activity and AP-1-dependent transcription regulates Drosophila motoneuron dendrite development. Development 140, 606–616. 10.1242/dev.089235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Hamby A. M., Zhou K., Feller M. B. (2011). Development of asymmetric inhibition underlying direction selectivity in the retina. Nature 469, 402–406. 10.1038/nature09600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. H., Osterwalder T. P., Yoon K. S., Joiner W. J., Whim M. D., Kaczmarek L. K., et al. (2001). Targeted attenuation of electrical activity in Drosophila using a genetically modified K+ channel. Neuron 31, 699–711. 10.1016/s0896-6273(01)00415-9 [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. (1963). Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J. Neurophysiol. 26, 978–993. [DOI] [PubMed] [Google Scholar]
- Winberg M. L., Mitchell K. J., Goodman C. S. (1998). Genetic analysis of the mechanisms controlling target selection: complementary and combinatorial functions of netrins, semaphorins and IgCAMs. Cell 93, 581–591. 10.1016/s0092-8674(00)81187-3 [DOI] [PubMed] [Google Scholar]
- Wong R. O. (1999). Retinal waves and visual system development. Annu. Rev. Neurosci. 22, 29–47. 10.1146/annurev.neuro.22.1.29 [DOI] [PubMed] [Google Scholar]
- Wong C. O., Chen K., Lin Y. Q., Chao Y., Duraine L., Lu Z., et al. (2014). A TRPV channel in Drosophila motor neurons regulates presynaptic resting Ca2+ levels, synapse growth, and synaptic transmission. Neuron 84, 764–777. 10.1016/j.neuron.2014.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Leinwand S. G., Dell A. L., Fried-Cassorla E., Raper J. A. (2010). The calmodulin-stimulated adenylate cyclase ADCY8 sets the sensitivity of zebrafish retinal axons to midline repellents and is required for normal midline crossing. J. Neurosci. 30, 7423–7433. 10.1523/JNEUROSCI.0699-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., López-Bendito G. (2012). Shaping brain connections through spontaneous neural activity. Eur. J. Neurosci. 35, 1595–1604. 10.1111/j.1460-9568.2012.08101.x [DOI] [PubMed] [Google Scholar]
- Yonehara K., Balint K., Noda M., Nagel G., Bamberg E., Roska B. (2011). Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature 469, 407–410. 10.1038/nature09711 [DOI] [PubMed] [Google Scholar]
- Zheng J. Q., Poo M. M. (2007). Calcium signaling in neuronal motility. Annu. Rev. Cell Dev. Biol. 23, 375–404. 10.1146/annurev.cellbio.23.090506.123221 [DOI] [PubMed] [Google Scholar]
- Zhong Y., Budnik V., Wu C. F. (1992). Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J. Neurosci. 12, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]