Abstract
Adiponectin demonstrates beneficial effects in various metabolic diseases, including diabetes, and in bowel cancer. Recent data also suggest a protective role in colitis. However, the precise molecular mechanisms by which adiponectin and its receptors modulate colitis and the nature of the adaptive immune response in murine models are yet to be elucidated. Adiponectin knock-out mice were orally administered dextran sulfate sodium for 7 days and were compared with wild-type mice. The severity of disease was analyzed histopathologically and through cytokine profiling. HCT116 colonic epithelial cells were employed to analyze the in vitro effects of adiponectin and AdipoR1 interactions in colonic injury following dextran sulfate sodium treatment. Adiponectin knock-out mice receiving dextran sulfate sodium exhibited severe colitis, had greater inflammatory cell infiltration, and an increased presence of activated B cells compared with controls. This was accompanied by an exaggerated proinflammatory cytokine profile and increased STAT3 signaling. Adiponectin knock-out mouse colons had markedly reduced proliferation and increased epithelial apoptosis and cellular stress. In vitro, adiponectin reduced apoptotic, anti-proliferative, and stress signals and restored STAT3 signaling. Following the abrogation of AdipoR1 in vitro, these protective effects of adiponectin were abolished. In summary, adiponectin maintains intestinal homeostasis and protects against murine colitis through interactions with its receptor AdipoR1 and by modulating adaptive immunity.
Keywords: adiponectin, autoimmunity, colitis, obesity, STAT3
Introduction
The intestinal epithelial cell (IEC)2 layer is in a state of constant renewal. New IECs are formed in the stem cell compartment at the base of intestinal crypts and then migrate to the villus tip and are subsequently shed after they undergo apoptosis. Simultaneously, these cells also react to inflammatory cues from gut bacteria and the host immune response. In healthy individuals, an intact IEC layer provides a protective barrier to limit the transfer of undigested intestinal contents and gut bacteria. In contrast, in disease, IEC apoptosis outweighs proliferation leading to disruption of the IEC layer as seen in inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn's disease (CD). Importantly, IBD is a significant cause of morbidity with near 51,000 deaths in 2013, increasing prevalence in Europe, Asia, and the United States, and a significant economic burden (1–4).
IBD, and importantly ulcerative colitis, have been labeled as immune-mediated diseases driven by autoreactive T cells due to dysfunction of tolerogenic mechanisms (5, 6). However, B cells also have a role in mucosal immune responses, secreting immunoglobulin A, which assists in the maintenance of barrier function, as well as reducing immune responses to the local microbiota (7–10). Additionally, evidence suggests that B cells promote intestinal epithelial damage (9).
The pathogenesis of IBD is complex and multifactorial, including both genes and environment and their interactions (11–13). Clinical studies demonstrate strong associations between increased body mass index (14–16) and IBD risk, as also for clinical complications (17, 18). Interestingly, inversely associated with body mass index levels are reduced serum levels of the adipocytokine, adiponectin (19). Adiponectin (APN) signals through two receptors AdipoR1 and AdipoR2 and numerous studies now establish a protective role for this protein against metabolic diseases such as type 2 diabetes and atherosclerosis (20–23). Adiponectin also modulates inflammatory responses in a number of gut diseases, including inflammatory bowel disease and colorectal cancer (24, 25). However, it remains unresolved how APN influences IEC survival and modulates immune responses during colitis.
Here, using the dextran sulfate sodium (DSS) model and APN knock-out (APN-KO) mice, we assessed the function of APN in colitis. Significantly, we observed in APN-KO mice increased IEC damage, B-cell accumulation, and up-regulated STAT3 signaling. These effects were modulated through its receptor AdipoR1. Our results suggest that limited levels of APN modulate colitis progression through multiple actions.
Results
APN absence promotes colitis and dysregulates epithelial homeostasis
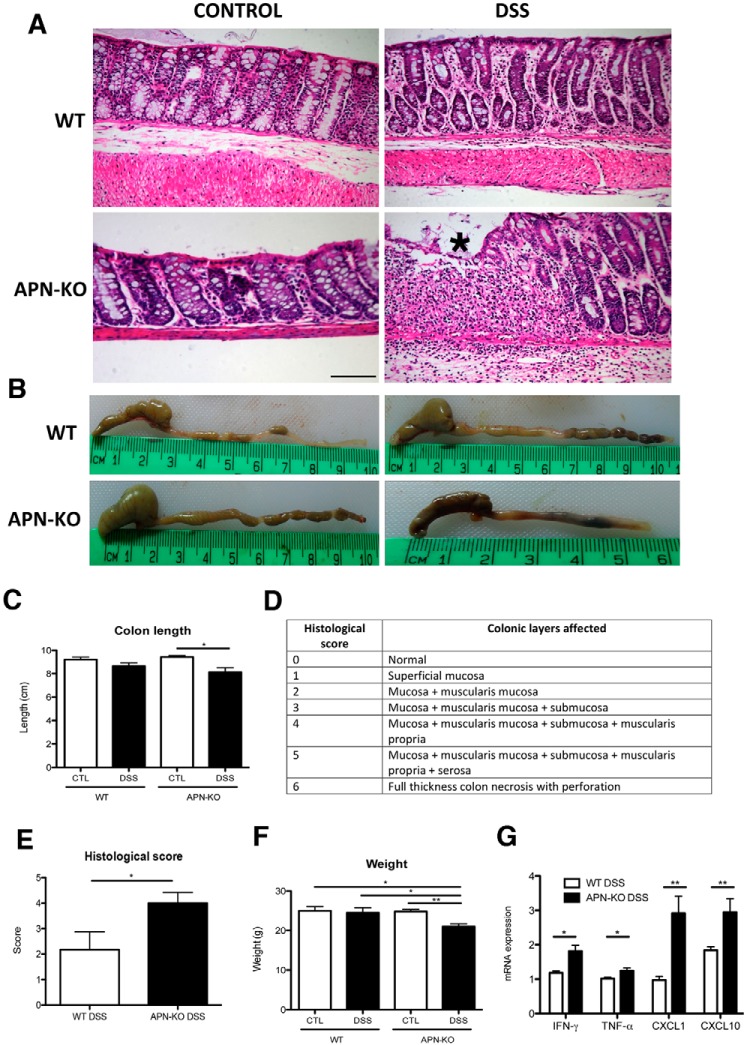
As would be expected from the known anti-inflammatory effects of adiponectin, colonic pathology analyses of APN-KO mice treated with DSS revealed distorted epithelial crypt architecture accompanied by a significant immune cell infiltrate compared with all other groups (WT control, WT DSS, and APN-KO control; Fig. 1A). A histological scoring system was developed and KO mice had 2-fold greater colon damage than their WT counterparts (Fig. 1, D and E). APN-KO mice had reduced colon length (p < 0.05) (Fig. 1B and C), reduced body weight (p < 0.01)(Fig. 1F), and increased rectal bleeding compared with controls (data not shown). Through quantitative real-time (qRT)-PCR it was found that APN-KO DSS-treated mice had increased expression of the proinflammatory cytokines IFN-γ (2-fold; p < 0.05), TNF-α (1.3-fold; p < 0.05), CXCL1 (3-fold; p < 0.01), and CXCL10 (1.5-fold; p < 0.01, Fig. 1G). Hence, APN-KO mice have greater susceptibility to DSS-induced colitis and an enhanced colonic inflammatory response.
Figure 1.
APN-KO mice are more susceptible to DSS-colitis than WT mice. A, H&E histology from WT and APN-KO of the descending colon, asterisk (*) shows inflammation and architectural distortion. B, representative images of colonic shortening in APN-KO colitic mice, and C, colonic length reduction following DSS between all groups. D, a histological scoring system used to evaluate the mouse cohorts, and E, APN-KO mice had 2-fold more damage that WT. F, reduced weight after DSS treatment in APN-KO colitic mice compared with other groups. G, proinflammatory cytokine profile of APN-KO colitic mice compared with WT colitic mice, showing an increase in IFN-γ and TNF-α (p < 0.05 for both groups), CXCL1 and CXCL10 (p < 0.01 for both groups). Scale bar, 100 μm. *, p < 0.05, ** p < 0.01.
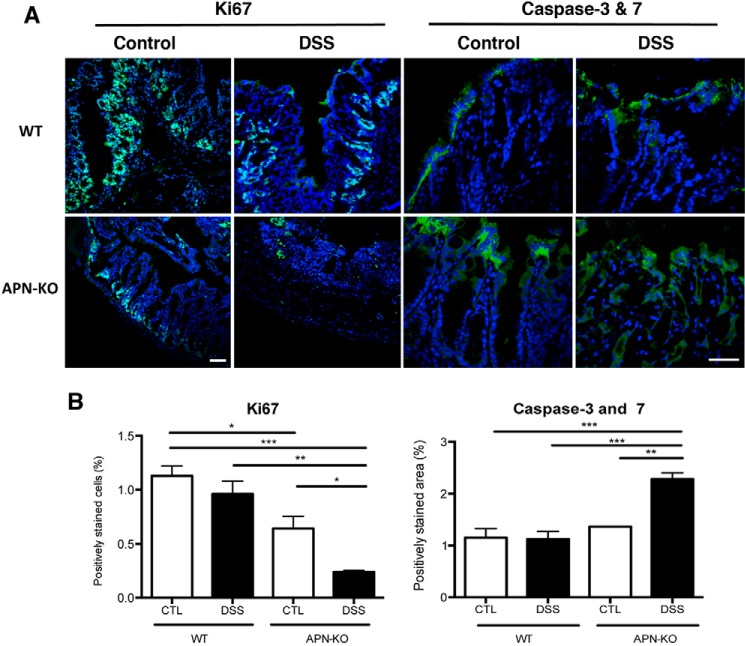
We next examined for markers of cell proliferation and apoptosis in WT and APN-KO mice treated with DSS. Immunofluorescence for proliferation with anti-Ki67 antibodies, and for apoptosis with FLICATM to detect caspase-3 and −7 activity showed a 5-fold decrease in Ki67 and a 2.3-fold increase in caspase-3 and -7 staining (p < 0.001 for both) in APN-KO DSS groups compared with WT controls (Fig. 2, A and B). Analysis of stress pathways by Western blot analysis confirmed increased phosphorylation of p38 MAPK (1.6-fold; p < 0.01) and ERK1/2 (5-fold; p < 0.05) in APN-KO DSS colons compared with controls. This was accompanied by reductions in PI3K (2.4-fold; p < 0.01) and Akt (3-fold; p < 0.001) compared with that in controls (Fig. 3, A–E). Taken together, these data suggest that APN absence, accompanied by DSS treatment, reduces proliferation and increases epithelial apoptosis and promotes cellular stress.
Figure 2.
APN-KO DSS colons have reduced proliferation and increased apoptosis. A, immunofluorescent images of Ki67 or caspases-3 and -7 (green) and DAPI (blue) in WT control, WT DSS, APN-KO control, and APN-KO DSS colons. B, analysis of Ki67 staining showing a reduction in APN-KO DSS-treated mice versus WT controls (p < 0.001), WT DSS (p < 0.01), and APN-KO controls (p < 0.05), as well as lower Ki67 in APN-KO control mouse colons compared with WT controls (p < 0.05). Analysis of caspase-3 and -7 with an increase in APN-KO colons compared with WT controls, WT DSS and APN-KO controls (p < 0.001 for all groups). Scale bar, 50 μm. *, p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 3.
APN-KO colitic mice exhibit increased stress signaling, an altered APN receptor profile, and APN co-localizes with AdipoR1. A, Western blots of proliferative and cellular stress markers in APN-KO DSS colons versus APN-KO controls. Densitometry analysis of: B, p-p38 MAPK demonstrates an increase in APN-KO DSS mice compared with APN-KO controls (p < 0.01); C, p-ERK1/2 showing an increase in APN-KO DSS mice versus APN-KO controls (p < 0.05); D, PI3K showing a reduction in APN-KO DSS colonic protein versus APN-KO controls (p < 0.01); E, p-Akt showing a reduction in APN-KO DSS-treated mice compared with APN-KO controls (p < 0.001); F, AdipoR1 showing an increase in APN-KO DSS versus APN-KO control (p < 0.001); and G, AdipoR2 showing a reduction in APN-KO DSS compared with APN-KO controls (p < 0.05). H, immunofluorescence ×60 image of the colon showing APN (green), AdipoR1 (red), and DAPI (blue). Scale bar, 25 μm. White arrows indicate co-localization of APN and AdipoR1. *, p < 0.05, ** p < 0.01, *** p < 0.001.
APN mediates protection through AdipoR1
To understand how APN affects cellular signaling in the colon, we next examined for expression of adiponectin receptors AdipoR1 and -R2. By Western blot analysis APN-KO mice with DSS colitis had a 3-fold (p < 0.001) increase in AdipoR1 protein, whereas AdipoR2 was reduced 2-fold compared with controls (p < 0.05; Fig. 3, A, F, and G). Considering the significant increase in total AdipoR1 protein expression in APN-KO colitic mouse colons, we assessed the association between APN and AdipoR1 by immunofluorescence to ascertain functional localization within the gut wall. As expected, APN and AdipoR1 co-localized to the luminal interface, and within the IECs (Fig. 3H).
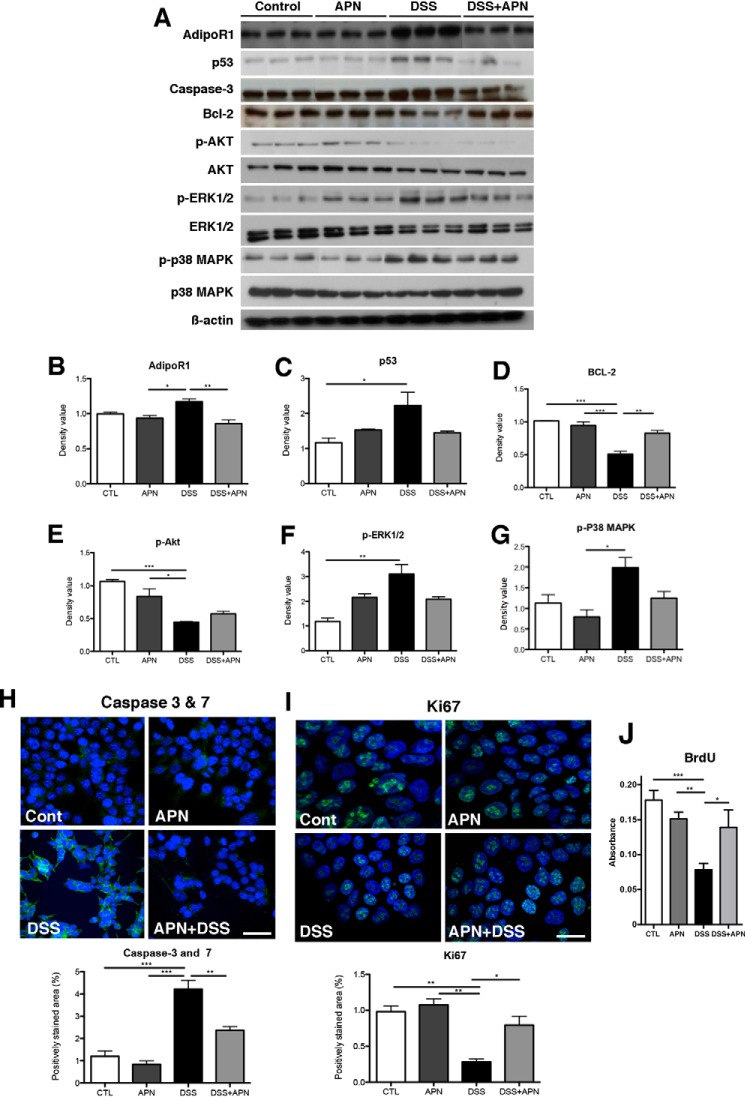
To test the importance of APN and AdipoR1 interactions in DSS-induced colitis, HCT116 epithelial cells were utilized in cellular assays. DSS treatment increased protein expression of AdipoR1 and the subsequent addition of APN reduced this 1.3-fold (p < 0.01). Likewise, protein levels of modulators of cellular stress and apoptosis: p53, p-ERK1/2, and p-p38 MAPK, increased following DSS treatment. The addition of APN reduced their levels by 1.8-, 1.6-, and 2.5-fold, respectively, compared with DSS treatment alone, although this failed to achieve statistical significance. Likewise, anti-apoptotic Bcl-2 levels decreased after DSS application and increased 1.8-fold after APN treatment (p < 0.05 in both instances; Fig. 4, A–G). Subsequent immunofluorescence analysis for Caspase-3 and -7, and Ki67, and BrdU incorporation confirmed that DSS promoted HCT116 apoptosis and reduced proliferation. The addition of APN reduced cell death and promoted mitosis (Fig. 4, H, I, and J).
Figure 4.
APN is important in mediating epithelial homeostasis in an in vitro model of DSS colitis. A, Western blots showing cellular stress markers following DSS treatment, and the restoration of balance in HCT116 colonic epithelial cells when APN is present with DSS. Densitometry analysis showing: B, AdipoR1, with an increase in DSS-treated cells compared with APN treatment (p < 0.05), and DSS + APN treatment (p < 0.01); C, p53, with an increase in DSS treatment compared with controls (p < 0.05); D, Bcl-2, with a significant reduction in DSS-treated cells compared with controls (p < 0.001), APN (p < 0.001), and DSS + APN-treated cells (p < 0.01); E, p-Akt, with a reduction in DSS treated cells compared with controls (p < 0.001) and APN- treated cells (p < 0.05); F, p-ERK1/2, with a significant increase in DSS treatment compared with controls (p < 0.01); and G, p-p38 MAPK, with an increase in DSS treatment compared with controls (p < 0.01). H, immunofluorescent images showing DAPI (blue) and caspases-3 and -7 (green) in HCT116 cells, where APN reduces apoptosis in the presence of DSS. Analysis of caspase-3 and -7 staining showing an increase in DSS treatment versus controls, and APN-treated cells (p < 0.01 for both groups), and a significant decrease in DSS + APN-treated cells, compared with DSS alone (p < 0.01). I, immunofluorescent images showing DAPI (blue) and Ki67 (green) in HCT 116 cells, where APN promotes proliferation in the presence of DSS. Analysis of Ki67 staining showing a reduction after DSS treatment versus controls and APN-treated cells (p < 0.01 for both groups), and a significant restoration in DSS + APN-treated cells, compared with DSS alone (p < 0.05). Scale bar, 25 μm. J, BrdU incorporation assays confirmed reduced proliferation after DSS treatment versus controls and APN-treated cells (p < 0.001 and 0.01, respectively), and that the addition of APN to DSS-treated cells rescued proliferation, compared with DSS alone (p < 0.05). *, p < 0.05, ** p < 0.01, *** p < 0.001.
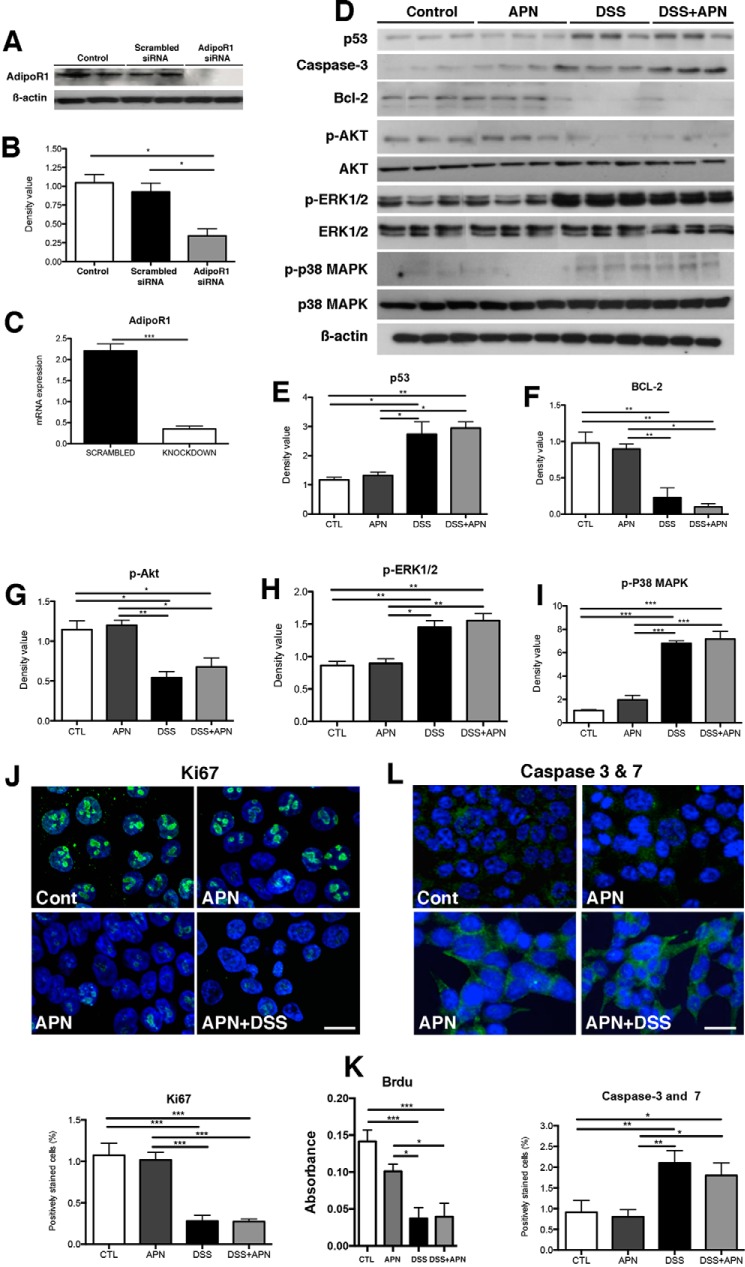
To further confirm that AdipoR1 was responsible for mediating the protective actions of APN, siRNA was used to knock-down this receptor in vitro. Western blot and qPCR for AdipoR1 confirmed reduced protein and gene expression by nearly 70% (Fig. 5, A–C). In the context of reduced levels of AdipoR1, APN could not counteract the actions of DSS with respect to p53, Bcl-2, p-AKT, p-ERK1/2, and p-p38 MAPK, as they were unchanged after APN treatment (Fig. 5, D–I). Furthermore, and consistent with the earlier data, immunofluorescence for Ki67 and Caspase-3 and -7, and BrdU incorporation confirmed that in the absence of AdipoR1, APN could not promote proliferation or limit apoptosis (Fig. 5, J–L). Taken together, these data suggest that in the DSS model, adiponectin, through AdipoR1, mediates HCT116 cell survival.
Figure 5.
APN mediates its homeostatic/restorative effects in vitro via interactions with its receptor, AdipoR1. A, Western blot confirming AdipoR1 knockdown via siRNA in HCT116 colonic epithelial cells. B, densitometry analysis of AdipoR1 following knockdown, showing a significant reduction in the siRNA-treated cells compared with scrambled conditions and controls (p < 0.05 for both groups). C, qRT-PCR analysis confirming knockdown of the AdipoR1 gene in the knockdown groups versus scrambled (p < 0.001). D, Western blots showing the lack of restorative effects of APN following AdipoR1 knockdown in HCT116 cells. Densitometry analysis following siRNA-mediated AdipoR1 knockdown in HCT116 colonic epithelial cells, showing p53 (E), with significant increases in DSS treatment compared with control and APN-treated cells (p < 0.05 for both), as well as in DSS + APN treatment compared with controls (p < 0.01) and APN-treated cells (p < 0.05); F, Bcl-2, with a significant reduction in DSS-treated cells compared with control and APN groups (p < 0.01 for both), as well as a reduction in DSS + APN-treated cells compared with controls and APN-treated cells (p < 0.01 and 0.05, respectively); G, p-Akt, with a significant reduction in DSS-treated cells compared with controls (p < 0.05) and APN-treated cells (p < 0.01), as well as DSS + APN-treated cells compared with controls and APN treatment alone (p < 0.05 for both groups); H, p-ERK1/2 with significant increases in DSS-treated cells compared with controls and APN-treated cells (p < 0.01 and 0.05, respectively), as well as DSS + APN-treated cells compared with controls and APN-treated groups (p < 0.01 for both comparisons); and I, p-p38 MAPK, with a significant increase in DSS-treated cells compared with control and APN treatment (p < 0.001 for both groups), as well as in DSS + APN treatment compared with controls and APN treatment alone (p < 0.001 for both groups). J, immunofluoresence images of DAPI (blue) and Ki67 (green) in HCT116 cells with AdipoR1 ablation, showing a reduction in proliferation with DSS treatment, and a lack of restoration with DSS + APN treatment. Analysis of immunofluorescence showing a reduction of Ki67 with DSS treatment compared with controls and APN treatment alone (p < 0.001 for both groups), as well as with DSS + APN conditions compared with controls and APN treatment alone (p < 0.001 for both groups). K, BrdU analyses confirmed reduced proliferation with DSS and DSS + APN treatment after AdipoR1 siRNA treatment. Proliferation with DSS treatment was reduced compared with controls and APN treatment alone (p < 0.001 for both groups), as well as with DSS + APN compared with controls and APN treatment alone (p < 0.001 and 0.05, respectively). L, immunofluorescence of DAPI (blue) and caspase-3 and -7 (green) illustrating an increase in apoptosis following DSS treatment in HCT 116 cells with AdipoR1 ablation, and the absence of a reduction in apoptosis following APN treatment in the presence of DSS. Analysis of immunofluorescence showing an increase in caspases-3 and -7 in DSS treatment compared with controls and APN-treated cells (p < 0.01 for both groups), as well as in DSS + APN groups compared with controls and APN-treated cells (p < 0.05 for both groups). Scale bar, 25 μm. *, p < 0.05, ** p < 0.01, *** p < 0.001.
APN-KO colons and spleens exhibit a predominant B cell infiltrate, suggesting immune-mediated disease
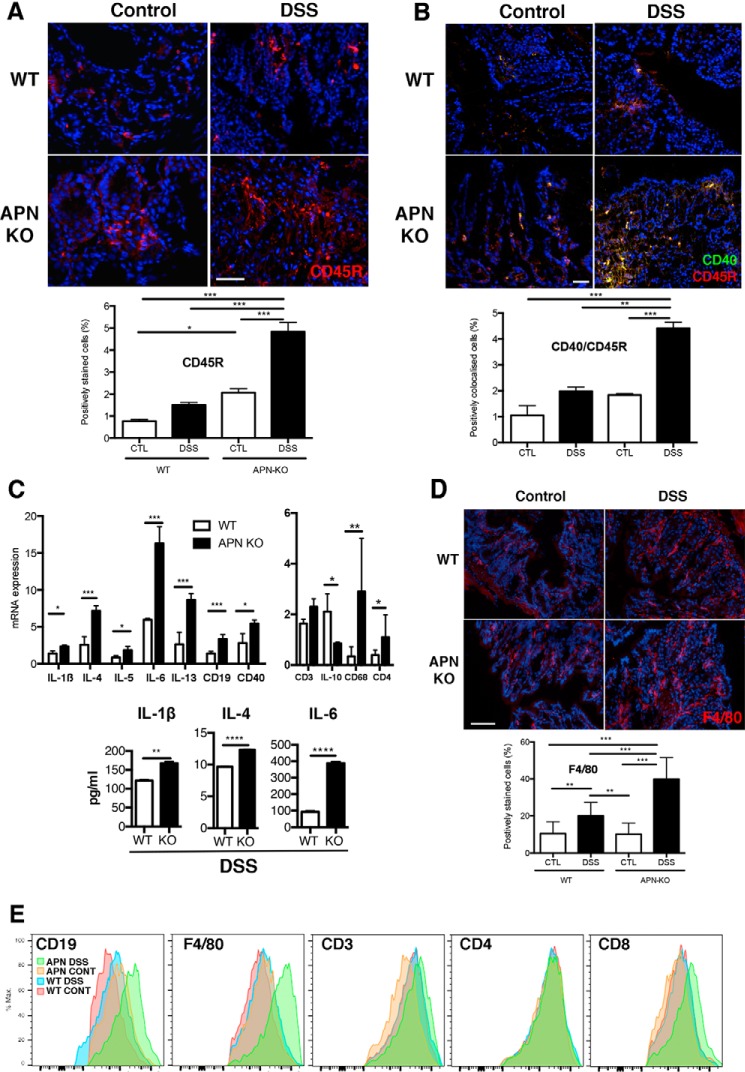
Adiponectin promotes anti-inflammatory responses in various disease models (26). We therefore characterized the colonic immune cell profile of APN-KO and WT littermates. Interestingly, as shown by CD45R staining and dual staining for CD45R and CD40, APN-KO DSS-treated mice had increased numbers of B cells and an activated B-cell infiltrate as indicated by CD45R and CD40 co-localization, which was 2-fold less in WT mice (Fig. 6, A and B). This observation was confirmed by qRT-PCR analysis showing significantly greater gene expression of CD19 and CD40 in APN-KO colitic mice compared with WT mice treated with DSS (p < 0.05 and p < 0.01, respectively; Fig. 6C). Moreover, additional characterization of inflammation showed a cytokine profile in APN-KO colitic mice that was suggestive of B-cell proliferation, with increased IL-1β (2-fold), IL-4 (4-fold), IL-5 (2-fold), and IL-13 (2.6-fold; p < 0.05, p < 0.001, p < 0.05, p < 0.001, respectively; Fig. 6C). Furthermore, ELISA of tissue homogenates confirmed increases in IL-1β (1.4-fold), IL-4 (1.3-fold), and IL-6 (5-fold; p < 0.01, p < 0.0001, p < 0.0001, respectively).
Figure 6.
B cell infiltration is associated with inflammation in APN-KO mice treated with DSS. A, CD45 staining (red) was increased in APN-KO DSS colons compared with controls and WT DSS groups (p < 0.001 for all comparisons). APN-KO control mice had increased CD45R staining compared WT control mice (p < 0.05). B, DAPI (blue), CD45 (red), and CD40 (green) co-localization in WT control, WT DSS, APN-KO control, and APN-KO DSS colons. Analysis showed increased CD40/CD45R colocalization in the APN-KO DSS treatment versus controls and WT DSS (p < 0.01 and 0.001). C, inflammatory cytokine profile by qRT-PCR analysis in WT and APN-KO mice treated with DSS, showing increases in IL-1β (p < 0.05), IL-4 (p < 0.001), IL-5 (p < 0.05), IL-6 (p < 0.001), IL-13 (p < 0.001), the B cell marker CD19 (p < 0.001), activation marker CD40 (p < 0.05), and CD68 (p < 0.001). CD3 was not significantly changed and IL-10 was reduced (p < 0.01). CD4, a T helper cell marker, was increased (p < 0.05). Tissue ELISA showed increases in IL-1β (p < 0.01), IL-4 (p < 0.0001), and IL-6 (p < 0.0001). D, F4/80 staining (red) was increased in APN-KO DSS mice compared with controls and WT DSS groups (**, p < 0.01; ***, p < 0.001). F4/80 staining was increased in WT DSS mice when compared with WT and APN control groups (p < 0.01 all groups). Scale bar, 25 μm. E, flow cytometry of digested colons showed increased staining for CD19, F4/80, CD3, CD4, and CD8. *, p < 0.05.
As colitis is associated with enhanced macrophage activity and has been suggested to be a T-cell-mediated disease, we next examined for these changes. Markers of macrophages were increased in APN-KO DSS mice as determined by CD68 gene expression (8.4-fold) and F4/80 immunofluorescence (2-fold) when compared with WT mice treated with DSS. Significantly, by qPCR we observed increases in CD3 and CD4 gene expression (1.4-fold, ns, and 2.5-fold, p < 0.05, respectively), and a significant decrease in IL-10 (2.4-fold; p < 0.05) gene expression in the APN-KO versus WT DSS groups (2-fold; p < 0.05; Fig. 6, C and D). Moreover, through flow cytometry increased staining was observed for immune cells expressing CD19, F4/80, CD3, CD4, and CD8 from APN-KO colons treated with DSS versus all groups (Fig. 6E).
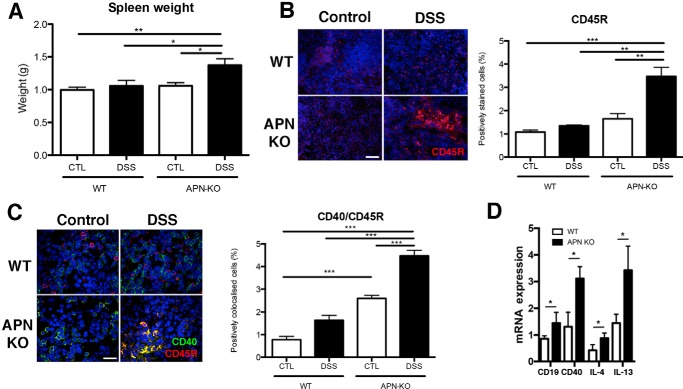
Given that B cell activation occurs in secondary lymphoid organs, we examined splenic size and B cell activation status. Importantly, we found that APN-KO DSS mice had spleens 1.3-fold heavier than WT DSS (p < 0.05; Fig. 7A). Moreover, by immunofluorescence, APN-KO colitic murine spleens expressed 3-fold more CD45 and had 2.8-fold greater colocalization of CD40 and CD45R, than the WT murine DSS group (Fig. 7, B and C; p < 0.01 and p < 0.001, respectively). Additionally, the gene expression of CD19, CD40, and IL-4 and IL-13 was increased in APN-KO DSS mice versus WT DSS mice (all p < 0.05). Taken together, these data suggest that APN-KO mice treated with DSS had an enhanced B cell inflammatory response.
Figure 7.
Enhanced splenic B cell response in DSS-colitis in APN-KO mice. A, spleens of APN-KO DSS-treated mice were heavier than WT controls, DSS-treated groups, and APN-KO controls. Immunofluorescent analyses of the spleens confirmed that: B, CD45R staining (red) was increased in APN-KO DSS mice compared with controls and WT DSS groups (p < 0.01 and p < 0.0001). C, CD40 (green) and CD45R (red) colocalization was increased in APN-KO DSS versus WT DSS colons (p < 0.001). APN-KO DSS colons had increased CD40/CD45R staining versus WT and APN-KO control groups (p < 0.001 all comparisons). Scale bar, 25 μm. D, splenic inflammatory cytokine profile by qRT-PCR analysis in WT and APN-KO treated with DSS, shows increases in CD19 (p < 0.05), CD40 (p < 0.001), IL-4 (p < 0.05), and IL-13 (p < 0.05). *, p < 0.05, ** p < 0.01, *** p < 0.001.
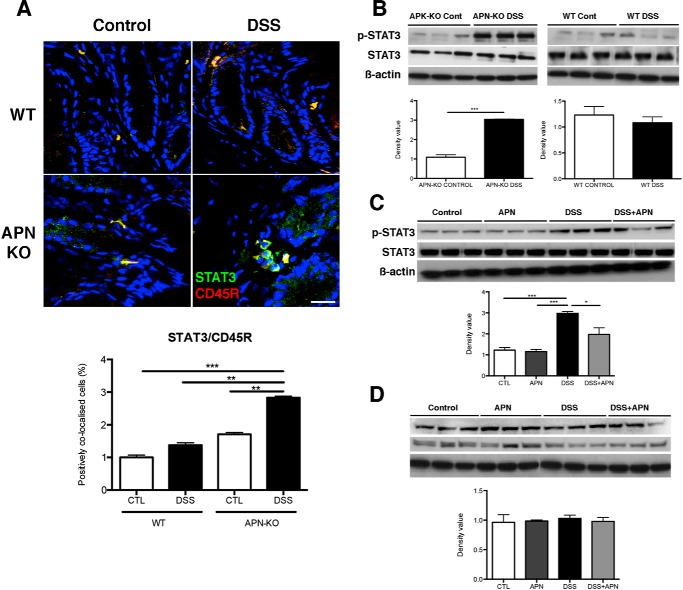
Analyses by immunofluoresence showed that STAT3 co-localized with CD45R and thus B cells in both WT and APN-KO mouse colons. Significantly, DSS-treated APN-KO colons were found to have 1.8-fold increased co-localization of STAT3 and CD45R versus WT mice receiving DSS (p < 0.001; Fig. 8A). Further evaluation by Western blot analysis showed that 3-fold more phosphorylated STAT3 protein was present in APK-KO colitic mice, compared with APN-KO controls (p < 0.001; Fig. 8B). In contrast, p-STAT3 and STAT3 protein levels were unchanged between WT control and DSS-treated mice (Fig. 8B). In support of this data, we observed increased IL-6 gene expression (3.2-fold) in APN-KO colitic mice, compared with WT mice treated with DSS (p < 0.001; Fig. 6C).
Figure 8.
B cell infiltration is accompanied by STAT3 activation in DSS-colitis in APN-KO mice, and is potentially mediated by AdipoR1. A, immunofluorescent images showing DAPI (blue), CD45 (red), and STAT3 (green) co-localization in WT control, WT DSS, APN-KO control, and APN-KO DSS colons. Analysis showed increased STAT3/CD45R colocalization in the APN KO DSS treatment versus controls and WT DSS (p < 0.01 and 0.001). Scale bar, 50 μm. B, Western blots of STAT3 colonic protein in WT and APN-KO control versus DSS colons showing an increase in p-STAT3 in APN-KO DSS colons versus APN-KO controls (p < 0.001). C, Western blots of p-STAT3 and STAT3 in the in vitro model of colitis showing increased p-STAT3 levels in DSS treatment versus controls and APN treatment (p < 0.001 for both groups), and a significant reduction following the addition of APN in the presence of DSS compared with DSS alone (p < 0.05). D, Western blots of p-STAT3 and STAT3 in the in vitro model of colitis following AdipoR1 knockdown, showing limited modulation of p-STAT3 levels (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
To confirm a role for STAT3 in APN signaling, we utilized the HCT116 in vitro model of colitis. Here, we found that DSS treatment increased p-STAT3 protein levels 3-fold compared with controls (p < 0.001). The addition of APN assisted in reducing p-STAT3 protein expression 1.5-fold (p < 0.05; Fig. 8C). Importantly, when AdipoR1 expression was ablated by siRNA, there was no change in p-STAT3 levels after DSS and APN treatment (Fig. 8D). These data suggest that APN, through AdipoR1, can modulate STAT3 signaling.
Discussion
We investigated the pathogenesis of DSS-induced colitis with respect to the functional role of APN. In summary, our in vivo and in vitro studies demonstrate that the absence of APN results in severe colonic inflammation and greater disease activity, with increased IEC apoptosis, colonic crypt disruption, and extensive B cell infiltration. Importantly, the protective effects of APN were dependent on the expression of AdipoR1.
We chose to use APN null mice generated from the Matsuzawa lab, because these mice reflect the known protective and anti-inflammatory functions of adiponectin (27–30). To account for differences in the intestinal microflora and genetic heterogeneity of the C57B/6 line, we performed pilot experiments with DSS concentrations ranging from 0.5 to 2.5% over the standard 7-day period (31)(data not shown). We found that 1.5% DSS induced colitis in WT and robustly in APN null mice, and at higher concentrations no additive effect on intestinal pathology. Next, to ensure that genetic differences were limited, only littermate controls were used from heterozygous breeding.
As expected, and in line with Nishihara et al. (32) we observed that APN null mice treated with DSS had higher clinical activity scores and inflammation. However, those investigators did not evaluate IEC mitosis and apoptosis, both critical determinants of colitis activity. We performed histology for Ki67 and Caspase-3 and -7, and confirmed that less proliferation and more apoptosis was present in DSS-treated APN KO IEC layers. Because DSS is toxic to IECs and promotes apoptosis, we complemented these data using a novel system by treating HCT116 cells in vitro with DSS and APN. We saw that DSS increased apoptosis and reduced proliferation, whereas in the presence of APN, this was reversed and coincided with normalization of the apoptosis and stress markers p53, Bcl-2, ERK, and p38.
Previous studies have not mechanistically addressed the role of adiponectin receptors in DSS-induced colitis. Here we observed in APN-KO mice that the protein expression of AdipoR1 was up-regulated and in contrast, AdipoR2 protein expression was down-regulated. In WT mice treated with DSS, it has been reported that AdipoR1 was more prevalent in the murine colon (32), and through confocal analyses we observed that APN colocalized with AdipoR1 in the colon, confirming their interaction. Studies using HCT116 cells have shown that APN acting through AdipoR1 and -R2 can repress HCT116 proliferation, whereas receptor knockdown equated with increased proliferation (33). Thus, we chose to knockdown AdipoR1 and evaluated the action of APN during in vitro DSS treatment. As expected, AdipoR1 absence followed by APN treatment did not rescue HCT116 cells, suggesting that this interaction is important for promoting IEC survival. These data are in agreement with other cellular stress systems where AdipoR1 is necessary to reduce cellular damage and apoptosis (34, 35). Importantly, it has been recently demonstrated that intracolonic injection of AdipoR1 siRNA worsens colitis in mice, thus reinforcing our observations (36). Taken together, these data suggest that APN through AdipoR1 is critical for maintaining IEC survival.
Previous studies concerning the role of APN in DSS-induced colitis have not adequately investigated all facets of the immune response, leaving open possible roles for APN in other inflammatory responses. Moreover, if these studies were not performed with littermate controls and clean animals, inappropriate data would be generated. Therefore using our experimental protocols, we examined a spectrum of inflammatory markers by qPCR, immunofluorescence, and flow cytometry. We found a modest increase in T-cell numbers as represented by CD3 and CD4 gene expression and flow cytometry. We assessed for other factors and saw up-regulation of CD19 and CD40, and increased co-localization of CD45R and CD40 suggesting that more activated B cells are present in APN-KO DSS colons. This coincided with up-regulation of the proinflammatory cytokines IL-1β, IL-4, IL-5, and IL-13, which have in turn been implicated in colitis, B cell, and T helper cell activation. With regards to B cells, IL-1β loss results in aggressive colitis and can be derived from B-cells and promotes IL-6 production (37). It has also been reported that IL-4 knock-out mice are protected from DSS-induced colitis and can facilitate B cell division and proliferation (38, 39). Likewise, IL-5 can support the growth and differentiation of B cells and increase the secretion of immunoglobulins (40). Finally, IL-13 has been shown to induce the apoptosis and dissociation of the IEC barrier during murine colitis (41) and to induce B cell proliferation (42).
Given these data, there are some plausible roles for APN in modulating B cell function. First, reduced levels of APN through a B cell-mediated mechanism has been shown to facilitate the entry of T cells into inflamed tissues and thereby to promote autoimmunity (43). Taking into account that T cell numbers are modestly increased, and CD4 gene expression is elevated, it is possible that this mechanism is operative. Second, APN inhibits B lymphopoiesis in long-term bone marrow cultures, suggesting that limited APN may promote the expansion of B cells and/or a selected lineage (44). Subsequently, autoimmunity can result when negative selection is unable to remove the potentially pathogenic autoreactive B cells during development or as a result of B cell antigen receptor somatic mutations during affinity maturation in germinal centers. This can occur due to alterations in B cell signaling, apoptosis, activation, and/or proliferation, contributing to the chance that a reactive B cell that recognizes a self-antigen from the colon can escape, expand, and contribute to disease. It remains to be determined at what level APN regulates B cell activation, maturation, and expansion and what is the identity of the antigen, and how the other immune subsets contribute to the phenotype. Nevertheless, our data suggest a new role for adiponectin in regulating immune B cell responses during DSS-induced colitis. It also needs to be questioned as to what is the relevance of our findings to patients? Previous studies have shown elevated and activated B cells in human IBD (45, 46). However, a relationship between B cell numbers, IBD, and obesity, where serum adiponectin levels are lower, has not been examined in any study.
IL-6 null mice and intestinal epithelial IL-6 conditional knock-out mice are highly susceptible to experimental colitis, indicating that STAT3 modulates intestinal homeostasis (47, 48). In the mouse intestine, lamina propria and macrophages are the major sources of IL-6. Consistent with the work of Nishihara et al. (32) we find in the absence of APN, there is increased macrophage numbers and IL-6, and independent of that report, reduced IL-10 gene expression in APN-KO mice treated with DSS. Previous studies have shown that APN can limit IL-6-induced macrophage proinflammatory signaling through reduced STAT3 phosphorylation (49). The most likely explanation for our phenotype then, is that the absence of adiponectin does not restrain macrophage activation and IL-6 release. STAT3 is also important in determining IEC cell fate and we find in our in vitro model that DSS promotes both apoptosis and increased STAT3 phosphorylation. Given the relationship between APN and IL-6 and that no previous study had addressed how APN could regulate STAT3 signaling in IECs, we treated HCT116 cells with DSS, specific AdipoR1 siRNA, and recombinant APN. Significantly, we find that STAT3 activity was reduced when APN was applied and this was dependent on the presence of AdipoR1. Thus, a conceivable role for IL-6 is to act on IECs, induce STAT3 activity to promote their proliferation and survival in the absence of adiponectin, and a sustained inflammatory response.
Finally, it must be discussed that other studies examining the role of APN in mouse models of DSS-induced colitis are contradictory. Nishihara et al. (32) observed that APN-KO mice are susceptible to disease, with increased inflammation and elevated mRNA levels of IL-1β, IL-6, and TNFα. Significantly, application of adenovirus overexpressing APN protected both WT and APN-KO against colitis. Likewise, in another independent study, WT mice treated with DSS and an APN adenovirus were protected (50). In marked contrast, the work of Fayad et al. (51, 52) reported that DSS treatment and APN absence was protective against colitis, and a reduced inflammatory response was observed. These differences could be explained by variations in methods, the source of KO mice, the form of recombinant APN, and the pathogen status of the animal facilities. For example, for Nishihara et al. (32) control WT mice of the same background were attained from an external provider and APN-KO mice were sourced from the Matsuzawa lab (27). Whereas, Fayad et al. (53) used littermate controls, but included heterozygotes in the WT group and sourced APN-KO mice from the Chan lab. Such discrepancies between the lines of APN null mice have been previously observed as they have different biological responses (27, 53).
In summary, our data suggest that APN is protective against DSS-induced colitis and its action is mediated most likely by binding to AdipoR1 and concomitantly suppressing B cell-induced inflammatory responses. Together, these mechanisms protect the IEC layer from damage.
Experimental procedures
Mice
APN-KO mice were bred for at least 12 generations on a C57B/6 background. Subsequent studies were performed with littermate male C57B/6 mice (wild type) and APN-KO mice (n = 9 per group). Mice were checked for all known pathogens and determined disease free. They were maintained on a standard diet until reaching experimental age (6–12 weeks) in accordance with the Western Sydney Local Health District Animal Ethics Committee guidelines.
Dextran sulfate sodium-induced colitis
Male mice aged 6–12 weeks were given 1.5% DSS (molecular mass 36–50 kDa; MP Biomedicals; catalog number 0216011080) in autoclaved drinking water for 7 days, with fresh DSS being replaced on days 3 and 5. Control mice were provided standard autoclaved drinking water; all animals were sacrificed on day 8 of the study.
Histology
At harvest, the colons were excised and the length measured, and then flushed with ice-cold PBS to remove fecal matter, dissected, and embedded in OCT and snap frozen. Other samples were fixed in 70% ethanol and embedded in paraffin. Sections of 5 μm were cut and stained with hematoxylin and eosin for histological analysis, and blinded pathology scoring of crypt inflammation was performed (by P. B.).
Immunofluorescence
Frozen 7-μm colonic sections were fixed with acetone and stained with antibodies against: Ki67 (Abcam; catalog number 15580), CD3 (Santa Cruz; catalog number sc-1127), CD45R (Abcam; catalog number ab4100), CD40 (Santa Cruz; catalog number sc-975), F4/80 (Invitrogen; catalog number MF48000), STAT3 (Cell Signaling; catalog number 9132), and caspases-3 and -7 (ImmunoChemistry Technologies FAMFLICATM; catalog number 93). HCT116 colonic epithelial cells were also stained with caspase-3 and 7 (FAMFLICATM; catalog number 93). All slides were counterstained with DAPI and image acquisition was performed on a Leica DM LB confocal microscope (Leica, Germany) and analyzed with ImageJ.
RNA isolation and qRT-PCR
Total RNA from cells and tissues was isolated with RNeasy (Qiagen). A260/280 ratios and RNA quantity were determined by a NanoDrop ND-1000 spectrophotometer. 1000 ng of total RNA was reverse transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen). Quantification of mRNA expression was performed by qRT-PCR using a Corbett 6000 rotor gene platform (Corbett). Analyses consisted of 6 samples for each group. The primer pairs are listed in Table 1, and were normalized to GAPDH.
Table 1.
Primer sequences for RT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| AdipoR1 (human) | GCCATGGAGAAGATGGAGGA | AGCACGTCGTACGGGATGA |
| CD19 (mouse) | GGAGGCAATGTTGTGCTGC | ACAATCACTAGCAAGATGCCC |
| CD40 (mouse) | GCTATGGGGCTGCTTGTTGA | ATGGGTGGCATTGGGTCTTC |
| IL-1β (mouse) | GGAGAACCAAGCAACGACAATA | TGGGGAACTCTGCAGACTCAAAC |
| IL-4 (mouse) | ATCGGCATTTTGAACGAGGT | CATCGAAAAGCCCGAAAGAGTCT |
| IL-5 (mouse) | TCACCGAGCTCTGTTGACAA | CCACACTTCTCTTTTTGGCG |
| IL-6 (mouse) | TAGTCCTTCCTACCCCAATTT | TTGGTCCTTAGCCACTCCTTC |
| IL-13 (mouse) | GGCCATC CTGCAAAATAGTG | ACAGCGTCGGCAAGAACA |
| CD68 (mouse) | TGACCTGCTCTCTCTAAGGCTACA | TCACGGTTGCAAGAGAAACATG |
| IL-10 (mouse) | CACAAAGCAGCCTTGCAGAA | AGAGCAGGCAGCATAGCAGTG |
| CD3 (mouse) | ATGCGGTGGAACACTTTCTG | GCACGTCAACTCTACACTGGT |
| CD4 mouse | TCTGGCAACCTGACTCTGAC | TCATCACCACCAGGTTCACT |
Protein extraction and Western blotting
Cell culture as well as protein lysates from colonic tissue were separated using polyacrylamide gels and run at 110 V in 1× Running buffer (25 mm Tris, 250 mm glycine, 0.1% (v/v) SDS). Gels were transferred to Immobilon-P PVDF membranes (Millipore). Membranes were then blocked in TBST (0.1% Tween 20) with 5% skim milk for 1 h, and subsequently incubated in primary antibody against: adiponectin (Affinity Bioreagent; catalog number PA1–84881), Caspase-3 (Santa Cruz; catalog number sc-7148), AdipoR1 (Santa Cruz; catalog number sc-46748); and from Cell Signaling: Akt (catalog number 9272), p-Akt (catalog number 9271), ERK1/2 (catalog number 9102), p-ERK1/2 (catalog number 9101), PI3k (catalog number 4249), p-STAT3 (catalog number 9131), p38 MAPK (catalog number 9212), p-p38 (catalog number 9212) and p53 (catalog number 9282), and β-actin (Sigma; catalog number A2228) in 4% skim milk solution overnight at 4 °C. Membranes were then washed, and incubated in secondary antibodies diluted in 4% skim milk for 1 h. Probed membranes were visualized using SuperSignal West Pico/femto chemiluminescent kits, and exposed using ChemiDocTM (Bio-Rad). Western blots were performed in triplicate and protein expression was quantified using ImageJ (National Institutes of Health, Bethesda, MD) and normalized according to the respective β-actin bands.
Flow cytometry and ELISA
2–3 Colons of each line were flushed with PBS and treated sequentially with 5 mm EDTA in PBS and collagenase (20 units/ml) and DNase (0.5 mg/ml) for 30 min, filtered through a 70-μm cell strainer, and stained with 7-aminoactinomycin D, anti-F4/80 (clone BM8), CD19 (clone 1D3), CD3 (clone 145-2C11), CD4 (clone GK1.5), and CD8 (clone 53-67; BioLegend). Flow cytometry was performed on a BD LSRFortessa and analyzed with FlowJo. The ELISA was preformed with colon tissue from 5 to 6 mice pooled and homogenized in PBS containing protease inhibitors and normalized to 1 mg/ml and IL-1β, IL-4, and IL-6 (BioLegend).
In vitro colonic epithelial cell colitis
In vitro colonic epithelial cell colitis was induced as previously reported (54) in HCT116 cells cultured in McCoys 5A modified medium (Sigma; catalog number M4892) containing 10% fetal calf serum (FCS). For experiments, cells were treated with 1.5% DSS, 3 μg/ml of recombinant APN (Biovendor; catalog number RD172023100), or a combination of the two in serum-free media in triplicates. Cell proliferation was assessed using a bromodeoxyuridine (BrdU) kit (Roche Applied Science). Human AdipoR1 small interfering RNA (siRNA; Life Technologies) was used to knockdown AdipoR1 according to the manufacturer's instructions. Scrambled siRNA and untreated cells were used as controls.
Statistical analysis
All values are reported as mean ± S.E. p values were determined using a one-way analysis of variance for experiments with greater than two groups, and Bonferroni post testing. The Student's t test was employed for statistical comparison between two groups (GraphPad Prism). All experiments were performed three times and p < 0.05 was considered as statistically significant.
Author contributions
S. O. performed experiments and prepared the manuscript. B. C., M. W., J. S., R. K., S. E., M. R. M., C. D., S. R., and P. B. performed ancillary experiments. G. O., L. Q., and A. L. assisted in revisions. J. G. and L. H. wrote and revised the manuscript.
This work was supported by The Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney, National Health and Medical Research Council of Australia (NHMRC) Program Grant APP1053206, NHMRC project Grants 1047417 (to L. Q.) and 1087297 (to J. G. and L. H.), Cancer Council NSW Project Grants 1070076 (to L. Q.) and 1069733 (to L. H.), Cancer Council Queensland Project Grant 1123436 (to L. H., J. G., and L. Q.), and James Cook Development Grant 2016 (to L. H.). The authors declare that they have no conflicts of interest with the contents of this article.
- IEC
- intestinal epithelial cell
- IBD
- inflammatory bowel disease
- CD
- Crohn's disease
- APN
- adiponectin
- DSS
- dextran sulfate sodium
- qRT
- quantitative real-time.
References
- 1. GBD 2013 Mortality and Causes of Death, Collaborators. (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burisch J., and Munkholm P. (2015) The epidemiology of inflammatory bowel disease. Scand. J. Gastroenterol. 50, 942–951 [DOI] [PubMed] [Google Scholar]
- 3. Kappelman M. D., Moore K. R., Allen J. K., and Cook S. F. (2013) Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig. Dis. Sci. 58, 519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molodecky N. A., Soon I. S., Rabi D. M., Ghali W. A., Ferris M., Chernoff G., Benchimol E. I., Panaccione R., Ghosh S., Barkema H. W., and Kaplan G. G. (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54,e42; quiz e30 [DOI] [PubMed] [Google Scholar]
- 5. Snook J. A., de Silva H. J., and Jewell D. P. (1989) The association of autoimmune disorders with inflammatory bowel disease. Q. J. Med. 72, 835–840 [PubMed] [Google Scholar]
- 6. Francisco L. M., Sage P. T., and Sharpe A. H. (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berkowitz D., Peri R., Lavy A., and Kessel A. (2013) Increased Toll-like receptor 9 expression by B cells from inflammatory bowel disease patients. Hum. Immunol. 74, 1519–1523 [DOI] [PubMed] [Google Scholar]
- 8. Mann E. R., and Li X. (2014) Intestinal antigen-presenting cells in mucosal immune homeostasis: crosstalk between dendritic cells, macrophages and B-cells. World J. Gastroenterol. 20, 9653–9664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cupi M. L., Sarra M., Marafini I., Monteleone I., Franzè E., Ortenzi A., Colantoni A., Sica G., Sileri P., Rosado M. M., Carsetti R., MacDonald T. T., Pallone F., and Monteleone G. (2014) Plasma cells in the mucosa of patients with inflammatory bowel disease produce granzyme B and possess cytotoxic activities. J. Immunol. 192, 6083–6091 [DOI] [PubMed] [Google Scholar]
- 10. Alexander K. L., Targan S. R., and Elson C. O. 3rd (2014) Microbiota activation and regulation of innate and adaptive immunity. Immunol. Rev. 260, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Podolsky D. K. (2002) Inflammatory bowel disease. N. Engl. J. Med. 347, 417–429 [DOI] [PubMed] [Google Scholar]
- 12. Hanauer S. B. (2006) Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 12, S3–S9 [DOI] [PubMed] [Google Scholar]
- 13. Abraham C., and Cho J. H. (2009) IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 60, 97–110 [DOI] [PubMed] [Google Scholar]
- 14. Mendall M. A., Gunasekera A. V., John B. J., and Kumar D. (2011) Is obesity a risk factor for Crohn's disease? Dig. Dis. Sci. 56, 837–844 [DOI] [PubMed] [Google Scholar]
- 15. Harpsøe M. C., Basit S., Andersson M., Nielsen N. M., Frisch M., Wohlfahrt J., Nohr E. A., Linneberg A., and Jess T. (2014) Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int. J. Epidemiol. 43, 843–855 [DOI] [PubMed] [Google Scholar]
- 16. Steed H., Walsh S., and Reynolds N. (2009) A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes. Facts 2, 370–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blain A., Cattan S., Beaugerie L., Carbonnel F., Gendre J. P., and Cosnes J. (2002) Crohn's disease clinical course and severity in obese patients. Clin. Nutr. 21, 51–57 [DOI] [PubMed] [Google Scholar]
- 18. Hass D. J., Brensinger C. M., Lewis J. D., and Lichtenstein G. R. (2006) The impact of increased body mass index on the clinical course of Crohn's disease. Clin. Gastroenterol. Hepatol. 4, 482–488 [DOI] [PubMed] [Google Scholar]
- 19. Scherer P. E., Williams S., Fogliano M., Baldini G., and Lodish H. F. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 [DOI] [PubMed] [Google Scholar]
- 20. Motoshima H., Wu X., Mahadev K., and Goldstein B. J. (2004) Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem. Biophys. Res. Commun. 315, 264–271 [DOI] [PubMed] [Google Scholar]
- 21. Ouchi N., Kihara S., Arita Y., Maeda K., Kuriyama H., Okamoto Y., Hotta K., Nishida M., Takahashi M., Nakamura T., Yamashita S., Funahashi T., and Matsuzawa Y. (1999) Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100, 2473–2476 [DOI] [PubMed] [Google Scholar]
- 22. Wang Z. V., and Scherer P. E. (2008) Adiponectin, cardiovascular function, and hypertension. Hypertension 51, 8–14 [DOI] [PubMed] [Google Scholar]
- 23. Hebbard L., and Ranscht B. (2014) Multifaceted roles of adiponectin in cancer. Best Pract Res. Clin. Endocrinol. Metab. 28, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saxena A., Chumanevich A., Fletcher E., Larsen B., Lattwein K., Kaur K., and Fayad R. (2012) Adiponectin deficiency: role in chronic inflammation induced colon cancer. Biochim. Biophys. Acta 1822, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saxena A., Baliga M. S., Ponemone V., Kaur K., Larsen B., Fletcher E., Greene J., and Fayad R. (2013) Mucus and adiponectin deficiency: role in chronic inflammation-induced colon cancer. Int. J. Colorectal Dis. 28, 1267–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolf A. M., Wolf D., Rumpold H., Enrich B., and Tilg H. (2004) Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 323, 630–635 [DOI] [PubMed] [Google Scholar]
- 27. Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., Komuro R., Ouchi N., Kihara S., Tochino Y., Okutomi K., Horie M., Takeda S., Aoyama T., Funahashi T., and Matsuzawa Y. (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 [DOI] [PubMed] [Google Scholar]
- 28. Denzel M. S., Scimia M. C., Zumstein P. M., Walsh K., Ruiz-Lozano P., and Ranscht B. (2010) T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Invest. 120, 4342–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Konter J. M., Parker J. L., Baez E., Li S. Z., Ranscht B., Denzel M., Little F. F., Nakamura K., Ouchi N., Fine A., Walsh K., and Summer R. S. (2012) Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J. Immunol. 188, 854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamada Y., Tamura S., Kiso S., Matsumoto H., Saji Y., Yoshida Y., Fukui K., Maeda N., Nishizawa H., Nagaretani H., Okamoto Y., Kihara S., Miyagawa J., Shinomura Y., Funahashi T., and Matsuzawa Y. (2003) Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 125, 1796–1807 [DOI] [PubMed] [Google Scholar]
- 31. Wirtz S., Neufert C., Weigmann B., and Neurath M. F. (2007) Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2, 541–546 [DOI] [PubMed] [Google Scholar]
- 32. Nishihara T., Matsuda M., Araki H., Oshima K., Kihara S., Funahashi T., and Shimomura I. (2006) Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology 131, 853–861 [DOI] [PubMed] [Google Scholar]
- 33. Kim A. Y., Lee Y. S., Kim K. H., Lee J. H., Lee H. K., Jang S. H., Kim S. E., Lee G. Y., Lee J. W., Jung S. A., Chung H. Y., Jeong S., and Kim J. B. (2010) Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol. Endocrinol. 24, 1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song J., Kang S. M., Kim E., Kim C. H., Song H. T., and Lee J. E. (2015) Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study. Cell Death Dis. 6, e1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nigro E., Scudiero O., Sarnataro D., Mazzarella G., Sofia M., Bianco A., and Daniele A. (2013) Adiponectin affects lung epithelial A549 cell viability counteracting TNFα and IL-1ss toxicity through AdipoR1. Int. J. Biochem. Cell Biol. 45, 1145–1153 [DOI] [PubMed] [Google Scholar]
- 36. Sideri A., Stavrakis D., Bowe C., Shih D. Q., Fleshner P., Arsenescu V., Arsenescu R., Turner J. R., Pothoulakis C., and Karagiannides I. (2015) Effects of obesity on severity of colitis and cytokine expression in mouse mesenteric fat: potential role of adiponectin receptor 1. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G591–G604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bersudsky M., Luski L., Fishman D., White R. M., Ziv-Sokolovskaya N., Dotan S., Rider P., Kaplanov I., Aychek T., Dinarello C. A., Apte R. N., and Voronov E. (2014) Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut 63, 598–609 [DOI] [PubMed] [Google Scholar]
- 38. Stevceva L., Pavli P., Husband A., Ramsay A., and Doe W. F. (2001) Dextran sulphate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Genes Immunity 2, 309–316 [DOI] [PubMed] [Google Scholar]
- 39. Rush J. S., and Hodgkin P. D. (2001) B cells activated via CD40 and IL-4 undergo a division burst but require continued stimulation to maintain division, survival and differentiation. Eur. J. Immunol. 31, 1150–1159 [DOI] [PubMed] [Google Scholar]
- 40. Takatsu K., Kouro T., and Nagai Y. (2009) Interleukin 5 in the link between the innate and acquired immune response. Adv. Immunol. 101, 191–236 [DOI] [PubMed] [Google Scholar]
- 41. Kawashima R., Kawamura Y. I., Oshio T., Son A., Yamazaki M., Hagiwara T., Okada T., Inagaki-Ohara K., Wu P., Szak S., Kawamura Y. J., Konishi F., Miyake O., Yano H., Saito Y., Burkly L. C., and Dohi T. (2011) Interleukin-13 damages intestinal mucosa via TWEAK and Fn14 in mice: a pathway associated with ulcerative colitis. Gastroenterology 141, 2119–2129.e8 [DOI] [PubMed] [Google Scholar]
- 42. Cocks B. G., de Waal Malefyt R., Galizzi J. P., de Vries J. E., and Aversa G. (1993) IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int. Immunol. 5, 657–663 [DOI] [PubMed] [Google Scholar]
- 43. Chimen M., McGettrick H. M., Apta B., Kuravi S. J., Yates C. M., Kennedy A., Odedra A., Alassiri M., Harrison M., Martin A., Barone F., Nayar S., Hitchcock J. R., Cunningham A. F., Raza K., et al. (2015) Homeostatic regulation of T cell trafficking by a B cell-derived peptide is impaired in autoimmune and chronic inflammatory disease. Nat. Med. 21, 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yokota T., Meka C. S., Kouro T., Medina K. L., Igarashi H., Takahashi M., Oritani K., Funahashi T., Tomiyama Y., Matsuzawa Y., and Kincade P. W. (2003) Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J. Immunol. 171, 5091–5099 [DOI] [PubMed] [Google Scholar]
- 45. Noronha A. M., Liang Y., Hetzel J. T., Hasturk H., Kantarci A., Stucchi A., Zhang Y., Nikolajczyk B. S., Farraye F. A., and Ganley-Leal L. M. (2009) Hyperactivated B cells in human inflammatory bowel disease. J. Leukoc. Biol. 86, 1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roberts-Thomson I. C., Fon J., Uylaki W., Cummins A. G., and Barry S. (2011) Cells, cytokines and inflammatory bowel disease: a clinical perspective. Expert Rev. Gastroenterol. Hepatol. 5, 703–716 [DOI] [PubMed] [Google Scholar]
- 47. Grivennikov S., Karin E., Terzic J., Mucida D., Yu G. Y., Vallabhapurapu S., Scheller J., Rose-John S., Cheroutre H., Eckmann L., and Karin M. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., Lehr H. A., Hirth S., Weigmann B., Wirtz S., Ouyang W., Neurath M. F., and Becker C. (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Folco E. J., Rocha V. Z., López-Ilasaca M., and Libby P. (2009) Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J. Biol. Chem. 284, 25569–25575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arsenescu V., Narasimhan M. L., Halide T., Bressan R. A., Barisione C., Cohen D. A., de Villiers W. J., and Arsenescu R. (2011) Adiponectin and plant-derived mammalian adiponectin homolog exert a protective effect in murine colitis. Dig. Dis. Sci. 56, 2818–2832 [DOI] [PubMed] [Google Scholar]
- 51. Fayad R., Pini M., Sennello J. A., Cabay R. J., Chan L., Xu A., and Fantuzzi G. (2007) Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology 132, 601–614 [DOI] [PubMed] [Google Scholar]
- 52. Kaur K., Saxena A., Larsen B., Truman S., Biyani N., Fletcher E., Baliga M. S., Ponemone V., Hegde S., Chanda A., and Fayad R. (2015) Mucus mediated protection against acute colitis in adiponectin deficient mice. J. Inflamm. (Lond.) 12, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ma K., Cabrero A., Saha P. K., Kojima H., Li L., Chang B. H., Paul A., and Chan L. (2002) Increased β-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J. Biol. Chem. 277, 34658–34661 [DOI] [PubMed] [Google Scholar]
- 54. Araki Y., Sugihara H., and Hattori T. (2006) In vitro effects of dextran sulfate sodium on a Caco-2 cell line and plausible mechanisms for dextran sulfate sodium-induced colitis. Oncol. Rep. 16, 1357–1362 [PubMed] [Google Scholar]