Abstract
Ciprofloxacin is a quinolone antibiotic used to treat Klebsiella pneumoniae infections in the clinic. Previous studies have demonstrated that berberine exhibits antibacterial activity and less acquired resistance related to efflux pumps. The multidrug efflux pump acrAB-tolC can be stimulated to expel as much toxic material as possible from the cells, but a detrimental effect can be produced owing to an overcrowded periplasm with excess expression products, which inhibits bacterial growth. In this study, the in vitro antibacterial activities of ciprofloxacin in combination with berberine were evaluated and compared with those of ciprofloxacin and berberine alone by evaluating the MIC, MBC and summation fractional IC against 20 clinical multidrug-resistant K. pneumoniae isolates, 1 quality control bacterium and 1 induced-resistance bacterium. Susceptibility tests showed that the MIC for the combination of berberine and ciprofloxacin was 1/2 that of the individual agents or less. Antimicrobial activities of 18.18% synergy and 77.27% additivity were found. Furthermore, synergism was verified through a time-kill assay, which suggested that the synergistic antibacterial effect of the two-drug combination may, to some extent, be related to the high expression of the acrAB-tolC and acrR multidrug efflux pumps. Indeed, the expression of these genes was increased >14-fold in the isolates affected by ciprofloxacin–berberine combination synergism.
Introduction
Klebsiella pneumoniae is a Gram-negative opportunistic bacterium that is usually found in the human mouth, skin and intestines. This bacterium commonly causes infections of the respiratory tract, urinary tract, lower biliary tract, gastrointestinal tract and wound sites.1 The massive use of antibiotics and invasive devices has increased the rate of nosocomial infections and multidrug resistance in K. pneumoniae. The US Center for Disease Control and Prevention reported that, during the past 10 years, the number of resistant strains of Klebsiella species has increased faster than that of other bacteria (from 1.6 to 10.4%).2 During the past 8 years in Switzerland, the rate of infection with extended-spectrum cephalosporin-resistant K. pneumoniae has increased significantly, from 1.1 to 4.4%.3 In China, the Mohnarin 2011 annual report indicated that the rate of detection of K. pneumoniae capable of producing extended-spectrum beta-lactamases was 50.3% in all clinical specimens.4 Multidrug-resistant K. pneumoniae is a rapidly growing issue worldwide. Concerning the mechanism of resistance in K. pneumoniae, efflux systems have an essential role in rendering pathogens resistant to a variety of antibiotics by extruding these drugs.5 acrAB-tolC has been identified as a major multidrug efflux pump and a structural component of enterobacteria.6 In K. pneumoniae, acrAB-tolC dominates efflux-mediated resistance to fluoroquinolones.7 Berberine is an alkaloid that is extracted from various plants used in traditional Chinese medicine.8 Previous studies have shown that berberine exhibits activity against bacteria, viruses, yeast, parasites, fungi and Candida albicans.9 Berberine is considered an antibacterial agent in both Chinese and Western medicine.10 Some studies have indicated that berberine can inhibit the reproduction of pathogens if used in combination with other antibiotics.11, 12, 13, 14 Moreover, Karaosmanoglu et al.15 reported that the transcription of acrEF was upregulated 17-fold in the presence of berberine in Escherichia coli, and acrEF was found to be highly homologous to the acrAB portion of the multidrug efflux system. Synergistic and additive effects against pathogenic microbes have been observed between berberine and antibiotics or antifungal drugs.12, 16, 17, 18, 19 However, the combined effects of berberine and ciprofloxacin against multidrug-resistant K. pneumoniae isolates rarely appear in mechanistic studies.
In the present study, the interactions between berberine and ciprofloxacin and their activity against multidrug-resistant K. pneumoniae isolates were examined in vitro, and the effects of berberine and ciprofloxacin on the expression of acrAB-tolC were investigated in K. pneumoniae at the molecular level to elucidate the underlying antibacterial activity.
Materials and methods
Bacterial isolates
Twenty nonduplicated, multidrug-resistant clinical K. pneumoniae isolates (R1–R20) were collected from the Clinical Microbiology Laboratory of the First Affiliated Hospital of Guangzhou Medical University, Guangdong, China, during 2013 and 2014. The strains included ESBLs+ that were resistant to three types of penicillin, quinolones, cephalosporins, aminoglycosides, macrolides and sulfonamides according to the Performance Standards for Antimicrobial Disk Susceptibility Tests.20 All isolates were identified and confirmed using a Vitek-60 system (bioMérieux, Lyon-Gerland, France). The K. pneumoniae ATCC700603 strain was included as a quality control strain and was referred to as ATCC. All strains were stored at −80 °C and subcultured in Mueller−Hinton broth and Mueller−Hinton agar (Oxoid, Basingstoke, UK). Highly purified lines of active monoclonal strains were then selected for further study. The ATCC strain was induced by ciprofloxacin at concentrations of 0.5−256 μg ml−1,21 which was then referred to as RATCC.
Antimicrobial agents
Ciprofloxacin powders (Guangzhou Nanxin Pharmaceutical, Guangzhou, China) that were obtained as gifts were diluted to concentrations of 5–120 μg ml−1 in ddH2O. Berberine was diluted to concentrations of 10–240 μg ml−1 in ddH2O (purity ⩾98% Shanghai Yuanye Bio-Technology, Shanghai, China). Each sample was stored at −20 °C for <2 weeks before use.
Antimicrobial susceptibility testing
The macrodilution broth method was used in accordance with the methodology of the Clinical and Laboratory Standards Institute.22 The density of the inocula of isolated colonies was standardized to 0.5 McFarland standard turbidity (~1–2 × 108 CFU ml−1) with sterile saline, diluted with cation-adjusted Mueller−Hinton broth and adjusted to a bacterial concentration of 1 × 105 CFU ml−1 in each of several sterile 13 × 100 mm test tubes, with a final volume of 1 ml. The final concentrations were prepared in serial twofold dilutions; the ciprofloxacin solutions ranged from 0.0625 to 512 μg ml−1 and the berberine solution from 128 to 1024 μg ml−1. The macrodilution tubes were incubated at 37 °C in an ambient air incubator for 16–18 h. Turbidity was compared by eye. Under the MIC, visible growth of the bacterium was completely inhibited. Samples were then pipetted onto Mueller−Hinton agar. After 24 h of incubation in a 37 °C forced air incubator, the inoculum with the lowest concentration of an antimicrobial agent that still demonstrated no visible colonies was considered to represent the MBC of that agent. MBC is defined here as the concentration capable of killing 99.9% of the bacterium in the final inoculum.
Checkerboard method
A checkerboard method was used to assess the antibacterial interactions between ciprofloxacin and berberine, as described by Pillai et al.23 Blank control groups with no drugs were also tested. In 96-well microtiter plates containing ~1 × 105 CFU ml−1 of inoculum, ciprofloxacin at 1/8 to 1/2 the MIC was added to the wells and the concentration of ciprofloxacin was plotted on the x-axis. Berberine was plotted on the y-axis in the same way. After 16–18 h of incubation at 37 °C, the lowest possible concentration of the agents associated with no visible turbidity represented the MIC of the checkerboard results.
Synergistic effects
The fractional IC (FIC) index and the summation fractional IC (ΣFIC) were used to assess the synergistic effects of these two antibacterial agents in accordance with the checkerboard MIC results:23 ΣFIC=FICA+FICB=FICCIP+FICBER=MICCIP(combine)/MICCIP(alone)+MICBER(combine)/MICBER(alone). ΣFIC ⩽0.5 was considered synergistic and 0.5<ΣFIC⩽1 was considered to indicate an additive effect; 1<ΣFIC⩽2 indicated indifference, and antagonism of ΣFIC was indicated by values >2.24, 25, 26, 27 When these values exceeded the highest fixed concentration (>512 μg ml−1), the experimental concentration was changed to the next twofold dilution (1024 μg ml−1) and was used to calculate the ΣFIC.
Time-kill assay
The time-kill assay was used to evaluate the antimicrobial activity of the combinations of the two drugs from another perspective. A modified version of the guidelines for the time-kill assay was used.28, 29 Approximately 1 × 106 CFU ml−1 of an initial inoculum of five isolates (R5, R12, R15, ATCC and RATCC) was exposed to a fixed final concentration of a single drug or a drug combination in each test tube under the same conditions and time periods in duplicate. Thus, three experimental groups, including a ciprofloxacin group (⩽1/4 MIC), a berberine group (1024 μg ml−1) and a ciprofloxacin ⩽1/4 MIC+berberine 1024 μg ml−1 group, as well as a blank control group (without agents) were set up. The test tubes were incubated at 37 °C. At 0, 1, 2, 4, 6, 8, 12 and 24 h, 10 μg of the bacterial cultures was removed and transferred to Mueller−Hinton agar. After 24 h, viable bacterial colonies were counted and compared with the single most active agent after 24 h of incubation with both agents. Synergy was defined as a ⩾2 log10 decrease in the number of bacterial colonies after the combination treatment. Additivity was defined as a 1–2 log10 decrease and antagonism as a ⩽1 log10 increase.
Reverse transcription polymerase chain reaction
Total RNA was extracted from five multidrug-resistant clinical K. pneumoniae isolates (R5, R12, R15, ATCC and RATCC) using the RNeasy Protect Bacteria Mini Kit (Qiagen China, Shanghai, China) and lysozyme (Sigma, Sigma-Aldrich China, Shanghai, China) following the manufacturer's protocol. acrA, acrB, acrR, tolC, ramA and marA were detected via RT-PCR using primers to examine gene expression (Table 1). 16S ribosomal RNA was used as an internal control. The PrimeScript RT reagent kit (Takara Bio, Kyoto, Japan) was used for complementary DNA synthesis. Reverse transcription polymerase chain reaction (RT-PCR) was performed in an RT-PCR system (Applied Biosystems, CA, USA) according to the protocol provided with the SYBR Select Master Mix reagent (Applied Biosystems by Life Technologie, CA, USA), with the following standard thermal-cycling conditions: 4 min holding stage, followed by a cycling stage of 40 repetitive cycles including denaturation at 95 °C for 15 s, annealing at 58 °C for 30 s and extension at 72 °C for 30 s. The final melt curve was run at 95 °C for 15 s, 60 °C for 1 min, 95 °C for 30 s and 60 °C for 15 min. The 2−ΔΔCT method was used to analyze the RT-PCR data, quantifying the relative differences in gene expression.30 In the current study, four experimental groups with five strains were used, and three test groups were included: the ciprofloxacin (target), berberine (target) and ciprofloxacin+berberine (target) groups, along with one control group (the blank control (reference)). The CT value was used to determine the fluorescence threshold cycle for the amplification of target or reference molecules, and the amplification efficiencies were kept approximately equal (the absolute value of the slope was always positive, even when it approached 0). ΔCT acrA was determined using the formula ΔCT acrA=CT,acrA−CT,16s-RNA and similar formulae. The 2−ΔΔCT equation was used to calculate the ΔΔCT value (ΔCT-target−ΔCT-reference), which was assumed to be the relative quantified expression of variable amplified RNA.
Table 1. Primer sequences and annealing temperatures used for RT-PCR.
| Gene(s) | Sequence (5′→3′) | TM (°C) | GenBank accession no. |
|---|---|---|---|
| acrA-F | GACTTGGTTTGTTCTGATGGCG | 58 | CP000647.1 |
| acrA-R | CCGTCTGGAAGAAGGGATTAACC | ||
| acrB-F | CCAAACAGTGCAGAACACCGT | 57 | AJ318073.1 |
| acrB-R | GCCACGTAGTCCGAAATATCGT | ||
| tolC-F | ATGAGCCAGGCGGAAAACC | 57 | CP000647.1 |
| tolC-R | TTCGGTATAGGAAAGCGTGTCG | ||
| acrR-F | TATGTTCTTGAAGCGACAGTGACA | 57 | CP000647.1 |
| acrR-R | TTAAGCTGACAAGCTCTCCGG |
Abbreviations: RT-PCR, reverse transcription polymerase chain reaction; TM, melting temperature.
Statistical analysis
Each experiment was performed in triplicate. Time-kill curves and RT-PCR data are expressed as the means±SD. The differences were calculated and compared using Student's t-test or ANOVA. The threshold for statistical significance was set as a two-tailed P-value <0.05. Data were analyzed using GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA). Time-kill curves were drawn as line charts, which were plotted with time (h) on the horizontal axis and log10 CFU ml−1 on the vertical axis. The RT-PCR data are presented as a histogram. The ΔΔCT value was determined in accordance with the ordinate and abscissa from the four experimental groups for the five strains.
Results
Characterization of antimicrobial susceptibility
The assay to determine the in vitro antibacterial activity of ciprofloxacin and berberine against multidrug-resistant K. pneumoniae was performed via the macrodilution method and checkerboard microdilution analysis. The results, measured in terms of the MIC and MBC, are summarized in Table 2. The MICs for ciprofloxacin against K. pneumoniae ranged from 4 to >512 μg ml−1 and the MICs for berberine were ⩾512 μg ml−1. The MBCs for both ciprofloxacin and berberine were ⩾the value of twofold MICs. These results demonstrated that the MICs for the combination of berberine and ciprofloxacin were at least twofold lower than those for the individual agents. When combined with sub-MICs of ciprofloxacin, the concentration of berberine was lower than that for berberine alone. The FICs of berberine ranged from 0.125 to 0.5 and the FICs of ciprofloxacin ranged from 0.125 to 1. The ΣFIC data showed that the values for the combination of berberine and ciprofloxacin ranged from 0.375 to 1, with 4 of the 22 (18.18%) showing synergy, 17 of the 22 (77.27%) showing additivity and 1 isolate showing no difference.
Table 2. MICs and MBCs (μg ml−1) of CIP and BER alone against 20 clinical strains of multidrug-resistant K. pneumoniae, ATCC and RATCCa.
|
MIC |
MBC |
|||||
|---|---|---|---|---|---|---|
| Strain no. | CIP | BER | CIP | BER | ΣFICb | Effect |
| R1 | 256 | >512 | 512 | >1024 | 0.625 | add |
| R2 | 64 | >512 | 128 | >1024 | 1. 5 | ind |
| R3 | 512 | >512 | >512 | >1024 | 0.75 | add |
| R4 | 4 | 512 | 4 | 512 | 1 | add |
| R5 | 128 | >512 | 128 | >1024 | 0. 5 | syn |
| R6 | >512 | >512 | >512 | >1024 | 0.75 | add |
| R7 | 32 | >512 | 32 | >1024 | 0.75 | add |
| R8 | 512 | >512 | >512 | >1024 | 0.75 | add |
| R9 | 512 | >512 | 512 | >1024 | 0.75 | add |
| R10 | 256 | >512 | 256 | >1024 | 1 | add |
| R11 | 256 | >512 | 256 | >1024 | 1 | add |
| R12 | 256 | >512 | 256 | >1024 | 0.375 | syn |
| R13 | 64 | >512 | 64 | >1024 | 0.75 | add |
| R14 | 4 | 512 | 8 | 1024 | 1 | add |
| R15 | 256 | >512 | 512 | >1024 | 0.375 | syn |
| R16 | 8 | >512 | 8 | 1024 | 1 | add |
| R17 | 512 | >512 | >512 | >1024 | 0.75 | add |
| R18 | 128 | >512 | 256 | >1024 | 1 | add |
| R19 | >512 | >512 | >512 | >1024 | 1 | add |
| R20 | 128 | >512 | 128 | >1024 | 1 | add |
| ATCC | 0.5 | >512 | 1 | >1024 | 0.375 | syn |
| RATCC | 256 | >512 | 256 | >1024 | 0.75 | add |
Abbreviations: BER, berberine; CIP, ciprofloxacin; ΣFIC, summation fractional IC.
The experimental maximum concentration of the agents (ciprofloxacin/berberine) was 512 μg ml−1.
FICI ⩽0.5, synergy (syn); 0.5<FICI⩽1, additivity (add); 1<FICI⩽2, no difference (ind). MICs beyond the maximum scale were converted to the next twofold highest concentration.
In accordance with the ΣFIC data for K. pneumoniae, there were five typical isolates, including three clinical strains (synergy): R5, R12 and R15; one control strain (synergy): ATCC; and one induced strain (additivity): RATCC.
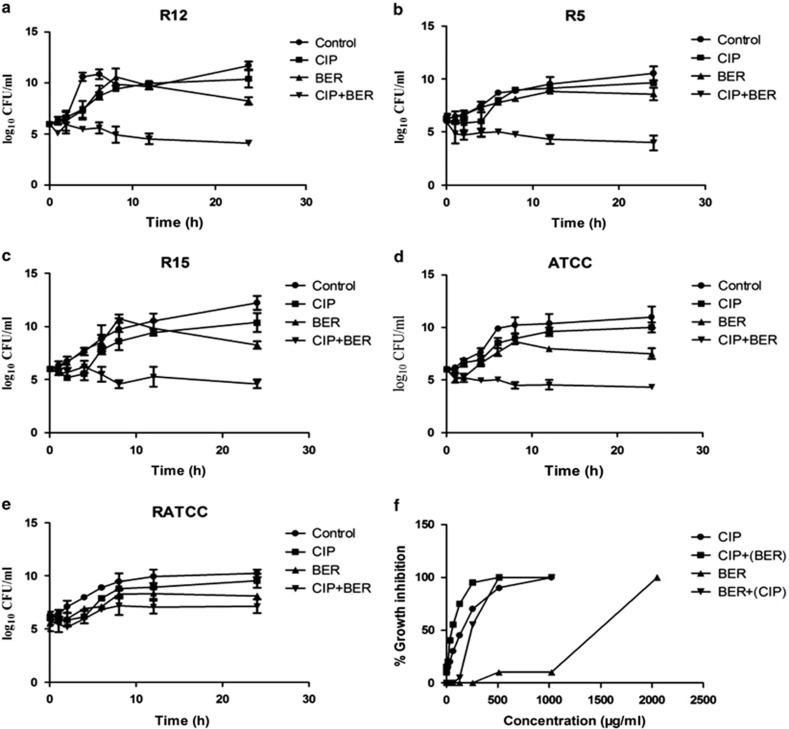
The results regarding the synergistic and additive interactions were reproduced using time-kill assays (Figure 1). Figure 1 shows that ciprofloxacin (⩽1/4 MIC) did not affect bacterial growth or viability after 24 h (P>0.05). As anticipated for berberine alone at 1024 μg ml−1, no appreciable antibacterial activity was found (0.9–1.79 log10 CFU ml−1, P>0.05). However, the antibacterial activity of ciprofloxacin was markedly enhanced in the presence of berberine, with bacterial growth being markedly reduced after 24 h when ciprofloxacin was combined with berberine at 1024 μg ml−1, compared with ciprofloxacin at ⩽1/4 MIC (2.41−5.7 log10 reduction in CFU ml−1, P<0.05) or berberine at 1024 μg ml−1 alone (1.51−4.17 log10 reduction in CFU ml−1, P<0.05). The results showed that this combination produced synergistic/additive bactericidal effects. These results were consistent with those of the checkerboard analyses (ΣFIC data). The results of the time-kill assay showed that against R12, R5, R15 and ATCC, the combination of ciprofloxacin and berberine exerted a synergistic effect on bacterial yields ⩾2 log10 reduction in CFU ml−1, with stronger antibacterial effects being observed in all groups after 24 h (P<0.05). The exception was the RATCC strain, which was considered indicative of an additive effect (<2 log10 CFU ml−1 lower than in any other group, P>0.05).
Figure 1.
The time-kill curves (a−e) of K. pneumoniae isolates (R12, R5, R15, ATCC and RATCC);  , growth control;
, growth control;  , CIP (a, b, c and e: ciprofloxacin 32 μg ml−1, d: ciprofloxacin 0.0625 μg ml−1);
, CIP (a, b, c and e: ciprofloxacin 32 μg ml−1, d: ciprofloxacin 0.0625 μg ml−1);  , BER (berberine 1024 μg ml−1);
, BER (berberine 1024 μg ml−1);  , CIP+BER (a, b, c and e: ciprofloxacin 32 μg ml−1, d ciprofloxacin 0.0625 g ml−1 combined with berberine 1024 μg ml−1). Dose−response inhibition curve of cell growth (f) of 22 K. pneumoniae isolates.
, CIP+BER (a, b, c and e: ciprofloxacin 32 μg ml−1, d ciprofloxacin 0.0625 g ml−1 combined with berberine 1024 μg ml−1). Dose−response inhibition curve of cell growth (f) of 22 K. pneumoniae isolates.  , berberine 1024 μg ml−1 was combined with various concentrations of ciprofloxacin.
, berberine 1024 μg ml−1 was combined with various concentrations of ciprofloxacin.  , a sub-MIC level of ciprofloxacin was combined with various concentrations of berberine.
, a sub-MIC level of ciprofloxacin was combined with various concentrations of berberine.
An assessment of the dose-response inhibition of K. pneumoniae by berberine or ciprofloxacin is shown in Figure 1f. It can be seen that, compared with single agents, the curves for the berberine–ciprofloxacin combination were both shifted to the left. The dose of ciprofloxacin was at least fourfold lower, while that of berberine was at least twofold lower. Berberine alone at a concentration <512 μg ml−1 exhibited no detectable antibacterial activity, and at 1024 μg ml−1 it showed poor antibacterial effects. Similar results were obtained at a concentration <4 μg ml−1 for ciprofloxacin alone, where the growth of K. pneumoniae was not significantly inhibited. However, when ciprofloxacin was combined with berberine, the growth of K. pneumoniae was partially inhibited. Therefore, berberine functionally complemented the antibacterial activity of ciprofloxacin at low concentrations. Moreover, when combined with sub-MIC concentrations of ciprofloxacin, the concentration of berberine was reduced (<256 μg ml−1). These results indicated that the combination of berberine and ciprofloxacin can be used to reduce the use of single agents, thus supplementing the inadequate antimicrobial activity of single agents at subinhibitory concentrations.
Expression of acrA, acrB, tolC and acrR in K. pneumoniae in response to ciprofloxacin and berberine
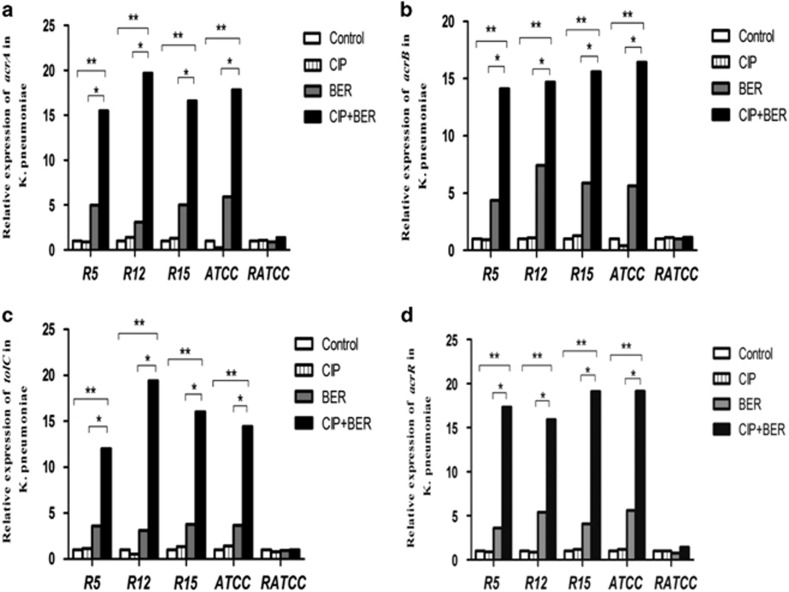
The gene expression levels in the R12, R5, R15, ATCC and RATCC strains were confirmed in the control group (untreated cultures), the berberine group (1024 μg ml−1), the ciprofloxacin ⩽1/4 MIC group and the ciprofloxacin ⩽1/4 MIC+berberine 1024 μg ml−1 group through RT-PCR analysis. For the ciprofloxacin ⩽1/4 MIC group, the following concentration was used: ciprofloxacin at 32 μg ml−1 (R12, R5, R15 and RATCC strains) and 0.0625 μg ml−1 (ATCC strain). The following methods were used for RNA extraction: bacteria were grown until they reached the exponential growth or stationary phase and were then exposed to ciprofloxacin, berberine and the combination of the two for 3 h. The rate of transcription of acrAB was several times higher in the stationary phase in E. coli and E. amylovora than in other phases.31, 32 The gene expression results are shown in Figure 2. All assays were inspected for single unique peaks in the melting curves; the melting temperatures were close to 89 °C. The amplification curves were linear and cycle threshold (CT) values of nearly 18–28 indicated specific amplification. According to the concentrations of the two-drug combination in the time-kill assay, at the messenger RNA level, the analyses indicated that the expression of acrA, acrB, tolC and acrR was significantly higher than that in untreated cultures and was >14-fold higher in four strains (R12, R5, R15 and ATCC) in the ciprofloxacin–berberine combination group (**P<0.05). In the berberine-treated groups, the expression levels of these genes were upregulated nearly fourfold on average (*P<0.05). However, the expression of acrA, acrB, tolC and acrR in the ciprofloxacin-treated group was not significantly different from that in the untreated control group (P>0.05). The single exception was the RATCC strain, which showed no inhibition of bacterial growth when treated with either the single or combined agents. In addition, this strain did not show inhibition of growth and did not exhibit increased transcription in the different treatment groups (P>0.05).
Figure 2.
The relationship between the expression of acrA, acrB, tolC and acrR messenger RNA in five strains of multidrug-resistant K. pneumoniae (R5, R12, R15, ATCC and RATCC). (a) Analysis of the expression of acrA, (b) acrB, (c) tolC and (d) acrR. White bars indicate the control group in untreated culture; striped bars, CIP (ciprofloxacin 32 μg ml−1 in all cases except 0.0625 μg ml−1 against the ATCC strain); gray bars, BER (berberine 1024 μg ml−1); black bars, CIP+BER (ciprofloxacin 32 μg ml−1 in all cases except 0.0625 μg ml−1 against the ATCC strain combined with berberine 1024 μg ml−1). For the five strains not including RATCC, the expression of acrA, acrB, tolC and acrR was higher in the berberine and ciprofloxacin+berberine groups than in the controls. This effect was more pronounced in the ciprofloxacin+ berberine group (**P<0.05).
Discussion
As previously described, the development of drug resistance in K. pneumoniae is a serious clinical problem. Molecular biology has shown that there are several causes of drug resistance.33, 34, 35, 36 A combination of agents is one means of solving the problem of resistance. This study demonstrated that berberine specifically potentiates the bactericidal activity of ciprofloxacin against multidrug-resistant K. pneumoniae. Berberine is a plant secondary metabolite that does not produce drug resistance. In the current study, as indicated by the MIC value of ciprofloxacin in combination with berberine, the addition of berberine to ciprofloxacin reduced the required concentration of ciprofloxacin. In addition, 77.27% of the tested K. pneumoniae isolates showed an additive bactericidal effect.
The concentration of ciprofloxacin in the presence of berberine was decreased by 1/2 to 3/4 compared with the single agent. Synergistic effects were observed in 18.18% of isolates and the concentration of ciprofloxacin could be decreased by >3/4 through the use of the combination. Moreover, the results showed that ciprofloxacin combined with berberine resulted in bacterial growth arrest in the time-kill assay. These findings suggest that the presence of berberine enhanced bacterial susceptibility to ciprofloxacin and potentiated the inhibitory activity of ciprofloxacin. Therefore, berberine is considered an antibiotic potentiator and merits further study in vivo.
However, the molecular mechanism by which berberine increases the antimicrobial activity of ciprofloxacin against multidrug-resistant K. pneumoniae is not yet understood. It is possible that berberine increases ciprofloxacin uptake via channel formation in bacterial membranes, as observed in aminoglycosides.37 It has long been reported that berberine is a substrate for multidrug efflux pumps.13, 38 Several efflux pumps belonging to the resistance-nodulation-division (RND) superfamily are found in multidrug-resistant Gram-negative bacteria.39 Many types of RND family efflux systems have been characterized in K. pneumoniae. The acrAB efflux pump is homologous to the system in E. coli.35, 40 As reported by Karaosmanoglu et al.,16 the transcription of acrEF is upregulated in E. coli after inhibition of growth in the presence of berberine over time. In the presence of sub-MIC concentrations, compared with norfloxacin and a blank control, the transcription levels of acrAB and acrR were found to remain the same in Erwinia amylovora but were higher in the presence of berberine than in the blank control.32 Accordingly, the results of the present study demonstrated that K. pneumoniae activity was inhibited owing to the synergistic effect of the ciprofloxacin–berberine combination, and the transcription levels of the efflux pumps acrA, acrB, tolC and acrR were upregulated. These findings suggest that regular transcription of acrAB may be used to identify protective mechanisms in bacteria. Some of these mechanisms may have been stimulated to expel as much berberine as possible from the cells. One of the reasons that bacterial growth is affected may be that berberine upregulates acrAB transcription. The inner membrane transporter acrB forms a complex with the periplasmic fusion protein acrA, which coordinates with the outer membrane channel tolC.41, 42 Previous studies have indicated that the inner membrane transport protein acrB is essential for mediating contact-dependent growth inhibition.43 In this regard, overexpression of acrB might inhibit bacterial cell growth via cell-to-cell-contact-mediated mechanisms, and hence acrB overexpression is harmful to bacterial growth.44 Moreover, when acrAB transcription is increased, the periplasm becomes overcrowded with excess expression products that can cause detrimental effects on cell fate.45
In summary, this study demonstrates that the combination of ciprofloxacin and berberine exhibits definite antibacterial effects that could help decrease the dose of ciprofloxacin, decrease the production of ciprofloxacin-resistant K. pneumoniae and reduce the incidence of drug resistance. Berberine shows a synergistic/additive bactericidal effect that is likely to be useful for the treatment of clinical infections. Berberine may be important for the future development of antibiotics against K. pneumoniae, especially in multidrug-resistant and refractory infections.
Acknowledgments
We thank the Guangzhou Municipal Key Laboratory of Allergy & Clinical Immunology, Allergy Research Branch of the State Key Laboratory Respiratory Disease and the Second Affiliated Hospital of Guangzhou Medical University for providing technical support. The experimental strains were provided by the Bacteriology Department of the First Affiliated Hospital of Guangzhou Medical University. This research was supported by the Science and Technology Program of Guangzhou, China (No. [2012]J4100022).
Footnotes
The authors declare no conflict of interest.
References
- Ryan, K. J. & Ray, C. G. Sherris Medical Microbiology 4th edn (McGraw-Hill Medical Publishing, New York, NY, USA, 2004).
- Jesse, T. et al. Vital signs: carbapenem-resistant Enterobacteriaceae. Morbid. Mortal. Wkly. Rep. 62, 165–170 (2013). [PMC free article] [PubMed] [Google Scholar]
- Kronenberg, A. et al. Temporal trends of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates in in- and outpatients in Switzerland, 2004 to 2011. Eurosurveillance 18, 1–9 (2013). [PubMed] [Google Scholar]
- Yong-hong, X. et al. Mohnarin report of 2011: monitoring of bacterial resistance in China. Chin. J. Nosocomiol 22, 4946–4952 (2012). [Google Scholar]
- Piddock, L. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19, 382–402 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davin-Regli, A. et al. Membrane permeability and regulation of drug “influx and efflux” in enterobacterial pathogens. Curr. Drug Targets 9, 750–759 (2008). [DOI] [PubMed] [Google Scholar]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 56, 20–51 (2005). [DOI] [PubMed] [Google Scholar]
- Asai, M. et al. Berberine alters the processing of Alzheimer's amyloid precursor protein to decrease Aß secretion. Biochem. Biophys. Res. Commun. 352, 498–502 (2007). [DOI] [PubMed] [Google Scholar]
- Birdsall, T. C. & Kelly, G. S. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 2, 94–103 (1997). [Google Scholar]
- Kong., D. X et al. How many traditional Chinese medicine components have been recognized by modern Western medicine? A chemoinformatic analysis and implications for finding multicomponent drugs. Chem. Med. Chem 3, 233–236 (2008). [DOI] [PubMed] [Google Scholar]
- Xu, Y. et al. Proteomic analysis reveals a synergistic mechanism of fluconazole and berberine against fluconazole-resistant Candida albicans: endogenous ROS augmentation. J. Proteome Res. 8, 5296–5304 (2009). [DOI] [PubMed] [Google Scholar]
- Yu, H. H. et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J. Med. Food. 8, 454–461 (2005). [DOI] [PubMed] [Google Scholar]
- Stermitz, F. R. et al. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5'-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl Acad. Sci. USA 97, 1433–1437 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu-lin, Z. et al. Inhibitory effects of golden thread (Coptis chinensis) and berberine on Microcystis aeruginosa. Water Sci. Technol. 61, 763– (2010). [DOI] [PubMed] [Google Scholar]
- Karaosmanoglu, K. et al. Assessment of Berberine as a multi-target antimicrobial: a multi-omics study for drug discovery and repositioning. Omics 18, 42–53 (2014). [DOI] [PubMed] [Google Scholar]
- Stermitz, F. R. et al. 5'-Methoxyhydnocarpin-D and pheophorbide A: Berberis species components that potentiate berberine growth inhibition of resistant Staphylococcus aureus. J. Nat. Prod. 63, 1146–1149 (2000). [DOI] [PubMed] [Google Scholar]
- Han, Y. & Lee, J. H. Berberine synergy with amphotericin B against disseminated candidiasis in mice. Biol. Pharm. Bull. 28, 541–544 (2005). [DOI] [PubMed] [Google Scholar]
- Quan, H. et al. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazol. Antimicrob. Agents Chemother. 50, 1096–1099 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, G. X., Xu, X. & Wu, C. D. In vitro synergism between berberine and miconazole against planktonic and biofilm Candida cultures. Arch. Oral Biol. 56, 565 (2011). [DOI] [PubMed] [Google Scholar]
- Cockerill, F. R. et al. Performance Standards for Antimicrobial Susceptibility Testing; 21st Informational Supplement. CLSI M100-S22 (Clinical and Laboratory Standards Institute, Wayne, PA, USA, 2012).
- Gui-jian, L. et al. Enterococcus induced resistance: a laboratory study of antibacterial agents by multiple-step method. Chin. J. Nosocomiol. 15, 601–604 (2005). [Google Scholar]
- Wayne, P. A. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; 9th approved standard. CLSI M07–A9 (Clinical and Laboratory Standards Institute, 2012).
- Pillai, S. K., Moellering, R. C. Jr & Eliopoulos, G. M. in Antibiotics in Laboratory Medicine 5th edn (ed. Lorian, V.) 365–440 (Lippincott Williams & Wilkins, Philadelphia, PA, USA, 2005).
- Mosquera, J. et al. In vitro interaction of terbinafine with itraconazole, fluconazole, amphotericin B and 5-flucytosine against Aspergillus spp. J. Antimicrob. Chemother. 50, 189–194 (2002). [DOI] [PubMed] [Google Scholar]
- Naghmouchi, K. et al. Nisin A and polymyxin B as synergistic inhibitors of gram-positive and gram-negative bacteria. Probiot. Antimicrob. Proteins 2, 98–103 (2010). [DOI] [PubMed] [Google Scholar]
- An, J. et al. Antibacterial and synergy of a flavanonol rhamnoside with antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). Phytomedicine 18, 990–993 (2011). [DOI] [PubMed] [Google Scholar]
- Schmitt, P. et al. Expression, tissue localization and synergy of antimicrobial peptides and proteins in the immune response of the oyster Crassostrea gigas. Dev. Comp. Immunol. 37, 363–370 (2012). [DOI] [PubMed] [Google Scholar]
- Wayne, P. A. Performance Standards for Antimicrobial Susceptibility Testing; 19th Informational Supplement. CLSI M100-S19 (Clinical and Laboratory Standards Institute, 2009).
- Isenberg, H. Clinical Microbiology Procedures Handbook 2nd edn (American Society for Microbiology, Washington, DC, USA, 2004).
- Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 22DDCT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Ma, D. et al. Genes acrA and acrB encode a stress-induced efflux system in Escherichia coli. Mol. Microbiol. 16, 45–55 (1995). [DOI] [PubMed] [Google Scholar]
- Burse, A., Weingart, H. & Ullrich, M. The phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen Erwinia amylovora. Mol. Plant Microbe Interact. 17, 43–54 (2004). [DOI] [PubMed] [Google Scholar]
- Hernandez-Alles, S. et al. Porin expression in clinical isolates of Klebsiella pneumoniae. Microbiology 145, 673–679 (1999). [DOI] [PubMed] [Google Scholar]
- Martinez-Martinez, L. et al. Energy-dependent accumulation of norfloxacin and porin expression in clinical isolates of Klebsiella pneumoniae and relationship to extended-spectrum lactamase production. Antimicrob. Agents Chemother. 46, 3926–3932 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzariol, A. et al. AcrAB efflux system: expression and contribution to fluoroquinolone resistance in Klebsiella spp. Antimicrob. Agents Chemother. 46, 3984–3986 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley, B., Benedi, V. J. & Domenech-Sanchez, A. Expression of SHV-2 b-lactamase and of reduced amounts of OmpK36 porin in Klebsiella pneumoniae results in increased resistance to cephalosporins and carbapenems. Antimicrob. Agents Chemother. 46, 3679–3682 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, B. D. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 51, 341–350 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, G. D. Resisting resistance: new chemical strategies for battling superbugs. Chem. Biol. 7, 127–132 (2000). [DOI] [PubMed] [Google Scholar]
- Piddock, L. J. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4, 629–636 (2006). [DOI] [PubMed] [Google Scholar]
- Padilla, E. et al. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 54, 177–183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgurskaya, H. I. & Nikaido, H. Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 37, 219–225 (2000). [DOI] [PubMed] [Google Scholar]
- Zgurskaya, H. I. & Nikaido, H. Cross-linked complex between oligomeric periplasmic lipoprotein AcrA and the inner-membrane-associated multidrug efflux pump AcrB from Escherichia coli. J. Bacteriol. 182, 4264–4267 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, S. K. et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol. MIcrobiol. 70, 323–340 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuka, Y. & Nikaido, H. Covalently linked trimer of the AcrB multidrug efflux pump provides support for the functional rotating mechanism. J. Bacteriol. 191, 1729–1737 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, S. Y. & Zgurskaya, H. I. Cell division defects in Escherichia coli deficient in the multidrug efflux transporter AcrEF-TolC. J. Bacteriol. 187, 7815–7825 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]