Abstract
Objective Growth hormone (GH) deficiency has recently been reported as a cause of nonalcoholic fatty liver disease (NAFLD), and GH supplementation has been shown to improve the histology of NAFLD. The aim of the present study was to clarify the relationship between the histological severity of NAFLD and production of the GH/insulin-like growth factor 1 (IGF-1) axis.
Methods A total of 222 Japanese patients with liver biopsy-confirmed NAFLD and 55 patients with hepatitis C virus (HCV)-related chronic liver disease (CLD) were enrolled in the present study. The serum levels of GH, IGF-1, and IGF-binding protein 3 (IGFBP-3) were measured and their relationships with the histological severity of liver disease were assessed. To exclude age- and sex-related differences, the IGF-1 standard deviation score (IGF-1:SDS) was determined for each patient.
Results With respect to the stage of fibrosis in patients with NAFLD, the serum GH levels were higher and the serum IGFBP-3 levels and IGF-1:SDSs were lower in patients with cirrhosis (grade F4 fibrosis) than in patients grade F1-F3 fibrosis; moreover, these differences were statistically significant (all p<0.01). The GH, IGF-1, and IGFBP-3 levels were not correlated with fibrosis in patients with HCV-related CLD. Furthermore, the GH levels were lower and the IGFBP-3 levels were significantly higher in patients with severe steatosis (S3) than in patients with mild to moderate steatosis (S1-S2) (p<0.05).
Conclusion Increased GH levels and decreased IGF-1 and IGFBP-3 levels might contribute to the progression of NAFLD. The GH/IGF-1 axis may be important in the development of NAFLD, but not in patients with HCV-related CLD.
Keywords: nonalcoholic fatty liver disease (NAFLD), growth hormone (GH), insulin-like growth factor 1 (IGF-1), insulin-like growth factor-binding protein 3 (IGFBP-3)
Introduction
Untreated growth hormone (GH) deficiency in adults presents symptoms that mimic those of metabolic syndrome, including underlying insulin resistance, dyslipidemia, hypertension, and visceral obesity (1). Nonalcoholic fatty liver disease (NAFLD) is thought to be the hepatic manifestation of metabolic syndrome. GH replacement therapy improves the histological features of NAFLD (2-4) and metabolic disease (5). Thus, the relationship between GH and NAFLD has been a focus of research.
GH is secreted by the anterior pituitary gland and is responsible for increasing the bone length, bone density, and muscle mass during childhood and adolescence (6). Moreover, it has important effects on the metabolism of lipids, carbohydrates, and body water throughout life (6). GH exerts some of its effects by binding to receptors on target cells, and it stimulates the production and secretion of insulin-like growth factor 1 (IGF-1) from many tissues, mainly the liver (7). Besides the direct effects of GH, IGF-1, which is thought to be the main mediator of the biological action of GH, is involved in metabolism (8).
The biological effects of IGF-1 are modulated by several IGF-specific binding proteins (IGFBP-1 to -6), such as IGFBP-3, which is the major binding protein for IGF-1 (9). Eighty to 90% of the IGF-1 in circulation forms trimer complexes that are composed of IGF-1, IGFBP-3, and acid-labile subunit (ALS) (10). The formation of these trimer complexes restricts the IGFs to the circulation and prolongs their half-lives (11). The circulating complexes are believed to partially dissociate to allow IGF-1 to reach the tissues from the bloodstream (12). IGFBP-3 is mainly produced by the Kupffer cells in the liver. GH and IGF-1 directly and indirectly modulate the IGFBP-3 levels (13,14) and they are affected by nutritional deficiencies (including anorexia nervosa) (15) and liver function (16).
Since the 1980s, several studies have reported that patients with liver cirrhosis due to hepatitis virus infection, autoimmune liver disease, and alcoholic liver disease have low levels of IGF-1, IGFBP-3, and ALS, despite having high serum levels of GH (16,17). This is thought to be a consequence of reduced hepatic IGF-1, IGFBP-3, and ALS production and an increase in the level of GH due to the loss of negative feedback (18). However, these studies did not include patients with NAFLD.
In recent years, several studies have been published on the relationship between the GH/IGF-1 system and NAFLD in patients without GH deficiency. However, few studies have investigated the relationship between the histology of the liver and abnormalities of the GH/IGF-1 axis. The aim of the present study was to clarify the relationship between the histological severity of NAFLD and hepatitis C virus (HCV)-related chronic liver disease (CLD) and the level of GH/IGF-1 production, in order to evaluate the involvement of the GH/IGF-1 axis in the progression of NAFLD.
Materials and Methods
Patients
The present retrospective study included a total of 222 Japanese patients with liver biopsy-confirmed NAFLD. These consecutively biopsied patients were examined at the Department of Gastroenterology and Internal Medicine of Tokyo Women's Medical University (TWMU) from October 2002 to May 2012. NAFLD was diagnosed on the basis of a liver biopsy that showed steatosis in at least 5% of the hepatocytes. The exclusion criteria were the consumption of more than 20 g of alcohol per day; hepatitis B or C virus, autoimmune hepatitis, metabolic liver disease, or other liver diseases; and treatment with drugs or the presence of endocrine abnormalities that are known to cause steatohepatitis. In addition, 55 patients in whom serum hepatitis C virus (HCV) RNA was detectable by a polymerase chain reaction and who showed histological findings that were compatible with chronic hepatitis or liver cirrhosis were enrolled as a comparison group. This study was approved by the TWMU Ethics Committee. Written informed consent was obtained from all of the patients.
Clinical and laboratory analysis
This was a retrospective and observational study. The age, clinical history, height, body weight, blood samples for biochemistry, hematology data, and hormonal data were collected from all patients at the time of liver biopsy. The platelet count and the serum concentrations of aspartate aminotransferase, alanine transaminase, γ-glutamyltransferase, hemoglobin A1c, total cholesterol, triglycerides, and GH were determined using standard laboratory techniques. The plasma levels of IGF-1 and IGFBP-3 were measured using an immunoradiometric assay kit (IGF-1 assay; Fuji Rebio Inc., Tokyo, Japan) and an IGFBP-3 enzyme-linked immunosorbent assay kit (Mediagnost GmbH, Reutlingen, Germany). Because the IGF-1 level is related to age and sex, we calculated the standard deviation of IGF-1 score (IGF-1:SDS) according to age and sex of the patients (19).
All of the liver tissue specimens were evaluated by a single pathologist. Specimens from patients with NAFLD were evaluated to determine the grades and stages of steatosis (S0-S3), activity (A0-A3), ballooning (B0-B2), and fibrosis (F0-F4) according to the modified criteria of Kleiner (20). Specimens from patients with HCV were evaluated according to the histology activity index scoring system of Knodell et al. (21) with the modifications proposed by Schvarcz et al. (22) (for the categories of periportal necrosis and portal inflammation) and by Scheuer (23) (for the categories of lobular necroinflammation and fibrosis). The grading score for histological activity of chronic hepatitis represents the sum of the scores for their periportal, lobular, and portal lesions (24).
Statistical analysis
The Wilcoxon signed-rank test and Kruskal-Wallis H-test were performed as appropriate, using the JMP Pro 11 software program (SAS Institute Inc., Cary, NC, USA). The data are presented as the median (range). p values of <0.05 and <0.01 were considered to indicate statistical significance.
Results
The correlation between fibrosis and the GH, IGF-1, and IGFBP-3 axis
The clinical and laboratory data of the patients are shown in Table 1. The mean age was significantly lower and the body mass index (BMI) values and the prevalence of diabetes mellitus, hypertension, and dyslipidemia were significantly higher among the patients with NAFLD (p<0.05).
Table 1.
The Characteristics of the Patients.
| NAFLD (n = 222) | HCV (n = 55) | p value | |
|---|---|---|---|
| Age | 53(14-85) | 61 (30-76) | <0.05 |
| Sex (M/F, %) | 58/42 | 44/56 | N.S. |
| Body mass index | 26.9 (19.7-43.3) | 22.7 (15.7-29.6) | <0.05 |
| Diabetes mellitus | 106 (48%) | 8 (15%) | <0.05 |
| Hypertension | 89 (40%) | 12 (22%) | <0.05 |
| Dyslipidemia | 149 (67%) | 3 (5%) | <0.05 |
| Alanine aminotransferase (IU/L) | 71 (8-483) | 65 (10-306) | N.S. |
| Aspartate transaminase (IU/L) | 45 (13-231) | 51 (17-276) | N.S. |
| Fibrosis (F0-1/2/3/4, %) | (21/32/28/19) | (25/29/20/25) | N.S. |
| Activity (A0/A1/A2/A3, %) | (0/13/42/44) | (0/20/67/13) | <0.01 |
| Steatosis (S1/S2/S3, %) | (17/21/61) | (36/2/0) | <0.01 |
| Ballooning (B0/B1/B2, %) | (3/63/28) | (96/4/0) | <0.01 |
NAFLD: nonalcoholic fatty liver disease, HCV: hepatitis C virus, M/F: male/female
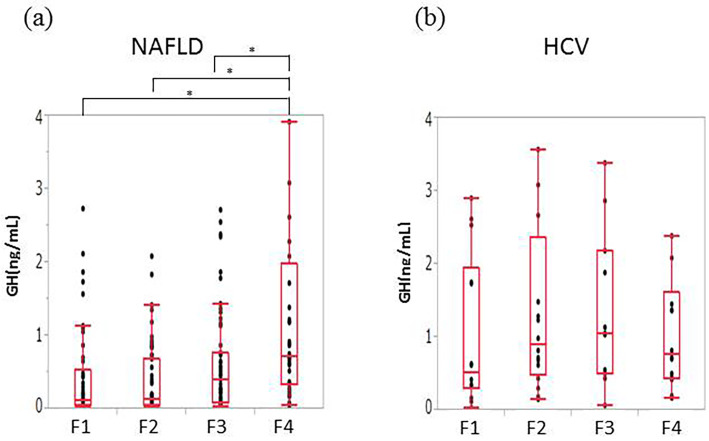
We assessed the relationships between the histological severity of liver disease and the GH, IGF-1 and IGFBP-3 levels, the IGF-1:SDS and the IGF-1/IGFBP-3 ratio. The patients with NAFLD showed higher grades of fibrosis tended to have higher levels of GH. Patients with F4 had significantly higher levels of GH than patients with F1-F3 (median GH [ng/mL]: F1, 0.12; F2, 0.14; F3, 0.30; F4, 0.71) (Fig. 1a). No correlation was found between the GH level and fibrosis in patients with HCV-related CLD (Fig. 1b).
Figure 1.
The correlations between fibrosis and GH. (a) NAFLD. Patients with F4 had significantly higher levels of GH than patients with F1 to F3. (b) HCV. No correlation between the GH level and fibrosis was evident in patients with HCV-related chronic liver disease. GH: growth hormone, F: fibrosis stage, NAFLD: nonalcoholic fatty liver disease, HCV: hepatitis C virus. *p<0.01 **p<0.05.
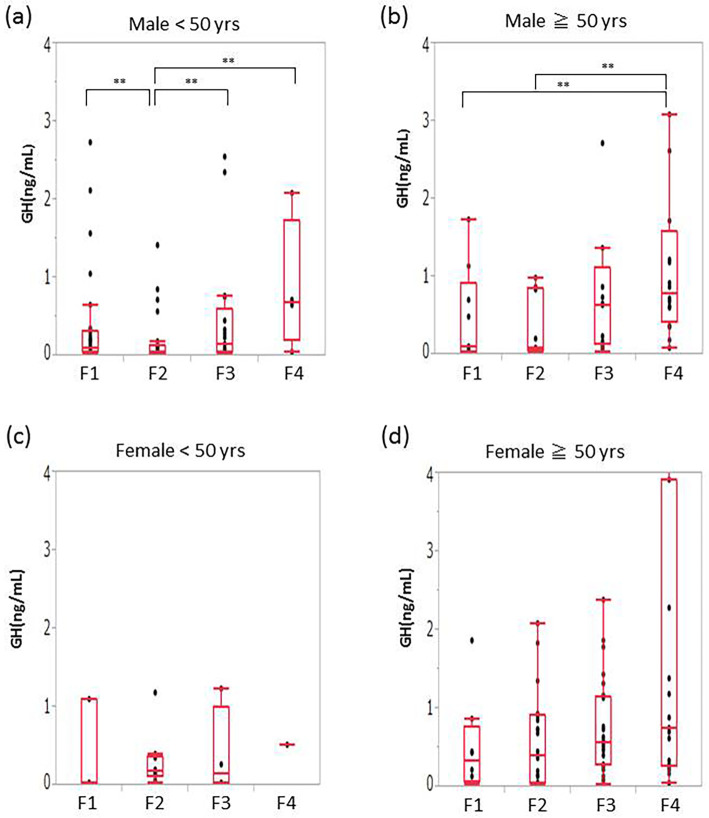
To assess the age- and sex-related differences in the GH levels, we classified the patients of each sex into two age-based categories: <50 years of age and ≥50 years of age. Among men who were <50 years of age, patients with F2 were found to have significantly lower levels of GH than patients at other stages (mean GH [ng/mL]: F1, 0.11; F2, 0.05; F3, 0.12; F4, 0.68) (Fig. 2a). Among men who were ≥50 years of age, the patients with F4 had significantly higher levels of GH than those with F1-F2 (mean GH [ng/mL]: F1, 0.11; F2, 0.08; F3, 0.63; F4, 0.79) (Fig. 2b). Female patients showed a similar tendency, but the results did not reach statistical significance (Fig. 2c and d).
Figure 2.
The sex- and age-specific correlations between fibrosis and GH in NAFLD patients. (a) Men of <50 years of age, (b) women of <50 years of age, (c) men of ≥50 years of age (d) women of ≥50 years of age. (a) Among the men of <50 years of age with NAFLD, patients with F2 had significantly lower levels of GH in comparison to patients of other stages. (b) Among men of ≥50 years of age, patients with F4 had significantly higher levels of GH than patients with F1 to F2 (b). Female patients showed a similar tendency, but the result did not reach statistical significance (c, d). GH: growth hormone, F: fibrosis stage, NAFLD: nonalcoholic fatty liver disease, HCV: hepatitis C virus, yrs: years. *p<0.01 **p<0.05.
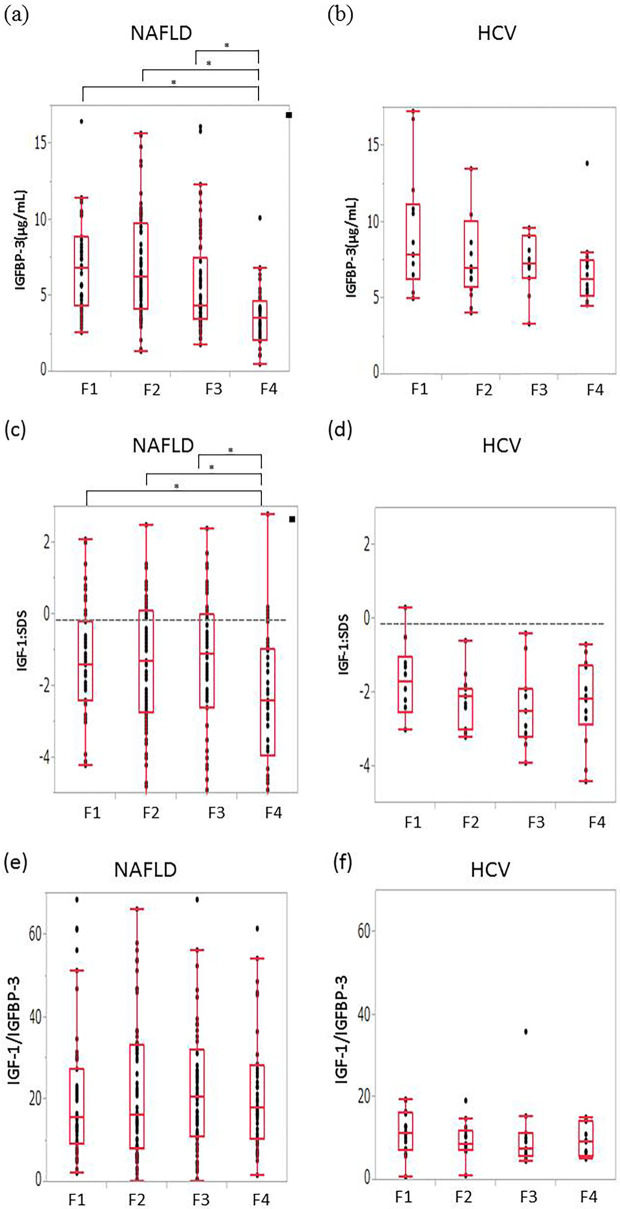
Patients with F4 had significantly lower IGFBP-3 levels and IGF-1:SDS values than patients with F1-F3 (mean IGFBP-3 [μg/mL]: F1, 6.84; F2, 6.30; F3, 4.34; F4, 3.59 and mean IGF-1:SDS: F1, -1.4, F2, -1.3, F3, -1.1; F4, -2.4) (Fig. 3a and c). HCV-related CLD did not show these findings according to the grade of fibrosis (Fig. 3b and d). The IGF-1/IGFBP-3 ratio was not correlated with the grade of fibrosis in any subset of patients (Fig. 3e and f).
Figure 3.
The correlation between fibrosis and the IGFBP-3 level, the IGF-1: SDS, and the IGF-1/IGFBP-3 ratio. (a) The IGFBP-3 level in NAFLD, (b) the IGFBP-3 level in HCV, (c) the IGF-1: SDS in NAFLD, (d) the IGF-1: SDS in HCV, (e) the IGF-1/IGFBP-3 ratio in NAFLD, and (f) the IGF-1/IGFBP-3 ratio in HCV. Patients with F4 NAFLD had significantly lower levels of IGFBP-3 and a lower IGF-1: SDS than in comparison to patients with F1 to F3 (Fig. 3a, c). Patients with HCV-related CLD did not show these findings according to the grade of fibrosis (Fig. 3b, d). The IGF-1/IGFBP-3 ratio was not correlated with the grade of fibrosis (Fig. 3e, f). IGFBP-3, insulin-like growth factor-specific binding proteins; IGF-1: SDS: IGF-1: standard deviation score, F: fibrosis stage, NAFLD: nonalcoholic fatty liver disease, HCV: hepatitis C virus. *p<0.01 **p<0.05.
The correlation of the histological activity/steatosis with the GH, IGF-1, and IGFBP-3 axis
We next analyzed the relationship between the histological activity and the GH, IGF-1, and IGFBP-3 axis and found that none of these factors were significantly correlated with the histological activity.
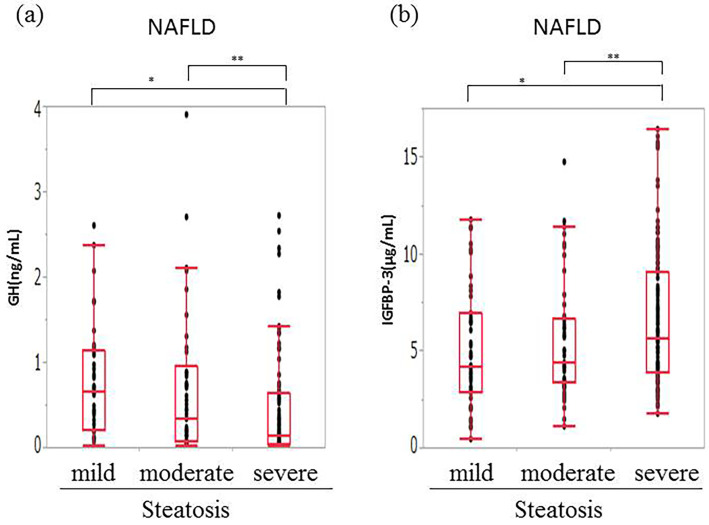
NAFLD patients with severe steatosis (S3) had significantly lower levels of GH and higher levels of IGFBP-3 in comparison to patients with mild to moderate steatosis (S1-S2) (Fig. 4). NAFLD patients with higher grades of steatosis tended to have higher IGF-1:SDS values. The IGF-1/IGFBP-3 ratio was not correlated with the grade of steatosis. Ballooning was associated with significantly lower levels of IGFBP-3 in grade 2 NAFLD patients than it was in grade 0 to 1 patients. The GH, and IGF-1 levels, the IGF-1:SDS, and the IGF-1/IGFBP-3 ratio were not correlated with ballooning. Neither severe steatosis nor ballooning was observed in the HCV-CLD patients. The levels of GH, IGF-1, and IGFBP-3 did not differ to a statistically significant extent between patients with no or mild-to-moderate steatosis and ballooning.
Figure 4.
The correlation between GH and IGFBP-3 and steatosis in NAFLD. (a) GH and (b) IGFBP-3. Patients with severe steatosis (S3) had significantly lower levels of GH and higher levels of IGFBP-3 in comparison to patients with mild-moderate steatosis (S1-S2). GH: growth hormone, IGFBP-3: insulin-like growth factor-specific binding proteins, NAFLD: nonalcoholic fatty liver disease, HCV: hepatitis C virus. *p<0.01 **p<0.05.
The characteristics of patients with low levels of IGF-1 and an IGF-1:SDS of <-6 SD
Six (2.7%) of the patients with NAFLD had notably low levels of IGF-1 and an IGF-1:SDS of <-6 SD (Table 2). All were female patients of 20-70 years of age. One patient had cirrhosis with a high level of GH. In the other five patients, however, the GH levels were within the normal limit or low and the levels of cortisol and thyroid-stimulating hormone were within the normal limits. All of these five patients had dyslipidemia, four had diabetes mellitus, and two had hypertension. They had no medical history of endocrinal abnormalities or intracranial lesions, nor had they undergone intracranial radiation therapy. Two had grade F2 liver fibrosis, three had grade F3 liver fibrosis, one had mild liver steatosis, and four had severe steatosis. We were unable to find a clinical feature that was common to all five patients; thus, the cause of their decreased IGF-1 levels was unclear.
Table 2.
Patients with NAFLD with Low Levels of IGF-1: SDS (<-6 SD).
| Case | Sex | Age (years) | IGF-1: SDS | IGF-1 (ng/mL) | GH (ng/mL) | BMI (kg/m2) | Height (m) | Fibrosis | Steatosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 51 | -9.6 | 4 | 0.727 | 34.1 | 1.47 | 2 | 3 |
| 2 | F | 57 | -9.8 | 4 | 0.731 | 24.2 | 1.48 | 3 | 3 |
| 3 | F | 73 | -6.5 | 10 | 0.058 | 24.0 | 1.49 | 2 | 2 |
| 4 | F | 72 | -8.1 | 5 | 0.580 | 26.0 | 1.51 | 3 | 3 |
| 5 | F | 60 | -6.2 | 18 | 18.670 | 21.0 | 1.56 | 4 | 1 |
| 6 | F | 26 | -14.5 | 7 | 0.264 | 33.3 | 1.56 | 3 | 3 |
NAFLD: nonalcoholic fatty liver disease, IGF-1:SDS: insulin-like growth factor 1: standard deviation score, GH: growth hormone, BMI: body mass index, F: female
Discussion
This study shows that patients with NAFLD and advanced fibrosis had high levels of GH and low levels of IGF-1 and IGFBP-3. It was paradoxically observed that the levels of GH, IGF-1, and IGFBP-3 were correlated with the severity of steatosis. In patients with HCV-related CLD, however, the levels were not affected by either fibrosis or steatosis. We hypothesize that abnormal GH and IGF-1 levels may contribute to the development of NAFLD.
A relationship between NAFLD without GH deficiency and GH or IGF-1 expression has been reported. Patients with NAFLD have significantly lower levels of serum GH in comparison to controls without NAFLD (25). Moreover, patients with NAFLD had lower peak levels of GH after a GH stimulation test in comparison to age- and BMI-matched controls (26). Studies concerning the state of the GH/IGF-1 axis in patients with NAFLD report conflicting results.
The secretion of human GH declines progressively with age, as do the levels of IGF-1 and IGFBP-3. In addition, because the IGF-1/IGFBP-3 ratio declines with age, the level of bioactive dissociated IGF-1 also declines. Thus, the IGF-1/IGFBP-3 ratio has been used as an index of the bioavailability of IGF in the circulation of humans (27). In the present study, the IGF-1/IGFBP-3 ratio was not correlated with the histological features of NAFLD. IGFBPs are present as six subclasses (1-6); thus, the calculation of the IGF/IGFBP-3 ratio does not involve the concentration of free IGF-1.
In contrast, a paradoxical elevation of the serum GH level has been reported in patients with cirrhosis (16). The GH secretion rate was found to be increased in patients with cirrhosis, and the GH level was correlated with high Child-Pugh classes (18). In healthy subjects, IGF-1 and IGFBP-3 are dependent on the secretion of GH; however, the liver produces reduced amounts of IGF-1 in patients with cirrhosis, which results in an increase in the GH level because of the loss of negative feedback. The IGF-1 and IGFBP-3 levels were shown to be low in cirrhosis patients, but to improve after liver transplantation (28). These observations suggest that the liver function is involved in the regulation of the IGF-1 and IGFBP-3 levels. IGF-1 is principally secreted by the hepatocytes. In this study, we showed that the GH level was high and that the IGF-1 and IGFBP-3 levels were low in patients with NAFLD and advanced fibrosis, including patients with cirrhosis. However, we did not identify a relationship between the liver histology and the GH/IGF-1 axis in HCV patients. This was likely because of the low number of patients in this study or because the correlation was specific to NAFLD. We hypothesize that the IGF-1 level was possibly reduced in HCV patients, but that it was more influenced by the progression of disease in NAFLD patients. Further studies with larger numbers of patients will be required to confirm this hypothesis.
Low levels of IGF-1 have been associated not only with NAFLD, but also with type 2 diabetes (29), insulin resistance (30), metabolic syndrome (31), and ischemic heart disease (32). These results are consistent with our study. The discrepancy between previous studies and our own are likely because liver biopsies were not performed and cirrhotic patients were not included in the previous studies.
Few studies have evaluated the association between the GH/IGF-1 axis and the severity of steatosis in NAFLD. Furthermore, these few studies have reported conflicting results (33,34). Ichikawa et al. (33) reported that the levels of GH were higher in patients with advanced steatosis, but that there was no correlation between the IGF-1 or IGFBP-3 levels and steatosis. However, Sumida et al. (34) reported that the levels of IGF-1 were lower in patients with advanced steatosis; however, there was no correlation between the IGF-1:SDS and steatosis. In our study, the levels of GH were lower and the levels of IGF-1 and IGFBP-3 were higher in patients with advanced steatosis. The levels of GH were found to be decreased in obese patients and weight loss was followed by the restoration of the normal spontaneous and stimulated release of GH (35). In patients with abdominal obesity, GH treatment improved insulin sensitivity and reduced the level of abdominal visceral fat and the total cholesterol and low-density lipoprotein cholesterol concentrations (36). In our study, the mean BMI of patients with more advanced steatosis was higher than that of patients with mild steatosis. Our results are therefore consistent with previous reports.
Six (2.7%) patients with NAFLD showed remarkably low levels of IGF-1 and an IGF-1:SDS of <-6 SD. These patients were all women of various ages. One of the patients had cirrhosis and had a high GH level. However, the levels of GH were within the normal limits or low in the other five patients. It is possible that GH and IGF-1 abnormalities played a role in the development of NAFLD in these five patients. Further examinations, including a GH stimulation test, will be needed to determine the mechanisms underlying the progression of NAFLD.
Although the precise mechanisms by which GH and IGF-1 contribute to liver fibrosis and steatosis remain unclear, the genetic transfer of IGF-1 into hepatic tissue improved the liver histology and function in cirrhotic rats (37). In addition, in a GH-deficient rat model, the administration of IGF-1 improved the degree of liver steatosis and fibrosis to levels that were similar to those achieved with GH supplementation (38). It was hypothesized that IGF-1 reduced the levels of reactive oxygen species (ROS), thus improving the mitochondrial function and contributing, in turn, to the improvement of liver steatosis and fibrosis (38). The degree of improvement in fibrosis that was induced by IGF-1 was greater than that induced by GH. These results indicate that low levels of IGF-1 are not only due to liver dysfunction, but that they are also a cause of liver impairment. Moreover, IGF-1 treatment is effective for patients with Laron syndrome (primary growth hormone insensitivity) (39). Conchillo et al. (40) reported that IGF-1 therapy significantly increased the serum albumin level and tended to increase the resting energy expenditure in patients with cirrhosis. In the future, IGF-1 therapy could be a treatment option for patients with end-stage liver disease.
In conclusion, a higher GH level and lower IGF-1 and IGFBP 3 levels may contribute to the progression of fibrosis in patients with NAFLD. A further study is required to establish a new treatments that correct abnormal GH and IGF-1 levels to treat the progression of fibrosis in patients with NAFLD.
Author's disclosure of potential Conflicts of Interest (COI).
Katsutoshi Tokushige: Research funding, Eisai, Chugai Pharmaceutical, Daiichi Sankyo Pharmaceutical and Takeda Pharmaceutical.
References
- 1. Johannsson G, Bengtsson BA. Growth hormone and the metabolic syndrome. J Endocrinol Invest 22: 41-46, 1999. [PubMed] [Google Scholar]
- 2. Ichikawa T, Hamasaki K, Ishikawa H, et al. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult-onset growth hormone deficiency. Gut 52: 914, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takahashi Y, Iida K, Takahashi K, et al. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology 132: 938-943, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Nishizawa H, Iguchi G, Murawaki A, et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur J Endocrinol 167: 67-74, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Salomon F, Cuneo RC, Hesp R, et al. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 321: 1797-1803, 1989. [DOI] [PubMed] [Google Scholar]
- 6. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM In: Williams Textbook of Endocrinology. 13th ed Elsevier, 2015: . [Google Scholar]
- 7. Schwander JC, Hauri C, Zapf J, et al. Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology 113: 297-305, 1983. [DOI] [PubMed] [Google Scholar]
- 8. Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nature Rev Endocrinol 6: 515-525, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine Rev 16: 3-34, 1995. [DOI] [PubMed] [Google Scholar]
- 10. Baxter RC, Martin JL, Beniac VA. High molecular weight insulin-like growth factor binding protein complex. Purification and properties of the acid-labile subunit from human serum. J Biol Chem 264: 11843-11848, 1989. [PubMed] [Google Scholar]
- 11. Guler HP, Zapf J, Schmid C, et al. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol 121: 753-758, 1989. [DOI] [PubMed] [Google Scholar]
- 12. Booth BA, Bar RS, Boes M, et al. Intrinsic bioactivity of insulin-like growth factor-binding proteins from vascular endothelial cells. Endocrinology 127: 2630-2638, 1990. [DOI] [PubMed] [Google Scholar]
- 13. Scharf J, Ramadori G, Braulke T, et al. Synthesis of insulin like growth factor binding proteins and of the acid-labile subunit in primary cultures of rat hepatocytes, of Kupffer cells, and in cocultures: regulation by insulin, insulinlike growth factor, and growth hormone. Hepatology (Baltimore, MD) 23: 818-827, 1996. [DOI] [PubMed] [Google Scholar]
- 14. Villafuerte BC, Koop BL, Pao CI, et al. Coculture of primary rat hepatocytes and nonparenchymal cells permits expression of insulin-like growth factor binding protein-3 in vitro. Endocrinology 134: 2044-2050, 1994. [DOI] [PubMed] [Google Scholar]
- 15. Fichter MM, Pirke KM, Holsboer F. Weight loss causes neuroendocrine disturbances: experimental study in healthy starving subjects. Psych Res 17: 61-72, 1986. [DOI] [PubMed] [Google Scholar]
- 16. Donaghy A, Ross R, Gimson A, et al. Growth hormone, insulinlike growth factor-1, and insulinlike growth factor binding proteins 1 and 3 in chronic liver disease. Hepatol (Baltimore, MD) 21: 680-688, 1995. [PubMed] [Google Scholar]
- 17. Moller S, Juul A, Becker U, et al. The acid-labile subunit of the ternary insulin-like growth factor complex in cirrhosis: relation to liver dysfunction. J Hepatol 32: 441-446, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Cuneo RC, Hickman PE, Wallace JD, et al. Altered endogenous growth hormone secretory kinetics and diurnal GH-binding protein profiles in adults with chronic liver disease. Clin Endocrinol (Oxf) 43: 265-275, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Isojima T, Shimatsu A, Yokoya S, et al. Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocr J 59: 771-780, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313-1321, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology (Baltimore, MD) 1: 431-435, 1981. [DOI] [PubMed] [Google Scholar]
- 22. Schvarcz R, Glaumann H, Weiland O, et al. Histological outcome in interferon alpha-2b treated patients with chronic posttransfusion non-A, non-B hepatitis. Liver 11: 30-38, 1991. [DOI] [PubMed] [Google Scholar]
- 23. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 13: 372-374, 1991. [DOI] [PubMed] [Google Scholar]
- 24. Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology (Baltimore, MD) 19: 1513-1520, 1994. [PubMed] [Google Scholar]
- 25. Xu L, Xu C, Yu C, et al. Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS One 7: e44136, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fusco A, Miele L, D'Uonnolo A, et al. Nonalcoholic fatty liver disease is associated with increased GHBP and reduced GH/IGF-I levels. Clin Endocrinol (Oxf) 77: 531-536, 2012. [DOI] [PubMed] [Google Scholar]
- 27. Juul A, Main K, Blum WF, et al. The ratio between serum levels of insulin-like growth factor (IGF)-I and the IGF binding proteins (IGFBP-1, 2 and 3) decreases with age in healthy adults and is increased in acromegalic patients. Clin Endocrinol (Oxf) 41: 85-93, 1994. [DOI] [PubMed] [Google Scholar]
- 28. Castro GR, Coelho JC, Parolin MB, et al. Insulin-like growth factor I correlates with MELD and returns to normal level after liver transplantation. Ann Transplant 18: 57-62, 2013. [DOI] [PubMed] [Google Scholar]
- 29. Sandhu MS, Heald AH, Gibson JM, et al. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet 359: 1740-1745, 2002. [DOI] [PubMed] [Google Scholar]
- 30. Succurro E, Andreozzi F, Marini MA, et al. Low plasma insulin-like growth factor-1 levels are associated with reduced insulin sensitivity and increased insulin secretion in nondiabetic subjects. Nutr Metab Cardiovasc Dis 19: 713-719, 2009. [DOI] [PubMed] [Google Scholar]
- 31. Sesti G, Sciacqua A, Cardellini M, et al. Plasma concentration of IGF-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care 28: 120, 2005. [DOI] [PubMed] [Google Scholar]
- 32. Juul A, Scheike T, Davidsen M, et al. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 106: 939-944, 2002. [DOI] [PubMed] [Google Scholar]
- 33. Ichikawa T, Nakao K, Hamasaki K, et al. Role of growth hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol Int 1: 287-294, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sumida Y, Yonei Y, Tanaka S, et al. Lower levels of insulin-like growth factor-1 standard deviation score are associated with histological severity of non-alcoholic fatty liver disease. Hepatol Res 45: 771-781, 2015. [DOI] [PubMed] [Google Scholar]
- 35. Scacchi M, Pincelli AI, Cavagnini F. Growth hormone in obesity. Int J Obes Relat Metab Disord 23: 260-271, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Franco C, Brandberg J, Lonn L, et al. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab 90: 1466-1474, 2005. [DOI] [PubMed] [Google Scholar]
- 37. Sobrevals L, Rodriguez C, Romero-Trevejo JL, et al. Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology (Baltimore, MD) 51: 912-921, 2010. [DOI] [PubMed] [Google Scholar]
- 38. Nishizawa H, Takahashi M, Fukuoka H, et al. GH-independent IGF-I action is essential to prevent the development of nonalcoholic steatohepatitis in a GH-deficient rat model. Biochem Biophys Res Commun 423: 295-300, 2012. [DOI] [PubMed] [Google Scholar]
- 39. Laron Z. Insulin-like growth factor-I treatment of children with Laron syndrome (primary growth hormone insensitivity). Pediatr Endocrinol Rev 5: 766-771, 2008. [PubMed] [Google Scholar]
- 40. Conchillo M, de Knegt RJ, Payeras M, et al. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol 43: 630-636, 2005. [DOI] [PubMed] [Google Scholar]