Abstract
Objective The National Kidney Foundation (NKF) Kidney Disease Outcome Quality Initiative (KDOQI) guidelines have recommended the use of arteriovenous fistula (AVF) at the initiation of dialysis. However, there are significant differences in the dialysis environments of Japan and the United States, and there are few people who receive hemodialysis via a central venous catheter (CVC) in Japan. The aim of the present study was to examine the association between the type of vascular access at the initiation of dialysis and the incidence of mortality in Japan.
Methods This study was a prospective, multicenter, cohort study. The data was collected by the Aichi Cohort study of Prognosis in Patients newly initiated into dialysis (AICOPP) in which 18 Japanese tertiary care centers participated. The present study enrolled 1,524 patients who were newly introduced to dialysis (the patients started maintenance dialysis between October 2011 and September 2013). After excluding 183 patients with missing data, 1,341 patients were enrolled. The Cox proportional hazards model was used to evaluate mortality based on the type of vascular access. The types of vascular access were divided into four categories: AVF, arteriovenous graft (AVG), CVC changed to AVF during the course (CAVF), CVC changed to AVG during the course (CAVG).
Results A multivariate analysis revealed that AVG, CAVF and CAVG were associated with a higher risk of mortality in comparison to AVF [hazard ratio (HR), 1.60; p=0.048; HR, 2.26; p= 0.003; and HR, 2.45; p=0.001, respectively].
Conclusion The research proved that the survival rate among patients in whom hemodialysis was initiated with AVF was significantly higher than that in patients in whom hemodialysis was initiated with AVG or CVC.
Keywords: hemodialysis, mortality risk, venous catheter, arteriovenous fistula, graft access
Introduction
The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines for Vascular Access (1) and the European guidelines for vascular access (2) state that the preferred type of permanent vascular access for hemodialysis patients is an arteriovenous fistula (AVF). Prior studies have reported that central venous catheters (CVCs) and arteriovenous grafts (AVGs) are associated with a higher risk of mortality in hemodialysis patients than AVFs (3-10). This has resulted in a large increase in AVF use in the United States (11). However, it has been reported that there are significant differences in the dialysis environments of Japan and the United States. In Japan, there is a high rate of AVF usage (91%) and a low rate of CVC usage (1% to 2%) (11,12), while the risk of mortality in U.S. hemodialysis patients is higher than that in Japanese patients (8). Japanese hemodialysis patients show excellent survival in comparison to patients from other countries (13). Robinson et al. reported that the low proportion of renal transplant patients and the higher number of young hemodialysis patients might contribute to the low mortality rate among hemodialysis patients in Japan (14).
In Japan, dialysis is performed using a low single-pool Kt/V long dialysis time with low blood flow and a dialyzer membrane with a low surface area; the rate of synthetic polymer membrane usage is low (15). These elements contribute to the low rate of mortality in Japanese dialysis patients.
The relationship between mortality and the type of initial vascular access in Japanese hemodialysis patients has not been reported. The aim of this study was to examine the association between the type of vascular access at the initiation of dialysis and mortality in Japan.
Materials and Methods
Study design, setting and participants
This study was a prospective, multicenter, cohort study. We examined 1,524 Chronic Kidney Disease patients who were >20 years of age and who had been newly introduced to dialysis between October, 2011, and September, 2013, at 17 institutions that were affiliated with our study. For the survival analysis, patients were followed-up until death or until March, 2015. Patients who stopped dialysis while in the hospital, died in the hospital, or who did not agree to be registered were excluded from the present study (Fig. 1).
Figure 1.
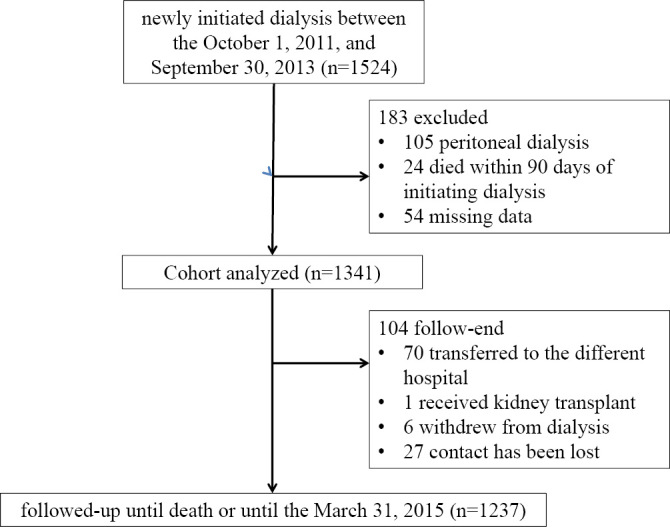
A flow diagram of the present study.
This study was registered at the University Hospital Medical Information Network-Clinical Trials Registry (UMIN-CTR) under trial identification no. UMIN C000007096. Permission for the use of medical records was obtained from the institutional review board of each institution.
Data collection
The data for each patient were collected by the dialysis provider at the initiation of dialysis and every six months thereafter. Blood and urine sampling was performed before the first dialysis session at the initiation of dialysis. The patients' medical data, including their age, sex, BMI, medical history, comorbidities, the length of nephrology follow-up, and hematological data were obtained from the medical records compiled by the participating nephrologists. The BMI was calculated using the height and weight values that were collected at the start of dialysis. The estimated glomerular filtration rate (eGFR) was calculated using the following equation: eGFR (mL/min/1.73 m2) = 194× serum creatinine-1.094× age-0.287 ×0.739 (if woman). Diabetes mellitus was defined by an HbAlc value of >6.5%, the use of medications to treat diabetes mellitus, or a past medical history of diabetes mellitus. Coronary artery disease was defined by a recorded or patient-reported history of myocardial infarction, percutaneous coronary intervention (PCI), or coronary artery bypass graft surgery (CABG). Peripheral artery disease (PAD) was defined by a recorded or patient-reported history of a resting ABI value of ≤0.90, a post-exercise ABI decrease of ≥20% in at least one leg, rest pain or gangrene/ulcers, with signs of intermittent claudication. The type of vascular access was divided into four groups: AVF, AVG, CVC changed to AVF during the course (CAVF), and CVC changed to AVG during the course (CAVG).
Statistical methods
Univariate analyses were carried out using the Pearson χ2 test or the Kruskall-Wallis test as appropriate. Kaplan-Meier plots were used to examine the association between the duration of vascular access and survival. Differences between the groups were analyzed using the log-rank test. A multivariate survival analysis was carried out using a Cox proportional hazards model. The explanatory variables that were included in the Cox regression analysis were age, sex, BMI, serum albumin, serum Hb, eGFR, primary renal diagnosis, the number of days from the first nephrology follow-up examination to the initial dialysis date. All of the statistical analyses were performed using the SPSS (version 22.0.0) software program. p values of <0.05 were considered to indicate statistical significance.
Results
From the total of 1,524 patients, 1,341 patients were enrolled and 183 patients were excluded for the following reasons: they received peritoneal dialysis (n=105); missing data (n=54); or the patient died within 90 days of the initiation of dialysis (n=24). One hundred four patients could not be followed until the completion of the study for the following reasons: the patient was transferred to a different hospital (n=70); the patient received a kidney transplant (n=1); the patient was withdrawn from dialysis (n=6); and loss of contact (n=27).
In the first session, hemodialysis was initiated with AVF (n=975; 72.7%), AVG (n=90, 6.7%), or CVC (n=276, 20.5%). Among the patients in whom hemodialysis was initiated with CVC, 58 patients were changed to AVG and 218 patients were changed to AVF.
Table 1 shows the baseline characteristics of the patients. The patients in the AVF and CAVF groups tended to be younger than those in the AVG and CAVG groups. The patients in the AVF group were more likely to be men. There was a significant association between the duration of nephrology care for pre-end-stage renal disease (pre-ESRD) and the type of vascular access (p<0.001). The patients with pre-ESRD in the AVF and AVG groups received nephrology care for a longer duration of time than those in the CAVG and CAVG groups.
Table 1.
The Baseline Characteristics of the Study Population at the Initiation of Hemodialysis.
| Characteristics | AVF (n=975) | AVG (n=90) | CAVF (n=218) | CVAVG (n=58) | p value |
|---|---|---|---|---|---|
| Age (years) (mean, SD) | 67.8 (12.8) | 69.9 (12.6) | 67.5 (13.4) | 73.2 (10.7) | 0.002 |
| Male sex (n, %) | 695 (71.3) | 45 (50.0) | 131 (60.1) | 30 (51.7) | <0.001 |
| Diabetes mellitus (n, %) | 525 (53.8) | 45 (50.0) | 102 (46.8) | 31 (53.4) | 0.279 |
| Peripheral artery disease (n, %) | 45 (4.6) | 7 (7.8) | 7 (3.2) | 4 (6.9) | 0.304 |
| Coronary artery disease (n, %) | 166 (17.0) | 19 (21.3) | 28 (12.8) | 12 (21.0) | 0.208 |
| Body mass index (mean, SD) | 23.6 (4.0) | 24.6 (5.0) | 22.6 (4.6) | 23.3 (4.8) | <0.001 |
| Hemoglobin(g/L) (mean, SD) | 9.5 (1.4) | 9.1 (1.5) | 8.7 (2.0) | 9.0 (1.6) | <0.001 |
| Creatinine (mg/dL) (mean, SD) | 9.0 (3.0) | 8.4 (2.5) | 9.3 (4.5) | 8.1 (3.2) | 0.017 |
| eGFR (mL/min per 1.73 m2) (mean, SD) | 5.3 (1.9) | 5.4 (1.9) | 5.5 (2.5) | 6.2 (4.4) | 0.850 |
| Serum albumin (g/L) (mean, SD) | 3.2 (0.6) | 3.2 (0.5) | 2.9 (0.7) | 2.8 (0.5) | <0.001 |
| Duration of ESRD nephrology care (days) (mean, SD) | 1,065.5 (1337.0) | 1,089.7 (1036.9) | 611.6 (873.0) | 610.5 (873.0) | <0.001 |
| Duration from the creation of blood access to the initiation of hemodialysis (days) (mean, SD) | 126.4 (201.8) | 80.63 (190.0) | 36.1 (96.0) | 10.15 (18.4) | <0.001 |
| Primary renal diagnosis (n, %) | 0.044 | ||||
| Glomerulonephritis | 160 (16.4) | 7 (7.8) | 37 (16.9) | 8 (13.8) | |
| Diabetes | 444 (45.5) | 42 (46.7) | 85 (39.0) | 27 (46.6) | |
| Hypertension/renal vascular disease | 253 (25.9) | 21 (23.3) | 58 (26.6) | 18 (31.0) | |
| Polycystic kidney disease | 37 (3.8) | 3 (3.3) | 2 (0.9) | 0 (0.0) | |
| Other | 49 (5.0) | 9 (10.0) | 19 (8.7) | 4 (6.9) | |
| Unknown | 32 (3.3) | 8 (8.9) | 17 (7.8) | 1 (1.7) |
ESRD: end stage renal disease
The median observation period was 819 days (mean, 812 days; standard deviation, 276). Two hundred thirty-four patients died over during the follow-up period.
The Kaplan-Meier survival curves are shown in Fig. 2. There was a significant difference among the groups (p<0.001, log-rank test). A Cox regression model was created to investigate the relationship between the type of vascular access and all-cause mortality. The following factors were included in the multivariable model: age, sex, primary renal diagnosis, BMI, serum Hb, serum Alb, eGFR and the duration of pre-ESRD nephrology care (Table 2).
Figure 2.
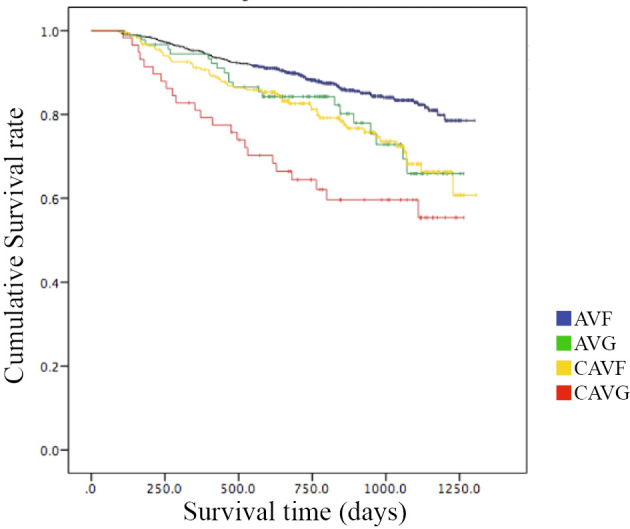
The Kaplan-Meier survival curves according to the type of vascular access. A Kaplan-Meier analysis showed that patients in the AVG, CAVF and CAVG group had significantly worse survival in comparison to those in the AVF group (p=0.00 log-rank test).
Table 2.
The Association between the Different Types of Vascular Access with Patient Mortality as Determined by a Cox Proportional Hazards Model Adjusted for Age, Sex, BMI, Primary Renal Diagnosis, Serum Albumin, Serum Hb, eGFR, Duration of pre ESRD Nephrology Care.
| HR (95% CI) | p value | |
|---|---|---|
| AVF | Reference | <0.001 |
| AVG | 1.600 (1.005-2.548 ) | 0.048 |
| CAVF | 1.652 (1.181-2.311 ) | 0.003 |
| CAVG | 2.264 (1.424-3.599 ) | 0.001 |
BMI: body mass index, ESRD: end stage renal disease
The multivariate analysis revealed that AVG was associated with a higher risk of mortality than AVF [hazard ratio (HR), 1.60; p=0.048]. CAVG and CAVF were also associated with a higher risk of mortality in comparison to AVF (HR, 2.26; p=0.001; and HR, 1.65; p=0.003, respectively). No significant differences were observed between AVG and CAVF (HR, 1.03; p=0.904) or CAVG (HR, 1.42; p=0.261).
The numbers of patients whose vascular access was created more than 30 days prior to the first hemodialysis session were as follows AVF (n=660); AVG (n=30); and CVC (n=12). Similarly, the patients in the AVG group also showed a higher risk of mortality in comparison to the AVF group in a multivariate analysis in which the patients in whom vascular access had been prepared before the initiation of dialysis were excluded (HR, 1.87; 95% CI, 1.07-3.27; p=0.027). Furthermore, CAVG and CAVF both had a higher risk of mortality than AVF (HR, 2.75; 95% CI, 1.65-4.57; p=0.001 and HR, 1.55; 95% CI, 1.04-2.31; p=0.031, respectively).
Discussion
The results of the present study proved that hemodialysis initiated with AVG or CVC was associated with a significantly higher risk of mortality than hemodialysis initiated with AVF. In addition, this study proved that in Japan, the proportion of people in whom dialysis was initiated by AVF was greater than that in other countries.
There are significant differences in the dialysis environments of Japan and the United States. The DOPPS study revealed that the hemodialysis patients in the United States have a higher prevalence of diabetes, cardiovascular disease and other complications in comparison to Japan. The use of CVC and AVG for vascular access is also quite high in the United States. Furthermore, some patients in the United States occasionally skip their dialysis treatments (16). This situation, which appears to be associated with a higher risk of mortality, does not happen in Japan. It is therefore unclear whether we can adapt the recommendation of ‘AVF first’ to dialysis patients in Japan.
This study proved that hemodialysis initiated with AVG and CVC was associated with a significantly higher risk of mortality in comparison to AVF. Although numerous studies have discussed the relationship between vascular access and mortality (17-20), the present study represents the first time an investigation into this relationship has been conducted in Japan. The reason why patients in whom hemodialysis is initiated with CVC and with AVG show a worse mortality rate is thought to involve the higher incidence of adverse effects (such as infectious disease, and vascular injury) that are associated with these modes of access (21,22). AVF is known to be associated with lower rates of infection than AVG and CVC, and vascular access infection has the potential to lead to death (21). AVG has been associated with higher rates of access thrombosis. Furthermore, AVG is associated with a higher relative risk of hospitalization than AVF (22). In this study, we could not obtain details on hospitalization, vascular access infection or access thrombosis. Thus we were unable to elucidate the reason(s) why AVF was associated with a lower rate of mortality. One possible reason for a poor prognosis in cases in which a catheter used in the first hemodialysis session was changed to AVF (in comparison to AVF alone) is that the catheter used in the first hemodialysis causes an increased risk of catheter-related complications, even after it is removed. There is a case in which a patient suffered delayed complications after standing, long after the hemodialysis catheter was removed (23). AVF was prepared before the initiation of dialysis, in an organized manner, according to the physicians' suggestion. It was therefore unclear whether AVF itself was superior to AVG/CAC or whether AVF was just one of the markers for patients with a high compliance to medical practice. In order to eliminate this concern, we excluded the patients whose vascular access was created more than 30 days prior to the first hemodialysis session and performed a multivariate analysis. However, we did not find any significant differences in the mortality rates of the groups. This result showed that vascular access might have a more important role in the prognosis than the timing of its creation.
The present study proved that in Japan, the proportion of patients in whom dialysis was initiated by AVF was larger than that in other countries. Ethier et al. reported that the proportion of patients in whom dialysis was initiated by AVF was larger than that in other European and North American countries (11). The present study supports the findings of the previous study. The fact that hemodialysis is initiated by AVF in a larger number of patients may be a reason why Japanese dialysis patients live longer than dialysis patients in other countries.
There present study is associated with a number of limitations. First, it was a prospective cohort study. Second, we could not determine the cause of death in the majority of the cases due to missing data. It is commonly thought that CVC and AVG are associated with a higher risk of infection (16,17); however, we could not be assess this point in the present study.
In summary, the present study proved that hemodialysis initiated with AVF was associated with a significantly higher survival rate than hemodialysis initiated with AVG and CVC.
The authors state that they have no Conflict of Interest (COI).
Participants in this study
Hideto Oishi (Komaki City Hospital), Hiroko Kushimoto (Chita City Hospital), Hiroshi Ogawa (Shinseikai Daiichi Hospital), Hirotake Kasuga (Nagoya Kyoritsu Hospital), Hisashi Kurata (Toyota Kosei Hospital), Hirofumi Tamai (Anjo Kosei Hospital), Isao Aoyama (Social Insurance Chukyo Hospital), Junichiro Yamamoto (Tsushima City Hospital), Kei Kurata (Tosei General Hospital), Shizunori Ichida (Red Cross Nagoya Daiichi Hospital), Taishi Yamakawa (Toyohashi Municipal Hospital), Takaaki Yaomura (National Hospital Organization Nagoya Medical Center), Tomohiko Naruse (Kasugai Municipal Hospital), Shigehisa Koide, (Fujita Health University Hospital), Yukio Yuzawa (Fujita Health University Hospital), Shoichi Maruyama (Nagoya University Graduate School of Medicine), Noritoshi Kato (Nagoya University Graduate School of Medicine) and Seiichi Matsuo (Nagoya University Graduate School of Medicine).
Acknowledgement
I thank the members of the Aichi cohort study of prognosis in patients newly initiated into dialysis (AICOPP) for their help and support.
References
- 1. National Kidney Foundation K/DOQI Clinical Practice Guidelines for Vascular Access, 2000. Am J Kidney Dis 37 (Suppl 1): S137-S181, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Tordoir JH, Mickley V. European guidelines for vascular access: clinical algorithms on vascular access for haemodialysis. Edtna Erca J 29: 131-136, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE. Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol 15: 1936-1942, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int 62: 620-626, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013-1019, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Thomson PC, Stirling CM, Geddes CC, Morris ST, Mactier RA. Vascular access in haemodialysis patients: a modifiable risk factor forbacteraemia and death. QJM 100: 415-422, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 15: 477-486, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Pisoni RL, Arrington CJ, Albert JM, et al. . Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 53: 475-491, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Astor BC, Eustace JA, Powe NR, et al. . Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol 16: 1449-1455, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 60: 1443-1451, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Ethier J, Mendelssohn DC, Elder SJ, et al. . Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 23: 3219-3226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pisoni RL, Young EW, Mapes DL, Keen ML, Port FK. Vascular access use and outcomes in the U.S., Europe, and Japan: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol News Issues 17: 38-47, 2003. [PubMed] [Google Scholar]
- 13. Goodkin DA, Bragg-Gresham JL, Koenig K, et al. . Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 3270-3277, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Robinson BM, Porf FK. International hemodialysis patient outcomes comparisons revisited: the role of practice patterns and other factors. Clin J Am Soc Nephrol 4 (Suppl 1): S12-S17, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Akiba T, Akizawa T, Fukuhara S, et al. . Results of the international DOPPS hemodialysis study in Japan. Journal of Japanese Society for Dialysis Therapy 37: 1865-1873, 2004(in Japanese, Abstract in English). [Google Scholar]
- 16. Saran R, Bragg-Gresham JL, Rayner HC, et al. . Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int 64: 254-262, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Hicks CW, Canner JK, Arhuidese I, et al. . Mortality benefits of different hemodialysis access types are age dependent. J Vasc Surg 61: 449-456, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Bray BD, Boyd J, Daly C, et al. . Vascular access type and risk of mortality in a national prospective cohort of haemodialysis patients. QJM 105: 1097-1103, 2012. [DOI] [PubMed] [Google Scholar]
- 19. DeSilva RN, Patibandla BK, Vin Y, et al. . Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol 24: 1297-1304, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perl J, Wald R, McFarlane P, et al. . Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 22: 1113-1121, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 60: 1-13, 2001. [DOI] [PubMed] [Google Scholar]
- 22. Churchill DN, Taylor DW, Cook RJ, et al. . Canadian hemodialysis morbidity study. Am J Kidney Dis 19: 214-234, 1992. [DOI] [PubMed] [Google Scholar]
- 23. Jin L, Wang J, Wu C, Shao C, Yu X, Lei W. Femoral arteriovenous fistula associated with leg swelling 6 months after removal of a hemodialysis catheter: a case report. Medicine (Baltimore) 94: e1738, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


