Abstract
Henoch Schönlein purpura (HSP), also known as IgA vasculitis (IgAV), is a systemic small-vessel vasculitis that predominantly affects adolescents and is rare in adults. In many cases, the onset of HSP has been causally linked to an infectious disease. We encountered a case of HSP with severe renal involvement diagnosed by renal biopsy following bacillus Calmette-Guerin (BCG) therapy for bladder cancer. This is of clinical relevance, as intravesical BCG administration is becoming an established therapy for superficial bladder cancer and is supposed to be safe. It is important for all clinicians to recognize that BCG therapy has this rare but potentially serious systemic complication.
Keywords: Henoch Schönlein purpura, IgA vasculitis, crescentic glomerulonephritis, bacillus Calmette-Guerin, bladder cancer
Introduction
Intravesical administration of bacillus Calmette-Guerin (BCG) has been used in the treatment of non-muscle invasive bladder cancer for nearly 40 years and is one of the most successful immunotherapies for this condition (1). However, since it contains a live attenuated strain of Mycobacterium bovis and is based on an immune reaction to it, this therapy can occasionally cause serious side effects, such as BCG infection and autoimmunity. We herein report a recent case of Henoch Schönlein purpura (HSP) nephritis associated with this therapy.
Case Report
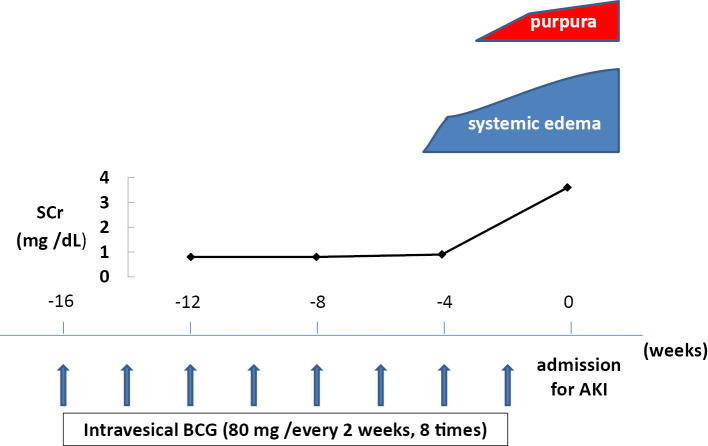
An 80-year-old man was hospitalized for acute kidney injury (AKI) presenting with systemic edema, diarrhea, and purpura on lower legs. Approximately 1 year prior to this presentation, he had been diagnosed with transitional carcinoma of the bladder and underwent endoscopic resection 8 months before admission, and bi-weekly administrations of Connaught strain BCG (80 mg) had been initiated 4 months before admission (Fig. 1). He first noticed lower leg edema and abdominal distention 1 month before admission. No history of an upper respiratory infection, visible hematuria, pain on urination during BCG therapy, or intake of new medications was noted.
Figure 1.
The clinical course before admission. BCG: bacillus Calmette-Guerin, AKI: acute kidney injury, SCr: serum creatinine
He had no fever, arthralgia, or abdominal pain. A physical examination revealed moderate pitting edema and palpable purpura on both lower legs (Fig. 2). Blood test results showed a white blood cell count of 17,800/mm3, a C-reactive protein (CRP) concentration of 7.9 mg/dL, and a platelet count of 33.3×104/mm3. The plasma coagulation factor XIII (F XIII) activity was 47% (normal 70-140%).
Figure 2.
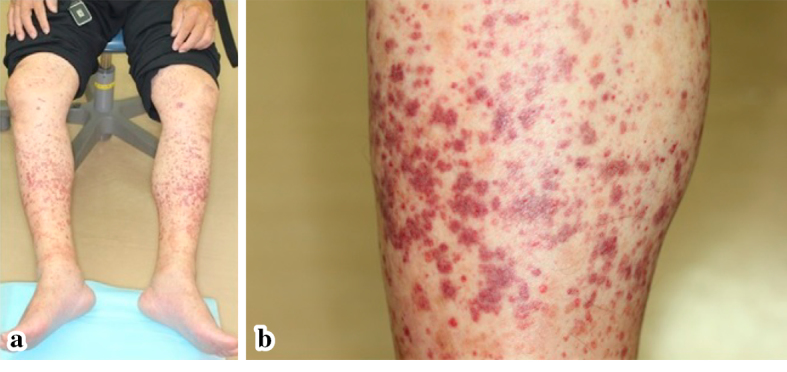
(a), (b) purpuric lesions of both lower legs.
His creatinine level had risen to 3.6 mg/dL from 0.9 mg/dL 1 month prior. His serum albumin level was 2.6 g/dL, and his electrolytes were normal. The urine protein-to-creatinine ratio was 4.1 g/gCr with several red blood cells (RBCs) visible on urine microscopy. Anti-nuclear antibody and anti-neutrophil cytoplasmic antibodies were negative, and serum complement levels were normal, while IgA was 654 mg/dL (normal 110-410 mg/dL). The blood and urinary cultures, including for mycobacteria, were negative.
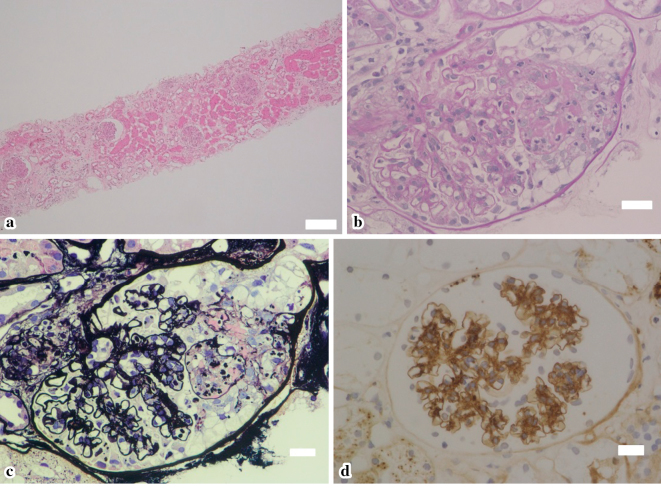
Renal biopsy showed a crescentic and fibrinoid necrotizing glomerulonephritis in 20% of glomeruli(Fig. 3a-c), and immunohistochemistry revealed IgA deposits mainly in the mesangium (Fig. 3d). Skin biopsy of the lower leg lesion showed leukocytoclastic vasculitis. We made a diagnosis of HSP nephritis in conjunction with the clinical course, which was classified as ISKDC IIIb according to the classification criteria of the International Study of Kidney Disease in Children.
Figure 3.
The renal biopsy findings. (a) Hematoxylin and Eosin staining (×25), showing a crescentic glomerulonephritis in 20% of glomeruli. (b) PAS stain (×400) and (c) silver stain (×400), showing a crescentic and fibrinoid necrotizing glomerulonephritis with focal rupture of the capillary walls. (d) Immunohistochemstry (×400), demonstrating granular staining for IgA deposits mainly in the mesangium. Scale bars: 200 µm (a) and 20 µm (b, c, d).
Based on the clinical and pathological severity, he was started on 3 sessions of plasmapheresis and hemodialysis, with pulsed methyl prednisolone (500 mg/day for 3 days) followed by oral prednisolone (30 mg/day). After that, his symptoms and renal function gradually improved, with his creatinine level decreasing to 2.8 mg/dL. However, he developed catheter-related blood stream infection due to methicillin-resistant Staphylococcus aureus (MRSA) after 30 days and died of MRSA septicemia after 32 days despite aggressive antibiotic treatment.
Discussion
Henoch Schönlein purpura (HSP), also termed IgA vasculitis (IgAV), is a systemic small-vessel vasculitis characterized by immune complexes containing IgA. It predominantly affects children; 90% of HSP patients are under 10 years of age (2). The clinical features of adult case are often atypical, and significant renal involvement is more likely to develop than in children, thereby leading to a poorer prognosis (3). It is now believed that individuals who have high levels of underglycosylated IgA1 with an uncertain second hit aggregate IgA1 accompanied by anti-glycan autoantibody, which is then deposited in the small vessels and mesangium, causing inflammation. What constitutes this second hit is unclear, but more than 50% of cases are preceded by infectious disease (4).
In the present case, the evidence for BCG-induced HSP nephritis is circumstantial but strengthened by the following: First, from a clinical perspective, there was no obvious exposure to an infection other than BCG administration. Second, our patient was undergoing intravesical BCG therapy and developed HSP nephritis 4 months after starting the treatment. This clinical course and the temporal relationship between the therapy and the onset of HSP suggested that BCG treatment might be the cause of the HSP. While HSP is occasionally reported after BCG therapy (5,6) or associated with Mycobacterium avium-intracellilare complex (MAC) (7), our case differs from these previous reports in that this case of HSP nephritis after intravesical BCG therapy was diagnosed by renal biopsy. In addition, the pathological findings and decreased F XIII activity of this patient implied strong renal involvement.
With respect to the etiology of AKI, we speculated that AKI was caused by hypersensitivity vasculitis, i.e., HSP nephritis, rather than direct lesions of BCG. This is because the lesions indicating acid-fast bacillus infection were not detected by renal biopsy, such as acute interstitial nephritis and granulomatous inflammation.
The most disappointing point in this case was the outcome, namely the patient's death due to catheter-related blood stream infection. In a randomized, double-blind, placebo-controlled trial for children with HSP in 2013, the authors found no evidence to suggest that early treatment with prednisolone reduces the prevalence of proteinuria 12 months after disease onset; they therefore do not support the routine use of prednisolone to prevent nephropathy in early HSP (8). Considering this report, the indication of immunosuppressive therapy should be carefully judged in cases of HSP to prevent renal involvement, particularly in patients who have risk factors for infection.
In conclusion, clinicians should be aware of HSP nephritis as a potential complication associated with BCG therapy, since a considerable number of patients receive intravesical BCG administration. Conversely, in adult cases of HSP nephritis, clinician should take a thorough history regarding recent BCG therapy, paying attention to renal involvement.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer: a current perspective. Nat Rev Urol 3: 153-162, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Saulsbury FT. Clinical update: Henoch-Schönlein purpura. Lancet 369: 976-978, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 13: 1271-1278, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Couser WG, Johnson RJ. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int 86: 905-914, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Hirayama T, Matsumoto K, Tsuboi T, et al. Anaphylactoid purpura after intravesical therapy using Bacillus Calmette-Guerin for superficial bladder cancer. Hinyokika Kiyo 54: 127-129, 2008(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 6. Nan DN, Fernández-Ayala M, García-Ibarbia C, Gutiérrez-Santiago M, Hernández JL. Henoch-Schönlein purpura after intravesical administration of bacillus Calmette-Guérin. Scand J Infect Dis 37: 613-615, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Yano S. Henoch-Schönlein purpura associated with pulmonary Mycobacterium avium-intracellilare complex (MAC). Intern Med 43: 843-845, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Dudley J, Smith G, Llewelyn-Edwards A, Bayliss K, Pike K, Tizard J. Randomised, double-blind, placebo-controlled trial to determine whether steroids reduce the incidence and severity of nephropathy in Henoch-Schönlein Purpura (HSP). Arch Dis Child 98: 756-763, 2013. [DOI] [PubMed] [Google Scholar]