Abstract
Short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) are noninvasive transcranial magnetic stimulation (TMS) measures of GABAA receptor-mediated inhibition and glutamatergic excitatory transmission, respectively. Conventionally these measures have been restricted to the motor cortex. We investigated whether SICI and ICF could be recorded from the dorsolateral prefrontal cortex (DLPFC) using combined TMS and electroencephalography (TMS–EEG). We first characterized the neural signature of SICI and ICF in M1 in terms of TMS-evoked potentials (TEPs) and spectral power modulation. Subsequently, these paradigms were applied in the DLPFC to determine whether similar neural signatures were evident. With TMS at M1, SICI and ICF led to bidirectional modulation (inhibition and facilitation, respectively) of P30 and P60 TEP amplitude, which correlated with MEP amplitude changes. With DLPFC stimulation, P60 was bidirectionally modulated by SICI and ICF in the same manner as for M1 stimulation, whereas P30 was absent. The sole modulation of early TEP components is in contradistinction to other measures such as long-interval intracortical inhibition and may reflect modulation of short latency excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs). Overall, the data suggest that SICI and ICF can be recorded using TMS–EEG in DLPFC providing noninvasive measures of glutamatergic and GABAA receptor-mediated neurotransmission. This may facilitate future research attempting to ascertain the role of these neurotransmitters in the pathophysiology and treatment of neurological and psychiatric disorders.
INTRODUCTION
Transcranial magnetic stimulation (TMS) provides a noninvasive method to study human cortical neurophysiology. The integrity of various cortical circuits can be measured using different conditioning-test stimulus (TS) protocols. Short-interval intracortical inhibition (SICI) describes the suppression of a TS given at short intervals of 1–6 ms following a subthreshold conditioning stimulus (CS), first identified by Kujirai et al (1993). This is followed by a period of motor-evoked potential (MEP) facilitation at longer CS-TS ISIs of ~7–25 ms, known as intracortical facilitation (ICF) (Claus et al, 1992; Kujirai et al, 1993). SICI is mediated by fast ionotropic GABAA receptors, specifically those subtypes bearing the α2 or α3 subunit (Di Lazzaro et al, 2006a; Ilic et al, 2002; Ziemann et al, 1996). SICI is thus thought to arise because the CS activates a low threshold inhibitory system, which generates a hyperpolarizing inhibitory postsynaptic potentials (IPSPs) inhibiting the cortical output evoked by a subsequent TS (Ilic et al, 2002; Kujirai et al, 1993). ICF is mediated by glutamate (Liepert et al, 1997; Schwenkreis et al, 2000) and may be evoked by the summation of excitatory postsynaptic potentials (EPSPs) from CS and TS. ICF is thought to reflect excitatory transmission largely through the NMDA receptor (Mori et al, 2011; Schwenkreis et al, 1999; Ziemann et al, 1998). SICI and ICF are common noninvasive measures of cortical excitability and are abnormal in a number of movement and cognitive disorders (Chen and Curra, 2004).
Previous TMS neurophysiological studies have been largely restricted to the primary motor cortex (M1), owing to the ease of recording electromyographic (EMG) motor output in target muscles. However, the ability to noninvasively investigate SICI and ICF in other areas would be highly desirable, for example, in prefrontal areas, which are more directly involved in cognitive disorders (Julkunen et al, 2011). Recent technical advances have enabled the concurrent recording of electroencephalographic (EEG) responses to TMS (Ilmoniemi and Kicic, 2010), thus providing a potentially more direct measure of induced changes in neuronal activity and extending the use of TMS to non-motor areas (Daskalakis et al, 2008; Fitzgerald et al, 2008). Such studies have demonstrated EEG neurophysiological correlates of cortical inhibitory circuits such as GABAB-mediated long-interval intracortical inhibition (LICI) in motor and dorsolateral prefrontal cortex (DLPFC) (Daskalakis et al, 2008; Fitzgerald et al, 2008). Changes in this paradigm have been associated with schizophrenia (Farzan et al, 2010; Radhu et al, 2015) and predict treatment response to magnetic seizure therapy in depression (Sun et al, 2016). Changes are typically characterized by modulation in the amplitude of components of the TMS-evoked potential (TEP) and modulation of oscillatory power at different frequencies. TEP components are thought to reflect changes in cortical excitability potentially related to longer lasting underlying IPSPs and EPSPs rather than neural firing (Premoli et al, 2014a).
Studies have explored the electrophysiological TMS–EEG correlates of SICI and ICF in M1 (Ferreri et al, 2011; Manganotti et al, 2012; Paus et al, 2001). One of these studies explored SICI and ICF at sufficiently high temporal resolution and with adequate power to identify changes in TEP components (Ferreri et al, 2011). SICI attenuated and ICF enhanced the amplitude of positive deflections at a latency of 30 ms (P30) and 60 ms (P60), whereas the amplitude of the negative trough at 45 ms (N45) was partially affected. The influence of SICI and ICF on cortical TEPs in prefrontal cortex and on oscillatory power remains unknown.
In the present study we first characterized the TMS–EEG neural signature of SICI and ICF in M1 in terms of TEP component amplitude and spectral power modulation. Subsequently these paradigms were applied in DLPFC to determine whether similar neural signatures were evident, with the aim of enabling the future use of these paradigms as novel measures of GABAA and glutamate receptor-mediated neurotransmission in prefrontal cortex.
MATERIALS AND METHODS
Participants
Twelve right-handed adults (six female, aged 22–57 years; mean age 39±12 years) participated in the main component of the present study to investigate SICI from the M1 (M1–SICI) and DLPFC (DLPFC–SICI) as well as ICF from the DLPFC (DLPFC–ICF). The influence of ICF with M1 stimulation (M1–ICF) was tested in 21 right-handed adults (14 female, aged 23–56 years; mean age 32±10 years) in a separate session owing to time constraints. Exclusion criteria included a self-reported comorbid medical illness, smoking, use of prescription medication, or a history of drug or alcohol abuse. Psychopathology was ruled out using the Structured Clinical Interview for DSM–IV Axis I Disorders. Participants abstained from caffeine intake prior to commencing the study, although it is thought that caffeine does not influence SICI and ICF (Orth et al, 2005). Written informed consent was obtained from each participant. The experiment was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Centre for Addiction and Mental Health.
Experimental Design and Procedures
EMG recordings
EMG was recorded from the relaxed first dorsal interosseous of the dominant (right) hand according to previously published methods (Daskalakis et al, 2002).
TMS
Monophasic TMS pulses were administered to the left M1 hotspot using a 70 mm figure-of-eight coil, and two Magstim 200 stimulators (Magstim Company Ltd., UK) connected via a Bistim module. MEP data were collected using Signal (Cambridge Electronics, UK). DLPFC stimulation was performed with the coil centered between F3 and F5 electrodes, which provides the most accurate estimation of left DLPFC (border of BA9 and BA46) and low inter-subject variability in the absence of MRI-guided neuronavigational equipment (Fitzgerald et al, 2009; Rusjan et al, 2010).
Measurement of SICI and ICF
SICI was studied according to established methods (Kujirai et al, 1993). An ISI of 2 ms was used to avoid contamination by excitatory interneurons (Peurala et al, 2008), and as this ISI optimally suppresses late I-waves and MEP amplitude (Di Lazzaro et al, 1998). CS intensity was 80% RMT. TS intensity was set to evoke a MEP of 1 mV peak-to-peak amplitude when delivered alone, which is considered optimal for evoking SICI, whereas lower TS intensities do not reliably evoke SICI (Daskalakis et al, 2002; Sanger et al, 2001; Wagle-Shukla et al, 2009). In fact, SICI delivered with a lower TS at ~RMT would have the potential to elicit facilitation rather than inhibition (c.f. Figure 1b, Ilic et al, 2002). At last, the use of near threshold TS intensities would not allow investigation of the relationship between TMS–EEG and TMS–EMG measures of SICI and ICF. ICF was measured using the same CS and TS intensities and an ISI of 10 ms (Kujirai et al, 1993). Hundred trials were delivered per condition.
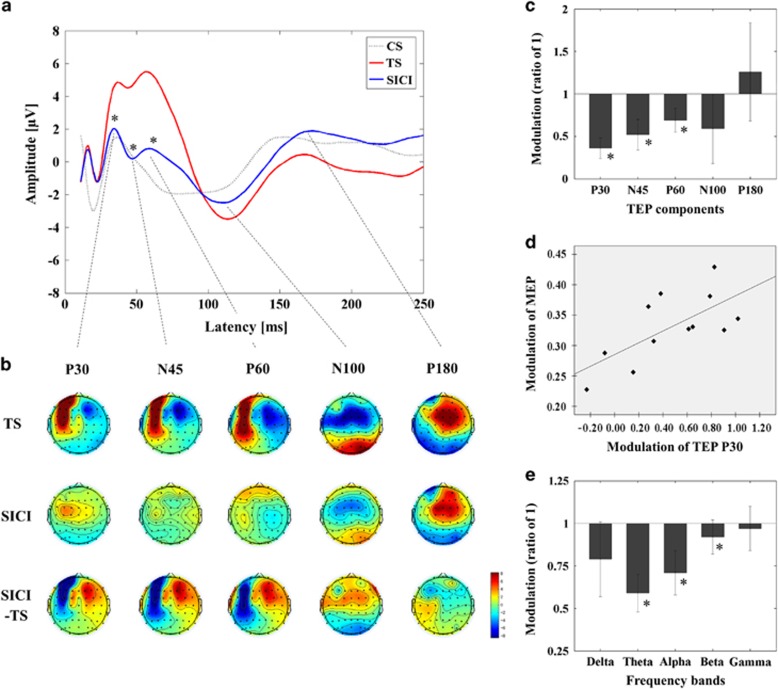
Figure 1.
Inhibitory influence of SICI on TEPs with TMS over M1. (a) Group averaged TEPs following TS (red; delivered at a time equal to 0 ms), SICI (CS.TS) (blue) and CS alone (dotted line; delivered at –-2 ms, that is, 2 ms prior to TS). P30, N45, and P60 were significantly reduced in amplitude by SICI. (b) Topographical display of the suppression of TEP components by SICI. (c) Amplitude suppression of TEP components by SICI (mean±SEM) is displayed as a ratio of 1 (1 is equivalent to no change relative to the TEP amplitude of TS alone; *P<0.05). Note that TEP latency varied according to individual, TEP and condition leading to phase cancellation in the average trace and voiding direct comparison between (a and c) in all figures. (d) The suppression of MEP amplitude by SICI and of P30 TEP was significantly positively correlated (Pearson's correlation, r=0.678, p=0.015, N=12). (e) Modulation of cortical oscillatory power. M1–SICI was associated with a significant decrease in power in the theta, alpha, and beta bands (*P<0.05). A full color version of this figure is available at the Neuropsychopharmacology journal online.
EEG recording and signal preprocessing
EEG was recorded as previously described (Daskalakis et al, 2008), and preprocessing was performed in line with published methodology (Radhu et al, 2015; Rogasch et al, 2014) as outlined in supplementary information.
Data Analysis
Analysis of SICI and ICF
Averages are expressed as mean±(SEM). MEPs evoked by single and paired pulse (conditioned–test) stimulation for each subject were averaged in each condition. For EMG and EEG, SICI and ICF were calculated from MEP or TEP amplitudes as ((CS.TS)/TS) and expressed as a percentage or ratio. For TMS–EEG, first, the area under the TEP curve (time window 20–200 ms) was computed with the Hilbert transform method for each ROI (Supplementary Figure 1). The Hilbert transform is a measure of the amplitude envelope and power changes in cortical oscillatory activity and thus identifies the cortical region in which these changes in neural activity evoked by SICI and ICF are greatest. Subsequently, the influence of SICI and ICF on the amplitude of individual TEP components (P30, N45, P60, N100, and P180) was computed for each ROI. Although some (Premoli et al, 2014b), but not all (Daskalakis et al, 2008; Ferreri et al, 2011, 2012; Paus et al, 2001) studies have subtracted the influence of CS alone, the present data for SICI and conversely ICF clearly indicate bidirectional changes in the amplitude of TEP components that are clearly unrelated to the influence of CS alone. Thus, for simplicity and greater transparency in this case, CS amplitude was not subtracted from the CS.TS waveform.
Modulation of the specific frequencies with SICI/ICF paradigms were investigated from the delta to gamma band range (ie, delta: 0.3–3 Hz, theta: 4–7 Hz, alpha: 8–14 Hz, beta: 14–30 Hz, gamma: 30–50 Hz) in a time window of 2000 ms following TS.
Statistical analyses
Statistical analysis on main effects (and sub-effects) was performed using repeated (and multivariate) measure analysis of variance (ANOVA; MANOVA) and two-tailed paired t-tests with SPSS (version 19.0). Additional details are provided in supplementary information.
RESULTS
MEP Amplitude Modulation
MEP amplitude was significantly attenuated by SICI (32.3±3.7% of TS alone, mean±SEM; t11=–8.506, P<0.0001). For SICI, TS alone gave an MEP amplitude of 1.13±0.32 mV, which was attenuated to 0.36±0.11 (n=12). For ICF, MEP amplitude was significantly facilitated (168.4±8.0% of TS alone; t15=–5.532, P=0.01). TS alone evoked an MEP amplitude of 1.03±0.03 mV, which was facilitated to 1.73±0.09 mV (n=21).
TEP Power and Amplitude Changes
M1–SICI
Results are displayed in Figure 1 and statistics are summarized in Supplementary Table 1. The ANOVA for TEP power showed significant main effects of ROI and condition (TS vs SICI (CS.TS)) as well as a significant ROI-by-condition interaction. MANOVA indicated that for TEP Power the significant interaction of ROI × Condition (TS and SICI) was restricted to the left central ROI, with TEP power significantly decreased by SICI (see Supplementary Table 1 and Supplementary Figure 2A). ANOVA of TEP amplitude at this ROI revealed a significant interaction of condition (TS vs SICI) and TEP component. Post hoc analyses indicated that M1–SICI suppressed the amplitude of several early TEP components (Figure 1(a–c): (i) TEP P30 (SICI<TS), (ii) TEP N45 (SICI<TS), and (iii) TEP P60 (SICI<TS) (Supplementary Table 1).
DLPFC–SICI
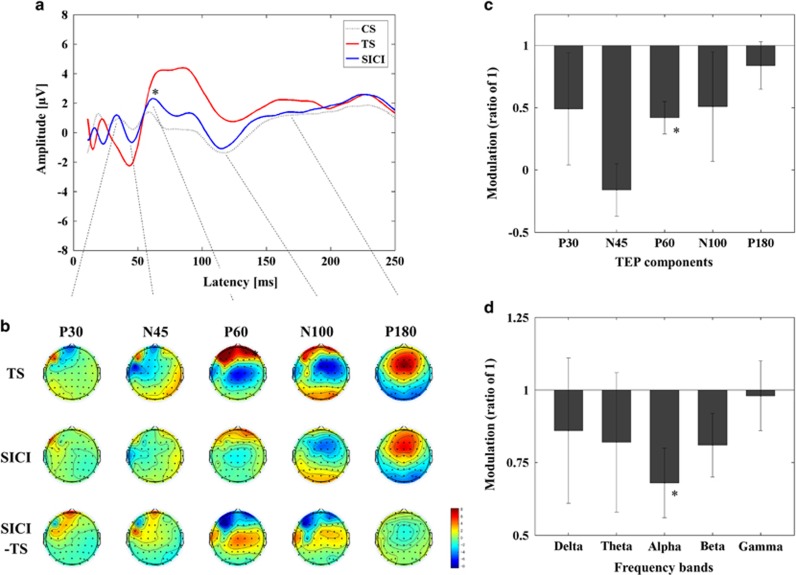
Results are displayed in Figure 2 and statistics summarized in Supplementary Table 2. ANOVA and post hoc analysis for TEP power showed that the influence of DLPFC–SICI was significant and specific to the left frontal ROI (Supplementary Figure 2B). Further, ANOVA and post hoc analyses for TEP values indicated that SICI significantly suppressed P60 amplitude at the left frontal ROI (Figure 2a; Supplementary Table 2) and in its topographical distribution (Figure 2b).
Figure 2.
Inhibitory influence of SICI on TEPs with TMS over DLPFC. (a) Group averaged TEPs following TS (red; delivered at a time equal to 0 ms), SICI (CS.TS) (blue) and CS alone (dotted line; delivered at -2 ms, ie, 2 ms prior to TS). P60 was significantly reduced in amplitude by SICI, whereas P30 was not apparent in DLPFC. (b) Topographical display of the suppression of TEP components by SICI. (c) Amplitude suppression of TEP components by SICI (mean±SEM) is quantified as a ratio of 1 (1 is equivalent to no change relative to the TEP amplitude of TS alone; *P<0.05). The modulation of TEP components by SICI (mean±SEM) is displayed as a ratio of 1 (1 is equivalent to no change; *P<0.05). (d) Modulation of cortical oscillatory power. DLPFC–SICI was associated with a significant decrease in power specific to the alpha band (*P<0.05). A full color version of this figure is available at the Neuropsychopharmacology journal online.
M1–ICF
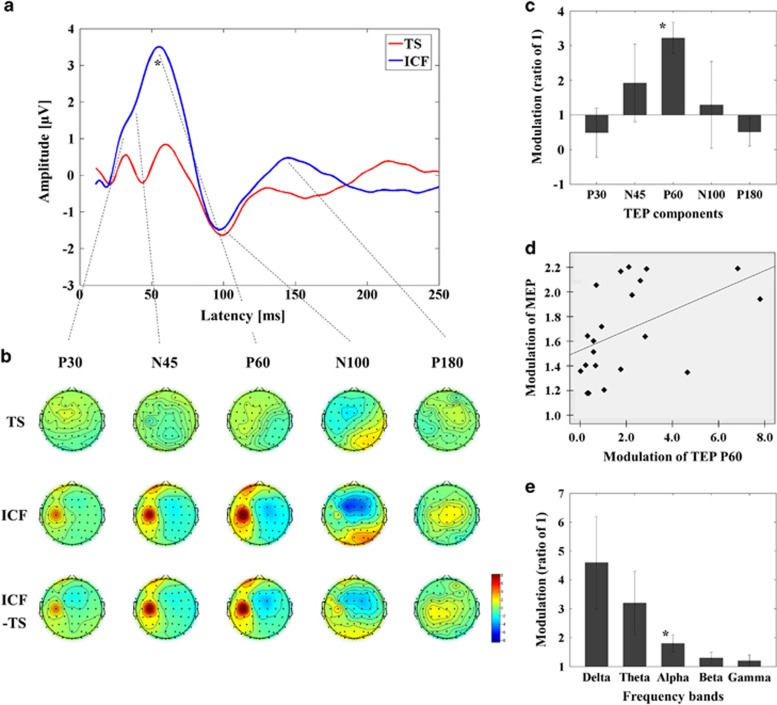
As a complementary analysis, we studied the M1–ICF paradigm to validate the DLPFC–ICF paradigm (Figure 3; Supplementary Table 3). ANOVA and post hoc analyses for TEP power revealed that the influence of M1–ICF was most significant at the left central ROI (Supplementary Figure 2(C)). Further, the ANOVA and post hoc analyses for TEP values demonstrated that M1–ICF induced significant facilitation of P60 at the left central ROI (TS<ICF) (see Figure 3a–c and Supplementary Table 3).
Figure 3.
Facilitatory influence of ICF on TEPs with TMS over M1, recorded in a separate group of 21 individuals. (a) Group averaged TEPs following TS (red; delivered at a time equal to 0 ms) and ICF (CS.TS) (blue). P60 was significantly increased in amplitude by ICF. P30 appeared to be increased in amplitude but was not evident as a distinct peak. (b) Topographical display of the facilitation of TEP components by ICF. (c) The modulation of TEP components by ICF (mean±SEM) is displayed as a ratio of 1 (1 is equivalent to no change relative to the TEP amplitude of TS alone; *P<0.05). (d) The facilitation of the MEP amplitude by ICF and of P60 TEP was significantly positively correlated (Pearson's correlation, r=0.543, p=0.011, N=21). (e) Modulation of cortical oscillatory power. M1–ICF was associated with a significant increase in power in the alpha band (*P<0.05). A full color version of this figure is available at the Neuropsychopharmacology journal online.
DLPFC–ICF
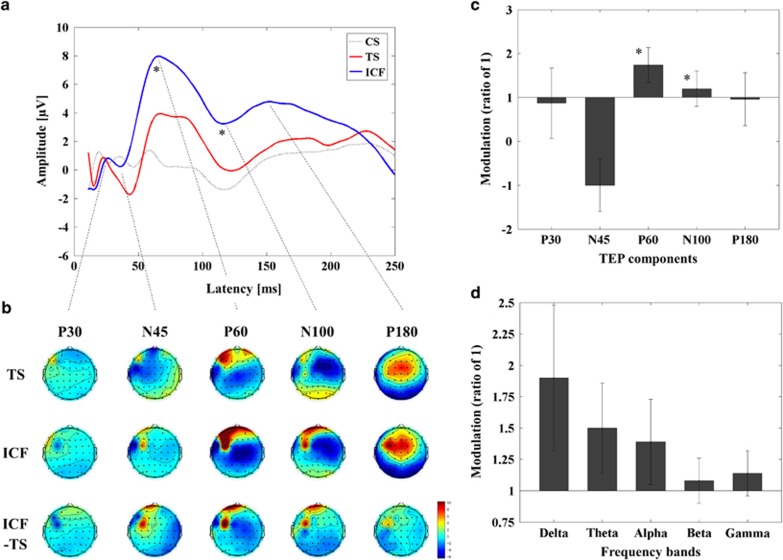
Results are displayed in Figure 4 and Supplementary Table 4. ANOVA and post hoc analyses for TEP power revealed that the influence of DLPFC–ICF was most significant at the left frontal ROI (Supplementary Figure 2D). Further, ANOVA and post hoc analyses for TEP values demonstrated that DLPFC–ICF induced significant modulation on TEP P60 and N100 at the left frontal ROI (TS<ICF) (Figure 4a–c; Supplementary Table 4).
Figure 4.
Facilitatory influence of ICF on TEPs with TMS over DLPFC. (a) Group averaged TEPs following TS (red; delivered at a time equal to 0 ms), ICF (CS.TS) (blue) and CS alone (dotted line; delivered at -10 ms, ie, 10 ms prior to TS). P60 was significantly increased in amplitude similar to M1–ICF, whereas P30 was not evident in DLPFC. In addition, there was a longer lasting increase in EEG-positive potential with a modulation of N100 amplitude. (b) The topographical distribution of TEP components, and their modulation by ICF, is displayed. Note that the topographical distributions of TEPs from TS for DLPFC–ICF and DLPFC–SICI are equivalent, but that the scaling has been adjusted in each case to better visualize the respective changes. (c) The modulation of TEP components by ICF (mean±SEM) is displayed as a ratio of 1 (1 is equivalent to no change relative to the TEP amplitude of TS alone; *P<0.05). Note that DLPFC–ICF increases the positivity of N45 (consistent with M1–ICF), but that this is equivalent to a decrease the amplitude of N45 in DLPFC–ICF, resulting in a negative ratio. (d) DLPFC–ICF did not modulate power in any frequency band. A full color version of this figure is available at the Neuropsychopharmacology journal online.
Topological Distributions of TEPs
For the M1–SICI/ICF paradigms, P30 and P60 showed a slightly lateralized and somewhat frontal topographical distribution extending from the site of stimulation, whereas N100 and P180 showed a bilateral distribution, in broad agreement with previous studies (Ferreri et al, 2011; Premoli et al, 2014a, 2014b). With a TS intensity of ~1mV as used here, TEPs may become somewhat dominated by the P60–N100 complex (Lioumis et al, 2009), and this may explain why the first TEP components appear dominated by a broad positive peak (Figure 1b and Figure 3b). This is a limitation of using a TS of 1mV to reliably elicit SICI and to compare the modulation of TMS–EEG and TMS–EMG amplitude. Nonetheless, P30 and N45 are evident as superimposed on these more dominant TEP components (Figure 1a), and modulation of these components by SICI and ICF is evident topographically (Figures 1,2b), in agreement with the results of Ferreri et al (2011, c.f. Table 1). With DLPFC–SICI/ICF paradigms, early TEPs (P30, N45, and P60) showed a more frontal distribution, whereas N100 and P180 had a more central/bilateral distribution (Figure 2b and Figure 4b).
Modulation of Cortical Oscillatory Power in Different Frequency Bands
ANOVA and post hoc analyses for the M1–SICI paradigm indicated SICI significantly decreased power in theta (t11=5.446, p<0.0001; SICItheta<TStheta), alpha (t11=3.297, p=0.007; SICIalpha<TSalpha), and beta (t11=4.222, p=0.001; SICIbeta<TSbeta) bands. For DLPFC–SICI, ANOVA and post hoc analyses revealed a significant decrease in alpha band power (t11=3.680, p=0.004; SICIalpha<TSalpha). M1–ICF induced a significant increase in the alpha band (t20=-3.300, p=0.004; ICFalpha>TSalpha), whereas no significant modulation occurred in the DLPFC–ICF paradigm.
Correlated Modulation of MEP and TEP Amplitude
A significant positive correlation for M1 measures was observed between SICI of MEP and P30 TEP amplitude (r=0.678, p=0.015, N=12) (Figure 1d and for ICF of MEP and TEP P60 amplitude (r=0.543, p=0.011, N=21) (Figure 3d) at the left central ROI.
DISCUSSION
Summary
Using simultaneous TMS and EEG, we demonstrated that SICI and ICF are associated with bidirectional modulation of neuronal activity, and that this is consistent for TMS applied to motor and prefrontal regions. With M1 stimulation, there was a significant association between the level of modulation of TEP and MEP amplitude, suggesting that the TMS–EEG measures were representative of neural excitability changes evoked by SICI and ICF. These findings pave the way for future noninvasive investigation of inhibitory and excitatory physiological activity in prefrontal cortex for patients with neurologic and psychiatric disorders and distinction between aberrant GABAA and GABAB receptor-mediated neurotransmission.
Influence of SICI and ICF on TEP and MEP Amplitude with M1 TMS
The modulation of TEP components by SICI and ICF has been investigated previously however the results were inconsistent. Paus et al (2001) found no influence of SICI on TEPs however that study may have been underpowered as only five participants survived rejection. Manganotti et al (2012) explored the influence of SICI on TMS–EEG potentials on a broader time-scale of seconds rather than TEP components in the milliseconds immediately following stimulation. The present results in motor cortex are consistent with those of Ferreri et al (2011) who used similar methodology. SICI attenuated and ICF enhanced the amplitude of positive deflections at a latency of 30 ms (P30) and 60 ms (P60), whereas the amplitude of the negative trough at 45 ms (N45) was moderately affected by SICI. It appeared as if P30 was potentially increased in amplitude during ICF; however, it was not possible to definitively measure this change as P30 was generally occluded by a marked increase in P60 amplitude during ICF. The masking of P30 by P60 during ICF is consistent with the same phenomenon occurring at higher intensities with single pulse TMS (Lioumis et al, 2009). Nonetheless, a shared modulation of the amplitude of these components would be consistent with correlated inhibition of P30 and P60 amplitude in the LICI paradigm (Rogasch et al, 2013). The change in P30 and P60 amplitude is consistent with previous studies indicating that these components are particularly sensitive to changes in excitability, gaining in amplitude as stimulus intensity is increased with both M1 and DLPFC stimulation (Kahkonen et al, 2005a, b; Komssi et al, 2004; Lioumis et al, 2009), and being inhibited by LICI (Rogasch et al, 2013), the CSP (Farzan et al, 2013), and SAI (Ferreri et al, 2012). Critically, the degree of modulation of TEP components P30 and P60, respectively, was related to the modulation of MEP amplitude by SICI and ICF in the present study (Figure 1d and Figure 3d) and elsewhere (Rogasch et al, 2013), suggesting that these TEP measures reflect similar neural excitability changes to the MEP. Together, these results suggest that P30 and P60 provide sensitive measures of changes in cortical excitability, and provide evidence of a direct relationship between neural activity measured by modulation of P30 and P60 TEP amplitude and more conventional MEP based measures of cortical excitability changes associated with SICI and ICF.
Interestingly N100 TEP amplitude was not influenced by SICI or ICF, in contradistinction to the modulation of N100 by LICI (Rogasch et al, 2013) and the CSP (Farzan et al, 2013; Kimiskidis et al, 2008). N100 is thought to be related to GABAB-receptor mediated long lasting IPSP (Premoli et al, 2014b), whereas earlier TEP components could be related to a shorter latency EPSP and GABAA receptor-mediated IPSP (Premoli et al, 2014a). In this sense, the sole modulation of early components may represent a relatively distinct neural signature of SICI or ICF.
Influence of SICI and ICF on TEP Amplitude in DLPFC
In DLPFC, SICI and ICF were characterized by inhibition and facilitation, respectively, of P60 TEP amplitude, consistent with the results from M1 stimulation, whereas P30 was generally absent in DLPFC with single pulse stimulation. For ICF, it appeared that following P60 there was a longer lasting increase in positive potentials, influencing N100 amplitude. In data sets where N100 has a negative potential, rather than solely representing a trough, this could perhaps be simply explained by the finding that P60 and N100 amplitude may be negatively correlated and P60 may be inversely linked to inhibition (Rogasch et al, 2013). The absence of P30 in DLPFC is consistent with previous findings that P30 is expressed to a lesser extent in DLPFC compared with M1 (c.f. Figure 6A,B and 10A,B Rogasch et al, 2014; Van Der Werf and Paus, 2006). Overall early components, in particular P60 TEP amplitude, were most consistently modulated by SICI and ICF across M1 and DLPFC stimulation, and in a bidirectional manner, which was linked to their modulation of MEP amplitude with M1 stimulation. It appears that P60 TEP amplitude may most robustly reflect neural excitability and its modulation by SICI and ICF across M1 and DLPFC. The results provide the first evidence that it might be possible to measure GABAA receptor-mediated inhibition or glutamatergic activity with SICI and ICF in non-motor regions using TMS–EEG. In addition, these findings enable direct comparison in future studies of changes in GABAB (Daskalakis et al, 2008) and GABAA receptor-mediated inhibition in prefrontal cortex, which are differentially implicated in psychiatric disorders including major depressive disorder (MDD) and schizophrenia (Deng and Huang, 2006). MDD is associated with region-specific modulation of GABAA receptor subunit composition and GABA concentration (Pehrson and Sanchez, 2015), but functional effects on inhibitory strength have been difficult to assess in human using existing techniques. We anticipate that the present noninvasive measures will be of significant benefit for investigation of inhibitory and excitatory strength in neurological disorders, and their modulation by treatment.
As noted above, SICI and ICF showed the same bidirectional modulation of TEP amplitude in M1 and DLPFC. We anticipated that there would be a stronger association between MEPs and M1 TEPs compared with MEPs and DLPFC TEPs. We performed an exploratory correlational analysis between MEP measures and TEP amplitude changes in DLPFC, however no additional correlations were observed. Similarly, other studies have reported an absence of correlations between modulation of specific TEP components in M1 compared to other areas (Farzan et al, 2009, 2013; Noda et al, 2016), and in the present study this may reflect a difference in the strength of SICI and ICF in M1 compared to DLPFC. This could perhaps be due to differences in GABAA or glutamatergic tone. Such differences could suggest that SICI and ICF measured in DLPFC provide unique information compared to SICI and ICF in M1.
Evidence for a Cortical Origin of ICF
One further aspect of the present findings deserves mention. There is substantial evidence that SICI is mediated at the cortical level, and although ICF is widely assumed to be cortically mediated, to date direct evidence for this has been considerably weaker. Using paired electrical and magnetic stimulation, with the former thought to activate pyramidal cells directly, Kujirai et al (1993) demonstrated that SICI involved cortical interneuronal activity and could only be evoked using paired pulse magnetic stimulation. In contrast, ICF could still be evoked with paired electrical and magnetic stimulation. Later, Di Lazzaro et al (1998) demonstrated that SICI reduces the amplitude of descending I-waves evoked by the test pulse, providing direct evidence of inhibition at the cortical level, whereas the ICF conditioning pulse had no effect on the amplitude of descending I-wave activity evoked by the test pulse (Di Lazzaro et al, 2006b). Although systemic administration of pharmacological agents has suggested that ICF is mediated by glutamate (for review, see Paulus et al, 2008), it was not demonstrated whether this occurred in the spinal or cortical CNS. The present results provide clear evidence that ICF modulates neuronal activity at the cortical level. Additional EPSP summation at the spinal level cannot be excluded (Di Lazzaro et al, 2006b; Kujirai et al, 1993), however the tight correlation with TEP amplitude suggests that the spinal contribution may be modest.
Modulation of Spectral Power
The present findings indicate that multiple frequency bands were modulated by SICI in M1, but that modulation was specific to the alpha band with DLPFC–SICI. Similarly, suppression of multiple frequency bands occurs with LICI (Farzan et al, 2009) and the cortical silent period (Farzan et al, 2013). Differential modulation of spectral properties was also observed previously according to the site of stimulation (Farzan et al, 2009), although the present results indicate a greater specificity to lower frequency bands.
As noted above, a consistent finding across DLPFC and M1 was the reduction in alpha power following SICI. Oscillatory activity in the alpha band preceding a task has been associated with functional inhibition (for review, see Jensen and Mazaheri, 2010), and whereas activity of inhibitory interneurons is often associated with the gamma rhythm (Bartos et al, 2007), GABAergic feedback from interneurons is strongly implicated in the physiological mechanism generating the alpha rhythm (for review, see Mazaheri and Jensen, 2010). Little is known about the modulation of alpha power following stimulation of GABAA mediated inhibition; however, the present results appear consistent with the decrease in cortical alpha power that occurs following administration of benzodiazepines (positive allosteric modulators of the GABAA receptor) (Berchou et al, 1986; Fingelkurts et al, 2004; Link et al, 1991; Schreckenberger et al, 2004).
Modulation in the beta band was specific to M1–SICI. Beta band activity is more dominant in the rolandic area and associated with movement execution and the motor network. Previous studies found that beta power (ie, amplitude) and phase-synchrony are increased following single pulse TMS (Fuggetta et al, 2005; Paus et al, 2001; Van Der Werf and Paus, 2006), and this increase is specific to M1 stimulation and not induced by stimulation of dorsal premotor cortex (Van Der Werf and Paus, 2006). Beta activity is suppressed by GABA receptor-mediated inhibitory paradigms LICI (Farzan et al, 2009) and SAI (Ferreri et al, 2012). Beta band activity and SICI have both been closely linked to stopping and response inhibition (Coxon et al, 2006; for review, see Schall and Godlove, 2012). Together these findings provide a possible basis for the specificity of beta band modulation to the motor domain and its link to inhibitory activity.
With regard to the theta band, this frequency is more commonly associated with cognitive tasks and it is surprising that this frequency was more modulated in M1 than DLPFC. Interestingly, however, the theta rhythm appears particularly responsive to, and is suppressed following, low frequency rTMS to M1, which has an inhibitory impact on excitability (Van Der Werf and Paus, 2006).
Limitations
Accuracy could have potentially been enhanced and variability reduced by using MRI rather than EEG-guided neuronavigation. Nonetheless, the TEPs evoked in the present study are consistent with those previously reported using either method. Indeed, both methods produce highly comparable results (compare Lioumis et al, 2009; Rogasch et al, 2014). Non-MRI-guided neuronavigation is commonly employed and was used in all but one study in a recent review of TMS–EEG studies by Hone-Blanchet et al (2015).
The results may be contaminated by the auditory-evoked potential (AEP) from the TMS click, which has peaks ~90 and 180 ms (Rogasch et al, 2014). However, these peaks are too broad to explain the bidirectional differences between SICI and ICF—ie, polarity reversal associated with the AEP would require a shift in ISI of ~90 ms, whereas the shift in ISI was just 8 ms. Thus auditory contamination is unlikely to account for the present findings in both motor and frontal cortex, which relate to relatively early components.
In the present study we used a CS intensity of 80% RMT, as this is commonly applied for SICI and ICF in M1 (Kujirai et al, 1993). It is not yet clear whether this intensity would be optimal for activating inhibitory and excitatory circuits in DLPFC and this could be explored in future studies. Menstrual status can influence excitability and should be controlled for in future studies (Smith et al, 2002).
At last, somatosensory potentials may be induced TMS; however, previous analysis in a similar context indicated that muscle activation is unlikely to influence TMS-elicited potentials (Paus et al, 2001). Certainly, the significant decrease in P30, which directly correlated with SICI MEP amplitude changes, occurs too early to be influenced by sensory feedback (minimum ~45 ms). In addition, it is important to note that DLPFC stimulation does not evoke MEPs, and thus the finding that SICI and ICF produced consistent modulation of P60, regardless of the stimulated domain, indicates that these changes are unrelated to sensory feedback and instead related to SICI and ICF. The bidirectional changes in P60 TEP amplitude with SICI and ICF are further suggestive of bidirectional modulation of neural excitability, and are consistent with prior studies of their association with cortical excitability (Farzan et al, 2013; Ferreri et al, 2012; Kahkonen et al, 2005a; Rogasch et al, 2013).
In conclusion, these findings provide the first evidence that excitatory and inhibitory cortical circuits SICI and ICF share a similar neural signature across M1 and DLPFC, and suggest that neural excitability changes associated with GABAA receptor-mediated inhibition and glutamatergic transmission can be recorded in prefrontal areas. We anticipate that these results will facilitate several advances in our understanding of the pathophysiology and treatment of a variety of neurological and psychiatric disorders.
Funding And Disclosure
YN receives a postdoctoral fellowship from the Centre for Addiction and Mental Health (CAMH) Foundation. RFCH was supported by a Canadian Institutes of Health Research (CIHR)—Dystonia Medical Research Foundation Fellowship award. NR is supported by the Parkinson Canada postdoctoral research fellowship. FF receives funding from NARSAD, Slaight Family Centre for Youth in Transition at the CAMH, Natural Sciences and Engineering Research Council of Canada (NSERC), the Ontario Brain Institute (OBI). TKR received research support from Brain Canada, Brain and Behavior Research Foundation, Canada Foundation for Innovation, the CIHR, Ontario Ministry of Health and Long–Term Care, Ontario Ministry of Research and Innovation, the US National Institute of Health (NIH), and the W Garfield Weston Foundation. PBF is supported by a NHMRC Practitioner Fellowship (606907). PBF has received equipment for research from MagVenture A/S, Medtronic, Cervel Neurotech and Brainsway and funding for research from Cervel Neurotech. ZJD received research and equipment in kind support for an investigator–initiated study through Brainsway, and a travel allowance through Merck. ZJD has also received speaker funding through Sepracor, and AstraZeneca, served on advisory boards for Hoffmann–La Roche Limited and Merck, and received speaker support from Eli Lilly. DMB receives research support from CIHR, NIH, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He receives research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He receives in-kind equipment support from Magventure for an investigator-initiated study. He receives medication supplies for an investigator-initiated trial from Invidior. The authors declare no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the assistance of all study volunteers and thank Ms Stacey Shim and Ms Felicity Backhouse for the recruitment of participants.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bartos M, Vida I, Jonas P (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56. [DOI] [PubMed] [Google Scholar]
- Berchou R, Chayasirisobhon S, Green V, Mason K (1986). The pharmacodynamic properties of lorazepam and methylphenidate drugs on event-related potentials and power spectral analysis in normal subjects. Clin Electroencephalogr 17: 176–180. [PubMed] [Google Scholar]
- Chen R, Curra A (2004). Measures of cortical inhibition in health and disease. Suppl Clin Neurophysiol 57: 691–701. [DOI] [PubMed] [Google Scholar]
- Claus D, Weis M, Jahnke U, Plewe A, Brunholzl C (1992). Corticospinal conduction studied with magnetic double stimulation in the intact human. J Neurol Sci 111: 180–188. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD (2006). Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95: 3371–3383. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R (2002). The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543(Pt 1): 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB (2008). Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology 33: 2860–2869. [DOI] [PubMed] [Google Scholar]
- Deng C, Huang XF (2006). Increased density of GABAA receptors in the superior temporal gyrus in schizophrenia. Exp Brain Res 168: 587–590. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P et al (2006. a). GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol 575(Pt 3): 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P et al (2006. b). Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol 96: 1765–1771. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A et al (1998). Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119: 265–268. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Hoppenbrouwers SS, Fitzgerald PB, Chen R, Pascual-Leone A et al (2013). The EEG correlates of the TMS-induced EMG silent period in humans. Neuroimage 83: 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB et al (2010). Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain 133(Pt 5): 1505–1514. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Wong W, Chen R, Fitzgerald PB, Daskalakis ZJ (2009). Suppression of gamma-oscillations in the dorsolateral prefrontal cortex following long interval cortical inhibition: a TMS-EEG study. Neuropsychopharmacology 34: 1543–1551. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Pasqualetti P, Maatta S, Ponzo D, Ferrarelli F, Tononi G et al (2011). Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage 54: 90–102. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Ponzo D, Hukkanen T, Mervaala E, Kononen M, Pasqualetti P et al (2012). Human brain cortical correlates of short-latency afferent inhibition: a combined EEG-TMS study. J Neurophysiol 108: 314–323. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Kivisaari R, Pekkonen E, Ilmoniemi RJ, Kahkonen S (2004). The interplay of lorazepam-induced brain oscillations: microstructural electromagnetic study. Clin Neurophysiol 115: 674–690. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Daskalakis ZJ, Hoy K, Farzan F, Upton DJ, Cooper NR et al (2008). Cortical inhibition in motor and non-motor regions: a combined TMS-EEG study. Clin EEG Neurosci 39: 112–117. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Maller JJ, Hoy KE, Thomson R, Daskalakis ZJ (2009). Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul 2: 234–237. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Fiaschi A, Manganotti P (2005). Modulation of cortical oscillatory activities induced by varying single-pulse transcranial magnetic stimulation intensity over the left primary motor area: a combined EEG and TMS study. Neuroimage 27: 896–908. [DOI] [PubMed] [Google Scholar]
- Hone-Blanchet A, Ciraulo DA, Pascual-Leone A, Fecteau S (2015). Noninvasive brain stimulation to suppress craving in substance use disorders: Review of human evidence and methodological considerations for future work. Neurosci Biobehav Rev 59: 184–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U (2002). Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545(Pt 1): 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Kicic D (2010). Methodology for combined TMS and EEG. Brain Topogr 22: 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A (2010). Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkunen P, Jauhiainen AM, Kononen M, Paakkonen A, Karhu J, Soininen H (2011). Combining transcranial magnetic stimulation and electroencephalography may contribute to assess the severity of Alzheimer's disease. Int J Alzheimers Dis 2011: 654794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahkonen S, Komssi S, Wilenius J, Ilmoniemi RJ (2005. a). Prefrontal TMS produces smaller EEG responses than motor-cortex TMS: implications for rTMS treatment in depression. Psychopharmacology (Berl) 181: 16–20. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Komssi S, Wilenius J, Ilmoniemi RJ (2005. b). Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. Neuroimage 24: 955–960. [DOI] [PubMed] [Google Scholar]
- Kimiskidis V, Papagiannopoulos S, Kazis D, Vasiliadis G, Oikonomidi A, Sotirakoglou K et al (2008). Silent period (SP) to transcranial magnetic stimulation: the EEG substrate. Brain Stimul 1: 315–316. [Google Scholar]
- Komssi S, Kahkonen S, Ilmoniemi RJ (2004). The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp 21: 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A et al (1993). Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Schwenkreis P, Tegenthoff M, Malin JP (1997). The glutamate antagonist riluzole suppresses intracortical facilitation. J Neural Transm 104: 1207–1214. [DOI] [PubMed] [Google Scholar]
- Link CG, Leigh TJ, Fell GL (1991). Effects of granisetron and lorazepam, alone and in combination, on the EEG of human volunteers. Br J Clin Pharmacol 31: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioumis P, Kicic D, Savolainen P, Makela JP, Kahkonen S (2009). Reproducibility of TMS-Evoked EEG responses. Hum Brain Mapp 30: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Formaggio E, Storti SF, De Massari D, Zamboni A, Bertoldo A et al (2012). Time-frequency analysis of short-lasting modulation of EEG induced by intracortical and transcallosal paired TMS over motor areas. J Neurophysiol 107: 2475–2484. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Jensen O (2010). Rhythmic pulsing: linking ongoing brain activity with evoked responses. Front Hum Neurosci 4: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Ribolsi M, Kusayanagi H, Siracusano A, Mantovani V, Marasco E et al (2011). Genetic variants of the NMDA receptor influence cortical excitability and plasticity in humans. J Neurophysiol 106: 1637–1643. [DOI] [PubMed] [Google Scholar]
- Noda Y, Cash RF, Zomorrodi R, Garcia Dominguez L, Farzan F, Rajji TK et al (2016). A combined TMS-EEG study of short-latency afferent inhibition in the motor and dorsolateral prefrontal cortex. J Neurophysiol (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Orth M, Amann B, Ratnaraj N, Patsalos PN, Rothwell JC (2005). Caffeine has no effect on measures of cortical excitability. Clin Neurophysiol 116: 308–314. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen LG, Large CH, Di Lazarro V, Nitsche M et al (2008). State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimulation 1: 151–163. [DOI] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP (2001). Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: an EEG study. J Neurophysiol 86: 1983–1990. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C (2015). Altered gamma-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Design Dev Ther 9: 603–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peurala SH, Muller-Dahlhaus JF, Arai N, Ziemann U (2008). Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clin Neurophysiol 119: 2291–2297. [DOI] [PubMed] [Google Scholar]
- Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C et al (2014. a). TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci 34: 5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premoli I, Rivolta D, Espenhahn S, Castellanos N, Belardinelli P, Ziemann U et al (2014. b). Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG. Neuroimage 103: 152–162. [DOI] [PubMed] [Google Scholar]
- Radhu N, Garcia Dominguez L, Farzan F, Richter MA, Semeralul MO, Chen R et al (2015). Evidence for inhibitory deficits in the prefrontal cortex in schizophrenia. Brain 138(Pt 2): 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Daskalakis ZJ, Fitzgerald PB (2013). Mechanisms underlying long-interval cortical inhibition in the human motor cortex: a TMS-EEG study. J Neurophysiol 109: 89–98. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Thomson RH, Farzan F, Fitzgibbon BM, Bailey NW, Hernandez-Pavon JC et al (2014). Removing artefacts from TMS-EEG recordings using independent component analysis: importance for assessing prefrontal and motor cortex network properties. Neuroimage 101: 425–439. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB et al (2010). Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp 31: 1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R (2001). Interactions between two different inhibitory systems in the human motor cortex. J Physiol 530(Pt 2): 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Godlove DC (2012). Current advances and pressing problems in studies of stopping. Curr Opin Neurobiol 22: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreckenberger M, Lange-Asschenfeldt C, Lochmann M, Mann K, Siessmeier T, Buchholz HG et al (2004). The thalamus as the generator and modulator of EEG alpha rhythm: a combined PET/EEG study with lorazepam challenge in humans. Neuroimage 22: 637–644. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Liepert J, Witscher K, Fischer W, Weiller C, Malin JP et al (2000). Riluzole suppresses motor cortex facilitation in correlation to its plasma level. A study using transcranial magnetic stimulation. Exp Brain Res 135: 293–299. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M et al (1999). Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett 270: 137–140. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM (2002). Effects of ovarian hormones on human cortical excitability. Ann Neurol 51: 599–603. [DOI] [PubMed] [Google Scholar]
- Sun Y, Farzan F, Mulsant BH, Rajji TK, Fitzgerald PB, Barr MS et al (2016). Indicators for remission of suicidal ideation following magnetic seizure therapy in patients with treatment-resistant depression. JAMA Psychiatry 73: 337–345. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Paus T (2006). The neural response to transcranial magnetic stimulation of the human motor cortex. I. Intracortical and cortico-cortical contributions. Exp Brain Res 175: 231–245. [DOI] [PubMed] [Google Scholar]
- Wagle-Shukla A, Ni Z, Gunraj CA, Bahl N, Chen R (2009). Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J Physiol 587(Pt 23): 5665–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M (1998). Dextromethorphan decreases the excitability of the human motor cortex. Neurology 51: 1320–1324. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W (1996). The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res 109: 127–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.