Abstract
Background
Lymphangioleiomyomatosis (LAM) is included within group 5 of the current PH classification (unclear multifactorial mechanisms). However, data regarding the occurrence of PH in LAM are scarce. The aims of the study were to describe the prevalence and characteristics of PH in a large cohort of LAM patients with different levels of severity, and to evaluate the role of echocardiography and carbon monoxide diffusion capacity (DLCO) as screening methods for PH in LAM.
Methods
One hundred five LAM patients underwent transthoracic echocardiography, pulmonary function tests (PFTs) and 6-min walk test (6MWT). Patients with a suspicion of PH on echocardiography, defined by the presence of estimated systolic pulmonary artery pressure (PAP) over 35 mmHg or PFT showing DLco below 40% of the predicted value, underwent right heart catheterisation to confirm the diagnosis of PH.
Results
Eight patients (7.6%) had PH confirmed on right heart catheterisation, six patients (5.7%) had a pre-capillary pattern and two patients (1.9%) had a post-capillary profile. Only one patient (1%) had mean PAP over 35 mmHg. Patients with PH had lower FEV1 and DLCO in PFTs and greater oxygen desaturation and dyspnea intensity during 6MWT compared with those without PH. In 63% of the patients with confirmed PH, the right heart catheterisation was performed based only on DLCO result.
Conclusions
The prevalence of PH is low in LAM patients. Pulmonary hypertension in LAM is typically mild and significantly associated with pulmonary parenchymal involvement. Carbon monoxide diffusion capacity significantly improved the identification of PH in LAM patients.
Keywords: Echocardiography, Lymphangioleiomyomatosis, Prevalence, Pulmonary hypertension, Right heart catheterisation
Background
Lymphangioleiomyomatosis (LAM) is a rare low-grade neoplasm characterised by proliferation of atypical muscle cells (LAM cells) predominantly around airways, blood vessels and lymphatics, leading to the development of diffuse pulmonary cysts [1–3]. Clinically, it is characterised by progressive dyspnea, recurrent spontaneous pneumothorax, dry cough, hemoptysis and chylothorax, and by extrapulmonary manifestations, such as renal angiomyolipoma and lymphangioleiomyomas [1, 4–6]. Dyspnea and lower exercise capacity may be associated with several factors, such as dynamic hyperinflation, worsening gas exchange and, potentially, pulmonary hypertension (PH). Hypoxemia may occur at rest, during exercise or even during sleep, mainly in those patients with a higher degree of impairment in pulmonary function tests (PFTs) [5, 7, 8].
Pulmonary hypertension is a known complication of LAM, classified among other diseases with unclear multifactorial mechanisms within group 5 of the current PH classification [9]. Several pathophysiological processes might be implicated in the development of PH in LAM. Dysregulation of the mammalian target of rapamycin pathway, a major factor associated with the atypical proliferation of LAM cells, could be related to endothelial dysfunction; furthermore, chronic hypoxic vasoconstriction and even pulmonary artery wall infiltration by LAM cells could also contribute to the increase in pulmonary vascular resistance [10–13].
Nevertheless, data regarding PH in LAM are still scarce. Previous studies evaluating the association of PH and LAM included only patients with impaired lung function and/or were based solely on echocardiographic evaluation to determine the prevalence of PH. To our knowledge, the prevalence and characterisation of PH in patients with different levels of disease severity, including those with normal lung function, have not been completely determined in LAM. The aims of our study are to describe the prevalence and characteristics of PH, and to evaluate the role of echocardiography and carbon monoxide diffusion capacity (DLCO) in predicting the presence of PH in a large cohort of patients with LAM followed in a national reference centre.
Methods
Study design and participants
This was a cross-sectional, single-centre study conducted at a national reference centre in São Paulo, Brazil. All patients with LAM attending the outpatient clinic of the Pulmonary Division of Hospital das Clínicas, University of São Paulo, were evaluated for inclusion in the study. Patients were required to meet the following criteria: definitive diagnosis of LAM according to European Respiratory Society, American Thoracic Society and Brazilian Thoracic Society guidelines, and clinical stability, defined as the absence of exacerbations for a minimum of 6 weeks [1, 6, 14]. Patients who underwent lung transplant were not enrolled in the study. The protocol was approved by the local research ethics committee, and all patients provided written informed consent before enrollment (protocol number 759.676). All patients performed PFTs, echocardiogram and six-minute walk test (6MWT) at the baseline evaluation; when indicated, right heart catheterisation was performed within 30 days of the initial visit.
Measurements
Pulmonary function tests
All measurements were obtained based on the recommended standards [15–17]. Spirometry was performed using a calibrated pneumotachograph (Medical Graphics Corporation, St, Paul, MN), and lung volumes and DLCO measurements were obtained with a body plethysmograph (Elite Dx, Elite Series; Medical Graphics Corporation). The following variables were obtained: forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), total lung capacity (TLC), residual volume (RV) and DLCO. Predicted values were derived from the Brazilian population [18–20].
Transthoracic echocardiography
All patients underwent two-dimensional transthoracic Doppler echocardiography using IE 33 equipment, Philips Medical Systems, Botthel, USA, to evaluate the following variables: tricuspid regurgitation jet velocity, estimated systolic pulmonary artery pressure (PAP), obtained from the tricuspid regurgitation jet velocity and the inferior vena cava collapsibility index; and left ventricular ejection fraction [21, 22].
Six-minute walk test
Patients with LAM performed the 6MWT according to the American Thoracic Society guidelines [23]. Heart rate (HR) and oxygen saturation (SpO2) were measured at rest, every minute, and at the end of exercise. Breathlessness was evaluated using a modified Borg scale before and at the end of exercise.
Right heart catheterisation
All patients with estimated systolic PAP > 35 mmHg at echocardiography and/or DLco < 40% of the predicted value during the PFT, underwent right heart catheterisation to confirm the diagnosis of PH [24–27].
The measurements from the right heart catheterisation were obtained using a pulmonary artery catheter 7 F inserted through the jugular vein for diagnostic evaluation of potential patients with PH [28, 29]. The following variables were recorded: cardiac output (CO), mean pulmonary artery pressure (mPAP), pulmonary artery occlusion pressure (PAOP) and pulmonary vascular resistance (PVR). Cardiac output was measured by thermodilution, considering the average of three consecutive measurements with a maximum variation of 10% among them. Pulmonary hypertension was defined by the presence of mean pulmonary artery pressure ≥ 25 mmHg [9]. Patients with PH were also divided according to the PAOP level into pre-capillary PH (when PAOP ≤ 15 mmHg) or post-capillary PH (when PAOP > 15 mmHg) [30, 31].
Statistical analysis
Continuous variables are reported as the mean ± SD for those with normal distribution or median (interquartile range) for those with non-normal distribution, whereas categorical variables are presented as proportions. The prevalence of PH is reported as proportion with 95% confidence interval. Unpaired t-tests or Mann-Whitney U test was used for comparison of continuous variables. Categorical variables were compared using the Chi-squared test. Differences were considered significant if P < 0.05. Data were analysed with SigmaStat version 3.5 (Systat Software, Inc; San Jose, California).
Results
One hundred six patients with LAM were followed in our outpatient clinic between January 2014 and July 2016; one patient was excluded due to the presence of a large chylothorax, therefore, 105 patients with LAM were enrolled in the study (Fig. 1). Patients had a mean age of 41 ± 13 years and a median time from diagnosis of 5 years (IQR 1 to 9 years). Eighteen patients (17%) had tuberous sclerosis (Table 1).
Fig. 1.
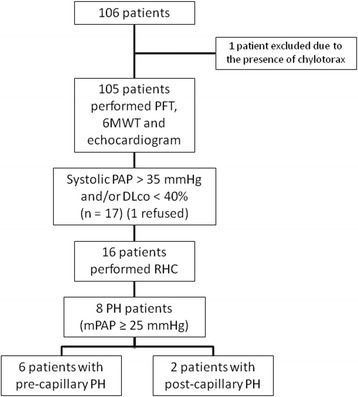
Patient disposition. Definition of abbreviations: 6MWT: six-minute walk test; DLCO: lung diffusing capacity for carbon monoxide; mPAP: mean pulmonary arterial pressure; PAP: pulmonary arterial pressure; PFT: pulmonary function tests; PH: pulmonary hypertension; RHC: right heart catheterisation
Table 1.
Clinical, functional and echocardiographic characteristics (n = 105)
| Clinical variables | |
| Age (years) | 41 ± 13 |
| Time from LAM diagnosis (years, IQR) | 5 (1 – 9) |
| Smoker (former or current) (n, %) | 10 (9.5%) |
| BMI > 35 kg.m−2 (n, %) | 2 (2%) |
| Tuberous sclerosis (n, %) | 18 (17%) |
| Renal angiomyolipoma (n, %) | 48 (45%) |
| Dyspnea (n, %) | 74 (70%) |
| Pneumothorax (n, %) | 48 (46%) |
| Hemoptysis (n, %) | 14 (13%) |
| Use of sirolimus (n, %) | 34 (32%) |
| Duration of use of sirolimus (months) | 27 ± 18 |
| Pulmonary function tests | |
| FEV1 (L; % predicted) | 2.08 ± 0.72 L; 73 ± 24% |
| FVC (L; % predicted) | 3.01 ± 0.73 L; 86 ± 19% |
| FEV1/FVC | 0.68 ± 0.17 |
| RV (L; % predicted) | 138 ± 57% |
| TLC (L; % predicted) | 5.08 ± 1.02 L; 103 ± 18% |
| RV/TLC | 0.43 ± 0.15 |
| DLco (mL/min/mmHg; % predicted) | 16.7 ± 7.1; 68 ± 28% |
| Six-minute walk test | |
| Distance (m; % predicted) | 480 ± 114 m; 82 ± 19% |
| Minimum SpO2 (%) | 90 ± 8 |
| Change in SpO2 (%) | 7 ± 5 |
| Peak HR, beats/min | 115 ± 19 |
| Final Borg dyspnea score (IQR) | 2 (0 – 5) |
| Final Borg leg discomfort score (IQR) | 1 (0 – 3) |
| Transthoracic echocardiography | |
| Estimated systolic PAP (mmHg) | 27 ± 6 |
| Left ventricular ejection fraction (%) | 67 ± 2 |
Values are the mean ± SD, median (interquartile range) or percentage
Definition of abbreviations: BMI body mass index, DL CO lung diffusing capacity for carbon monoxide, FEV 1 forced expiratory volume in the first second, FVC forced vital capacity, HR heart rate, LAM lymphangioleiomyomatosis, PAP pulmonary arterial pressure, RV residual volume, SpO 2 oxygen saturation, TLC total lung capacity
All patients performed PFTs, 6MWT and echocardiography (Table 1). With respect to the PFTs, FEV1 was 2.08 ± 0.72 L (73 ± 24% of predicted value), whereas DLCO was 16.7 ± 7.1 mL/min/mmHg (68 ± 28% of predicted value). Fifty-five patients (52%) presented DLCO below 75%, whereas 14 patients (13%) had DLCO below 40%.
The mean distance walked during the 6MWT was 480 ± 114 m (82 ± 19% of predicted values), whereas the reduction in the SpO2 and the minimum SpO2 were, respectively, 7 ± 5% and 90 ± 8%. The median Borg dyspnea score at the end of the 6MWT was 2 (IQR 0 to 5).
Based on echocardiography results, estimated systolic PAP was 27 ± 6 mmHg and left ventricular ejection fraction was 67 ± 2%. Six (5.7%) patients had estimated systolic PAP over 35 mmHg.
Of the 105 patients included, 16 patients underwent right heart catheterisation based on DLco and/or echocardiography: two patients had only estimated systolic PAP over 35 mmHg, 11 patients had only DLCO below 40%, and three patients presented both abnormalities. One patient with elevated systolic PAP refused to undergo the procedure. Eight patients (7.6%; 95% CI: 4–14%) had PH confirmed during the right heart catheterisation; six patients (5.7%; 95% CI 2.6–11.9%) presented a pre-capillary pattern and 2 patients (1.9%; 95% CI 0.5 - 6.7%) with a post-capillary profile. Nonetheless, only one patient (1%; 95% CI 0.2–5.2%) had a mean PAP over 35 mmHg, with a post-capillary pattern. In five patients (63%) with confirmed PH, the right heart catheterisation was performed based only on DLCO results.
Comparison between PH and non-PH groups
When comparing patients with and without PH, there was no significant difference in terms of age and time from diagnosis. Patients with PH had a higher frequency of use of sirolimus, worse functional impairment, characterised by lower FEV1 and DLCO, and diminished exercise performance, greater oxygen desaturation and higher dyspnea intensity during 6MWT, compared with the non-PH group (Table 2 and Fig. 2).
Table 2.
Clinical, functional and echocardiographic variables, and data obtained from right heart catheterisation: comparison between PH and non-PH groups
| Clinical, functional and echocardiographic variables | PH (n = 8) | Non-PH (n = 97) | P |
| Age (years) | 44 ± 6 | 41 ± 13 | 0.60 |
| Time from LAM diagnosis (years, IQR) | 2 (1.5–11.5) | 5 (1–9) | 0.75 |
| Use of sirolimus (n, %) | 7 (87%) | 27 (28%) | 0.002 |
| Duration of use of sirolimus (months) | 23 ± 7 | 28 ± 20 | 0.57 |
| FEV1 (% predicted) | 33 ± 18 | 76 ± 21 | <0.001 |
| DLCO (% predicted) | 24 ± 14 | 72 ± 26 | < 0.001 |
| 6MWD (m) | 371 ± 113 | 488 ± 110 | 0.01 |
| Minimum SpO2 | 82 ± 6 | 91 ± 8 | 0.01 |
| Final Borg dyspnea score | 5 (5–7) | 2 (0–5) | 0.048 |
| Estimated systolic PAP | 38 ± 7 | 26 ± 5 | < 0.001 |
| Right heart catheterisation | PH (n = 8) | Non-PH (n = 8) | |
| mPAP (mmHg) | 29 ± 5 | 21 ± 2 | <0.001 |
| PAOP (mmHg) | 14 ± 4 | 10 ± 5 | 0.11 |
| CO (L/min) | 4.8 ± 1.2 | 5.0 ± 0.6 | 0.70 |
| PVR (IU) | 3.4 ± 1.2 | 2.3 ± 0.8 | 0.06 |
Values are the mean ± SD or median (interquartile range)
Definition of abbreviations: 6MWD six-minute walk distance, CO cardiac output, DL CO lung diffusing capacity for carbon monoxide, FEV 1 forced expiratory volume in the first second, LAM lymphangioleiomyomatosis, mPAP mean pulmonary arterial pressure, PH pulmonary hypertension, PAOP pulmonary artery occlusion pressure, PAP pulmonary arterial pressure, PVR pulmonary vascular resistance, SpO 2 oxygen saturation
Fig. 2.
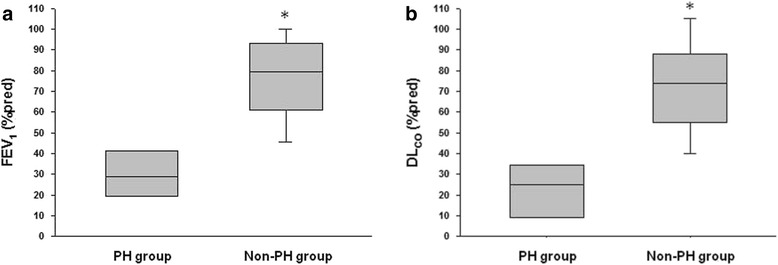
Comparison of FEV1 and DLCO between PH vs. non-PH groups. Definition of abbreviations: DLCO: lung diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in the first second; PH: pulmonary hypertension. *p < 0.001. Box plots show the quartiles (box limits), the 10th and 90th percentiles (error bars) and the median (line)
We also compared data obtained from right heart catheterisation between PH and non-PH groups (Table 2); as expected, mPAP was higher in the PH group but with similar levels of CO.
Discussion
To our knowledge, this is the first study that has evaluated the prevalence of PH in patients with LAM with different severity levels, including patients with normal PFTs, comparing the clinical, functional and echocardiographic characteristics, and variables obtained from right heart catheterisation between those with and without PH. The main findings of this study include the following: 1) The prevalence of PH was low in patients with LAM; 2) PH is of mild severity in LAM and may be pre- or post-capillary; 3) PH was associated with greater impairment of pulmonary function, which suggests that the elevation in PAP is likely associated with the degree of pulmonary parenchymal involvement; 4) Patients with PH had lower exercise performance and greater dyspnea intensity and desaturation during 6MWT; and 5) Reduced DLCO added sensitivity in predicting PH in LAM.
Cottin et al. described the findings of 20 patients with LAM and with pre-capillary PH. However, this previous study was not designed to define the prevalence of PH in LAM, because echocardiography was performed at the discretion of the physicians and the routine was to perform it in those patients with impaired lung function. In addition, as DLCO was not used as an associated tool for screening of PH, the number of patients with confirmed PH may have been underestimated. We performed echocardiography even in patients with normal PFTs, which resulted in a more precise prevalence of PH. Our study, by prospectively evaluating a large cohort of LAM patients, determined a prevalence of pre-capillary PH of 5.7%. It is noteworthy that no patients with pre-capillary PH presented with mean PAP > 35 mmHg, a threshold suggesting PH to be important in the setting of lung diseases. Patients with PH presented with worse pulmonary function and exercise capacity despite preserved CO [11].
Taveira-DaSilva et al. evaluated 95 patients with echocardiography and identified elevation of systolic PAP at rest in less than 10% of patients with LAM, with only mild increase in PAP. The results obtained in this previous study suggested hypoxemia as an important mechanism for the increase in pulmonary vascular resistance in LAM. However, the authors did not perform invasive hemodynamic evaluation to confirm the presence of PH and there was a failure to estimate PAP on echocardiography in approximately 20% of the patients [10]. Our study reinforced that the use of echocardiography as the single tool for the screening of PH in LAM might be of limited sensitivity, underestimating the prevalence of PH, due to the lack of an adequate chest window in a significant proportion of patients, potentially as a consequence of lung hyperinflation. Therefore, we decided to include DLCO as a secondary screening tool to determine the need for invasive hemodynamic evaluation in order to increase the sensitivity of screening. Most patients with confirmed PH during right heart catheterisation were referred to the test only because of the DLCO levels (five out of eight patients, 63%), raising the question about the appropriateness of echocardiography as the main screening tool in patients with parenchymal lung diseases, as suggested in the current guidelines [32].
As there are only few studies of PH in LAM, we chose the methods of screening of PH in our study based on data obtained in studies that evaluated PH in other diseases, such as chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis (IPF), systemic sclerosis, sickle cell disease and schistosomiasis. Echocardiography is a widespread screening method for PH, established in many clinical scenarios, and the presence of estimated systolic PAP > 35 mmHg at echocardiography has mostly acceptable sensitivity for the indication of right heart catheterisation [21, 22, 33–35]. In addition, other studies have shown that low DLCO may predict the presence of PH [26–28]. In patients with IPF the presence of low DLCO added sensitivity to the echocardiographic findings in the screening for PH and is associated with a higher risk of PH [25–27]. Nathan et al. showed that DLCO below 40% of the predicted value had high sensitivity to predict the diagnosis of PH [25]. Raghu et al. recently showed that patients with IPF and PH had lower DLCO than those without PH (39% vs. 44%, respectively, P = 0.002) [27]. In the DETECT study, 466 patients with systemic sclerosis underwent right heart catheterisation to confirm the diagnosis of PH and those with PH had lower DLCO than those without PH [24]. Therefore, based on our findings and in previous studies, we considered that DLCO could be added as a reasonable screening method of PH in patients with parenchymal lung diseases.
In patients with systemic sclerosis, the presence of mild elevations in mPAP is associated with a higher risk of future development of PH [36]; however, the significance of such finding in other clinical scenarios is still unknown. The non-PH group in our study presented mPAP of 21 ± 2 mmHg; close follow-up of our patients that presented this haemodynamic profile will determine the relevance of this finding in predicting the development of PH in LAM patients.
Previous reports of LAM cells involving pulmonary vessels and vascular remodeling could suggest a direct pulmonary vascular involvement as the cause of PH in LAM [11, 12]. However, these previous findings were observed mostly in patients with severe parenchymal lung disease, and our study also showed that PH was associated with a degree of parenchymal involvement in LAM, as in other lung diseases, which explains the higher prevalence of use of sirolimus in those with PH. This suggests that PH associated with LAM should be considered among the other parenchymal lung diseases, as part of the group 3 of the current PH classification, instead of its current position in group 5, which comprises PH-associated diseases with unclear multifactorial mechanisms [9].
Despite our important findings, this study has limitations that should be acknowledged. Although this was a single-reference-centre study, it included all patients followed in our centre, which is a national reference centre receiving patients from all over the country. Therefore, it is reasonable to generalise the results of our study for all patients with LAM in Brazil. Another limitation is the use of echocardiographic and pulmonary function criteria based on other diseases, such as IPF and scleroderma; however, this was the only way to question the sensitivity of echocardiography as a single screening method without submitting all patients to right heart catheterisation, which would not be acceptable or ethical in this setting. In this context, although our strategy of combining DLCO below 40% of the predicted value and an echocardiographic criteria as screening tools has added sensitivity in predicting PH in LAM, we cannot completely exclude the presence of mild PH in those with DLCO equal or greater than 40% of the predicted value, since right heart catheterisation was not performed in all patients.
Conclusions
Our study demonstrated that the prevalence of PH in a large cohort of LAM patients with different levels of severity is low and with mild hemodynamic impairment. Furthermore, PH was associated with more pronounced pulmonary function impairment, suggesting that LAM associated PH should be better considered among the other parenchymal lung diseases associated with the development of PH, which compose group 3 of the current classification of PH. In addition, DLCO significantly improved the identification of PH in LAM patients.
Acknowledgments
Not applicable.
Funding
No financial support for this study.
Availability of data and materials
All available data have been included in this manuscript.
Authors’ contributions
CSGF contributed to the study design, data collection, data analysis, writing and review of the manuscript. BGB contributed to the study design, data analysis, writing and final manuscript review. CJ contributed to the data analysis and review of the manuscript. MSA contributed to the data analysis and writing. JBS contributed to the study with data collection, performing echocardiography and writing. GIH contributed to the data collection, writing and review of the manuscript. RAK contributed to the study design and writing. RS contributed to the study design, writing and final manuscript review. CRRC contributed to the study design, data analysis, writing and final manuscript review. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the local research ethics committee (University of Sao Paulo Medical School) and conducted according to the Declaration of Helsinki Principle. All patients provided written informed consent before enrollment (protocol number 759.676).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- 6MWD
Six-minute walk distance
- 6MWT
Six-minute walk test
- BMI
Body mass index
- CO
Cardiac output
- DLCO
Carbon monoxide diffusion capacity
- FEV1
Forced expiratory volume in the first second
- FVC
Forced vital capacity
- HR
Heart rate
- LAM
Lymphangioleiomyomatosis
- mPAP
Mean pulmonary artery pressure
- PAOP
Pulmonary artery occlusion pressure
- PAP
Pulmonary artery pressure
- PFT
Pulmonary function test
- PH
Pulmonary hypertension
- PVR
Pulmonary vascular resistance
- RHC
Right heart catheterisation
- RV
Residual volume
- SpO2
Oxygen saturation
- TLC
Total lung capacity
References
- 1.Johnson SR, Cordier JF, Lazor R, Review Panel of the ERS LAM task Force et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 2.McCormack FX, Travis WD, Colby TV, et al. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med. 2012;186:1210–2. doi: 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Nicholson AG, WHO Panel et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 4.Ryu JH, Moss J, Beck GJ, NHLBI LAM Registry Group et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–11. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldi BG, Freitas CS, Araujo MS, et al. Clinical course and characterization of lymphangioleiomyomatosis in a Brazilian reference centre. Sarc Vasc Diffuse Lung Dis. 2014;31:129–35. [PubMed] [Google Scholar]
- 6.McCormack FX, Gupta N, Finlay GR, ATS/JRS Committee on Lymphangioleiomyomatosis et al. Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis Diagnosis and Management. Am J Respir Crit Care Med. 2016;194:748–61. doi: 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldi BG, Albuquerque AL, Pimenta SP, et al. Exercise performance and dynamic hyperinflation in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2012;186:341–8. doi: 10.1164/rccm.201203-0372OC. [DOI] [PubMed] [Google Scholar]
- 8.Medeiros P, Jr, Lorenzi-Filho G, Pimenta SP, et al. Sleep desaturation and its relationship to lung function, exercise and quality of life in LAM. Respir Med. 2012;106:420–8. doi: 10.1016/j.rmed.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Taveira-DaSilva AM, Hathaway OM, Sachdev V, et al. Pulmonary artery pressure in lymphangioleiomyomatosis: an echocardiographic study. Chest. 2007;132:1573–8. doi: 10.1378/chest.07-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottin V, Harari S, Humbert M, Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) et al. Pulmonary hypertension in lymphangioleiomyomatosis: characteristics in 20 patients. Eur Respir J. 2012;40:630–40. doi: 10.1183/09031936.00093111. [DOI] [PubMed] [Google Scholar]
- 12.Baldi BG, Pimenta SP, Kawassaki Ade M, et al. Pulmonary arterial involvement leading to alveolar hemorrhage in lymphangioleiomyomatosis. Clinics (Sao Paulo) 2011;66:1301–3. doi: 10.1590/S1807-59322011000700031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krymskaya VP, Snow J, Cesarone G, et al. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J. 2011;25:1922–33. doi: 10.1096/fj.10-175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldi BG, Pereira CA, Rubin AS, et al. Highlights of the Brazilian Thoracic Association guidelines for interstitial lung diseases. J Bras Pneumol. 2012;38:282–91. doi: 10.1590/S1806-37132012000300002. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, ATS/ERS Task Force et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 17.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 18.Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33:397–406. doi: 10.1590/S1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 19.Duarte AA, Pereira CA, Rodrigues SC. Validation of new brazilian predicted values for forced spirometry in caucasians and comparison with predicted values obtained using other reference equations. J Bras Pneumol. 2007;33:527–35. doi: 10.1590/S1806-37132007000500007. [DOI] [PubMed] [Google Scholar]
- 20.Neder JA, Andreoni S, Peres C, et al. Reference values for lung function tests. III. Carbon monoxide diffusing capacity (transfor factor) Braz J Med Biol Res. 1999;32:729–37. doi: 10.1590/S0100-879X1999000600008. [DOI] [PubMed] [Google Scholar]
- 21.Milan A, Magnino C, Veglio F. Echocardiographic indexes for the noninvasive evaluation of pulmonary hemodynamics. J Am Soc Echocardiogr. 2010;23:225–39. doi: 10.1016/j.echo.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013;26:1–14. doi: 10.1016/j.echo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 23.ATS Statement Guidelines for the six minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 24.Coghlan JG, Denton CP, Grunig E, DETECT study group et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73:1340–9. doi: 10.1136/annrheumdis-2013-203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan SD, Shlobin OA, Barnett SD, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2008;102:1305–10. doi: 10.1016/j.rmed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lettieri CJ, Nathan SD, Barnett SD, et al. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–52. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 27.Raghu G, Nathan SD, Behr J, et al. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild-to-moderate restriction. Eur Resp J. 2015;46:1370–7. doi: 10.1183/13993003.01537-2014. [DOI] [PubMed] [Google Scholar]
- 28.McGoon M, Gutterman D, Steen V, American College of Chest Physicians et al. Screening, early detection and diagnosis of pulmonary arterial hypertension. ACCP evidence-based clinical practice guideline. Chest. 2004;126(1 Suppl):14S–34. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 29.Minai OA, Fessler H, Stoller JK, NETT Research Group et al. Clinical characteristics and prediction of pulmonary hypertension in severe emphysema. Respir Med. 2014;108:482–90. doi: 10.1016/j.rmed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Costa EL, Jardim C, Bogossian HB, et al. Acute vasodilator test in pulmonary arterial hypertension: evaluation of two response criteria. Vascul Pharmacol. 2005;43:143–7. doi: 10.1016/j.vph.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Gavilanes F, Alves JL, Jr, Fernandes C, et al. Left ventricular dysfunction in patients with suspected pulmonary arterial hypertension. J Bras Pneumol. 2014;40:609–16. doi: 10.1590/S1806-37132014000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–75. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 33.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery and lung transplantation. Chest. 2005;127:1531–6. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 34.Fonseca GH, Souza R, Salemi VM, et al. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39:112–8. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 35.Lapa M, Dias B, Jardim C, et al. Cardiopulmonary manifestations of hepatosplenic schistosomiasis. Circulation. 2009;119:1518–23. doi: 10.1161/CIRCULATIONAHA.108.803221. [DOI] [PubMed] [Google Scholar]
- 36.Bae S, Saggar R, Bolster MB, et al. Baseline characteristics and follow-up in patients with normal haemodynamics versus borderline mean pulmonary arterial pressure in systemic sclerosis: results from the PHAROS registry. Ann Rheum Dis. 2012;71:1335–42. doi: 10.1136/annrheumdis-2011-200546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data have been included in this manuscript.


