Abstract
Background
Calcific aortic stenosis (CAS) is the most common heart valve disease in the elderly, representing an important economic and social burden in developed countries. Currently, there is no way to predict either the onset or progression of CAS, emphasizing the need to identify useful biomarkers for this condition.
Methods
We performed a multi-proteomic analysis on different kinds of samples from CAS patients and healthy donors: tissue, secretome and plasma. The results were validated in an independent cohort of subjects by immunohistochemistry, western blotting and selected reaction monitoring.
Results
Alpha 1 antichymotrypsin (AACT) abundance was altered in the CAS samples, as confirmed in the validation phase. The significant changes observed in the amounts of this protein strongly suggest that it could be involved in the molecular mechanisms underlying CAS. In addition, our results suggest there is enhanced release of AACT into the extracellular fluids when the disease commences.
Conclusions
The significant increase of AACT in CAS patients suggests it fulfils an important role in the physiopathology of this disease. These results permit us to propose that AACT may serve as a potential marker for the diagnosis of CAS, with considerable clinical value.
Keywords: Calcific aortic stenosis, Multi-proteomic, Alpha 1 antichymotrypsin, Biomarker
Background
Calcific aortic valve stenosis (CAS) is the most prevalent heart valve disease in Europe and North America, and it is currently responsible for most of the aortic valve replacement procedures that are performed [1, 2]. CAS is age-related and given the aging population in occidental countries, its prevalence is expected to increase in the forthcoming decades [3]. The natural history of CAS involves a long clinically silent phase of valve calcification and hardening (valve sclerosis) that generally requires at least a decade before heralding the clinical disease. CAS is characterized by an initial stage that has a pathogenesis similar to atherosclerosis, and an active pathology that is characterized by lipid accumulation, inflammation and calcification [4]. Hemodynamically, CAS involves obstructed blood flow at the level of the aortic valve, with a pressure gradient between the left ventricular (LV) and the ascending aorta. The increased LV pressure enhances myocardial wall stress, which is at first compensated by LV hypertrophy, but that is followed by LV dilation and systolic dysfunction during the later stages of the disease [5].
At present, aortic valve replacement is the only effective treatment for severe symptomatic CAS, whereas in most asymptomatic patients the risk of surgery outweighs that of disease monitoring. Therefore, asymptomatic patients with CAS are managed through regular clinical and echocardiography check-ups, as well as basic education to modify their lifestyle and to provide awareness of cardiac risk factors [6]. Nevertheless, it is necessary to develop markers that can guide clinicians to classify patients at high or low risk of disease progression and subsequently tailor their follow-up frequency, thereby optimizing healthcare resources and reducing costs [7]. The possibility of predicting the development of CAS in patients would be tremendously useful.
Here, we have adopted an omics strategy to perform an unbiased, non-targeted study of CAS. Our data show the importance of the alpha 1 antichymotrypsin (AACT) protein in CAS, for the first time describing its potential as a future diagnostic marker of this disease. This protein is associated with the acute-phase of the disease, placing the focus on inflammatory processes in relation to the mechanisms underlying CAS. Moreover, the potential predictive value of AACT was validated through a selected reaction monitoring (SRM) analysis in plasma samples, which could facilitate its future implementation in clinical practice (Fig. 1).
Fig. 1.
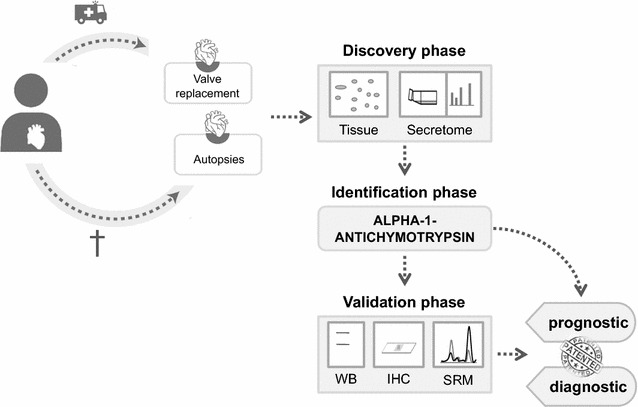
Schematic representation of the workflow. Samples were collected from valve replacement surgeries (AS) and autopsies (controls). During the discovery phase the whole tissue and secretome were analyzed and AACT was identified. Finally, AACT was validated as a potential biomarker in WBs, and by IHC and SRM
Methods
Patient and control samples
Stenotic heart valves (n = 20) were obtained from severe symptomatic CAS patients (55% male, 45% female), with an average age of 74 (SD = 4.0) years, who underwent aortic valve replacement. All patients had hypertension, 50% suffered hyperlipidemia and 60% diabetes mellitus. Patients with a medical history of significant aortic regurgitation, rheumatic fever or morphological valve alterations consistent with rheumatic heart disease, we’re excluded from the study (Table 1). The valves were classified according to a macroscopic inspection performed blind by cardiovascular surgeons. Normal valves (n = 20) from the autopsies of subjects who had died due to non-cardiovascular diseases were obtained within 4 h of death and used as controls (Table 2). In all the cases, the structure of both the stenotic and non-stenotic valves was 3-cuspid.
Table 1.
Clinical characteristics of AS patients
| Patient number | Age/gender | AHT | Diabetes | Dyslipidemia |
|---|---|---|---|---|
| 1 | 74/F | Yes | No | Yes |
| 2 | 73/M | Yes | Yes | No |
| 3 | 68/M | Yes | No | Yes |
| 4 | 81/M | Yes | No | Yes |
| 5 | 69/M | Yes | Yes | Yes |
| 6 | 79/F | Yes | Yes | No |
| 7 | 73/F | Yes | Yes | Yes |
| 8 | 77/M | Yes | No | No |
| 9 | 79/F | Yes | Yes | No |
| 10 | 75/M | Yes | No | Yes |
| 11 | 74/M | Yes | Yes | Yes |
| 12 | 74/F | Yes | Yes | No |
| 13 | 79/F | Yes | Yes | No |
| 14 | 79/F | Yes | Yes | No |
| 15 | 72/F | Yes | No | No |
| 16 | 63/M | Yes | No | Yes |
| 17 | 72//M | Yes | No | Yes |
| 18 | 75/F | Yes | Yes | Yes |
| 19 | 78/M | Yes | Yes | No |
| 20 | 74/M | Yes | Yes | No |
| Mean | 74 ± 0/45% F–55% M | 100.00% | 60.00% | 50.00% |
F female, M male, AHT arterial hypertension
Table 2.
Clinical characteristics of control subjects
| Control number | Age/gender | Cause of the death | AHT | Diabetes | Dyslipidemia |
|---|---|---|---|---|---|
| 1 | 64/M | Bronchopneumonia | No | No | No |
| 2 | 51/F | Septic shock | Yes | No | No |
| 3 | 47/H | Sepsis | No | No | No |
| 4 | 47/F | Bacterial pneumonia | No | No | No |
| 5 | 47/F | Carcinogenesis | No | No | No |
| 6 | 34/M | Acute Pancreatitis | Yes | No | No |
| 7 | 64/F | Bacterial Sepsis | No | No | No |
| 8 | 78/F | Bronchopneumonia | No | No | No |
| 9 | 42/F | Lymphoma | No | No | No |
| 10 | 67/M | Renal insufficiency | No | No | No |
| 11 | 74/M | COPD | No | No | No |
| 12 | 87/F | Carcinoma | No | No | No |
| 13 | 48/F | Pulmonary thromboembolism | No | No | No |
| 14 | 55/M | Pneumonia | No | No | No |
| 15 | 87/F | Cholelithiasis | No | No | No |
| 16 | 76/M | Carcinoma | No | No | No |
| 17 | 80/F | Atrial fibrillation | Yes | Yes | No |
| 18 | 49/F | Respiratory insufficiency | No | No | No |
| 19 | 74/M | Septic shock | Yes | No | Yes |
| 20 | 77/F | COPD | Yes | No | No |
| Mean | 69 ± 7.07/40% M–60% F | 25.00% | 5% | 5% |
F female, M male, AHT arterial hypertension, COPD chronic obstructive pulmonary disease
For the SRM validation analysis, peripheral blood samples were collected from CAS patients (n = 8) and control subjects (n = 8) selected to avoid significant differences between the groups in terms of age (p = 0.6), gender (p = 0.32), obesity (p = 0.5), hypertension (p = 0.5), dyslipidemia (p = 0.5) and diabetes (p = 0.7: Table 3). This study was carried out in accordance with the recommendations of the Helsinki Declaration and it was approved by the ethics committee at the Hospital “Virgen de la Salud” (Toledo, Spain). Signed informed consent was obtained from all the subjects or their relatives (in case of necropsies) prior to their inclusion in the study.
Table 3.
Clinical characteristics of the subjects used in the validation phase
| Subject number | Age/gender | AHT | Diabetes | Dyslipidemia |
|---|---|---|---|---|
| 1 Control | 62/M | Yes | No | Yes |
| 2 Control | 82/M | Yes | No | No |
| 3 Control | 86/M | Yes | No | Yes |
| 4 Control | 96/M | No | Yes | Yes |
| 5 Control | 78/F | Yes | Yes | No |
| 6 Control | 81/M | Yes | No | No |
| 7 Control | 40/F | No | No | No |
| 8 Control | 30/M | No | No | No |
| 9 AS | 77/F | Yes | Yes | No |
| 10 AS | 86/M | Yes | No | No |
| 11 AS | 69/F | Yes | No | No |
| 12 AS | 77/M | No | No | No |
| 13 AS | 60/F | Yes | No | No |
| 14 AS | 75/F | Yes | No | Yes |
| 15 AS | 74/M | No | No | Yes |
| 16 AS | 74/F | Yes | Yes | No |
| Mean control | 75% M | 62.75% | 25% | 37.75% |
| Mean AS | 37.5% M | 75 | 25% | 25% |
F female, M male, AHT arterial hypertension
Tissue sample preparation
Aortic valves were preserved and transported in PBS, and processed within a maximum of 2 h after extraction. The valves were washed immediately in PBS to reduce blood contaminants. For 2D-DIGE and western blotting, one aortic valve leaflet was ground into a powder in liquid N2 in a mortar. Protein extracts were then prepared from the valve as described previously [8, 9] and the total protein concentration was measured by the Bradford-Lowry method (Bio-Rad protein assay) [10].
For secretome analysis, the second valve leaaflet was cultured as described elsewhere [11, 12] using medium supplemented with antibiotics and amphotericin B (Fungizone®) to avoid contamination. Samples were transferred to a Petri dish (Cell Star®), cut into pieces and incubated at 37 °C in lysine-arginine free 1640 RPMI medium (Cell Culture Technologies Invitrus) supplemented with 5 mg/ml fungizone, 250 mg/ml amikacin, 2 mg/ml l-lysine 2HCl (U-13C6, 97–99%) and 10 mg/ml l-Arginine HCl (U-13C6, 97–98%: Cambridge Isotope Laboratories Inc., Andover, MA) in a humidified atmosphere of 5% CO2. The valves were cultured for 96 h and the medium collected was stored at −80 °C until analysis. Finally, the valves were fixed in formalin at 4 °C, decalcified in Shandon-TBD1 (Thermo Scientific) and embedded in OCT for subsequent immunohistochemistry.
Plasma sample preparation
Blood samples were collected in tubes containing EDTA and centrifuged at 1125×g for 15 min. The resulting supernatant was frozen immediately at −80 °C until analysis.
2D-DIGE separation, image acquisition and analysis
Tissue proteins were labeled according to the manufacturer´s instructions (GE Healthcare), and as described by Gil-Dones and Martin-Rojas [8, 9]. In all experiments an internal standard was added containing equal amounts of each protein extract. The internal standard and protein extracts from one stenotic and one control valve were combined and run on a single gel (150 μg of total protein). The proteins were separated by isoelectric focusing in the first dimension and the strips were then equilibrated via two consecutive incubations in SDS-equilibration buffer (1.5 M Tris∙HCl [pH 8.8], 6 M Urea, 87% Glycerol and 2% SDS) plus dithiothreitol and iodoacetamide, respectively. Subsequently, the proteins were separated on 12% Acrylamide/Bisacrylamide gels using an EttanDalt Six device (GE Healthcare), which were then scanned using a Typhoon 9400 fluorescence gel scanner (GE Healthcare). Relative protein quantification of Aortic Stenosic (AS) and healthy valves was performed with DeCyder software v6.5 (GE Healthcare) and with the multivariate statistical module EDA (Extended data analysis). Only proteins with >1.5-fold differences in abundance were considered significant. A statistical analysis was then carried out to determine the changes in protein expression, with p values below 0.05 accepted as significant when the Student’s t test was applied.
Protein identification by MALDI-TOF/TOF
2D-DIGE gels were silver stained to visualize the protein spots, and all differentially expressed protein spots were then manually excised and identified at the Hospital Nacional de Paraplejicos’ Proteomics Unit. The proteins were automatically digested in an “Ettan Digester” (GE Healthcare) according to Shevchenko et al. [13], with minor modifications. An aliquot of each digestion was mixed with an aliquot of the matrix solution and this mixture was pipetted directly onto the stainless steel sample plate of the mass spectrometer.
The MALDI-MS/MS data were obtained in an automated analysis loop using a 4800 Plus MALDI TOF/TOF Analyzer (Applied Biosystems). The spectra were acquired and the mass data were analysed automatically with the 4000 Series Explorer Software version 3.5.3 (Applied Biosystems). MALDI-MS and MS/MS data were combined through the GPS Explorer Software (Version 3.6) to search a non-redundant protein database (Swissprot 56.5) using the Mascot software (version 2.2: Matrix Science) [14].
LC–MS/MS identification
Labeled peptide samples from secretome experiments were analyzed by LC–MS/MS using a C-18 reversed phase nano-column (75 µm I.D. × 50 cm, 2 µm particle size, nanoEASY 100 C18: Thermo Fisher Scientific) and a continuous acetonitrile gradient of: 0–30% B in 420 min, 30–43% A in 5 min, and 43–90% B in 1 min (where: A = 0.5% formic acid; B = 90% acetonitrile, 0.5% formic acid). A flow rate of 200 nL/min was used to elute the peptides from the reverse phase nano-column to an emitter nanospray needle for real time ionization and peptide fragmentation in a QExactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific).
Western blotting
Protein extracts from CAS and control valves were resolved by 12% SDS-PAGE using a Bio-Rad Miniprotean II electrophoresis unit run for 1 h at a constant current of 25 mA/gel. After SDS-PAGE, the proteins were transferred to a nitrocellulose membrane under a constant voltage of 15 V for 20 min. Ponceau S staining was performed to guarantee that equal amounts of aortic valve proteins were loaded onto the IPG strips (2-D western blotting) or onto polyacrylamide gels (regular western blotting). Subsequently, the membranes were blocked for 1 h and incubated overnight with the primary antibody at a concentration of 1 µg/ml (Abcam, Ref. ab9374) in PBS-Tween20 + 5% non-fat dry milk. After rinsing, the membranes were incubated with the specific HRP-conjugated secondary antibody in PBS-Tween20 + 5% non-fat dry milk, which were detected by enhanced chemiluminescence (ECL: GE Healthcare) following the manufacturers’ instructions. Protein band intensity was measured on a GS-800 Calibrated Densitometer (Bio-Rad).
Immunohistochemistry
Tissues were embedded in OCT and cryosections (6 µm) were obtained for immunohistochemical analyses. The sections were blocked with 10% BSA in PBS with 0.1% Tween 20 and incubated overnight at 4 °C with the primary antibody (10 µg/ml: Abcam, ab9374). The sections were then incubated with an IgG-HRP-conjugated secondary antibody (NORDIC Immunology) and the chromogenic reaction was developed using 3,3′-diaminobenzidine. Sections were counterstained with hematoxylin prior to dehydration and coverslipping. As a negative control, the complete immunohistochemical procedure was performed on adjacent sections without adding the primary antibody. To quantify the DAB stained area, an orthonormal transformation of the RGB images was applied using an ImageJ plugin based on Ruifrok and Johnston’s method for color deconvolution.
Selected reaction monitoring
Prior to mass spectrometry (MS) analysis in the LC–MS/MS system, crude plasma samples were reduced, digested and cleaned on Pep-Clean spin columns (Pierce) according to the manufacturer’s instructions. LC–MS/MS was performed on a TEMPO nano LC system (Applied Biosystems) combined with a nano LC Autosampler coupled to a modified triple quadrupole (4000 QTRAP LC/MS/MS, Applied Biosystems). Theoretical SRM transitions were designed using MRMpilot software v1.1 (ABSciex), and the MIDAS acquisition method and Skyline were used for the analysis, including the theoretical transitions.
Statistical analysis
Statistical analyses were performed using SPSS 15.0 software for Windows (SPSS Inc.). A Kolmogorov–Smirnov test was applied to evaluate the normal distribution of the continuous variables in the population analyzed. When a normal distribution was demonstrated, a comparison of the means was performed using a t-Student test. For populations with no normal distribution, the non-parametric Mann–Whitney U test was used. Discrete variables, such as sex or the presence/absence of risk factors, were compared using Fisher’s exact test. For all tests, statistical significance was accepted when p < 0.05.
Results
To identify proteins of interest in CAS, two complementary proteomic approaches were applied to two different types of CAS samples (tissue and secretome), the in-gel separation of proteins 2D-DIGE MALDI-MS/MS and liquid-chromatography separation of peptides (LC–MS/MS). The results obtained were validated in an independent cohort of plasma samples to support their translation to a clinical setting. In tissue 2D-DIGE analysis, we identified 3 spots in the CAS tissue that corresponded to AACT (Fig. 2). In all cases, the MASCOT score of these spots was higher than 70 and they were about twofold more intense in stenotic valves (Table 4).
Fig. 2.
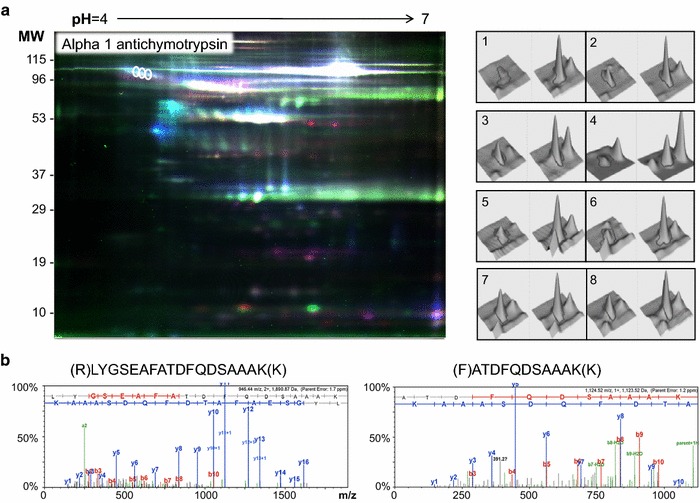
2D-DIGE results. a Representative fluorescence DIGE image, showing the differentially expressed spots corresponding to AACT. On the right, the spot intensity in 3D of one of the three spots corresponding to AACT is shown. The differences between controls and patients were consistent in the 8 gels studied. b Fragmentation spectra of the proteotypic peptides used for AACT identification by MALDI-MS/MS. The fragments of the y ion series are shown in blue, while the b ion series are shown in red
Table 4.
List of the alpha 1 antichymotrypsin isoforms identified in the 2D-DIGE analysis of AS patients versus controls
| Protein name | Accesion number | MASCOT score | Seq coverage | Match peptides | Expect score | Ratio CAS/C | Theo. pI | Theo. MW | Exp. pI | Exp. MW |
|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1-antichymotrypsin | P01011 | 394 | 23 | 13 | 1.6e−034 | 1.96 | 5.33 | 47.62 | 4.7 | 95 |
| 174 | 37 | 16 | 1.6e−012 | 1.94 | 5.33 | 47.62 | 4.65 | 104 | ||
| 77 | 23 | 7 | 0.0074 | 2.00 | 5.33 | 47.62 | 4.6 | 105 |
The Uniprot accession number is shown, along with the MASCOT score, sequence coverage, number of matched peptides, expect score, variation between the patients and the control groups (ratio AS/C), theoretical isoelectric point (pI) and molecular weight (MW), and experimental isoelectric point (pI) and molecular weight (MW) in kDa
We also performed a secretome analysis using l-lysine 2HCl (U-13C6, 97–99%) and 10 mg/ml l-Arginine HCl (U-13C6, 97–98%). AACT was one of the secreted proteins that incorporated the labeled amino acids in the AS group alone. AACT was analyzed using the SecretomeP software [15] and it was predicted to be a secreted protein. Its signal peptide indicated secretion by the classical pathway and in the absence of the signal peptide, it had an NN-score >0.5, indicative of non-classical secreted proteins. As such, it seems likely that AACT is secreted by the classical pathway.
We validated these results in an independent cohort of control and CAS subjects by immunohistochemistry, in western blots and by SRM. AACT accumulated most strongly in the tissue from the CAS patients (Fig. 3), primarily in the endothelium on the aortic side and in the fibrosa (controls mean = 5.41 ± 1.43; CAS mean = 14.35 ± 2.951: p = 0.023). As indicated, the proteomic results were also validated in 2-D western blots using three different CAS samples: tissue, plasma and secretome (Fig. 4). The relative intensity of this protein was 1.5 higher in CAS tissue than in the control samples and we also found a significant increase in the plasma from CAS patients (mean = 18.00 ± 1.75) relative to the controls (mean = 4.82 ± 0.63: p = 0.042). Similar results were obtained when analyzing the secretome (controls mean = 0.41 ± 0.01; CAS mean = 1.48 ± 0.42: p < 0.001).
Fig. 3.
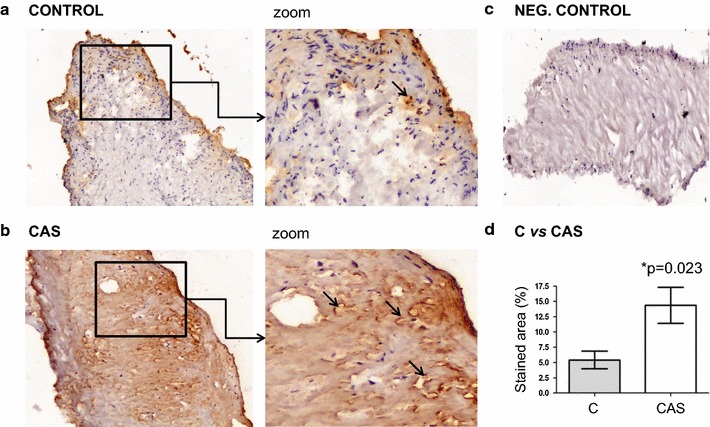
Validation of the AACT protein using IHC. Stronger expression of AACT is observed in the endothelium on the aortic side and, to a greater extent in the fibrosa of the stenotic valves when compared to the controls. DAB staining is brown and is indicated by the arrows. Statistical analyses showed significant differences (*p = 0.023). Amplified images at ×100 magnification
Fig. 4.
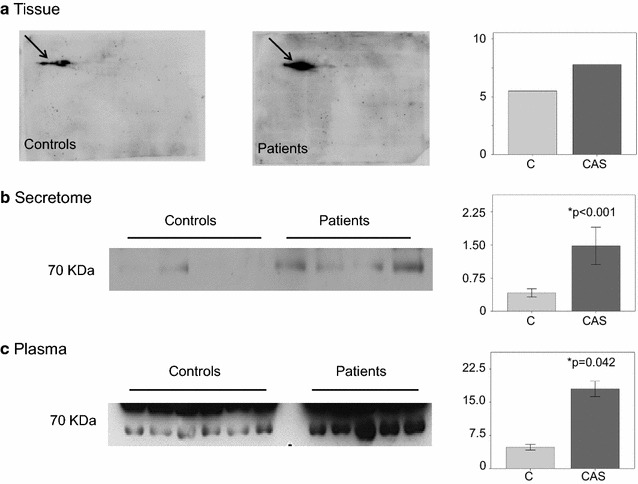
Validation of the AACT protein in Western Blots. a Bidimensional immunodetection analysis of tissue samples in which differences in the expression of the isoforms is indicated by arrows. b Immunodetection of the secretome samples. The band was more intense in the patient group than in the controls. c Immunodetection of the plasma samples. Again, the band in the plasma from the patients was more intense than in the controls. Quantification by densitometry is also shown in the figure and the AACT levels are clearly higher in the CAS patients in all cases
In terms of the validation of the SRM secretome samples, two different peptides and three transitions of each of these were measured (Fig. 5). The expression of both was significantly higher in the CAS patients than in the controls, and significant differences between the two study groups were also evident in the plasma samples (Table 5).
Fig. 5.
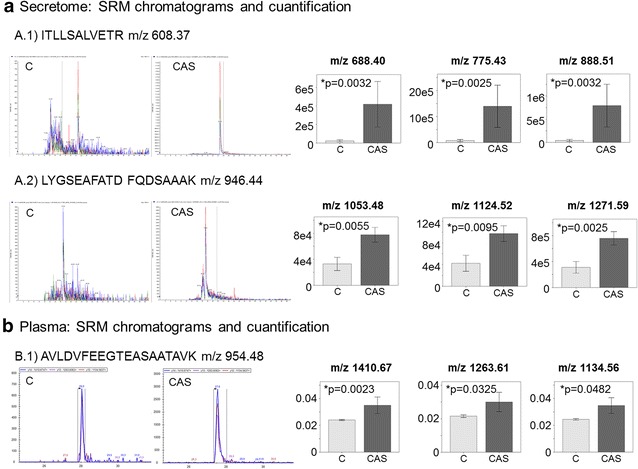
SRM validation of the differences in AACT found using LC-MS/MS. a Chromatograms of secretome samples showing the transitions of the 2 proteotypic peptides: ITLLSALVETR (A.1) and LYGSEAFATDFQDSAAAK (A.2). As it is shown in the figure, the m/z values selected in the first quadrupole were 608.37 and 946.44, respectively. On the right, quantification of the three transitions (area under the curve) that were monitored for each peptide. The different m/z values selected in the third quadrupole are indicated in the figure. b Chromatograms of plasma samples showing the transitions of the proteotypic peptide AVLDVFEEGTEASAATAVK (m/z 954.48) and quantification of each transition (area under the curve) in plasma samples. The different m/z values selected in the third quadrupole are indicated in the figure
Table 5.
Statistical analysis of the SRM
| Peptide | T | Mean ± SEM | p value | |
|---|---|---|---|---|
| 1 | ITLLSALVETR | T1 | Control: 22,938 ± 11,790; CAS: 418,327 ± 245,046 | 0.0032 |
| T2 | Control: 77,287 ± 41,645; CAS: 1,395,651 ± 811,858 | 0.0025 | ||
| T3 | Control: 42,469 ± 22,863; CAS: 792,453 ± 460,476 | 0.0032 | ||
| 2 | LYGSEAFATDFQDSAAAK | T1 | Control: 32,992 ± 10,156; CAS: 77,827 ± 11,396 | 0.0055 |
| T2 | Control: 42,505 ± 14,734; CAS: 97,228 ± 14,444 | 0.0095 | ||
| T3 | Control: 31,132 ± 8909; CAS: 75,803 ± 10,252 | 0.0025 | ||
| 3 | AVLDVFEEGTEASAATAVK | T1 | Control: 0.024 ± 0.001; CAS: 0.035 ± 0.006 | 0.0023 |
| T2 | Control: 0.022 ± 0.001; CAS: 0.030 ± 0.006 | 0.0325 | ||
| T3 | Control: 0.024 ± 0.001; CAS: 0.035 ± 0.006 | 0.0482 |
The peptide sequence is shown, along with the selected transitions (T) and the statistical significance (p value)
Discussion
Aortic stenosis is a progressive disease with aortic valve sclerosis as the first manifestation, which ultimately leads to valve dysfunction [4]. Over the last few years, great efforts have been made to understand the physiological and pathological mechanisms implicated in CAS. Calcification of the native aortic cups in this disease is a complex process, which appears to be related to inflammation and ossification [16]. Nevertheless, the mechanisms underlying degenerative heart valve disease still remains largely unclear, especially when compared with our understanding of the mechanisms underlying other cardiovascular diseases like heart failure and atherosclerotic disease. Due to the high social costs associated with CAS healthcare, there is a strong unmet clinical need to find markers that aid early diagnosis, estimate the long-term prognosis, monitor treatment response and predict potential adverse effects in CAS patients. By using multi-omics approaches, we hoped to define markers that may potentially serve to diagnose the disease earlier than traditional methods, such as echocardiography [11]. Novel tools for easier diagnosis will reduce the high morbidity and mortality associated to surgery, and they may provide a solution for those patients who cannot be operated on, as well as improving patient management.
The use of different proteomic techniques on distinct samples allowed us to identify AACT as a potential diagnostic marker for CAS. This was validated in an independent cohort of subjects using orthogonal techniques to analyze tissue, the secretome and plasma samples. Taking into account these results, AACT detection and its quantification could define AS, and as we believe that AACT may be a potential diagnostic marker of CAS, which have assessed its patentability (IP2145.5).
Although the exact role of AACT in CAS is unknown, it has been postulated to have several effects on the cardiovascular system. AACT is a serine protease inhibitor affecting acute phase proteins, and it induces tumor necrosis factor (TNF)-α and NF-κB expression [17], which both have anti-inflammatory properties. Additionally, AACT is thought to afford protection during ischemia reperfusion by inhibiting neutrophil accumulation into the ischemic-reperfused myocardium and by inactivating cytotoxic metabolites released from neutrophils [18]. This protein is a member of the serpin family [19] and it is also known as serpin 3. Although it is mainly synthesized in the liver [19–23], we have also found it expressed in aortic valves. It is well known that the levels of acute-phase proteins in plasma are elevated in response to inflammation and rupture [24, 25]. Thus, endothelial damage could explain the over expression of AACT in CAS, as well as the inflammation, which seems to be involved in the development of the disease [26–29].
Protein changes observed in tissue are important since they reveal the specific molecular processes taking place in the damaged organ. Nevertheless, valve tissue is not very accessible to clinicians and hence, it is significant that the increase in AACT is also evident in the secretome of CAS samples, as well as in the plasma of patients. The analysis of the secretome and plasma showed that this protein is released directly from the stenotic valve in response to the development of CAS, such that higher levels of ACCT imply further CAS development, which implies vascular surgery.
While, potential biomarkers found in plasma are less specific than those found in tissues, we have demonstrated the valvular origin of the increase in AACT in this biological fluid. This sample is easily accessible to clinicians, as extraction requires non-invasive methods and it is routinely used in clinical analysis. Besides, current methods such as echocardiography are time-consuming and expensive when compared with plasma analysis. Moreover, high qualified staff are needed to interpret images due to their complexity and thus it would be easier to measure AACT in a clinical setting in order to diagnose CAS. It is now important to perform a study with a larger number of patients with different grades of injury in order to confirm that AACT levels increase with the severity of the disease as a physiological response to the processes that take place in the stenotic aortic valve. This would allow us to establish ranges of protein concentrations in an attempt to predict which patients may develop a severe form of CAS, making it possible to apply more personalized treatment. In addition a prospective study would allow us to confirm the prognostic value of this protein.
Conclusions
Alpha-1-antichymotrypsin seems to be an important indicator for clinicians regarding the progression of CAS. These data are important considering the aging of the population where cardiovascular diseases, in this case CAS, are becoming an increasing economic and social burden in Western countries. Future studies with plasma samples taken at different time points and taken from patients with distinct disease progression will be necessary to analyze the potential role of AACT as a prognostic and diagnostic marker of CAS in more depth.
Authors’ contributions
TM and LM performed the 2D-DIGE and secretome studies, as well as the statistical analysis of the data obtained with these approaches. FG and JL performed the SRM analysis. FC and CL performed the validation in western blots and the statistical analysis. The selection of the controls from autopsies was performed by ER and MR, while the selection, study and follow-up of patients was carried out by LL in collaboration with FA. Finally, LP and MB designed and supervised the complete study. All authors read and approved the final manuscript.
Acknowledgements
We thank the CNIC (Centro Nacional Investigaciones Cardiovasculares) for assistance with the protein identification.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was carried out in accordance with the recommendations of the Helsinki Declaration and it was approved by the ethics committee at the Hospital “Virgen de la Salud” (Toledo, Spain). Signed informed consent was obtained from all subjects or their relatives (in the case of necropsies) prior to their inclusion in the study.
Funding
This work was supported by Grants from the Instituto de Salud Carlos III (FIS PI07/0537, PI11/02239, PI14/01917) and Redes Temáticas de Investigación Cooperativa (FONDOS FEDER, RD06/0014/1015, RD12/0042/0071). These results contribute to the Spanish initiative on the Human Proteome Project (SpHPP).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tatiana Martin-Rojas, Email: tatiana_martin_rojas@hotmail.com.
Laura Mourino-Alvarez, Email: lmourino@hotmail.com.
Felix Gil-Dones, Email: gildones@yahoo.es.
Fernando de la Cuesta, Email: ferdelacuesta@hotmail.com.
Esther Rosello-Lleti, Email: esther_rosello_lleti@hotmail.com.
Carlos M. Laborde, Email: laborde70@hotmail.com
Miguel Rivera, Email: miguelrivera492@gmail.com.
Luis Fernando Lopez-Almodovar, Email: lopezalmodovar@yahoo.es.
Juan Antonio Lopez, Email: jalopez@cnic.es.
Finn Akerstrom, Email: finnakerstrom@gmail.com.
Luis R. Padial, Email: lrpadial@secardiologia.es
Maria G. Barderas, Email: megonzalezb@sescam.jccm.es
References
- 1.Rajamannan NM, Gersh B, Bonow RO. Calcific aortic stenosis: from bench to the bedside–emerging clinical and cellular concepts. Heart. 2003;89:801–805. doi: 10.1136/heart.89.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/S0195-668X(03)00201-X. [DOI] [PubMed] [Google Scholar]
- 3.Rajamannan NM. Calcific aortic stenosis: a disease ready for prime time. Circulation. 2006;114:2007–2009. doi: 10.1161/CIRCULATIONAHA.106.657759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141–2151. doi: 10.1016/j.jacc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Akerström F, Barderas MG, Rodríguez-Padial L. Aortic stenosis: a general overview of clinical, pathophysiological and therapeutic aspects. Expert Rev Cardiovasc Ther. 2013;11:239–250. doi: 10.1586/erc.12.171. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, III, Guyton RA, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 7.Mourino-Alvarez L, Iloro I, de la Cuesta F, Azkargorta M, Sastre-Oliva T, Escobes I, et al. MALDI-imaging mass spectrometry: a step forward in the anatomopathological characterization of stenotic aortic valve tissue. Sci Rep. 2016;6:27106. doi: 10.1038/srep27106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil-Dones F, Martin-Rojas T, Lopez-Almodovar L, De la Cuesta F, Darde V, Alvarez-Llamas G, et al. Vascular aortic stenosis: a proteomic insight. Clin Med Insights Cardiol. 2010;4:1–7. doi: 10.4137/cmc.s3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Rojas T, Gil-Dones F, Lopez-Almodovar LF, Padial LR, Vivanco F, Barderas MG. Proteomic profile of human aortic stenosis: insights into the degenerative process. J Proteome Res. 2012;11:1537–1550. doi: 10.1021/pr2005692. [DOI] [PubMed] [Google Scholar]
- 10.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; May 7. [DOI] [PubMed]
- 11.Alvarez-Llamas G, Martin-Rojas T, de la Cuesta F, Calvo E, Gil-Dones F, Darde VM, et al. Modification of the secretion pattern of proteases, inflammatory mediators, and extracellular matrix proteins by human aortic valve is key in severe aortic stenosis. Mol Cell Proteomics. 2013;12:2426–2439. doi: 10.1074/mcp.M113.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez-Llamas G, Szalowska E, de Vries MP, Weening D, Landman K, Hoek A, et al. Characterization of the human visceral adipose tissue secretome. Mol Cell Proteomics. 2007;6:589–600. doi: 10.1074/mcp.M600265-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 14.Perkins DN, Pappin DJC, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Bendtsen JD, Jensen LJ, Blom N, von Heijne G, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng Des Sel. 2004;17:349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- 16.Rabuş MB, Kayalar N, Sareyyüpoğlu B, Erkin A, Kırali K, Yakut C. Hypercholesterolemia association with aortic stenosis of various etiologies. J Card Surg. 2009;24:146–150. doi: 10.1111/j.1540-8191.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 17.Braghin E, Galimberti D, Scarpini E, Bresolin N, Baron P. Alpha1-antichymotrypsin induces TNF-alpha production and NF-kappaB activation in the murine N9 microglial cell line. Neurosci Lett. 2009;467:40–42. doi: 10.1016/j.neulet.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 18.Murohara T, Guo JP, Lefer AM. Cardioprotection by a novel recombinant serine protease inhibitor in myocardial ischemia and reperfusion injury. J Pharmacol Exp Ther. 1995;274:1246–1253. [PubMed] [Google Scholar]
- 19.Travis J, Salvesen G. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 20.Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- 21.Brunetti ND, Padalino R, Gennaro L, Cuculo A, Ziccardi L, Pellegrino PL, et al. Acute phase proteins activation in subjects with coronary atherosclerosis and micro-vessel coronary circulation impairment. J Thromb Thrombolysis. 2008;28:50–56. doi: 10.1007/s11239-008-0248-4. [DOI] [PubMed] [Google Scholar]
- 22.Kang S-M, Chung N, Kim J-Y, Koo B-K, Choi D, Jang Y, et al. Relation of vasodilator response of the brachial artery to inflammatory markers in patients with coronary artery disease. Echocardiography. 2002;19:661–667. doi: 10.1046/j.1540-8175.2002.00661.x. [DOI] [PubMed] [Google Scholar]
- 23.Correale M, Brunetti ND, De Gennaro L, Di Biase M. Acute phase proteins in atherosclerosis (acute coronary syndrome) Cardiovasc Hematol Agents Med Chem. 2008;6:272–277. doi: 10.2174/187152508785909537. [DOI] [PubMed] [Google Scholar]
- 24.Abdullah NM, Kachman M, Walker A, Hawley AE, Wrobleski SK, Myers DD, et al. Microparticle surface proteins are associated with experimental venous thrombosis: a preliminary study. Clin Appl Thromb Hemost. 2008;15:201–208. doi: 10.1177/1076029608326753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Janciauskiene S. Multi-functional capability of proteins: alpha1-antichymotrypsin and the correlation with Alzheimer’s disease. J Alzheimers Dis. 2002;4:115–122. doi: 10.3233/JAD-2002-4206. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.ATV.16.4.523. [DOI] [PubMed] [Google Scholar]
- 27.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–1222. doi: 10.1161/01.ATV.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 28.Wallby L, Janerot-Sjöberg B, Steffensen T, Broqvist M. T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 2002;88:348–351. doi: 10.1136/heart.88.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren B, Yong J. Calcification of the aortic valve: its progression and grading. Pathology. 1997;29:360–368. doi: 10.1080/00313029700169315. [DOI] [PubMed] [Google Scholar]


