Abstract
Background
The aim of this study was to investigate the prevalence and prognostic significance of psychological distress in gastric cancer patients.
Methods
The study population included 229 gastric cancer patients visiting Yonsei Cancer Center between November 2009 and March 2011. The distress was measured by available tools including the Modified Distress Thermometer (MDT), Hospital Anxiety and Depression Scale (HADS), and Center for Epidemiologic Studies–Depression Scale (CES-D). Patients with psychological distress were defined as those who scored above the cut-off values in both the MDT and either one of the HADS or CES-D.
Results
The median age of patients was 56 (range, 20 to 86) and 97 (42.4%) patients were with stage IV disease status at enrollment. The overall prevalence of psychological distress was 33.6% (95% CI: 27.5–39.8%) in 229 gastric cancer patients. In multiple logistic regression analysis, lower education level (odds ratio [OR] 2.39; 95% confidence interval [CI] 1.11–5.17, P = 0.026) and higher disease stage (OR 2.72; 95% CI 1.47–5.03, P = 0.001) were associated with psychological distress. In stage I-III disease, patients with psychological distress had worse disease-free survival (DFS) (5-year DFS rate: 60% vs 76%, P = 0.49) compared with those without psychological distress. In stage IV disease (n = 97), patients with psychological distress showed poorer overall survival than those without psychological distress (median OS (Overall Survival): 12.2 vs. 13.8 months, P = 0.019).
Conclusion
Psychological distress is common in patients with all stages of gastric cancer and is associated with worse outcomes.
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-017-3260-2) contains supplementary material, which is available to authorized users.
Keywords: Psychological distress, Gastric cancer, Prognosis, Survival
Background
Cancer diagnosis and treatment is a significantly stressful event that generates psychological distress in a large number of cancer patients. Psychological distress is generally defined as a state of emotional suffering characterized by symptoms of depression and anxiety [1]. Approximately 20–40% of cancer patients show a significant level of psychological distress [2, 3]. Distress can exert a negative impact on their treatment adherence, quality of life (QOL), pain, and even on survival [4–8]. Recently, in most solid cancers including gastric cancer, the multidisciplinary approach is becoming more important for the decision of cancer treatment strategy and psychosocial support is one of the essential parts of the multidisciplinary approach [9, 10].
Gastric cancer is the second leading cause of cancer deaths in the world [11]. Most patients with gastric cancer have advanced to an incurable stage at the time of diagnosis, which induces tremendous psychological stress. Even if the patient is diagnosed with early-stage gastric cancer, they suffer from not only the diagnosis of cancer but also surgery itself [12]. Therefore, the importance of psychological distress in gastric cancer patients for the decision of treatment will continue to grow.
Few studies have reported on the prevalence or the nature of psychological distress in gastric cancer patients. Tavoli et al. reported that overall 47.2% and 57% of patients with gastrointestinal cancer scored high on both anxiety and depression respectively [13]. We previously reported the prevalence and associated factors of psychological distress in all types of cancer patients and found that 28.3% of gastric cancer patients had psychological distress [14]. Further, there is no report of the survival impact of psychological distress in gastric cancer patients.
The aim of this study was to evaluate the prevalence and prognostic impact of psychological distress in gastric cancer patients.
Methods
Study population
Patients were eligible for study participation if they met the following criteria: 1) histologically confirmed gastric adenocarcinoma; 2) age of >20 years; 3) Eastern Cooperative Oncology Group (ECOG) performance status 0–3; 4) the ability to read and understand the questionnaire; 5) the ability to communicate in written and spoken language; and 6) willing and able to provide written informed consent.
Patients with operable gastric cancer underwent surgery and then received adjuvant chemotherapy according to the final pathologic stage. Stage IV gastric cancer patients received a standard treatment of palliative systemic chemotherapy with or without palliative gastrectomy.
Patients with gastric cancer visiting Yonsei Cancer Center, Severance Hospital in Seoul, Korea between November 2009 and March 2011 were included. Patients were enrolled at the first visit to the medical oncology department. During the study period, 298 gastric cancer patients agreed to complete questionnaires for screening distress. Among them, 249 (83.5%) patients completed all questionnaires. We excluded 20 patients in the final analysis due to several reasons (such as neoadjuvant chemotherapy, follow-up loss, treatment refusal, etc.) Finally, we analyzed the data of 229 gastric cancer patients (Fig. 1).
Fig. 1.
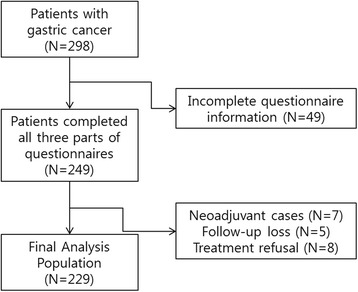
Study Population
Study procedure
An Oncology Certified Nurse (OCN) explained the purpose and procedure of the distress screening program to gastric cancer patients in the oncology outpatient clinic. Patients who signed informed consent forms completed questionnaires containing the Modified Distress Thermometer (MDT), Hospital Anxiety and Depression Scale (HADS), and Center for Epidemiologic Studies-Depression Scale (CES-D), and questions about socio-demographic and clinical status. Cancer-related information including cancer stage, disease-free survival, overall survival, and adjuvant chemotherapy were collected in electronic medical records. The patients who were identified as harboring psychological distress were referred to a psychiatrist for further evaluation and treatment for psychological distress by a medical oncologist. An independent psychiatric doctor reviewed the data without any clinical information and informed the oncologist about the status of psychological distress.
Measure of psychological distress
The following three self-administered questionnaires were used to evaluate the psychological distress of enrolled patients.
The MDT is an easily used screening tool to measure the severity of psychological symptoms including anxiety, insomnia, and depression, and the degree of functional impairment due to these symptoms. It contains three subscales: MDT-anxiety, MDT-insomnia, and MDT-depression. Patients are required to circle the number that corresponds to their severity of distress and the degree of functional impairment on an 11-point visual analogue scale ranging from 0 to 10 by referring to the previous week. More than 4 points in both the severity and impairment scales in each distress symptom indicates that it is necessary to refer to a psychiatrist [15].
The HADS is widely used to measure anxiety and depression in patients with medical illness. It is considered as an effective screening tool to evaluate psychological distress in cancer patients [16, 17]. It consists of two subscales (HADS-A and HADS-D) that evaluate anxiety and depression, respectively. We used the Korean-validated version of the HADS for this study. Scores for each subscale range from 0 to 21 and a cut-off score of 8 was used, which had been previously reported to show good sensitivity and specificity (89.2 and 82.5%, respectively) in a Korean population [18]. Patients who scored above the cut-off score in either the HADS-A or HADS-D scale were defined as one with psychological distress based on the HADS.
The CES-D is a 20-item tool used to evaluate depressive symptoms in the general population. The Korean version of the CES-D was used in this study and we defined a CES-D score of 21 as the cut-off score, which was reported to be the threshold for the purpose of estimating the prevalence of depressive symptoms in Korean patients [19].
In this study, patients with psychological distress were defined as those who scored above the cut-off value in both MDT scales and either one of the HADS or CES-D scales.
Statistical analysis
In order to compare psychological distress with regard to socio-demographic and clinical characteristics, chi-square tests were conducted for categorical and nominal variables, and independent samples t-test and analyses of variance (ANOVA) were performed for continuous variables. Standard univariate descriptive statistics were used to calculate the prevalence of distress. Logistic regression analysis was conducted to evaluate the factors that show the strongest association with psychological distress. Univariate and multivariate Cox proportional hazards models were used to determine the effect of independent predictors on survival times. Disease-free survival (DFS) was defined as the time from the date of surgery to the disease recurrence or death from any other causes. All statistical analysis was conducted using SPSS, software version 23.0 (SPSS Inc.).
Results
Patient characteristics
Table 1 shows the socio-demographic and clinical data of the evaluable 229 patients. The median age of the patients was 56 years (range: 20–86), and 167/229 (73%) were male. Most patients were married (196/229 [85.6%]), and more than half of the participants were high school educated or higher (178/229 [77.8%]) and unemployed (121/229 [52.8%]). Patients with metastatic or recurrent disease (stage IV) at enrollment were 42.4%. Most of the patients were non-smokers (79.9%) and physically active (ECOG 0 or 1: 94.7%). Among stage I-III disease patients (n = 132), 83 patients received adjuvant chemotherapy after surgery. The proportion of patients who received adjuvant chemotherapy in stage I, II, and III was 2.9%, 81.8%, and 83.1%, respectively. The most commonly used chemotherapy regimen was platinum-based doublet, for 67.5% with an adjuvant aim and 73.1% with a palliative aim. Of 97 patients with recurrent or metastatic disease at enrollment, more than half of the patients (53.6%) had peritoneal metastases at diagnosis and 84.5% received palliative chemotherapy in the first-line setting. Fifteen patients with recurrent or metastatic disease underwent palliative surgery for several purposes such as clinical trials (n = 4), good responder to palliative chemotherapy (n = 8), and palliation of symptoms (n = 3).
Table 1.
Baseline characteristics
| N = 229 | % | |
|---|---|---|
| Age | ||
| Median | 56 | |
| Range | 20–86 | |
| Gender | ||
| Male | 167 | 72.9 |
| Female | 62 | 27.1 |
| ECOG Performance | ||
| 0 | 121 | 52.8 |
| 1 | 96 | 41.9 |
| 2–3 | 12 | 5.2 |
| Smoking | ||
| Smoker | 46 | 20.1 |
| Non-Smoker | 183 | 79.9 |
| Marital status | ||
| Married | 196 | 85.6 |
| Single | 17 | 7.4 |
| Widowed | 12 | 5.2 |
| Divorced | 4 | 1.7 |
| Educational level | ||
| Elementary school | 24 | 10.5 |
| Middle school | 27 | 11.8 |
| High school | 86 | 37.6 |
| Undergraduate | 74 | 32.3 |
| Graduate school | 18 | 7.9 |
| Employment status | ||
| Full-time job | 82 | 35.8 |
| Part-time job | 26 | 11.4 |
| Unemployed | 82 | 35.8 |
| Housewife/Student | 39 | 17 |
| Histology | ||
| Tubular adenocarcinoma | 161 | 70.3 |
| Signet ring cell carcinoma | 58 | 25.3 |
| Mucinous carcinoma | 5 | 2.2 |
| Others | 5 | 2.2 |
| Adjuvant chemotherapy | ||
| Platinum-based doublet (SP or FP) | 56/83 | 67.5 |
| TS-1 monotherapy | 22/83 | 26.5 |
| Others | 5/83 | 6 |
| Initial metastasis site | ||
| Peritoneum | 52/97 | 53.6 |
| Distant LN | 41/97 | 42.3 |
| Liver | 28/97 | 28.9 |
| Bone | 12/97 | 12.4 |
| Lung | 9/97 | 9.3 |
| Brain | 2/97 | 2.1 |
| Others | 19/97 | 19.6 |
| Palliative chemotherapy | ||
| No | 15/97 | 15.5 |
| Yes | 82/97 | 84.5 |
| Palliative chemotherapy regimen | ||
| Platinum-based doublet (SP or FP) | 60/82 | 73.2 |
| Taxane-based regimen | 12/82 | 14.6 |
| Triplet (DCF) | 2/82 | 2.4 |
| Others | 8/82 | 9.7 |
| AJCC stage at enrollment | ||
| 1 | 34 | 14.8 |
| 2 | 33 | 14.4 |
| 3 | 65 | 28.4 |
| 4 | 97 | 42.4 |
Prevalence of psychological distress in gastric cancer patients
The results of distress screening through the questionnaires are shown in Table 2. Among the 229 patients, 77 (33.6%) were identified as patients with psychological distress. Using the MDT, 50 patients reported insomnia (21.8%), 69 anxiety (30.1%), or 68 depression (29.7%). The number of patients who scored above the cutoff value in HADS-A, HADS-D, and CES-D was 62 (27.1%), 92 (40.2%), and 76 (33.2%), respectively. Concordance between the parameters are displayed in Fig. 2. Among 77 patients with psychological distress, 61% had positive results for all three methods. Patients who are in the shaded area in Fig. 2c were defined as patients with psychological distress. The prevalence of psychiatric illness diagnosed by the psychiatrist is shown in Additional file 1: Table S1.
Table 2.
Prevalence of psychological distress by disease stage
| All Patients | Stage I-III | Stage IV | |||||
|---|---|---|---|---|---|---|---|
| N = 229 | % | N = 132 | % | N = 97 | % | P-value | |
| MDT | 93 | 40.6 | 46 | 34.8 | 47 | 48.5 | 0.038 |
| Insomnia | 50 | 21.8 | 28 | 21.2 | 22 | 22.7 | 0.79 |
| Anxiety | 69 | 30.1 | 30 | 22.7 | 39 | 40.2 | 0.004 |
| Depression | 68 | 29.7 | 31 | 23.5 | 37 | 38.1 | 0.016 |
| HADS | 106 | 46.3 | 52 | 39.4 | 54 | 55.7 | 0.015 |
| HADS-A | 62 | 27.1 | 29 | 22 | 33 | 34 | 0.043 |
| HADS-D | 92 | 40.2 | 45 | 34.1 | 47 | 48.5 | 0.028 |
| CES-D | 76 | 33.2 | 38 | 28.8 | 38 | 39.2 | 0.099 |
| Psychological distress | 77 | 33.6 | 35 | 26.5 | 42 | 43.3 | 0.008 |
MDT Modified Distress Thermometer, HADS Hospital Anxiety and Depression Scale, CES-D Center for Epidemiologic Studies-Depression Scale
Fig. 2.
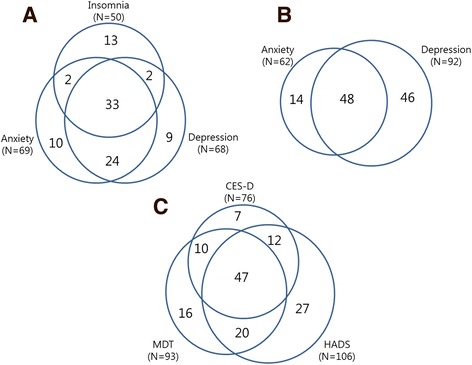
Concordance rate between distress measure parameters (a) MDT (b) HADS (c). All three distress parameters; Shaded area are the patient who was defined as psychological distress in this study
Risk factors of psychological distress
Table 3 describes the comparison of socio-demographic and clinical factors between patients with and without psychological distress. Patients with psychological distress were significantly higher in females (P = 0.024), the unemployed (P = 0.02), those with lower educational background (P = 0.021), and those at an advanced stage (P = 0.008). The logistic regression analysis showed that education, disease stage, and smoking status maintained a statistically significant association with psychological distress (Additional file 1: Table S2). The patients with low education levels were 2.39 times (95% CI, 1.11–5.17, P = 0.026) more likely to have psychological distress than those with high education levels. The patients with stage IV incurable disease stage were 2.72 times (95% CI, 1.47–5.03, P = 0.001) more likely to have psychological distress than those with a curable disease stage.
Table 3.
Comparison of socio-demographic characteristics between the patients with psychological distress and those without psychological distress
| Patients without psychological distress | Patients with psychological distress | ||||
|---|---|---|---|---|---|
| N = 152 | % | N = 77 | % | P-value | |
| Age | 0.289 | ||||
| Mean (SD) | 57 (13) | 55 (12.7) | |||
| Gender | 0.024 | ||||
| Male | 118 | 77.6 | 49 | 63.6 | |
| Female | 34 | 22.4 | 28 | 36.4 | |
| ECOG Performance | 0.055 | ||||
| 0 | 82 | 53.9 | 39 | 50.6 | |
| 1 | 66 | 43.4 | 30 | 39 | |
| 2–3 | 4 | 2.6 | 8 | 10.4 | |
| Smoking | 0.056 | ||||
| Smoker | 116 | 76.3 | 67 | 87 | |
| Non-Smoker | 36 | 23.7 | 10 | 13 | |
| Marital status | 0.247 | ||||
| Married | 133 | 87.5 | 63 | 81.8 | |
| Unmarried | 19 | 12.5 | 14 | 18.2 | |
| Educational status | 0.021 | ||||
| ≤ Middle school | 27 | 17.8 | 24 | 31.2 | |
| ≥ High school | 125 | 82.2 | 53 | 68.8 | |
| Employment status | 0.02 | ||||
| Employed | 80 | 52.6 | 28 | 36.4 | |
| Unemployed | 72 | 47.4 | 9 | 63.6 | |
| Disease stage at enrollment | 0.008 | ||||
| 1–3 | 97 | 63.8 | 35 | 45.5 | |
| 4 | 55 | 36.2 | 42 | 54.5 | |
| Disease status at enrollment | 0.072 | ||||
| Post-op status | 64 | 42.1 | 23 | 29.9 | |
| Pre-op or metastatic | 88 | 57.9 | 54 | 70.1 | |
SD Standard Deviation, ECOG Eastern Cooperative Oncology Group
Survival analysis
The median follow-up duration of the entire cohort was 42.5 months. We analyzed survival data divided into two subsets (curable disease, stage I-III vs. incurable disease, stage IV). DFS by TNM (Tumor/Node/Metastasis) sub-stage are shown in Additional file 1: Figure S1. In stage I-III disease, patients who have psychological distress had worse disease-free survival (5-year DFS rate: 60% vs 76%, P = 0.49, Fig. 3a). In stage IV disease, patients with psychological distress had worse OS than those without psychological distress (median OS: 12.2 vs. 13.8 months, P = 0.019, Fig. 3b).
Fig. 3.
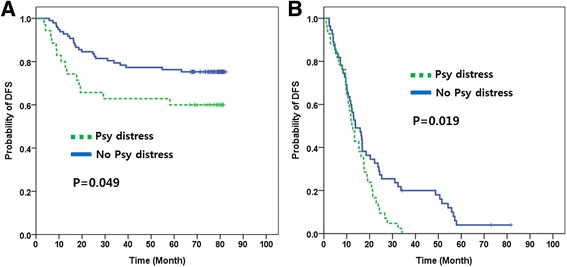
(a) DFS in Stage I-III disease and (b) OS in Stage IV disease by psychological distress
The Cox multivariate analysis model including age, gender, ECOG, adjuvant chemotherapy, marriage, education, employment, and psychological distress in stage III disease showed that adjuvant chemotherapy (Hazzard ratio [HR] 7.23, 95% CI 2.27–22.95, P < 0.001) and psychological distress (HR 2.47, 95% CI 1.07–5.68, P = 0.034) were associated with shorter disease free survival (Table 4).
Table 4.
Disease-free survival multivariate analysis in Stage III Disease
| Multivariate analysis | ||||
|---|---|---|---|---|
| Factor | N | Hazzard ratio | 95% CI | p-value |
| Gender | ||||
| Male | 39 | 1 | ||
| Female | 26 | 2.05 | 0.85–4.91 | 0.108 |
| Age | ||||
| < 65 | 32 | 1 | ||
| ≥ 65 | 33 | 1.34 | 0.47–3.79 | 0.585 |
| ECOG Performance | ||||
| 0 | 28 | 1 | ||
| > 1 | 37 | 1.27 | 0.54–2.97 | 0.584 |
| Adjuvant chemotherapy | ||||
| Yes | 54 | 1 | ||
| No | 11 | 7.23 | 2.27–22.95 | <0.001 |
| Psychological distress | ||||
| No | 45 | 1 | ||
| Yes | 20 | 2.47 | 1.07–5.68 | 0.034 |
| Marital status | ||||
| Married | 54 | 1 | ||
| Unmarried | 11 | 1.562 | 0.53–4.59 | 0.417 |
| Educational status | ||||
| ≤ Middle school | 18 | 1 | ||
| ≥ High school | 47 | 0.49 | 0.21–1.39 | 0.154 |
| Employment status | ||||
| Employed | 29 | 1 | ||
| Unemployed | 36 | 1.98 | 0.72–5.45 | 0.187 |
Discussion
Psychological support is an important part of the multidisciplinary approach, but there is no study that specifically evaluated the psychological distress in gastric cancer, which is the most common cancer in Korea. To our knowledge, this is the first study to explore the prevalence and prognostic impact of psychological distress among a large number of patients with gastric cancer. In our study cohort of gastric cancer patients, significant psychological distress was identified in 33.6% of patients. In addition, we found that psychological distress has a poor prognostic impact for gastric cancer patients.
The presence of psychological distress is a risk factor for treatment noncompliance. A meta-analysis showed that noncompliance was greater in patients with depression compared to non-depressed patients [20]. Therefore, it is important to identify the patients who may be vulnerable to psychological distress to improve treatment adherence. We found that the patients with advanced disease, low levels of education, and who were female were found to be significantly vulnerable to psychological distress. These findings are comparable to previous studies [21–24]. Several studies reported a higher prevalence of psychological distress in patients with lower education. Lower coping skills seem to contribute to the higher rate of psychological distress in those with little education [24].
Kuchler et al. reported that patients with gastrointestinal cancer including stomach, pancreatic, liver, or colorectal cancer who received a formal program of psychotherapeutic support during their hospital stay showed a better survival than those who did not [25]. In this study population, there were few recurrence cases in stage I or II disease; therefore, we performed a multivariate survival analysis in stage III disease. Although not statistically significant, patients who had psychological distress were less likely to receive adjuvant chemotherapy than those who did not. Treatment non-compliance related with adjuvant therapy could be one of the reasons for the poor survival in patients with psychological distress.
There are many screening tools with variable formats and lengths for evaluating psychological distress. However, it is not clear which screening method is appropriate for cancer patients. The Distress Thermometer (DT) is widely used due to its simplicity, but it is generally poor accuracy was pointed as a limitation [26]. To compensate this weakness, we added two other scales for the evaluation of psychological distress. In this study, we also have to consider the balance between minimizing the burden on patients and maximizing validity of data. We selected three short-length screening tools—MDT, HADS, and CES-D—that were validated by several studies [27]. We also previously reported the sensitivity and specificity of these tools in Korean cancer patients [14]. However, considering that 49 (16.5%) patients did not answer the questions completely, filling out all three questionnaires was clearly some burden to cancer patients. Further studies are warranted to develop efficient tools reflecting the distinct characteristics of Korean culture.
There are several limitations in our study. First, the timing of surveillance was not consistent in all populations. Some patients filled out the questionnaires after hearing the prognosis of their disease, and others did when not knowing their prognosis or treatment plan. Second, we could not follow the development of psychological distress over the trajectory of cancer because of our cross-sectional design. Third, these data were obtained by self-report, and it is possible that patients may have under- or overestimated their status. Fourth, systemic chemotherapy for metastatic gastric cancer was not standardized.
Despite these limitations, this study has important strengths. First, our study demonstrates that approximately one-third of gastric cancer patients have significant psychological distress, especially in low-educated patients with advanced stage disease. The patients with psychological distress showed poor survival outcomes that may be related with treatment non-compliance.
Conclusions
Psychological distress is common in patients with all stages of gastric cancer and is associated with worse outcomes. From these results, we conclude that we need to pay attention to the psychological status of gastric cancer patients. Ultimately, further research is needed to investigate whether psychotherapeutic interventions would decrease the distress and improve survival outcomes in gastric cancer patients.
Acknowledgements
None.
Funding
This research was supported by the Public welfare & Safety research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010–0020841). The role of funding was the study design and data collection.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Authors’ contributions
SYR and KRK designed the study concept. SKS collected the questionnaire results. GMK and SJK analyzed and interpreted the patient data. HRK, BDK, SHN, and HCC contributed to patient enrollment. GMK was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Institutional Review Board (IRB) approval was obtained at Severance Hospital. (4–2009-0312). Written informed consent was obtained from the study subjects.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ANOVA
Analyses of variance
- CES-D
Center for Epidemiologic Studies–Depression Scale
- CI
Confidence interval
- DFS
Disease-free survival
- DT
Distress Thermometer
- ECOG
Eastern Cooperative Oncology Group
- HADS
Hospital Anxiety and Depression Scale
- HR
Hazzard ratio
- MDT
Modified Distress Thermometer
- OCN
Oncology Certified Nurse
- OR
Odds ratio
- OS
Overall Survival
- QOL
Quality of life
- TNM
Tumor/Node/Metastasis
Additional file
Supplementary figure and tables. Disease free survival differences by TNM sub-stage, psychiatric illness by age group. (DOC 109 kb)
Contributor Information
Gun Min Kim, Email: gmkim77@yuhs.ac.
Seung Jun Kim, Email: nujeless@kyuh.ac.kr.
Su Kyung Song, Email: she11@yuhs.ac.
Hye Ryun Kim, Email: nobelg@yuhs.ac.
Beo Deul Kang, Email: wb0707@snubh.org.
Sung Hoon Noh, Email: sunghoonn@yuhs.ac.
Hyun Cheol Chung, Email: unchung8@yuhs.ac.
Kyung Ran Kim, Email: drgreat@yuhs.ac.
Sun Young Rha, Phone: +82-2-2228-8050, Email: rha7655@yuhs.ac.
References
- 1.Mirowsky J, Ross CE. Measurement for a human science. J Health Soc Behav. 2002;43(2):152–170. doi: 10.2307/3090194. [DOI] [PubMed] [Google Scholar]
- 2.Derogatis LR, Morrow GR, Fetting J, Penman D, Piasetsky S, Schmale AM, Henrichs M, Carnicke CL., Jr The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751–757. doi: 10.1001/jama.1983.03330300035030. [DOI] [PubMed] [Google Scholar]
- 3.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::AID-PON501>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel D, Sands S, Koopman C. Pain and depression in patients with cancer. Cancer. 1994;74(9):2570–2578. doi: 10.1002/1097-0142(19941101)74:9<2570::AID-CNCR2820740927>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356(9238):1326–1327. doi: 10.1016/S0140-6736(00)02821-X. [DOI] [PubMed] [Google Scholar]
- 6.Skarstein J, Aass N, Fossa SD, Skovlund E, Dahl AA. Anxiety and depression in cancer patients: relation between the hospital anxiety and depression scale and the European Organization for Research and Treatment of cancer Core quality of life questionnaire. J Psychosom Res. 2000;49(1):27–34. doi: 10.1016/S0022-3999(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 7.Prieto JM, Atala J, Blanch J, Carreras E, Rovira M, Cirera E, Espinal A, Gasto C. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol. 2005;23(25):6063–6071. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 8.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5(8):466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, Drysdale E, Hundleby M, Chochinov HM, Navarro M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345(24):1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 10.Carlsen K, Jensen AB, Jacobsen E, Krasnik M, Johansen C. Psychosocial aspects of lung cancer. Lung Cancer. 2005;47(3):293–300. doi: 10.1016/j.lungcan.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56(1):1–9. doi: 10.1016/S0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita T, Matsushima E, Maruyama M. Anxiety and depression of patients with digestive cancer. Psychiatry Clin Neurosci. 2005;59(5):576–583. doi: 10.1111/j.1440-1819.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 13.Tavoli A, Mohagheghi MA, Montazeri A, Roshan R, Tavoli Z, Omidvari S. Anxiety and depression in patients with gastrointestinal cancer: does knowledge of cancer diagnosis matter? BMC Gastroenterol. 2007;7:28. doi: 10.1186/1471-230X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SJ, Rha SY, Song SK, Namkoong K, Chung HC, Yoon SH, Kim GM, Kim KR. Prevalence and associated factors of psychological distress among Korean cancer patients. Gen Hosp Psychiatry. 2011;33(3):246–252. doi: 10.1016/j.genhosppsych.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Center . Recommendations for distress Management in Cancer Patients. 2008. [Google Scholar]
- 16.Moorey S, Greer S, Watson M, Gorman C, Rowden L, Tunmore R, Robertson B, Bliss J. The factor structure and factor stability of the hospital anxiety and depression scale in patients with cancer. Br J Psychiatry. 1991;158:255–259. doi: 10.1192/bjp.158.2.255. [DOI] [PubMed] [Google Scholar]
- 17.Ibbotson T, Maguire P, Selby P, Priestman T, Wallace L. Screening for anxiety and depression in cancer patients: the effects of disease and treatment. Eur J Cancer. 1994;30A(1):37–40. doi: 10.1016/S0959-8049(05)80015-2. [DOI] [PubMed] [Google Scholar]
- 18.Oh SM, Min KJ, Park DB. A study on the standardization of the hospital anxiety and depression scale for Koreans — a comparison of normal, depressed and anxious groups. J Korean Neuropsychiatr Assoc. 1999;38:289–296. [Google Scholar]
- 19.Cho MJ, Kim KH. The diagnostic validity of the CES-D (Korean version) in the assessment of DSM-III-R major depression. J Korean Neuropsychiatr Assoc. 1993;32:381–399. [Google Scholar]
- 20.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 21.Shim EJ, Shin YW, Jeon HJ, Hahm BJ. Distress and its correlates in Korean cancer patients: pilot use of the distress thermometer and the problem list. Psychooncology. 2008;17(6):548–555. doi: 10.1002/pon.1275. [DOI] [PubMed] [Google Scholar]
- 22.Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55(2):215–224. doi: 10.1016/j.lungcan.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen PB, Donovan KA, Trask PC, Fleishman SB, Zabora J, Baker F, Holland JC. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494–1502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- 24.Dalgard OS, Mykletun A, Rognerud M, Johansen R, Zahl PH. Education, sense of mastery and mental health: results from a nation wide health monitoring study in Norway. BMC Psychiatry. 2007;7:20. doi: 10.1186/1471-244X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchler T, Bestmann B, Rappat S, Henne-Bruns D, Wood-Dauphinee S. Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-year survival results of a randomized trial. J Clin Oncol. 2007;25(19):2702–2708. doi: 10.1200/JCO.2006.08.2883. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell AJ. Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders. J Clin Oncol. 2007;25(29):4670–4681. doi: 10.1200/JCO.2006.10.0438. [DOI] [PubMed] [Google Scholar]
- 27.Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101(21):1464–1488. doi: 10.1093/jnci/djp336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.


