Abstract
IMPORTANCE
Women with refractory urgency urinary incontinence are treated with sacral neuromodulation and onabotulinumtoxinA with limited comparative information.
OBJECTIVE
To assess whether onabotulinumtoxinA is superior to sacral neuromodulation in controlling refractory episodes of urgency urinary incontinence.
DESIGN, SETTING, AND PARTICIPANTS
Multicenter open-label randomized trial (February 2012–January 2015) at 9 US medical centers involving 381 women with refractory urgency urinary incontinence.
INTERVENTIONS
Cystoscopic intradetrusor injection of 200 U of onabotulinumtoxinA (n = 192) or sacral neuromodulation (n = 189).
MAIN OUTCOMES AND MEASURES
Primary outcome, change from baseline mean number of daily urgency urinary incontinence episodes over 6 months, was measured with monthly 3-day diaries. Secondary outcomes included change from baseline in urinary symptom scores in the Overactive Bladder Questionnaire Short Form (SF); range, 0–100, higher scores indicating worse symptoms; Overactive Bladder Satisfaction questionnaire; range, 0–100; includes 5 subscales, higher scores indicating better satisfaction; and adverse events.
RESULTS
Of the 364 women (mean [SD] age, 63.0 [11.6] years) in the intention-to-treat population, 190 women in the onabotulinumtoxinA group had a greater reduction in 6-month mean number of episodes of urgency incontinence per day than did the 174 in the sacral neuromodulation group (−3.9 vs −3.3 episodes per day; mean difference, 0.63; 95% CI, 0.13 to 1.14; P = .01). Participants treated with onabotulinumtoxinA showed greater improvement in the Overactive Bladder Questionnaire SF for symptom bother (−46.7 vs −38.6; mean difference, 8.1; 95% CI, 3.0 to 13.3; P = .002); treatment satisfaction (67.7 vs 59.8; mean difference, 7.8; 95% CI, 1.6 to 14.1; P = .01) and treatment endorsement (78.1 vs 67.6; mean difference; 10.4, 95% CI, 4.3 to 16.5; P < .001) than treatment with sacral neuromodulation. There were no differences in convenience (67.6 vs 70.2; mean difference, −2.5; 95% CI, −8.1 to 3.0; P = .36), adverse effects (88.4 vs 85.1; mean difference, 3.3; 95% CI, −1.9 to 8.5; P = .22), and treatment preference (92.% vs 89%; risk difference, −3%; 95% CI, −16% to 10%; P = .49). Urinary tract infections were more frequent in the onabotulinumtoxinA group (35% vs 11%; risk difference, −23%; 95% CI, −33% to −13%; P < .001). The need for self-catheterization was 8% and 2% at 1 and 6 months in the onabotulinumtoxinA group. Neuromodulation device revisions and removals occurred in 3%.
CONCLUSIONS AND RELEVANCE
Among women with refractory urgency urinary incontinence, treatment with onabotulinumtoxinA compared with sacral neuromodulation resulted in a small daily improvement in episodes that although statistically significant is of uncertain clinical importance. In addition, it resulted in a higher risk of urinary tract infections and need for transient self-catheterizations.
Urgency urinary incontinence is a sudden need to void resulting in uncontrollable urine loss. The prevalence of this disruptive condition is common and increases with age, from 17% of women older than 45 years to 27% older than 75 years in the United States.1 The US economic burden was projected to be $82.6 billion by 2020.2 When primary and secondary interventions such as pelvic floor muscle training, fluid restriction, and medication therapy do not result in adequate symptom relief, women with refractory urgency urinary incontinence are offered sacral neuromodulation, posterior tibial nerve stimulation, or onabotulinumtoxinA. A recent systematic review of these therapies found insufficient evidence to guide the choice between sacral neuromodulation and onabotulinumtoxinA.1
The Refractory Overactive Bladder: Sacral Neuromodulation vs Botulinum Toxin Assessment (ROSETTA) trial used a comparative effectiveness design to assess whether onabotulinumtoxinA is superior to sacral neuromodulation in controlling episodes of urgency incontinence in women with refractory symptoms.
Methods
Study Design and Procedures
Recruitment for this open-label randomized trial was conducted between February 2012 and January 2015 at 9 sites participating in the National Institutes of Health (NIH) – sponsored Pelvic Floor Disorders Network. Details of the study design and methods have been previously published.3 The protocol and statistical analysis plan are included in Supplement 1 and Supplement 2. The institutional review board of each clinical site and the coordinating center approved the protocol. All participants provided written informed consent. Race/ethnicity was ascertained by self-report, using fixed categories to describe the population per NIH guidelines. A data and safety monitoring board reviewed the progress, interim results, and safety of the study. A senior statistician verified data validity and adherence to the protocol.
Study eligibility required women to have refractory urgency urinary incontinence, defined as persistent symptoms despite at least 1 supervised behavioral or physical therapy intervention and the use of a minimum of 2 anticholinergics (or inability to tolerate or contraindications to the medication). To be included, women needed a minimum of 6 urgency incontinence episodes on a baseline 3-day diary. Exclusion criteria were relevant neurologic diseases, history of using either of the study interventions, or a postvoid residual of more than 150 mL (eTable 1 in Supplement 3). Participants were stratified by age (<65 years vs ≥65 years) and randomly assigned 1:1 in permuted blocks of size 2 or 4, with all participants other than a single data coordinating center statistician masked to block sequence either to undergo sacral neuromodulation (InterStim, Medtronic) or to receive 200 U of onabotulinumtoxinA (Botox A, Allergan). Surgeons had to have performed at least 10 InterStim procedures, be routinely performing the procedure in their practice, and were required to review an instructional video demonstrating optimal techniques and detailing placement of the lead in a standardized manner. Physicians administering onabotulinumtoxinA injections must have previously performed at least 10 injections and were required to view an instructional video detailing a standardized technique.
Participants randomized to sacral neuromodulation underwent a first-stage lead placement in the operating suite under local and monitored anesthesia care. Each electrode was assessed intraoperatively for both sensory and motor responses and criteria for the number of electrodes with intraoperative response and level of voltage intensity was set across sites. During the 7- to 14-day testing phase, participants were able to change programs to optimize treatment effect. Those participants with 50% or more reduction in mean episodes of urgency incontinence on a 3-day bladder diary on the same program were a priori defined as clinical responders and were eligible for the neurostimulator implant. A reduction of more than 50% in episodes from baseline is the threshold used in clinical practice to proceed with neurostimulator implants based on US Food and Drug Administration recommendations. Those without this improvement underwent lead removal. Those found to have a technical problem with the lead were allowed a second attempt at lead placement.
Participants randomized to onabotulinumtoxinA received a cystoscopic intradetrusor injection of 200 U performed in clinic. Women with a reduction of 50% or more in mean urgency incontinence episodes recorded in a 3-day bladder diary 1 month after injection were a priori defined as clinical responders. After injection, participants were followed up for urinary retention. Those with a postvoid residual of more than 300 mL or more than 200 mL and symptoms of incomplete voiding were instructed to perform clean intermittent catheterization after treatment.
Clinical responders were instructed not to receive additional urgency incontinence treatment (eg, medications, physical therapy, additional onabotulinumtoxinA) for the first 6 months after the intervention. Participants with sacral neuromodulation were allowed neurostimulator reprogramming, if required, at any time and 1 surgical revision prior to 6 months.
Outcomes
The primary outcome was change from baseline in mean number of daily episodes of urgency incontinence averaged over 6 months, as recorded for 3 consecutive days in monthly bladder diaries. Comparative secondary outcomes included change from baseline in bladder diary urinary frequency and nocturia through 6 months. Quality of life and symptom severity were assessed monthly with the Overactive Bladder Questionnaire Short Form (SF)4 (range, 0–100, higher scores indicate a better quality of life and higher scores on symptom severity, greater symptom severity). A minimal clinically important difference is considered a 10-point decrease from baseline scores.5
Other quality-of-life instruments assessed at baseline and 6 months included the Patient Global Impression of Improvement6 (range, 1, very much better, to 7, very much worse), improvement was defined as a rating of 1, 2, or 3. The Overactive Bladder Satisfaction of Treatment questionnaire7 (range, 0–100, higher scores indicate better satisfaction) included subcategories that measure treatment satisfaction, adverse effects, treatment endorsement, and convenience. Treatment preference was assessed as yes or no to the question “Do you prefer the treatment that you received since entering this study to the treatment you received before the study?” To assess incontinence severity, we used the Sandvik questionnaire,8 assessed on a scale of slight (1–2) to very severe (10–12) using the standard scoring algorithm; the Urinary Distress Inventory SF9 (range, 0–100; higher scores indicate greater distress); and the Incontinence Impact Questionnaire SF9 (range, 0–100; higher scores indicate worse quality of life). The Health Utility Index Mark-310 (0.00, death; 1.00, perfect health) was measured at 6 months. Bowel and sexual function outcomes will be reported later. Secondary descriptive measures of safety and adverse events were collected monthly, including the proportion of onabotulinumtoxinA participants requiring catheterization, the proportion of sacral neuromodulation participants requiring surgical revisions due to surgical site infection or pain and lead migration, and the proportion in each group with treated urinary tract infections, either culture positive, due to symptoms, or both.
Exploratory End Points
Exploratory end points included complete resolution of urgency incontinence as well as a 75% or greater and 50% or greater reduction from baseline in urgency incontinence recorded for 3 consecutive days in monthly diaries.
Statistical Analysis
The modified intention-to-treat population for the primary analysis included all eligible participants who provided at least 1 postbaseline bladder diary assessment. Planned comparative and descriptive secondary analyses were based on the clinical responders and per-protocol populations. The per-protocol analysis included participants receiving complete study therapy who, based on review masked to treatment group and outcome, did not substantially deviate from the protocol in a manner that affected the study outcome or treatment receipt. The planned 190 participants per treatment group provided at least 80% power to detect a mean between-group absolute difference in the reduction from baseline of 2 or more urgency incontinence episodes per day, assuming a common SD of 6.0 and 2-sided type I error rate of 5%, 10% loss to follow-up, and 20% initial nonresponder rate for each treatment group. For the primary analysis, missing monthly diary data were assumed to be missing at random. Because almost all participants had at least 4 diaries and the missing-data assumption conditions the relationship between the missing outcome and the reason for missingness on the observed data, this assumption is plausible. The safety analyses were performed on data from all randomized participants who initiated either onabotulinumtoxinA or sacral neuromodulation treatment as part of the study therapy.
Primary analyses used a linear mixed model with participant-month in the study (1 through 6) as the unit of analysis and monthly change from baseline in mean urgency incontinence episodes per day as the outcome, with terms for treatment group, month, interaction of treatment group with month, categorical covariates for age group (<65 years vs ≥65 years), and site consistent with randomization strata. Participants were treated as a random effect to account for within-person correlation in diary outcomes over time. The model generated adjusted estimates of change in the number of urgency incontinence episodes from baseline by treatment group and month and generated an F test of the hypothesis that the mean change from baseline averaged across 6 months differed between treatment groups. To confirm that the missing-at-random assumption adequately accounted for the missing diary data, the model was repeated using the multiple imputation approach described in the online appendix (Supplement 3). Similar models, with modifications accounting for measurement times, were used to evaluate treatment difference in continuous measures assessed over time.
Aggregate binary measures of efficacy and safety were evaluated using contingency tables, with differences between treatment groups assessed with the Mantel-Haenszel tests accounting for randomization strata. Analyses for binary outcomes with 0 events for either group used Fisher exact tests. Continuous measures assessed at a single follow-up time point were analyzed using analysis of covariance models accounting for randomization strata. Ordinal measures assessed only at 6 months were analyzed using Mantel-Haenszel mean score tests using modified ridit scores that control for randomization strata. The study was designed to conduct formal analyses for only the primary outcome at the .05 level of significance, and all other results and P values were considered exploratory. Consequently, no adjustments were made for multiple comparisons. Analyses were performed using SAS software, version 9.3 (SAS Institute Inc). All inferences and descriptive P values are based on 2-sided tests.
Results
Study Population and Assigned Treatment
Between February 2012 and June 2014, 480 women enrolled in the study, of whom 386 underwent randomization and 364 were available for primary outcome analysis (Figure 1). Of the 189 eligible participants randomized to sacral neuromodulation, 169 completed the first stage lead placement and 142 of 169 (84%) were categorized as clinical responders, of whom 139 received the permanent neurostimulator. Of the 192 women randomized to onabotulinumtoxinA, 189 received a complete dose during cystoscopic injection and 159 of 192 (83%) were similarly categorized as clinical responders (eFigure in the Supplement). No clinically meaningful differences in demographic or clinical characteristics were identified between groups (Table 1).
Figure 1. Flow Diagram of Progress Through Phases of a Randomized Trial Comparing OnabotulinumtoxinA With Sacral Neuromodulation Among Women With Refractory Urgency Urinary Incontinence.
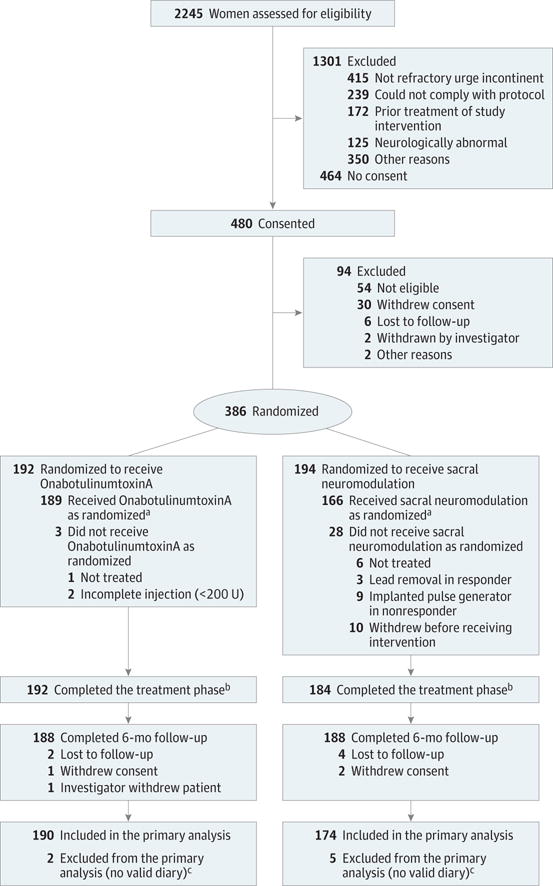
aReceipt of treatment as assigned per protocol for onabotulinumtoxinA includes complete first injection of 200 U and for sacral neuromodulation includes attempting the first-stage lead placement and if successful, continuing on to second-stage surgery: implantable pulse generator (IPG) implant for clinical responders and lead removal for nonresponders. Clinical response was defined as 50% or more reduction in mean episodes of urgency urinary incontinence on a 3-day bladder diary during the 7- to 14-day testing phase.
bThrough treatment phase includes all randomized individuals who continued the study through the treatment phase into study follow-up irrespective of whether they started or completed the assigned study treatment.
cFive baseline diaries were identified as being invalid during a data quality audit that occurred after randomization. All 5 individuals were treated and included in safety analyses but excluded from all analyses of efficacy.
Table 1.
Baseline Demographics for Intention-to-Treat Population Included in the Primary Efficacy Analyses
| OnabotulinumtoxinA (n = 190) | Sacral Neuromodulation (n = 174) | |
|---|---|---|
| Age, mean (SD), y | 62.9 (11.5) | 63.1 (11.8) |
| Hispanic ethnic group, No. (%) | 18 (9) | 10 (6) |
| Race, No. (%) | ||
| White | 154 (81) | 149 (86) |
| Black | 22 (12) | 16 (9) |
| Other | 14 (7) | 9 (5) |
| BMI, mean (SD) | 32.6 (8.7) | 31.7 (7.5) |
| Obese, No. (%) | 107 (56) | 87 (50) |
| Current smoker, No. (%) | 22 (12) | 18 (10) |
| Functional comorbidity index, mean (SD) | 3.8 (2.3) | 3.6 (2.3) |
| Postmenopausal, No. (%) | 162 (85) | 149 (86) |
| History of recurrent UTIs, No. (%) | 24 (13) | 25 (14) |
| Postvoid residual volume, median (IQR), mL | 20 (5–40) | 20 (7–50) |
| Urinary incontinence episodes, mean (SD), per day | ||
| Urge | 5.4 (2.7) | 5.2 (2.7) |
| Total | 6.0 (3.0) | 5.8 (3.0) |
| Urodynamic diagnosis of detrusor overactivity, No. (%) | 130 (68) | 101 (58) |
| Overactive Bladder Questionnaire SF, mean (SD)a | ||
| Symptom-bother | 74.6 (19.5) | 76.1 (16.8) |
| Quality of life | 38.2 (23.0) | 36.8 (21.6) |
| Urogenital Distress Inventory, mean (SD)b | 60.9 (18.3) | 59.2 (16.9) |
| Incontinence Impact Questionnaire, mean (SD)c | 52.7 (27.6) | 52.5 (25.8) |
| Sandvik, No. (%)d | ||
| Slight | 2 (1) | 1 (1) |
| Moderate | 27 (14) | 25 (14) |
| Severe | 52 (27) | 38 (22) |
| Very severe | 103 (54) | 105 (60) |
| Health Utility Index 3, mean (SD)e | 0.71 (0.3) | 0.74 (0.28) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; OAB-SF, Overactive Bladder Short Form; UTI, urinary tract infection.
Values range from 0 to 100, with higher scores on the symptom severity scale indicating greater severity of symptoms and higher scores on the quality-of-life scale indicating better quality of life.
Values range from 0 to 100, with higher scores indicating greater distress.
Values range from 0 to 100, with higher scores indicating worse quality of life.
Patient-reported measure of incontinence severity as assessed on a scale of slight (1–2) to very severe (10–12) using the standard scoring algorithm.7
Values are defined such that the score for dead is 0; the score for perfect health, 1.00.
Approximately 10% of monthly follow-up diaries were missing for the primary outcome; rates differed between treatment groups (7% for the onabotulinumtoxinA group vs 13% for the sacral neuromodulation group) with missing diaries from the 10 sacral neuromodulation participants who withdrew from the study prior to treatment accounting for the difference. Results of a sensitivity analysis based on multiple imputation of missing data were consistent with those of the primary analysis.
Outcomes
In the intention-to-treat population, participants treated with onabotulinumtoxinA had a greater 6-month mean reduction of 3.9 episodes of urgency incontinence per day than did the sacral neuromodulation group of 3.3 (mean difference, 0.63; 95% CI, 0.13–1.14; P = .01) with point estimates showing greater reductions for onabotulinumtoxinA at each month for 6 months (Figure 2).
Figure 2. Change From Baseline in Urgency Urinary Incontinence Episodes per Day by Treatment Group by Month.
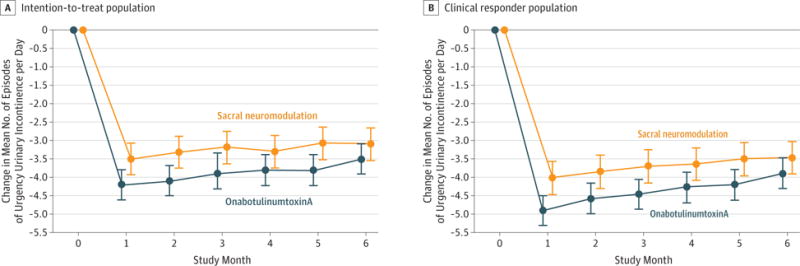
Values in the graph include adjusted mean estimates and associated 95% CIs (indicated by error bars) obtained from the linear mixed model controlling for randomization strata defined by age group (<65 years vs ≥65 years) and site. The number of diaries for each treatment group and month included in the model varies from 151 to 179 for the intention-to-treat population and 122 to 154 for the clinical responder population, but all values from all time points contribute to the mean and interval estimates at each time point. A clinical responder is defined as having 50% or more reduction in mean number of episodes of urgency urinary incontinence on a 3-day bladder diary duringthe 7- to 14-day testing phase for women randomized to receive sacral neuromodulation and 1 month after injection for women randomized to receive onabotulinumtoxinA.
Over 6 months, both groups improved on the Overactive Bladder SF symptom-bother score, with participants in the onabotulinumtoxinA group showing evidence of a greater improvement than the sacral neuromodulation group (−46.7 vs −38.6; mean difference, 8.1; 95% CI, 3.0 to 13.3; P = .002). Participants in the onabotulinumtoxinA group appeared to have greater improvements in the Overactive Bladder Satisfaction for treatment (mean difference, 7.8; 95% CI, 1.6 to 14.1; P = .01) and endorsement (mean difference, 10.4; 95% CI, 4.3 to 16.5; P < .001). However, there were no differences in convenience (67.6 vs 70.2; mean difference, −2.5; 95% CI, −8.1 to 3.0; P = .36), adverse effects (88.4 vs 85.1; mean difference, 3.3; 95% CI, −1.9 to 8.5; P = .22), and treatment preference (92% vs 89%; risk difference, −3%; 95% CI, −16 to 10; P = .49). Other quality-of-life measures showed no evidence of a difference between groups (Table 2). Over 6 months, of those receiving study treatment, 5 women in the onabotulinumtoxinA group and 14 in the sacral neuromodulation group received additional incontinence medication.
Table 2.
Efficacy and Quality of Life Outcomes of Intention-to-Treat Population at 6 Months
| Outcomes | OnabotulinumtoxinA (n = 190) | Sacral Neuromodulation (n = 174) | Treatment Group Difference (95% CI) | P Value |
|---|---|---|---|---|
| Change in mean daily urgency urinary incontinence episodes, adjusted mean (95% CI)a | −3.89 (−4.26 to −3.52) | −3.25 (−3.64 to −2.87) | 0.63 (0.13 to 1.14) | .01 |
| Resolution of Urinary Incontinence, No./Total (%)b | ||||
| ≥4 mo of diaries completed | ||||
| Complete resolution | 35/178 (20) | 6/166 (4) | −16 (−26 to −5) | <.001 |
| ≥75% reduction | 81/178 (46) | 43/166 (26) | −20 (−30 to −9) | <.001 |
| ≥50% reduction | 109/178 (61) | 84/166 (51) | −11 (−21 to 0) | .06 |
| All 6 mo of diaries completedb | ||||
| Complete resolution | 26/127 (20) | 2/99 (2) | −18 (−31 to −5) | <.001 |
| ≥75% reduction | 63/127 (50) | 27/99 (27) | −22 (−35 to −9) | .004 |
| ≥50% reduction | 85/127 (67) | 51/99 (52) | −15 (−28 to −2) | .05 |
| Change From Baseline in Urinary Incontinence, Adjusted Mean (95% CI)a | ||||
| Any | −4.02 (−4.44 to −3.61) | −3.50 (−3.94 to −3.06) | 0.52 (−0.04 to 1.09) | .07 |
| Nocturia | −0.40 (−0.56 to −0.24) | −0.26 (−0.43 to −0.10) | 0.13 (−0.08 to 0.35) | .22 |
| Voids | −1.12 (−1.53 to −0.70) | −0.84 (−1.28 to −0.41) | 0.28 (−0.29 to 0.84) | .34 |
| Pads/d | −2.02 (−2.31 to −1.73) | −1.64 (−1.94 to −1.34) | 0.38 (−0.01 to 0.77) | .06 |
| Overactive Bladder Questionnaire Change From Baseline, Adjusted Mean (95% CI) | ||||
| Questionnaire SF | ||||
| Symptom bother | −46.7 (−50.5 to −43.0) | −38.6 (−42.5 to −34.6) | 8.1 (3.0 to 13.3) | .002 |
| Quality of life | 41.6 (37.9 to 45.4) | 38.1 (34.1 to 42.0) | −3.6 (−8.7 to 1.5) | .17 |
| Satisfaction Questionnairec | ||||
| Treatment satisfaction | 67.7 (63.2 to 72.1) | 59.8 (55.0 to 64.7) | 7.8 (1.6 to 14.1) | .01 |
| Adverse effects | 88.4 (84.7 to 92.2) | 85.1 (81.1 to 89.2) | 3.3 (−1.9 to 8.5) | .22 |
| Endorsement | 78.1 (73.7 to 82.4) | 67.6 (62.9 to 72.3) | 10.4 (4.3 to 16.5) | <.001 |
| Convenience | 67.6 (63.7 to 71.6) | 70.2 (65.8 to 74.5) | −2.5 (−8.1 to 3.0) | .36 |
| Treatment preference, No. (%)d | 113 (92) | 89 (89) | −3 (−16 to 10) | .49 |
| Score at 6 Months, No. (%) | ||||
| PGI-Ie | ||||
| Urinary leakage | 101 (71) | 91 (68) | −2 (−14 to 10) | .82 |
| Bladder function | 100 (68) | 92 (70) | 2 (−10 to 13) | .54 |
| Sandvikf | .14 | |||
| Slight | 29 (23) | 23 (19) | ||
| Moderate | 33 (26) | 33 (27) | ||
| Severe | 28 (22) | 24 (19) | ||
| Very severe | 36 (29) | 44 (35) | ||
| Change From Baseline, Adjusted Mean (95% CI) | ||||
| Urinary Distress Inventory SFg | −10.0 (−12.2 to −7.8) | −8.6 (−10.9 to −6.3) | −1.4 (−4.4 to 1.6) | .36 |
| Incontinence Impact SFh | −12.4 (−14.9 to −9.9) | −10.4 (−13.0 to −7.8) | −2.0 (−5.4 to 1.4) | .25 |
| Health Utility Index 3i | −0.011 (−0.028 to 0.007) | −0.006 (−0.025 to 0.013) | −0.005 (−0.029 to 0.020) | .72 |
Abbreviations: PGI-I, Patient Global Impression of Improvement; SF, Short Form.
Values for any urinary incontinence, urgency urinary incontinence, nocturia, voids are based on mean number of episodes per day on a 3-day diary captured monthly and are generated from model-based estimates. The adjusted models controlled for the stratification variables of age and clinical site.
Represents individuals who had this degree of improvement on all their diaries.
Values for the Overactive Bladder Satisfaction questionnaire range from 0–100 and includes 5 subscales; treatment satisfaction, side effects, treatment endorsement, convenience, and patient preference, with higher scores reflecting better satisfaction.
Treatment preference is a binary outcome that is classified as yes if a participant answers either “Slight preference for the treatment I am receiving now” or “Definitely prefer the treatment I am receiving now” to the question: “Do you prefer the treatment that you received since entering this study to the treatment you received before the study?” from the Overactive Bladder Questionnaire SF.
The Patient Global Impression of Improvement (PGI-I) is a patient reported measure of perceived improvement with treatment on a scale of 1 (very much better) to 7 (very much worse). Included here are participants who had adequate improvement, defined as a rating of 1, 2 or 3 (better).
The Sandvik scale has a range of 1–12, with higher scores representing worse outcomes.
The Urinary Distress Inventory short form (UDI-SF) scale has a range of 0–100, with higher scores indicating greater distress.
The Incontinence Impact Questionnaire short form (IIQ-SF) scale has a range of 0–100, with higher scores representing a worse quality of life.
The Health Utility Index, Version 3 (HUI 3) scale has a range of 0–1, with higher scores representing better health.
Exploratory End Points
In the clinical responder population, participants treated with onabotulinumtoxinA had a mean 4.4 reduction in 6-month mean episodes of urgency incontinence per day compared with those in the sacral neuromodulation group, which had a mean reduction of 3.7 (mean difference, 0.68; 95% CI, 0.17–1.20; P = .01).
Of the participants who completed at least 4 monthly diaries over 6 months, 20% in the onabotulinumtoxinA group and 4% in the sacral neuromodulation group had complete resolution of urgency urinary incontinence (treatment difference, −16%; 95% CI, −26% to −5%; P < .001). Forty-six percent in the onabotulinumtoxinA group and 26% in the sacral neuromodulation group had at least a 75% reduction in the number of episodes of urgency incontinence (treatment difference, −20%; 95% CI, −30% to −9%; P < .001) (Table 2). Observed treatment effects were similar in the clinical responder and per-protocol populations (eTables 2 and 3 in Supplement 3).
Adverse Events
In the sacral neuromodulation group, 6 women (3%) had their device revised or removed during the 6-month period. In the onabotulinumtoxinA group, 8% required intermittent self-catheterization at 1 month, 4% at 3 months, and 2% at 6 months (Table 3). The median time of catheterization for any reason was 37 days (range, 2–203). By 6 months, risk of urinary tract infections appeared to be greater in the onabotulinumtoxinA group than in the sacral neuromodulation group (35% vs 11%; risk difference, −23%; 95% CI, −33% to −13%; P < .001). Thirty-nine percent of the 66 onabotulinumtoxinA participants and 30% of the 20 sacral neuromodulation participants who had a urinary tract infection during the 6-month period had multiple infections. However, multiple risk of urinary tract infections did not appear to differ between treatment groups.
Table 3.
Adverse Events
| Outcome | OnabotulinumtoxinA (n = 191) | Sacral Neuromodulation (n = 178) | Treatment Group Difference (95% CI) | P Value |
|---|---|---|---|---|
| Cumulative Urinary Tract Infection, No. (%) | ||||
| Through 1 mo | 22 (12) | 1 (1) | −11 (−21 to −1) | <.001 |
| Through 3 mo | 47 (25) | 10 (6) | −19 (−29 to −9) | <.001 |
| Through 6 mo | 66 (35) | 20 (11) | −23 (−33 to −13) | <.001 |
| Adverse surgical events revision or removal sacral neuromodulation through 6 mo | 6 (3) | |||
| Intermittent catheterization per-protocol criteria met, No./total (%) | ||||
| 2 wk | 29/191 (16) | |||
| 1 mo | 16/191 (8) | |||
| 3 mo | 8/191 (4) | |||
| 6 mo | 4/191 (2) | |||
| At any visit through 6 mo | 38/191 (20) |
Discussion
In this randomized study, over a 6-month assessment period, a single injection of 200 U of onabotulinumtoxinA provided a small but statistically significant greater reduction in episodes of urgency urinary incontinence than did sacral neuromodulation. Participants who received onabotulinumtoxinA had greater improvements in the Overactive Bladder Questionnaire SF symptom bother, as well as the subscales for treatment satisfaction and endorsement. However, there was no significant difference for quality of life or for the subscales for treatment preference, convenience, or adverse effects. Moreover, onabotulinumtoxinA increased the risk of urinary tract infections and need for self-catheterizations. Overall, these findings make it uncertain whether onabotulinumtoxinA provides a clinically important net benefit compared with sacral neuromodulation.
A one-time injection of 200 U of onabotulinumtoxinA was chosen in this severely affected population because published reports support 6-month durability and because there was wide variability in reported rates of clean intermittent self-catheterization of 0% to 52%.11–15 Enrollment for our study began March 2012, and the study design strategy was further strengthened in October 2012 with the publication of the only other randomized clinical trial comparing onabotulinumtoxinA with a second-line urgency urinary incontinence therapy anticholinergic medication.16 That study evaluated patients who were both drug naive and had received prior anticholinergic medication. It found no difference in their primary outcome of mean change in episodes of urgency incontinence over 6 months using a single injection of 100 U of onabotulinumtoxinA compared with daily anticholinergic medication use. Thus, increasing the onabotulinumtoxinA dose in a population of women refractory to second-line therapies was logical.
A lower rate of performing intermittent catheterization was ascertained in this study than the 21.2% reported in the 200-U group of the dose-finding study.12 This may be due to the difference in the threshold to require catheterization, ie, a residual volume after voiding of more than 200 mL irrespective of symptoms vs more than 200 mL with voiding symptoms or more than 300 mL without symptoms. Despite the higher tolerance for postvoid residual urine volumes in our trial, the onabotulinumtoxinA group had a lower urinary tract infection rate (35%) than the (48.1%) rate reported in the 200-U group of the dose-finding study and a similar urinary tract infection rate to those using 100 U (33%).16
The clinical responder rate of 84% during first-stage lead placement for sacral neuromodulation was similar to reported rates of 67% to 84%.17–19 Through 6 months, 51% of the participants in the sacral neuromodulation group reported 50% or more improvement in the number of episodes of urgency incontinence, and 4% had complete resolution of urgency incontinence. In these exploratory end points, the rates are lower than the 67% to 87% (≥50% improvement) and 39% to 56% complete continent rates at 6 months reported in the Cochrane review.20 However, the studies were small with a wide range of outcome measures. In the largest study,21 35% of the implanted group and 40% in the delayed group had missing primary data outcome at 6 months. A recent randomized trial of sacral neuromodulation vs standard medical therapy reported a 39% continence rate in the sacral neuromodulation group vs 21% in the standard medical therapy group (P = .06); however, the mean (SD) baseline leaks per day (2.4 [1.7]) for the sacral neuromodulation group in the study were lower than in our study (5.3 [2.7]), reflecting a less severe population.22 The 3% rate of surgical revision or removal during this period was similar to that of studies using the tined lead and smaller neurostimulators, which was 3% to 11%.23
Our study suggests that the small but statistically significant improved outcomes of onabotulinumtoxinA compared with sacral modulation may be due to the increased dosage of onabotulinumtoxinA used in the trial. Similar results found in the analyses of the intention-to-treat and per-protocol populations further validate the study’s conclusions. In addition, there was no participant study discontinuation due to a differential in treatment tolerance. Enrollment from multiple sites and standardized interventions by both urogynecologists and urologists increases the generalizability of the efficacy and safety findings. Although originally powered to detect a difference of 2 incontinence episodes per day between modalities, the estimated standard deviation was actually smaller than anticipated and a significant absolute difference of 0.64 episodes per day was determined. Although the difference resulted in an increased benefit in patient reported symptom bother and satisfaction, the clinical significance of this smaller difference in episodes is unclear.
Because a single injection of 1 formulation of botulinum toxin A was investigated, no conclusions can be reached on other botulinum toxin preparations or the effect of multiple injections of onabotulinumtoxinA compared with sacral neuromodulation therapy. Furthermore, this trial compared 2 active treatments, preventing any determination of a placebo effect. However, the observed rates of cure and improvement in episodes of urgency incontinence observed in the study for both therapies exceed the placebo effect observed in other randomized trials.12,14,21
Conclusions
Among women with refractory urgency urinary incontinence, treatment with onabotulinumtoxinA compared with sacral neuromodulation resulted in a small daily improvement in episodes that although statistically significant is of uncertain clinical importance. In addition, it resulted in a higher risk of urinary tract infections and need for transient self-catheterizations.
Supplementary Material
Key Points.
Question Is onabotulinumtoxinA superior to sacral neuromodulation in controlling symptoms of refractory urgency urinary incontinence?
Findings In this comparative effectiveness trial that included 386 women, onabotulinumtoxinA had a greater mean daily urgency urinary incontinence episode reduction over 6 months than did the sacral neuromodulation group, −3.9 vs −3.3 episodes per day, a statistically significant but small difference. Urinary tract infections and need for self-catheterization were more frequent among women receiving onabotulinumtoxinA.
Meaning Although onabotulinumtoxinA resulted in greater reduction in episodes of urgency urinary incontinence, this is limited by the small magnitude of the difference and greater likelihood of some adverse events.
Acknowledgments
Funding/Support: This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health at National Institutes of Health 3316 (U01 HD41249U10, HD41261U10 HD41267, U10 HD54215U10-HD41261-11, U10-HD069013, U10-HD054214-06, U10-HD054215-06, U10-HD041267-12, U10-HD069025-01, U10-HD069010, U10-HD054136, U10-HD054241, and U10-HD041250-11).
Role of the Funder/Sponsor: The Eunice Kennedy Shriver National Institute of Child Health and Human Development, Project Scientist for the PFDN, Susan Meikle, MD, MSPH, played a role in the design, conduct of the study; collection, analysis, and interpretation of the data; preparation, review and approval of the manuscript; and decision to submit for publication.
Footnotes
Author Contributions: Drs Nolan and Wallace had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Amundsen, Richter, Menefee, Komesu, Myers, Arya, Zyczinski, Vasavada, Wallace, Meikle.
Acquisition, analysis, or interpretation of data: All Authors.
Drafting of the manuscript: All Authors.
Critical revision of the manuscript for important intellectual content: Amundsen, Richter, Menefee, Komesu, Arya, Gregory, Myers, Zyczinski, Vasavada, Nolen, Meikle.
Statistical analysis: Richter, Nolen, Wallace, Meikle.
Administrative, technical, or material support: Amundsen, Komesu, Gregory, Zyczinski, Nolen, Meikle.
Study supervision: Amundsen, Richter, Arya, Wallace, Menefee, Komesu, Gregory, Myers, Zyczinski, Vasavada, Meikle.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Arya reported receiving a research grant from Pfizer. Dr Vasavada reported serving as a consultant for Medtronic, Allergan, and Axonics.
References
- 1.Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep) 2009;(187):94–95. [PMC free article] [PubMed] [Google Scholar]
- 2.Coyne KS, Wein A, Nicholson S, Kvasz M, Chen C-I, Milsom I. Economic burden of urgency urinary incontinence in the United States: a systematic review. J Manag Care Pharm. 2014;20(2):130–140. doi: 10.18553/jmcp.2014.20.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amundsen CL, Richter HE, Menefee S, et al. The Refractory Overactive Bladder: Sacral Neuromodulation vs Botulinum Toxin Assessment: ROSETTA trial. Contemp Clin Trials. 2014;37(2):272–283. doi: 10.1016/j.cct.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyne KS, Matza LS, Brewster-Jordan J. “We have to stop again?!” the impact of overactive bladder on family members. Neurourol Urodyn. 2009;28(8):969–975. doi: 10.1002/nau.20705. [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V. Determining the importance of change in the overactive bladder questionnaire. J Urol. 2006;176(2):627–632. doi: 10.1016/j.juro.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 6.Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189(1):98–101. doi: 10.1067/mob.2003.379. [DOI] [PubMed] [Google Scholar]
- 7.Margolis MK, Fox KM, Cerulli A, Ariely R, Kahler KH, Coyne KS. Psychometric validation of the overactive bladder satisfaction with treatment questionnaire (OAB-SAT-q) Neurourol Urodyn. 2009;28(5):416–422. doi: 10.1002/nau.20672. [DOI] [PubMed] [Google Scholar]
- 8.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19(2):137–145. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA, Continence Program for Women Research Group Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol Urodyn. 1995;14(2):131–139. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 10.Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40(2):113–128. doi: 10.1097/00005650-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kuo HC. Will suburothelial injection of small dose of botulinum A toxin have similar therapeutic effects and less adverse events for refractory detrusor overactivity? Urology. 2006;68(5):993–997. doi: 10.1016/j.urology.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 12.Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol. 2010;184(6):2416–2422. doi: 10.1016/j.juro.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Kuo HC. Comparison of effectiveness of detrusor, suburothelial and bladder base injections of botulinum toxin a for idiopathic detrusor overactivity. J Urol. 2007;178(4 Pt 1):1359–1363. doi: 10.1016/j.juro.2007.05.136. [DOI] [PubMed] [Google Scholar]
- 14.Tincello DG, Kenyon S, Abrams KR, et al. Botulinum toxin a versus placebo for refractory detrusor overactivity in women: a randomised blinded placebo-controlled trial of 240 women (the RELAX study) Eur Urol. 2012;62(3):507–514. doi: 10.1016/j.eururo.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 15.Sahai A, Dowson C, Khan MS, Dasgupta P, Group GKTBS; GKT Botulinum Study Group Repeated injections of botulinum toxin-A for idiopathic detrusor overactivity. Urology. 2010;75(3):552–558. doi: 10.1016/j.urology.2009.05.097. [DOI] [PubMed] [Google Scholar]
- 16.Visco AG, Brubaker L, Richter HE, et al. Pelvic Floor Disorders Network Anticholinergic therapy vs. onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367(19):1803–1813. doi: 10.1056/NEJMoa1208872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Voskuilen AC, Oerlemans DJ, Weil EH, van den Hombergh U, van Kerrebroeck PE. Medium-term experience of sacral neuromodulation by tined lead implantation. BJU Int. 2007;99(1):107–110. doi: 10.1111/j.1464-410X.2006.06508.x. [DOI] [PubMed] [Google Scholar]
- 18.Blandon RE, Gebhart JB, Lightner DJ, Klingele CJ. Re-operation rates after permanent sacral nerve stimulation for refractory voiding dysfunction in women. BJU Int. 2008;101(9):1119–1123. doi: 10.1111/j.1464-410X.2007.07426.x. [DOI] [PubMed] [Google Scholar]
- 19.Borawski KM, Foster RT, Webster GD, Amundsen CL. Predicting implantation with a neuromodulator using two different test stimulation techniques: a prospective randomized study in urge incontinent women. Neurourol Urodyn. 2007;26(1):14–18. doi: 10.1002/nau.20332. [DOI] [PubMed] [Google Scholar]
- 20.Herbison GP, Arnold EP. Sacral neuromodulation with implanted devices for urinary storage and voiding dysfunction in adults. Cochrane Database Syst Rev. 2009;2:CD004202. doi: 10.1002/14651858.CD004202.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt RA, Jonas U, Oleson KA, et al. Sacral Nerve Stimulation Study Group Sacral nerve stimulation for treatment of refractory urinary urge incontinence. J Urol. 1999;162(2):352–357. [PubMed] [Google Scholar]
- 22.Siegel S, Noblett K, Mangel J, et al. Results of a prospective, randomized, multicenter study evaluating sacral neuromodulation with InterStim therapy compared to standard medical therapy at 6-months in subjects with mild symptoms of overactive bladder. Neurourol Urodyn. 2015;34(3):224–230. doi: 10.1002/nau.22544. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui NY, Wu JM, Amundsen CL. Efficacy and adverse events of sacral nerve stimulation for overactive bladder: a systematic review. Neurourol Urodyn. 2010;29(S1 suppl 1):S18–S23. doi: 10.1002/nau.20786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


