Abstract
Verticillin A is a natural product isolated from fungal cultures and has displayed potent antibiotic, antiviral, nematocidal, and anticancer properties in vitro. While in vivo studies have been limited due to sparse supply, the in vivo efficacy data that does exist demonstrates potent anti-tumor activity in murine cancer models. The current study aims to investigate the pharmacokinetics and bioavailability of verticillin A in mice to provide guidance for further efficacy assessment in mouse models. A sensitive and specific liquid chromatography-tandem mass spectrometry method was developed and validated for the quantification of verticillin A in mouse plasma. Sample preparation was accomplished through protein precipitation, and chromatographic separation was achieved on an Agilent Zorbax Extend C18 column with a security guard cartridge C8 using a binary gradient with mobile phase A (water/0.1% formic acid) and B (ACN/0.1% formic acid) at a flow rate of 400 μl/min. Elution of verticillin A and internal standard, hesperetin, occurred at 4.87 and 2.06 min, respectively. The total chromatographic run time was 8 min., and the assay was linear in the concentration range of 1–1000 nM. The within- and between day precisions and accuracy were in the range of 2.58–8.71 and 90–105%, respectively. The assay was applied to determine plasma drug concentration in a mouse pharmacokinetic study. It was found that intraperitoneal dosing of 3 mg/kg resulted in high systemic exposure and achieved Cmax of 110 nM with plasma concentrations sustained above 10 nM for the 24-hour duration of the study. Intravenous and oral dosing achieved observed Cmax of 73 nM and 9 nM, respectively. Oral dosing resulted in an approximate 9% bioavailability. Comparing with previously published in vitro studies that demonstrated verticillin A is active in the 20 nM to 130 nM range, the pharmacokinetic data demonstrate similar levels are achieved in mouse plasma via intravenous or intraperitoneal dosing routes.
Keywords: Verticillin A, Epipolythiodioxopiperazine Alkaloids, Liquid Chromatography-Mass Spectrometry, mouse, plasma, pharmacokinetics
1. Introduction
The epipolythiodioxopiperazine alkaloids are a class of fungal secondary metabolites that have drawn interest of scientists from a range of disciplines, including natural products chemists [1–3], synthetic organic chemists [4–6], mycologists [7], and pharmacologists [8–11]. The first in this series, verticillin A, was isolated and characterized by Hitato and colleagues in the early 1970’s [12, 13]. The trivial name of this structural class was derived from the name of the fungus, Verticillium sp. [12]. In 2006, a research group crystalized verticillin A, thereby deriving its absolute configuration [14].
Verticillin A was found to be a potent antibiotic against Gram positive bacteria and mycobacteria [12]. Also, it has displayed antiviral [15] and nematocidal properties [16], with the latter being against Caenorhabditis elegans and Panagrellus redivivus. For most activities, the dimeric nature of the molecule, with a disulfide bridge in each monomer, is essential, as monomeric analogues of verticillin A are less potent [16].
The anticancer properties of verticillin A are the most investigated bioactivity. Even during its initial discovery, potent cytotoxicity against HeLa cells was noted, with an ED50 of 0.2 μg/mL (or 0.29 μM) [12]. In the mid 1990s, Chu et al. studied verticillin A as an inhibitor of the c fos proto oncogene [17], where antitumor potential could be due to prevention of signal transduction, blocking some genes involved in cell proliferation. More recently, Liu and colleagues discovered the ability of verticillin A to induce cell cycle arrest in the G2 phase, noted in colon cancer cells both in vitro and in an in vivo xenograft model [18]. This same group found verticillin A to be a selective HMTase inhibitor, and in probing these effects in vivo, showed that verticillin A could be used to overcome 5-fluorouracil resistance in a murine colon cancer model [10]. In the last twelve months, since the time we initiated these studies, two more studies on the in vivo efficacy of verticillin A have been published [19, 20].
As a natural product that is biosynthesized in low yield within fungal cultures, in vivo data on verticillin A is quite limited despite its promising biological activities. Nonetheless, the two in vivo studies that have been conducted demonstrated anti-tumor activity in mouse models of colon cancer [18]. Given the challenges associated with limited supplies of verticillin A and the growing interest in its evaluation in mouse models, we conducted the studies described herein to gain understanding of the pharmacokinetic properties of this agent in mice.
2. Materials and methods
2.1. Chemicals and reagents
Verticillin A was purified from culture MSX59553 from the Mycosynthetix fungal library, as detailed previously [2]. Hesperetin was used as internal standard (IS) and was purchased from Avanti Polar Lipids (Alabaster, AL). LC-grade acetonitrile (ACN), dimethyl sulfoxide (DMSO), water, methanol, cremophor EL, ethanol, and phosphate buffered saline (PBS, pH 7.4) were purchased from Fisher Scientific (Fair lawn, NJ). Formic acid was purchased from Sigma-Aldrich (St. Louis, MO). Blank mouse plasma was purchased from Lampire Biological Laboratories, Inc (Pipersville, PA).
2.2. Preparation of stock solutions and calibration standards
A stock solution of verticillin A (0.72 mM, or 0.5 mg/ml) was prepared in a mixture of DMSO and ACN (1:1, v/v) and stored at −80 °C. A series of calibration standard solutions (10 – 10,000 nM) were prepared freshly by diluting the stock solution stepwise using 50% ACN in H2O. A hesperetin stock solution (3.30 mM, or 1 mg/ml) was prepared in methanol and then diluted to make a working solution of 16.5 μM (or 5000 ng/ml).
2.3. Sample preparation
The standard and quality control (QC) samples were prepared by spiking 10 μl of serially diluted standard solutions of verticillin A and IS (5000 ng/ml) each into 100 μl blank mouse plasma to generate a calibration curve with final concentration of verticillin A ranging from 1 to1000 nM and QC samples with concentrations of 1, 3, 30, and 300 nM. The experimental samples of mouse plasma were prepared by spiking 10 μl of IS working solution to 100 μl plasma. All the standards, QC, and the experimental samples were extracted with 1 ml of ACN by vortex mixing for 1 min, and then were centrifuged for 10 min at 13,500 rpm. The supernatant was transferred into a glass tube and evaporated under a gentle stream of Nitrogen. The residue was then reconstituted with 200 μl of 50% ACN, followed by vortex mixing for 1 min. These solutions were transferred into microcentrifuge tubes and centrifuged at 13,500 rpm for 10 min at 4 °C. The supernatant was transferred into Thermo PAL autosampler vials and 20 μl was injected for LC-MS/MS analysis.
2.4. LC-MS/MS analysis
The assay was developed on a LC-MS system consisting of a Thermo Accela UHLPC pump, Thermo PAL autosampler and Thermo TSQ Discovery triple quadrupole mass spectrometer with XCalibur and LCquan software. The chromatographic separation of the analytes was achieved on an Agilent Zorbax Extend C18 analytical column (3.5 μm × 2.1 mm I.D. × 50 mm) with a security guard cartridge C8, and the column temperature was maintained at 40 °C. Linear gradient elution was performed at a flow rate of 400 μl/min with water/0.1% formic acid as mobile phase A, and ACN/0.1% formic acid as mobile phase B: 0.0 min (70% A and 30% B), 5.0 min (35% A and 65% B), 5.10 min (5% A and 95% B), 6.50 min (5% A and 95% B), 6.6 min (70%A and 30%B) and 8.0 min (70% A and 30%B) for 8 minutes. The autosampler temperature was set at 4 °C.
2.5. Method validation
The validation experiments were performed in accordance with the FDA guidance for bioanalytical methods. The experiments included selectivity, linearity, precision and accuracy, matrix effects, carryover, and stability tests. Standards and QC sample solutions were prepared as described in the sample preparation section to generate calibration curves with plasma concentrations of 1–1000 nM and QC samples at a concentration of 1, 3, 30, and 300 nM in mouse plasma. Within-day and between-day precision and accuracy were evaluated using replicate samples of 6 (n=6) at four QC concentration levels.
Carryover effect was evaluated by injecting a blank sample following the injection of the highest concentration standard sample (1000 nM). The recovery was calculated by comparing the peak areas of verticillin A in QC samples with those of corresponding neat solution added in the supernatant of the processed blank plasma samples. The matrix effect was assessed by comparing the peak areas of the spiked samples after extraction of blank plasma with those of neat solutions. The stability of verticillin A was performed at four concentrations indicated above in triplicates under all experimental conditions, including auto sampler at 4 °C for 12 h after extraction, and bench-top for 4 h, three cycles of freeze-thaw, and one month storage at −80 °C before extraction.
2.6. Animal study
Single-dose pharmacokinetics of verticillin A was evaluated in mice. All animal experiments were performed following the protocols approved by the Institutional Animal Care and Use Committees (IACUCs) of the Ohio State University. 5–6 week-old female ICR mice (22 to 28 g) were purchased from Harlan Laboratories, Inc. (Indianapolis, IN, USA) and housed in barrier rodent facility with free access to standard chow diet and water for one week prior to the experiment. Verticillin A was initially dissolved in DMSO to make 6 mg/ml stock solution and then diluted with a mixture of Cremophor EL-ethanol-PBS (10:5:85, v/v/v) by 20 fold according to the previously published animal study [10] and personal communication with the corresponding author. Prior to the start of the experiment, animals were randomly divided into three groups (n = 10/group). One animal per time point was included in this study due to the limited supply of verticillin A. The first group received verticillin A intravenously at 1 mg/kg (1.43 μmol/kg) by tail vein bolus injection (IV, dose volume = 3.3 mL/kg). The second and third groups were given verticillin A at a dose of 3 mg/kg (4.30 μmol/kg) by oral gavage (PO) and intraperitoneal (IP) administration, respectively (dose volume = 10 mL/kg). These doses were chosen due to the limited solubility of verticillin A and the maximum dosing volumes allowed. Blood samples were collected via cardiac puncture into the heparinized polypropylene tubes at 0.083, 0.17, 0.33, 0.5, 1, 2, 4, 6, 8, and 24 h after dosing. Plasma samples were harvested by centrifugation at 1000 g for 5 min and then processed for quantification of verticillin A by LC-MS/MS (described in sections 2.3. and 2.4.). Pharmacokinetic analyses were performed by non-compartmental methods using Phoenix WinNonlin version 6.3 (Pharsight Corporation, Mountain view, CA).
3. Results and discussion
3.1. Method development
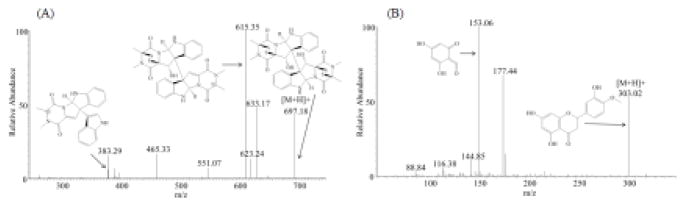
Chromatographic and mass spectrometric conditions were optimized to increase signal response of analytes and improve peak shape. Positive electrospray ionization was selected due to the superior signal intensity for both verticillin A and hesperetin. Transitions with maximum signal to noise ratios were chosen for the quantification of verticillin A and hesperetin; these were m/z 697.18→(383.29 + 615.35) and 303.06→153.01, respectively (Fig. 1), and a detailed analysis of the fragmentation pattern for verticillin A and related analogues was recently published [21]. The C18 column with a security guard cartridge C8, in combination with a gradient elution, resulted in the efficient separation and elution of verticillin A. Formic acid was added to the mobile phases to improve peak shape. Protein precipitation using acetonitrile was found to be appropriate with minimal matrix effects and high recoveries.
Fig. 1.
Chemical structures and mass fragmentation spectra of (A) verticillin A and (B) hesperetin (IS) along with their proposed product ion structures.
3.2. Assay validation
3.2.1 Selectivity, linearity, accuracy and precision
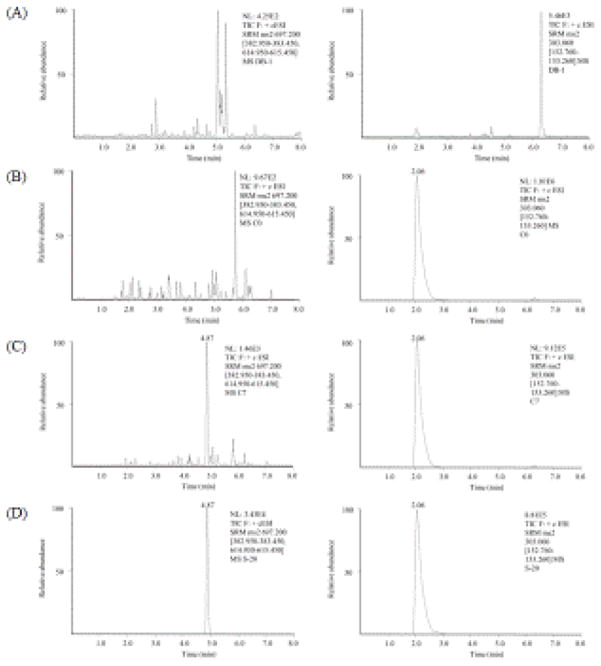
The chromatograms of blank mouse plasma spiked with LLOQ of verticillin A (1 nM) and IS are shown in Fig. 2 with retention times of 4.87 minutes for verticillin A and 2.06 minutes for IS. No interference peaks were observed in the mouse plasma matrix at the retention time of verticillin A and IS, indicating good selectivity of the method (Fig. 2). The linearity was calculated using 1/X2 weighting and a linear curve was obtained in the range of 1–1000 nM with correlation coefficient (r2) of 0.9926. The precision and accuracy for verticillin A were 2.58–8.71% and 90–105%, respectively, which are well within the acceptable limits (Table 1), suggesting that the developed method was precise and accurate for the quantification of verticillin A over the tested concentration range.
Fig. 2.
Representative SRM chromatograms of verticillin A (left) and hesperetin (right) in: A. blank (analyte and IS free) sample, B. zero sample (analyte-free plasma spiked with IS), C. plasma spiked with analytes (1 nM, LLOQ) and IS and D. plasma sample at 24 h after intraperitoneal administration of verticillin A (3 mg/kg). NL: Normalized intensity level
Table 1.
Within and between-day accuracy and precision for verticillin A in mouse plasma (n=6)
| Nominal conc. (nM) | Within-day | Between-day | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean measured conc. (nM) | Accuracy (%) | Precision (%CV) | Mean determined conc. (nM) | Accuracy (%) | Precision (%CV) | |
| 1 | 0.99 | 98.6 | 6.61 | 1.01 | 101 | 6.94 |
| 3 | 2.72 | 90.7 | 3.67 | 2.99 | 99.6 | 8.15 |
| 30 | 27.7 | 92.3 | 2.59 | 31.6 | 105 | 8.71 |
| 300 | 270 | 90.0 | 3.27 | 309 | 103 | 8.15 |
Accuracy (%) was determined as mean measured concentration/nominal concentration × 100% Precision (%CV) was determined as standard deviation/mean × 100%
3.2.2 Carry over, matrix effect and recovery
No carryover effects were observed as indicated by lack of signal response in the blank plasma sample injected following the injection of the highest standard sample (data not shown). Matrix effects evaluated at four QC levels ranged from 92–99%, suggesting that matrix components have little effects on verticillin A signal. The recovery ranged from 90–101% (Table 2) for four tested levels, indicating efficient and high recovery of the method.
Table 2.
Recovery & matrix effects for verticillin A in mouse plasma (n=3)
| Nominal conc. (nM) | Recovery | Matrix effect | ||
|---|---|---|---|---|
|
|
|
|||
| % nominal conc. | %CV | % nominal conc. | %CV | |
| 1 | 101 | 4.31 | 92 | 8.88 |
| 3 | 95 | 12.8 | 97 | 10.4 |
| 30 | 95 | 5.82 | 94 | 6.97 |
| 300 | 90 | 5.15 | 99 | 4.99 |
%CV was determined as standard deviation/mean × 100%
3.2.3. Stability
Verticllin A was found to be stable in mouse plasma under different tested conditions with the deviations of the measured concentration < 10% from the nominal concentration (Table 3), indicating no significant loss of verticillin A and IS under experimental conditions.
Table 3.
Stability of verticillin A in mouse plasma under different storage conditions (n=3)
| Nominal conc. (nM) | Measured concentration (nM)a | |||
|---|---|---|---|---|
|
| ||||
| Freeze-thaw | Benchtop on ice 4 h | Autosampler 12 h (4°C) | one month storage (− 80°C) | |
| 1 | 0.93 ± 0.04 | 0.91 ± 0.02 | 0.95 ± 0.03 | 1.05 ± 0.06 |
| 3 | 3.17 ± 0.06 | 2.87 ± 0.29 | 2.77 ± 0.19 | 3.32 ± 0.09 |
| 30 | 32.4 ± 0.88 | 28.4 ± 1.37 | 29.3 ± 1.42 | 32.2 ± 0.39 |
| 300 | 310 ± 6.8 | 280 ± 16 | 268 ± 19 | 324 ± 2.0 |
: Mean ± standard deviation of three measurements
3.3. Pharmacokinetic analysis and bioavailability
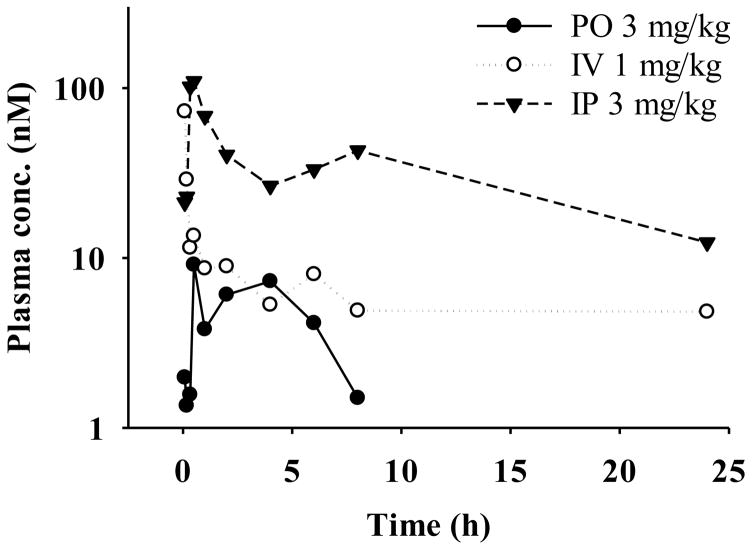
The plasma concentration-time profiles of verticillin A were determined after a single IV dose of 1 mg/kg or a single IP or PO dose of 3 mg/kg (Fig. 3), and the pharmacokinetic parameters were estimated by noncompartmental analysis (Table 4). Following IV administration, the maximum observed plasma concentration (Cmax) was 72.9 nM and occurred at our first sample time of 5 minutes. The IV data demonstrated a rapid distribution phase where plasma concentrations declined below 10 nM within 1 hour followed by a terminal phase with a long half-life of approximately 20 hours suggesting a slow elimination rate. IP dosing showed similar kinetics with a 110 nM Cmax and a terminal phase half-life of approximately 11 hours. Plasma levels were sustained above 10 nM throughout the 24 hour period after IP dosing. The observed Cmax levels with both IV and IP dosing, and the higher sustained plasma concentrations after IP dosing indicate active concentrations are at least achieved in plasma in vivo. This is based on prior in vitro studies demonstrating activity in the 20–50 nM range [10, 22] and 24-hour IC50s of 30–122 nM in an array of cancer cell types [2, 18]. Oral administration of verticillin A at 3 mg/kg resulted in Cmax of 9.13 nM at 0.5 h. The absolute oral bioavailability (F%) was determined to be approximately 9%, based on the areas under the concentration vs. time curves from time zero to last measurable time point (AUClast) values estimated from IV and PO administration. Following IP administration, the systemic availability was approximately 170%. The calculated bioavailability above 100% is likely a result of the limited data from single mice per time point and the fact that the earliest sample collected was at 5 minutes when plasma concentrations are rapidly declining after IV administration (i.e. a significant portion of the IV AUC likely occurs prior to the 5 minute time point). Despite the challenges of this study due to limited supply of this natural product, this study reports the first in vivo pharmacokinetic data for verticillin A.
Fig. 3.
Plasma concentration-time profiles of verticillin A in mice following IP, IV, and PO administration
Table 4.
Pharmacokinetic parameters of verticillin A following IP, IV, and PO administration to mice
| Parameter | IV, 1 mg/kg (1.43 μmol/kg) | IP, 3 mg/kg (4.30 μmol/kg) | PO, 3 mg/kg (4.30 μmol/kg) |
|---|---|---|---|
| Cmax (nM) | 72.9 | 110 | 9.13 |
| AUClast (nM·h) | 152 | 775 | 39.9 |
| AUC0-∞_obs | 290 | 970 | 43.7 |
| T1/2 (h) | 19.91 | 10.92 | 1.75 |
| Tmax (h) | 0.08 | 0.5 | 0.5 |
| CL (L/h/kg)a | 4.94 | 2.61 | 98.45 |
| Vd (L/kg)b | 142 | 70 | 249 |
| F (%) | 100 | 170 | 8.76 |
: Clearance; CL/F for IP and PO administration
: Volume of distribution; CL/F for IP and PO administration
AUC0-∞_obs, area under the observed concentration-time curve extrapolated to infinity
4. Conclusions
Verticillin A is a natural product isolated from fungal cultures. Despite the challenges in harvesting ample quantities of this agent, it has gained a great deal of interest from cancer researchers due to its potent anticancer activity in vitro [2] and in vivo [18–20] as well as its selectivity as an HMTase inhibitor to suppress colon cancer cell immune escape and chemoresistance [10]. Herein we present the development and validation of a sensitive LC–MS/MS assay for quantification of verticillin A in mouse plasma with linearity from 1 – 1000 nM. The assay demonstrates within- and between-day precision < 9% and accuracy ranging from 90% to 105%. Despite limited supplies of this agent, we harvested enough compound to administer 1 mg/kg (intravenous) or 3 mg/kg (oral and intraperitoneal) doses of verticillin A to 30 ICR mice and applied the LC-MS/MS assay to measure plasma concentrations after the mice were euthanized at 10 time points in each dosing group. While these data are limited, this study provides the first glimpse of the pharmacokinetic behavior of verticillin A after PO, IP and IV administration in mice. The relatively high exposures after IP and IV dosing suggest these are suitable routes of administration for further in vivo efficacy studies of verticillin A.
Highlights.
Verticillin A is a natural product in limited supply.
Verticillin A has demonstrated potent anti-infective and anticancer properties.
A sensitive and specific LC-MS/MS method was developed to quantify verticillin A.
The method was applied to characterize verticillin A pharmacokinetics in mice.
Verticillin A achieves active concentrations in vivo following IV or IP dosing
Acknowledgments
This research was supported in part by the National Institutes of Health/National Cancer Institute grant [P01 CA125066]. The authors thank Dr. Amninder Kaur, Soumia Amrine, and Blaise Darveaux for assistance in the re-isolation of verticillin A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Du L, Robles AJ, King JB, Mooberry SL, Cichewicz RH. Cytotoxic Dimeric Epipolythiodiketopiperazines from the Ascomycetous Fungus Preussia typharum. Journal of Natural Products. 2014;77:1459–1466. doi: 10.1021/np5002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Figueroa M, Graf TN, Ayers S, Adcock AF, Kroll DJ, Yang J, Swanson SM, Munoz-Acuna U, Carcache de Blanco EJ, Agrawal R, Wani MC, Darveaux BA, Pearce CJ, Oberlies NH. Cytotoxic epipolythiodioxopiperazine alkaloids from filamentous fungi of the Bionectriaceae. The Journal of antibiotics. 2012;65:559–564. doi: 10.1038/ja.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minato H. Studies on the metabolites of Verticillium sp. structures of Verticillins A, B, and C. Journal of the Chemical Society Perkin Transactions 1: Organic and Bio-organic Chemistry. 1973;17:1819. doi: 10.1039/p19730001819. [DOI] [PubMed] [Google Scholar]
- 4.Boyer N, Morrison KC, Kim J, Hergenrother PJ, Movassaghi M. Synthesis and anticancer activity of epipolythiodiketopiperazine alkaloids. Chemical Science. 2013;4:1646–1657. doi: 10.1039/C3SC50174D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Movassaghi M. Biogenetically inspired syntheses of alkaloid natural products. Chemical Society Reviews. 2009;38:3035–3050. doi: 10.1039/b819925f. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Movassaghi M. Biogenetically-Inspired Total Synthesis of Epidithiodiketopiperazines and Related Alkaloids. Accounts of Chemical Research. 2015;48:1159–1171. doi: 10.1021/ar500454v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox EM, Howlett BJ. Biosynthetic gene clusters for epipolythiodioxopiperazines in filamentous fungi. Mycological Research. 2008;112:162–169. doi: 10.1016/j.mycres.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Miao ZH, Zhao WM, Ding J. The p53 pathway is synergized by p38 MAPK signaling to mediate 11,11′-dideoxyverticillin-induced G2/M arrest. FEBS Letters. 2005;579:3683–3690. doi: 10.1016/j.febslet.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 9.Müllbacher A, Waring P, Tiwari-Palni U, Eichner RD. Structural relationship of epipolythiodioxopiperazines and their immunomodulating activity. Molecular Immunology. 1986;23:231–235. doi: 10.1016/0161-5890(86)90047-7. [DOI] [PubMed] [Google Scholar]
- 10.Paschall AV, Yang D, Lu C, Choi JH, Li X, Liu F, Figueroa M, Oberlies NH, Pearce C, Bollag WB, Nayak-Kapoor A, Liu K. H3K9 Trimethylation Silences Fas Expression To Confer Colon Carcinoma Immune Escape and 5-Fluorouracil Chemoresistance. Journal of immunology. 2015;195:1868–1882. doi: 10.4049/jimmunol.1402243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts Katharine R. Assessing the trypanocidal potential of natural and semi-synthetic diketopiperazines from two deep water marine-derived fungi. Bioorganic Medicinal Chemistry. 2010;18:2566. doi: 10.1016/j.bmc.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katagiri K. Verticillin A, a new antibiotic from Verticillium sp. Journal of Antibiotics The. 1970;23:420. doi: 10.7164/antibiotics.23.420. [DOI] [PubMed] [Google Scholar]
- 13.Minato H. Verticillin A, a new antibiotic from Verticillium sp. Journal of the Chemical Society C: Organic. 1971:44. [Google Scholar]
- 14.Liu F. Verticillin chloroform solvate. Acta Crystallographica Section E. 2006;62:974. [Google Scholar]
- 15.Jordan TW, Cordiner SJ. Fungal epipolythiodioxopiperazine toxins have therapeutic potential and roles in disease. Trends in Pharmacological Sciences. 1987;8:144–149. [Google Scholar]
- 16.Dong JY, He HP, Shen YM, Zhang KQ. Nematicidal Epipolysulfanyldioxopiperazines from Gliocladium roseum. Journal of Natural Products. 2005;68:1510–1513. doi: 10.1021/np0502241. [DOI] [PubMed] [Google Scholar]
- 17.Chu M. Inhibition of c-fos proto-oncogene induction by Sch 52900 and Sch 52901, novel diketopiperazines produced by Gliocladium sp. Journal of Antibiotics, The. 1995;48:1440. doi: 10.7164/antibiotics.48.1440. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Liu Q, Yang D, Bollag WB, Robertson K, Wu P, Liu K. Verticillin A overcomes apoptosis resistance in human colon carcinoma through DNA methylation-dependent upregulation of BNIP3. Cancer research. 2011;71:6807–6816. doi: 10.1158/0008-5472.CAN-11-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zewdu A, Lopez G, Braggio D, Kenny C, Constantino D, Bid HK, Batte K, Iwenofu OH, Oberlies NH, Pearce CJ, Strohecker AM, Lev D, Pollock RE. Verticillin A Inhibits Leiomyosarcoma and Malignant Peripheral Nerve Sheath Tumor Growth via Induction of Apoptosis. Clinical & experimental pharmacology. 2016;6 doi: 10.4172/2161-1459.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu C, Paschall AV, Shi H, Savage N, Waller JL, Sabbatini ME, Oberlies NH, Pearce C, Liu K. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. Journal of the National Cancer Institute. 2017;109 doi: 10.1093/jnci/djw283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paguigan Noemi D, Kao Diana, Raja Huzefa A, Pearce Cedric J, Oberlies Nicholas H. Enhanced dereplication of fungal cultures via use of mass defect filtering. The Journal of antibiotics. 2017 doi: 10.1038/ja.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paschall AV, Liu K. Epigenetic Regulation of Apoptosis and Cell Cycle Regulatory Genes in Human Colon Carcinoma Cells. Genomics data. 2015;5:189–191. doi: 10.1016/j.gdata.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]