Abstract
After unresolved endoplasmic reticulum stress, recovery of protein synthesis including increased expression of ribosomal components and translation factors may induce cell death. Using a mouse model of moderate contusive spinal cord injury (SCI) at the T9 level, upregulation of ribosomal biogenesis was observed in the injury epicenter at 24 h after trauma. Such upregulation coincided with endoplasmic reticulum stress response as previously reported in this model. It was also accompanied by changes in expression of many other genes associated with translational regulation. Systemic treatment with a pharmacological inhibitor of RNA-Polymerase-1, BMH-21 reduced rRNA transcription in the spinal cord. Moreover, in the injury epicenter, treatment with BMH-21 increased expression of oligodendrocyte-specific transcripts including Mbp and Cldn11 at 3 days post injury. Although such findings may suggest at least transient reduction of oligodendrocyte death, locomotor outcome was mostly unaffected except slightly accelerated recovery of hindlimb function at week 2 post-injury. Therefore, at least in mice, RNA-Polymerase-1 does not appear to be a robust target for therapies to protect spinal cord tissue after contusion. However, these findings raise an interesting possibility that altered rate of ribosomal biogenesis contributes to the apparent translational reprogramming after contusive SCI. Such a reprogramming could be a major regulator of SCI-induced gene expression.
Keywords: Ribosomal biogenesis, spinal cord injury, white matter loss, cell death, neuroprotection, pharmacotherapy
Introduction
Impairment of endoplasmic reticulum (ER) function leads to ER stress. Cells respond to such a challenge with the conserved ER stress response (ERSR) whose primary goal is to restore ER homeostasis. However, if that is not possible, the pro-apoptotic arm of the ERSR induces apoptosis. Recent work documented that the ERSR is activated after SCI and that its genetic or pharmacological modulation protects white matter, prevents oligodendrocyte apoptosis and improves functional recovery after trauma [20, 21].
The transcription factors CCAAT-enhancer-binding protein homologous protein (Chop/Ddit3) and activating transcription factor 4 (Atf4) are recognized as principal mediators of the ERSR-induced cell death. Recent work has identified an unorthodox mechanism by which Chop and Atf4 kill cells with a dysfunctional ER [13]. While global inhibition of protein synthesis is the early response to ER stress, relieving the ER from excessive protein overload, the subsequent activation of Atf4 and Chop increases global protein synthesis. If ER homeostasis has not been yet restored, the resulting protein overload of the ER induces oxidative stress, mitochondrial damage and cell death. Mechanisms that were implicated in such a toxic wave of translation include increased amino acid import, enhanced tRNA synthetase activity, activation of mTOR, and an increased supply of ribosomes [12, 17][13].
Protein synthesis is carried out by ribosomes whose cellular content appears to limit cellular capacity of translation [7, 9]. Thus, in many situations that require increased protein synthesis, like proliferation or cell hypertrophy, ribosome production is enhanced [5, 7, 9]. Intriguingly, many ribosomal biogenesis-associated genes are up-regulated by Atf4 and/or Chop, suggesting that increased ribosome supply contributes to the translational catastrophe after lethal ER stress [13]. Indeed, the potentially deleterious role of ribosomal biogenesis in ER stress-exposed cells has been documented in yeast [24].
The RNA polymerase I (Pol1)-driven transcription of ribosomal RNA (rRNA) initiates ribosome synthesis[15]. Pol1 is the major regulator of ribosome production. As cancer cells hijack ribosome biogenesis to fuel their growth, Pol1 became a target of novel anti-cancer drugs including the clinically tested CX-5461 and CX-3543 or the recently identified BMH-21 [6, 8, 10, 23]. If applied transiently, such agents kill cancer, but not normal, cells. While Pol1 activity is reduced immediately after induction of ER stress, ribosome synthesis resumes at later time points with many ribosomal component genes being upregulated by Atf4/Chop [11][13]. Although cells with reduced capacity of ribosome synthesis are more resistant to ER stress [13, 24], emerging pharmacological inhibitors of Pol1 have not been tested for their therapeutic potential in ER stress diseases such as SCI. The current study was initiated to examine whether ribosomal biogenesis is regulated after SCI and whether Pol1 inhibitors may affect SCI outcome.
Methods
Animals
Adult (8–10 weeks old) C57Bl/6 female mice were obtained from Harlan (Indianapolis, IN) and gentled for 5–7 days before experiments. All animal experiments strictly followed a protocol that was approved by University of Louisville Institutional Animal Care and Use Committee.
Spinal cord injury
Spinal cord injury was performed as described previously [21]. Briefly, animals were anaesthetized by an i.p. injection of 250 mg/kg body weight avertin. Gentamycin (50 mg/kg; Boehringer Ingelheim, St. Joseph, MO) was administered subcutaneously to reduce infection. Moderate contusion injuries (50 kdyn force/400–600 μm displacement) were performed using the IH impactor (Infinite Horizons, Lexington, KY) following a laminectomy at the T9 vertebrae. In SCI studies with BMH-21, comparison of actual injury force readings and tissue displacements revealed no significant differences between experimental groups (supplementary Table S1). Therefore, similar injury severity was consistently obtained using such a methodology. In the SCI pre-rRNA analysis experiment, controls included sham animals that received only a T9 laminectomy.
RNA extraction and analysis
RNA extraction and analysis was accomplished using standard techniques [21][16]. Briefly, total RNA was be extracted from spinal cord tissue at the injury epicenter (a 3 mm-long segment spanning the injury site) using Trizol (Invitrogen, Carlsbad, CA). Following cDNA synthesis with random hexamers qRT-PCR was performed using Syber-Green DNA dye (pre-rRNA/18S rRNA) or Taq-man universal PCR master mix (Mbp, Cldn11, Gfap, Gapdh). The following primers were used: pre-rRNA forward- ctcctctctcgcgctctctgtc, reverse- gcatggcttaatctttgagacaagca; 18S rRNA forward- gttggttttcggaactgaggc, reverse- gtcggcatcgtttatggtcg; Assay on Demand primers for Mbp, Claudin11, Gfap and Gapdh were described previously [21]. RNA levels were quantified using the ΔΔCT method; reference genes were as indicated.
BMH-21 treatment
BMH-21 was dissolved in 20 mM citrate buffer (pH 6.0) and administered by intraperitoneal injections (0.1–0.2 ml/injection) as indicated. Vehicle controls were also included. Detailed experimental design of SCI studies with BMH-21 is presented in supplementary Table S1.
Oligodendrocyte Precursor Cell (OPC) culture
Adult rat spinal cord OPCs were prepared and cultured as described [26]. Their survival was monitored by MTT assay following a standard protocol [14].
Nucleophosmin-1 (Npm1/B23) immunofluorescence
Nucleophosmin-1 (Npm1/B23) immunofluorescence was performed using a mouse monoclonal antibody (Sigma) as previously reported [16].
Locomotor function
Locomotor function was evaluated as previously reported [21]. Briefly, Open field Basso Mouse Scale (BMS)] locomotor analyses [3] were performed at baseline scores and weekly following SCI for 5–6 weeks. All raters were trained by Dr. Basso and colleagues at the Ohio State University and were blinded to animal groups.
Meta-analysis of RNA microarray data
Publicly available data from Affymetrix RNA microarray analysis of injury epicenter region after moderate contusive SCI (A. Faden, NCBI’s GEO GSE5296, all experiments in C57Bl6 female mice) were accessed and lists of 2167 (4 h post-SCI) or 2819 (24 h) or 4652 (72 h post SCI, data not shown) significantly affected genes (p<0.01) were retrieved by comparing injury epicenter regions (n=3) to sham controls (n=4 including 2 controls from the same time point as SCI + 2 additional controls from the closest time point to that of SCI; such strategy was used as only 2 sham controls were available for each time point). NCBI’s DAVID was used to analyze gene ontology term (GO) enrichment among affected genes. FDR (q value) was use as a primary measure of GO enrichment.
Statistical analysis
Result of qRT-PCR and MTT survival assay were analyzed using the non-parametric u-test as limited n number (3–4/group) precluded normality testing. BMS data were analyzed by repeated measure ANOVA and Tukey post-hoc tests; one-way ANOVA was used to analyze SCI force and tissue displacement data.
Results
Our prior work has documented contusive SCI-associated ER stress including increased mRNA expression of cytotoxic transcription factors Atf4 and Chop in the injury epicenter at 6- and 24 h after contusion [21]. We used the same total RNA material to investigate SCI effects on expression of pre-rRNA. Due to relatively rapid processing, pre-rRNA is a good indicator of Pol1 activity which, by transcribing rRNA genes, mediates the critical step of ribosomal biogenesis [9]. After SCI, higher pre-rRNA levels were observed at 24- and 72 h post injury (Fig. 1A). The maximal increase of 1.4-fold sham controls was seen 24 h post injury. Such a dynamic pattern fits a model of ribosomal biogenesis being under Atf4/Chop-dependent regulation following SCI.
Figure 1. SCI-associated upregulation of ribosomal biogenesis.
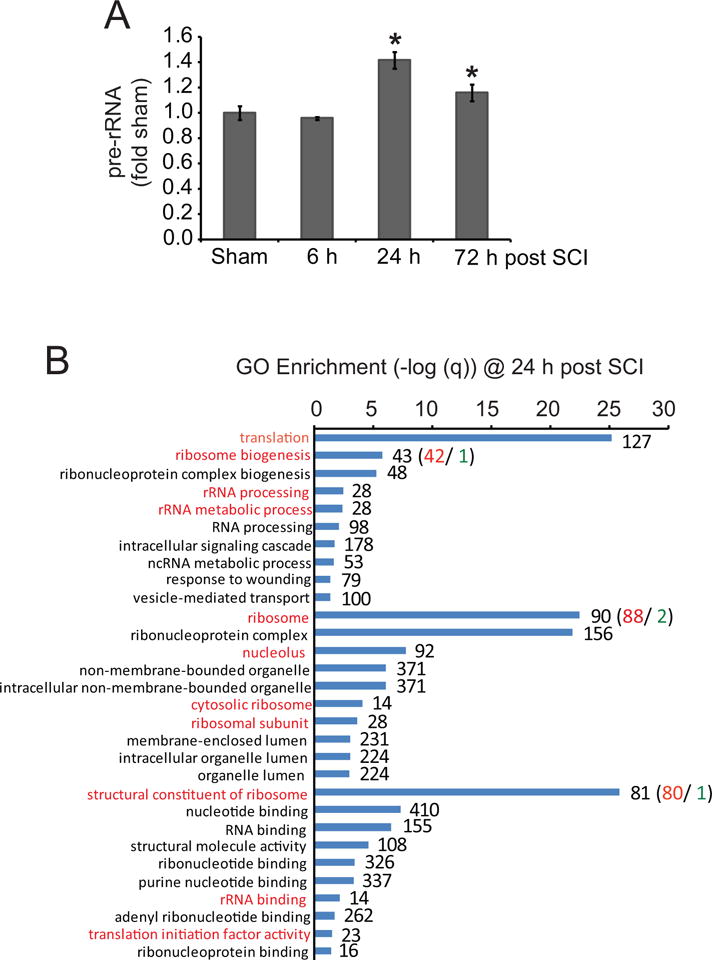
A. Moderate SCI was performed in female mice using IH Impactor, n=4 per group (50 kdyn). Pre-rRNA levels were determined by qRT-PCR with normalization against 18S rRNA. The increases at 24- and 72 h post-injury were significant (u-test, p<0.05, *). Error bars depict SEMs. B. Meta-analysis of microarray data from moderate contusive mouse SCI (T8 level, GEO GSE5296). In the injury epicenter, 2819 significantly affected mRNA transcripts were identified at 24 h (n=3 or 4 for SCI-, and, sham control animals, respectively, p<0.01). Gene ontology terms (GOs) related to translational regulation and ribosomal biogenesis were highly enriched (red-marked GOs). For ribosomal biogenesis-associated GOs, most affected transcripts were upregulated. The graph presents –log (q) values for 10 top enriched GOs from each of the following categories: “biological process”, “cellular component”, and “molecular function”; red or green numbers indicate numbers of upregulated or downregulated mRNAs, respectively. Results of similar analyses for 4 h and 72 h suggest that the ribosomal biogenesis enrichment is specific to 24 h (Fig. S1).
To further explore SCI effects on ribosomal biogenesis, meta-analysis of publicly available data from RNA microarray studies of mouse contusive SCI was performed. A data set from Dr. Alan Faden’s laboratory was chosen as it was obtained from similar animals (C57Bl6 mice, moderate contusion at the T8 level) and covered similar/identical time points to those of our study (accession number GSE5296) [22]. Thus, gene ontology (GO) analysis of significantly affected genes (p<0.01) from that data set revealed that translation, ribosomal biogenesis as well as ribosomal/nucleolar components were among top-enriched GO terms at 24 h post injury (Fig. 1B). When data were analyzed individually for each ribosome-related gene, upregulation was the dominant trend (Fig. 1B). While the GO term nucleolus was enriched at 4 h, no enrichment of ribosomal/nucleolar/translation-associated GOs was observed at 72 h post injury (Fig. S1). Therefore, in the injured spinal cord, ribosomal biogenesis appears to be transiently upregulated. At 24 h post injury, such upregulation is extensive with top enriched GOs being related to that process.
To investigate the significance of increased ribosomal biogenesis for SCI outcome, the Pol1 inhibitor BMH-21 was used. As functional deficits after thoracic SCI are mostly due to oligodendrocyte death and white matter damage [19–21], cultured OPCs were treated with BMH- 21 to assess its ability to affect oligodendrocytic Pol1. Consistent with robust inhibition of the latter enzyme, immunofluorescence staining for the nucleolar marker nucleophosmin-1 (Npm1/B23) revealed its nucleoplasmic diffusion in response to treatment with 50 nM BMH-21 (Fig. 2A). Such a release of nucleolar Npm1 is a well established morphological marker of Pol1 inhibition [6, 23]. Although in some cells, including immature neurons, inhibition of Pol1 may lead to apoptosis, BMH-21 was relatively non-toxic to OPCs (Fig S2). When injected intraperitoneally into naïve mice, BMH-21 lowered pre-rRNA levels in the spinal cord and the hippocampus suggesting its ability to cross the blood-brain/spinal cord barrier and inhibit ribosomal biogenesis in the CNS (Fig. 2B and data not shown). This is in contrast to another Pol1 inhibitor CX-5461 that was tested in a parallel experiment (data not shown).
Figure 2. Reduction of ribosomal biogenesis with a Pol1 inhibitor BMH-21 has only minor effects on locomotor recovery after moderate contusive SCI.
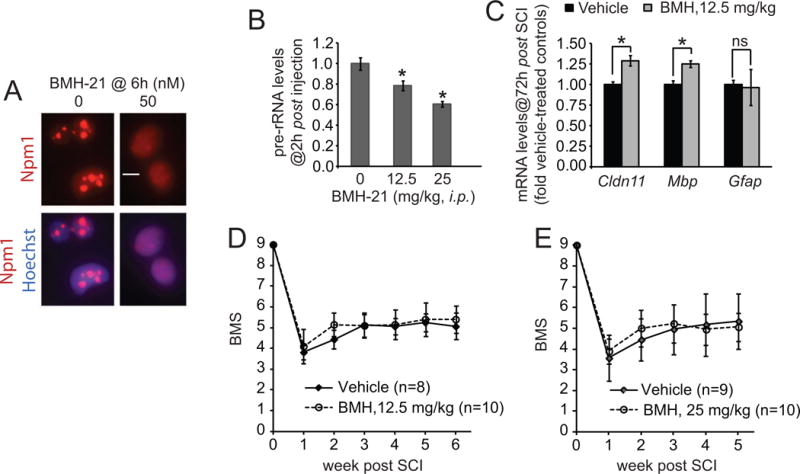
A, Rat OPCs were treated as indicated. Immunostaining for the nucleolar marker nucleophosmin (Npm1, red) revealed its dispersion in BMH-treated cells, confirming successful inhibition of Pol1. However, 24 h treatment with BMH-21 did not affect OPC survival (Fig. S2). B, A single i.p. injection of BMH-21 reduced the levels of pre-rRNA in the mouse spinal cord, n=4/group, 2 h after injection, 18S rRNA was used for normalization. C–E, C57Bl/6 females received a T9 50 kdyn SCI followed by i.p. injection of BMH-21 at 1 h and 24 h post SCI; in E, an additional BMH-21 injection was given at 48 h (detailed experimental design and SCI contusion parameters are presented in Table S1). C, Gapdh-normalized expression of glial-specific mRNAs in the injury epicenter was assessed by qRT-PCR. Note higher levels of oligodendrocytic mRNAs (Cldn11 and Mbp) in BMH-21-treated mice; n=3/group. D–E, In two separate studies, BMS analysis of hindlimb locomotion revealed no significant differences in recovery between vehicle- and BMH-treated mice (repeated measure two-way ANOVA, factor 1: time after injury, p<0.001, factor 2: BMH-21, p>0.05, factor 1*factor 2, p>0.05). However, when data from both studies were combined for weeks 1-5, the interaction between time after treatment and BMH-21 became significant suggesting slight acceleration of recovery as pointed by higher average BMS scores of BMH-21-treated groups at week 2 (see text for more details). Error bars represent SD; in B and C, *, p<0.05; ns, p>0.05, u-test.
To target SCI-associated stimulation of ribosomal biogenesis, mice were treated with i.p. injections of BMH-21 at 1 and 24 h post-SCI. These time points were chosen based on findings that SCI-mediated activation of ribosomal biogenesis peaks at 24 h after injury (Fig. 1 and Fig. S1). As 48–72 h post-SCI expression of oligodendrocyte specific mRNAs correlates well with oligodendrocyte protection [20, 21], BMH-21 effects on such marker mRNAs were evaluated. At least two of them, Cldn11 and Mbp, but not the astrocytic marker glial fibrillary acidic protein (Gfap), were significantly higher in the injury epicenter of BMH-21-treated animals. The observed increases of about 25% were similar in magnitude to those observed with the ERSR inhibitor salubrinal [20].
However, BMH-21 did not have any significant effects on recovery of hindlimb locomotion as evaluated in two independent studies using two different doses of the drug (Fig. 2D–E). In both cases, it was noted that BMH-21-treated animals appeared to recover slightly faster with about 0.5 differences of the BMS score at week two after injury. When combined data from both studies (weeks 1–5) was used for statistical analysis, that acceleration of recovery, although small, became significant (two-way repeated measure ANOVA, interaction for factor 1: BMH-21 treatment, factor 2: time after injury, F4,140=4.395, p<0.00223). Therefore, SCI-associated increase of ribosomal biogenesis does not play a major role in chronic locomotor outcome.
Discussion
Consistent with the concept that in the injury epicenter, the SCI-associated ER stress enhances ribosomal biogenesis, increased levels of pre-rRNA and upregulation of mRNAs for many ribosome components were observed. This SCI response was transient with a peak at 24 h post injury. Moreover, it followed induction of ER stress mediators including the pro-ribosomal transcription factors Atf4 and Chop whose mRNA levels were up as early as 6 h post injury [21]. Therefore, after SCI, increased ribosomal biogenesis is likely associated with ER stress-driven secondary injury. In this context, one should note that while it followed activation of the cytotoxic ER stress transcription factors, it occurred as spinal cord cells continued to die [4, 18]. Hence, one could consider a possibility that transient stimulation of ribosomal biogenesis is among cytotoxic mechanisms of SCI.
In support of the latter notion, an increase in oligodendrocyte-specific mRNAs was observed in animals, which, after moderate SCI, were treated with the Pol1 inhibitor BMH-21. However, although oligodendrocytes are highly sensitive to ER stress and their death/survival determines white matter sparing and locomotor outcome of contusive SCI [19–21], BMH-21 did not have major effects on locomotor recovery. Hence, one could speculate that BMH-21 merely delayed oligodendrocyte death without producing lasting white matter protection or persisting improvement of locomotor function. As multiple mechanisms were proposed to contribute to translational catastrophe after cytotoxic ER stress, it is conceivable that antagonizing Pol1 alone was insufficient to prevent it. However, optimization of the dosing regimen could improve the results.
Noteworthy, mechanisms of translational recovery from ER stress include Pp1/Gadd34-mediated removal of the inhibitory phosphorylation of eIF2α at the Ser-51 residue. Pharmacological inhibition of Pp1/Gadd34 with salubrinal improved oligodendrocyte survival and locomotor recovery in a mouse SCI model [20]. Therefore, one could wonder whether a combinatorial anti-translational therapy with salubrinal and BMH-21 could have greater beneficial effects that those of salubrinal alone.
While ribosomal biogenesis is an obvious therapeutic target in cancer, a concept of its therapeutic inhibition to improve outcome of neurological diseases has started to emerge only recently. Thus, RNA microarray analysis revealed suppression of the ribosomal biogenesis pathway in peripheral blood monocytes of patients with benign MS [1]. Based on these findings, it has been proposed that highly active ribosomal biogenesis fuels neuroinflammation. Indeed, in mice, the blood brain barrier non-permeable Pol1 inhibitor CX-5461 attenuated the severity of experimental autoimmune encephalomyelitis (EAE) improving locomotion and reducing expansion of the auto-reactive T cells [2]. In addition, reduced demyelination was also observed. As the blood-CNS barrier is often compromised in neuroinflammatory diseases such as MS or EAE, one can consider a possibility that in addition to reducing inflammatory phenotype of T cells, CX-5461 had also at least some direct beneficial effects on neuroinflammation-challenged oligodendrocytes including attenuation of the cytotoxic ER stress response [25].
Several factors may have contributed to different outcomes of the current SCI study and that on EAE. First, unlike neuroinflammation-driven EAE, contusive SCI has a complex pathogenesis that involves direct axonal damage, hypoxia-ischemia, excitotoxicity and neuroinflammation. Interplay of those additional pathogenic factors could affect which cytotoxic effector mechanisms are key players in injury-associated white matter loss. Second, in EAE study, CX5461 was administered chronically over a period of 2 weeks. Hence, 3 day treatment with BMH-21 may have not been long enough to produce a major beneficial effect in SCI.
Impairment of ribosomal biogenesis has been documented in various neurodegenerative diseases including Alzheimer’s, Parkinson’s, Huntington’s and, recently, ALS. Moreover it has been proposed that such impairment may contribute to neuronal death that is observed in these conditions. Therefore, a valid concern exists that Pol1 inhibitors may be neurotoxic. However, current work suggests relative safety of at least transient inhibition of spinal cord Pol1. Thus, despite BMH-21’s ability to reach the CNS, it did not worsen locomotor outcome of SCI. Moreover, although OPCs are known to be very sensitive to various toxins including anti-cancer drugs, BMH-21 was relatively non-toxic to these cells. While more studies are needed to fully evaluate effects of Pol1 inhibitors on CNS cells, these drugs could be considered as a therapy for CNS diseases. In particular, Pol1 inhibitor may be beneficial in conditions, in which hyperactivation of ribosomal biogenesis likely contributes to their pathology including brain tumors and/or epilepsy.
Besides testing restoration of ER homeostasis what could be other roles of SCI-associated increase in ribosome synthesis? An obvious possibility is that the increase is part of the tissue repair response including proliferation of reactive glial cells. However, consistent with the presented GO analysis of the data set (Fig. S1B), the latter response peaks at 3–4 day post injury after the peak changes in ribosomal biogenesis pathway [18]. Therefore, it is unlikely that the SCI-associated stimulation of ribosomal biogenesis is only a secondary component of the injury-induced cell proliferation program. As ribosomal diversification becomes a recognized regulatory mechanism of gene expression, one could consider a possibility that SCI stimulates production of new ribosome subtypes to enable optimal translation of transcripts that participate in such SCI responses as cell death, reactive gliosis, and/or neuronal plasticity [28]. A possibility that the SCI response involves sequential activation of gene transcription and then mRNA translation fits with predominant enrichment of transcription-related GOs at 4 h post injury (Fig. S1). Finally, one should note that meta-analysis of microarray data from thoracic trans-section rat SCI revealed similar transient upregulation of ribosomal biogenesis [27]. Hence translational reprogramming may be a conserved component of the SCI response.
Taken together, while ribosomal biogenesis is acutely upregulated after mouse contusive SCI, its transient general inhibition with the Pol1 inhibitor BMH-21 has minor effects on locomotor outcome. Therefore, although increased ribosome synthesis may contribute to cytotoxicity of ER stress, it does not appear as a major mediator of ER stress-associated white matter damage following mouse contusive SCI. Hence, more refined approaches are needed to evaluate pathogenic significance of SCI-induced ribosomal biogenesis including testing functional consequences of other anti-ribosomal drugs and defining their effects on the whole translome of the contused spinal cord.
Supplementary Material
Highlights.
Ribosomal biogenesis is acutely upregulated after mouse contusive spinal cord injury.
The upregulation coincided with spinal cord injury associated ER stress.
New ribosomes may support cytotoxic recovery of translation after ER stress.
However, inhibition of ribosomal biogenesis has minor effects on locomotor outcome.
Hence, ribosomal biogenesis is not the critical cytotoxic effector of spinal cord injury.
Acknowledgments
This work was supported by the National Institutes of Health (NS073584, 8P30GM103507 to MH and SRW; CA172069 and CA193637 to ML), and the Commonwealth of Kentucky Challenge for Excellence Fund. The authors wish to thank Ms. Jing-Juan Zheng for excellent technical assistance.
Abbreviations
- Atf4
activating transcription factor 4
- BMS
Basso mouse scale
- Chop
CCAAT-enhancer-binding protein homologous protein
- EAE
experimental autoimmune encephalomyelitis
- ERSR
endoplasmic reticulum stress response
- GO
gene ontology
- MS
multiple sclerosis
- mTOR
mechanistic target of rapamycin
- Npm1
nucleophosmin-1
- OPC
oligodendrocyte precursor cell
- Pol1
RNA-Polymersase-1
- qRT-PCR
quantitative reverse transcriptase PCR
- SCI
spinal cord injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Achiron A, Feldman A, Magalashvili D, Dolev M, Gurevich M. Suppressed RNA-polymerase 1 pathway is associated with benign multiple sclerosis. PLoS One. 2012;7:e46871. doi: 10.1371/journal.pone.0046871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achiron A, Mashiach R, Zilkha-Falb R, Meijler MM, Gurevich M. Polymerase I pathway inhibitor ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2013;263:91–97. doi: 10.1016/j.jneuroim.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 4.Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandenburger Y, Jenkins A, Autelitano DJ, Hannan RD. Increased expression of UBF is a critical determinant for rRNA synthesis and hypertrophic growth of cardiac myocytes. Faseb J. 2001;15:2051–2053. doi: 10.1096/fj.01-0853fje. [DOI] [PubMed] [Google Scholar]
- 6.Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, Wall M, Cluse L, Drygin D, Anderes K, Huser N, Proffitt C, Bliesath J, Haddach M, Schwaebe MK, Ryckman DM, Rice WG, Schmitt C, Lowe SW, Johnstone RW, Pearson RB, McArthur GA, Hannan RD. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51–65. doi: 10.1016/j.ccr.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaillou T, Kirby TJ, McCarthy JJ. Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. J Cell Physiol. 2014;229:1584–1594. doi: 10.1002/jcp.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drygin D, Lin A, Bliesath J, Ho CB, O’Brien SE, Proffitt C, Omori M, Haddach M, Schwaebe MK, Siddiqui-Jain A, Streiner N, Quin JE, Sanij E, Bywater MJ, Hannan RD, Ryckman D, Anderes K, Rice WG. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011;71:1418–1430. doi: 10.1158/0008-5472.CAN-10-1728. [DOI] [PubMed] [Google Scholar]
- 9.Drygin D, Rice WG, Grummt I. The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol. 2010;50:131–156. doi: 10.1146/annurev.pharmtox.010909.105844. [DOI] [PubMed] [Google Scholar]
- 10.Drygin D, Siddiqui-Jain A, O’Brien S, Schwaebe M, Lin A, Bliesath J, Ho CB, Proffitt C, Trent K, Whitten JP, Lim JK, Von Hoff D, Anderes K, Rice WG. Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Cancer Res. 2009;69:7653–7661. doi: 10.1158/0008-5472.CAN-09-1304. [DOI] [PubMed] [Google Scholar]
- 11.DuRose JB, Scheuner D, Kaufman RJ, Rothblum LI, Niwa M. Phosphorylation of eukaryotic translation initiation factor 2alpha coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol Cell Biol. 2009;29:4295–4307. doi: 10.1128/MCB.00260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan BJ, Krokowski D, Majumder M, Schmotzer CL, Kimball SR, Merrick WC, Koromilas AE, Hatzoglou M. Translational control during endoplasmic reticulum stress beyond phosphorylation of the translation initiation factor eIF2alpha. J Biol Chem. 2014;289:12593–12611. doi: 10.1074/jbc.M113.543215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hetman M, Kanning K, Smith-Cavanaugh JE, Xia Z. Neuroprotection by Brain-Derived Neurotrophic Factor Is Mediated by Extracellular-Signal-Regulated Kinase and Phosphatidylinositol-3 Kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- 15.Hetman M, Pietrzak M. Emerging roles of the neuronal nucleolus. Trends Neurosci. 2012;35:305–314. doi: 10.1016/j.tins.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalita K, Makonchuk D, Gomes C, Zheng JJ, Hetman M. Inhibition of nucleolar transcription as a trigger for neuronal apoptosis. J Neurochem. 2008;105:2286–2299. doi: 10.1111/j.1471-4159.2008.05316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krokowski D, Han J, Saikia M, Majumder M, Yuan CL, Guan BJ, Bevilacqua E, Bussolati O, Broer S, Arvan P, Tchorzewski M, Snider MD, Puchowicz M, Croniger CM, Kimball SR, Pan T, Koromilas AE, Kaufman RJ, Hatzoglou M. A self-defeating anabolic program leads to beta-cell apoptosis in endoplasmic reticulum stress-induced diabetes via regulation of amino acid flux. J Biol Chem. 2013;288:17202–17213. doi: 10.1074/jbc.M113.466920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lytle JM, Wrathall JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur J Neurosci. 2007;25:1711–1724. doi: 10.1111/j.1460-9568.2007.05390.x. [DOI] [PubMed] [Google Scholar]
- 19.Magnuson DS, Lovett R, Coffee C, Gray R, Han Y, Zhang YP, Burke DA. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J Neurotrauma. 2005;22:529–543. doi: 10.1089/neu.2005.22.529. [DOI] [PubMed] [Google Scholar]
- 20.Ohri SS, Hetman M, Whittemore SR. Restoring endoplasmic reticulum homeostasis improves functional recovery after spinal cord injury. Neurobiol Dis. 2013;58:29–37. doi: 10.1016/j.nbd.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59:1489–1502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pajoohesh-Ganji A, Knoblach SM, Faden AI, Byrnes KR. Characterization of inflammatory gene expression and galectin-3 function after spinal cord injury in mice. Brain Res. 2012;1475:96–105. doi: 10.1016/j.brainres.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltonen K, Colis L, Liu H, Trivedi R, Moubarek MS, Moore HM, Bai B, Rudek MA, Bieberich CJ, Laiho M. A targeting modality for destruction of RNA polymerase I that possesses anticancer activity. Cancer Cell. 2014;25:77–90. doi: 10.1016/j.ccr.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffen KK, McCormick MA, Pham KM, MacKay VL, Delaney JR, Murakami CJ, Kaeberlein M, Kennedy BK. Ribosome deficiency protects against ER stress in Saccharomyces cerevisiae. Genetics. 2012;191:107–118. doi: 10.1534/genetics.111.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone S, Lin W. The unfolded protein response in multiple sclerosis. Frontiers in Neuroscience. 2015;9:264. doi: 10.3389/fnins.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen T, Hou J, Wang F, Zhang Y, Zhang T, Sun T. Comparative analysis of molecular mechanism of spinal cord injury with time based on bioinformatics data. Spinal Cord. 2016;54:431–438. doi: 10.1038/sc.2015.171. [DOI] [PubMed] [Google Scholar]
- 28.Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol. 2012;13:355–369. doi: 10.1038/nrm3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


