Abstract
Background
Artemisinin-based combination therapy (ACT), together with other control measures, have reduced the burden of falciparum malaria in sub-Saharan countries, including Sudan. Sudan adopted ACT in 2004 with a remarkable reduction in mortality due to falciparum malaria. However, emergence of resistance to the first-line treatment artesunate and sulfadoxine/pyrimethamine (AS/SP) has created new challenges to the control of malaria in Sudan. A search for an alternative drug of choice for treating uncomplicated malaria has become inevitable. The objective of this study was to evaluate the therapeutic efficacies of dihydroartemisinin/piperaquine (DHA–PPQ) and AS/SP in an area of unstable transmission in Blue Nile State, Sudan in 2015–16.
Methods
A total of 148 patients with uncomplicated malaria were recruited in the study from November 2015 to end of January 2016. Seventy-five patients received DHA–PPQ while 73 received AS/SP. Patients were monitored for clinical and parasitological outcomes following the standard WHO protocol for a period of 42 days for DHA–PPQ and 28 days for AS/SP; nested PCR (nPCR) was performed to confirm parasite re-appearance from day 7 onwards.
Results
Fifty-five patients completed the DHA–PPQ arm protocol with success cure rate of 98.2% (95% CI 90.3–100%) and one late clinical failure 1.8% (95% CI 0.0–9.7%). The AS/SP showed adequate clinical and parasitological response (ACPR) of 83.6% (95% CI 71.9–91.8%), early treatment failure was 1.6% (95% CI 0.0–8.8%) and late parasitological failure (LPF) was 14.8% (95% CI 7–26.2%). The respective PCR uncorrected LPF was 20%.
Conclusion
DHA–PPQ is an efficacious ACT and candidate for replacement of first-line treatment in Sudan while AS/SP showed high treatment failure rate and must be replaced.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1817-9) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Dihydroartemisinin–piperaquine, Artesunate, Sulfadoxine–pyrimethamine, Sudan, Genotyping
Background
Malaria is endemic in Sudan and creates a burden to the health system. It has been reported that 7.5 million cases and 35,000 deaths per year were recorded before 2005 [1]. However, national efforts supported by WHO and partners have reduced the burden to one million cases in 2011 and 75% reduction of deaths [2]. Transmission is seasonal and unstable in most parts of the country following rainfall from May to October with peak transmission June to November [3].
Sudan adopted artemisinin-based combination therapy (ACT) in 2004 with artesunate and sulfadoxine/pyrimethamine (AS/SP) as the first-line of treatment, and artemether/lumefantrine (AL) as second-line [4]. SP continued to be used for prophylaxis in pregnancy. Reports from Sudan and other countries have indicated that there is resistance to the antifolate drugs sulfadoxine and pyrimethamine by in vivo efficacy studies and genotyping [5–7]. However, the initial trials of AS/SP justified its selection as the first-line treatment [8]. Successive in vivo trials, following the adoption of AS/SP, continued to show acceptable outcome from different parts of the country, including South Sudan [9, 10].
Dihydroartemisinin/piperaquine (DHA–PPQ) combination has been used in Southeast Asia and other countries in Africa with profound results. DHA is very effective in removal of the asexual stages of Plasmodium falciparum together with a gametocidal effect. However, it has a short half-life of 50–60 min [4, 11]. Its partner drug, piperaquine has a long half-life and is effective against asexual malaria parasite stages. Piperaquine has the advantage of not having been used as a monotherapy, except in China in the 1970s [4, 12]. This combination has been reported to provide longer prophylaxis from re-infection after treatment up to 65 days.
With reports of failure and reduced efficacy of AS/SP, especially in eastern Sudan [13], Sudan is seeking an alternative first-line efficacious and affordable treatment. Despite being an efficacious second line of treatment, AL has the disadvantage of being expensive [11]. AL will continue to be the second-line. DHA–PPQ, when found effective, will be a candidate first-line treatment.
Methods
Study design
This was an open-label, clinical trial according to WHO guidelines adopted by the National Federal Ministry of Health, Sudan. It is a non-randomized in vivo efficacy clinical trial conducted in two health centres in Dazamin. There was no intention of direct comparison of the patients and treatment outcomes of the two health centres.
Study area
This study was conducted in two health centres (Alzohor and Alsalam) in Damazin, the capital town of the Blue Nile State in southeastern Sudan. Its population is 281,403, while the population of the entire State is over one million. Rainfall is seasonal from June to October (average rainfall 558.5 ml per year) and malaria transmission follows this season. However Damazin has a second transmission season in December to February, similar to that reported from another area in eastern Sudan [14].
Seventy-five patients were studied for DHA–PPQ and 73 were studied for AS/SP combination therapy according to WHO guidelines [11] during the period from November 2015 to the end of January 2016. According to the protocol, patients presenting with fever or history of fever in the last 24 h and having a positive blood smear for asexual falciparum malaria were candidates for inclusion, after a written consent by patient or a guardian for children. A patient having asexual parasite count more than 1000 and not exceeding 100,000, would be given DHA–PPQ in clinic 1 and AS/SP in clinic 2 according to his/her weight, for three days. The administration of treatment was supervised in the clinics. Blood smears were collected and examined on days 2, 3, 7, 14, 21, 28, 35, and 42 for DHA–PPQ and up to day 28 for AS/SP. Thick and thin blood smears were stained by Giemsa according to standard protocol [15]. Parasite identification and count were performed by a skilled microscopist and subsequently revised by an independent expert microscopist. Where readings disagreed, the reading of the expert was considered final.
Patients were followed up in the above-stated days, when temperature and adverse events of treatment were recorded If parasites were not cleared or symptoms existed, a rescue treatment with AL combination of artemether and lumefantrine was administered.
The data from patient sheets were entered in a predesigned Excel sheet including gender, age, temperature, parasite counts until day 42 for DHA–PPQ and day 28 for AS/SP. Treatment outcomes were based on parasitological and clinical responses according the WHO guidelines [11] and were recorded as adequate clinical and parasitological response (ACPR), early treatment failure (ETF), late parasitological failure (LPF), withdrawal of patient (W) or lost for follow-up (LFU).
Genotyping of Plasmodium falciparum msp1 and msp2 genes
Blood from finger pricks was collected on filter paper (FTA® Whatman paper) on day 0 and the follow-up days, dried and stored in sealed plastic bags. Samples with positive asexual parasitaemia on/or after the seventh day were subjected for molecular analysis. Briefly, DNA was extracted from day 0 and the respective days of re-appearance of parasites using the QIAamp® DNA Mini Kit (Qiagen, Hilden, Germany) for dried blood according to manufacturer protocol. Nested PCR (nPCR) was performed for identification of P. falciparum MSP1 and MSP2 allele variants, as described previously [16]. The results were indicated as recrudescence, re-infection or unknown for two lost samples according to WHO guidelines [17].
Ethical statement
Ethical approval for the study was obtained from the National Research Ethics Review Committee, Federal Ministry of Health, Sudan and a permission to conduct the research was obtained from the Blue Nile State Ministry of Health. Written informed consent was obtained from patients or guardians of minor patients prior to enrollment. Anonymity and confidentiality of patients’ information were maintained throughout this study.
Data analysis
Data were analysed by Kaplan–Meier analysis using a WHO developed Excel sheet that included the classification of the treatment outcome according to WHO definition, with and without PCR [18].
Results
A total of 148 patients with uncomplicated falciparum malaria were enrolled in the study, of which 75 received DHA–PPQ and 73 received AS/SP in the two health centres Alzohor and Alsalam, respectively. The mean age of the patients who received DHA–PPQ was 10.8 years and for those who received AS/SP 8.3 years, ranging from one-20 years. The characteristics of the patients of both groups at day 0 are shown in Table 1. The response to DHA–PPQ was adequate. The drug was well tolerated and no adverse events were recorded.
Table 1.
Characteristics of the study patients in two centres in Damazin, Sudan
| DHA–PPQ patients | AS/SP patients | |
|---|---|---|
| Age years (range) | 10.8 (1–50) | 8.3 (1–20) |
| <5 | 22 | 11 |
| 5–15 | 39 | 57 |
| >15 | 14 | 5 |
| Weight kg (SD) | 28.6 (17) | 23.8 (11.1) |
| Temperature °C (range) | 36.9 (35–38) | 37.3 (36.6–38) |
| Parasite Gmean (range) | 23,007 (1000–100,000) | 9611 (1600–100,000) |
| Gametocytes | 7 (9.6%) | 7 (10%) |
On follow-up (Table 2) with PCR uncorrected, only those patients who were present at day 3 were included in the follow-up: DHA–PPQ was 73 patients and AS/SP was 70 patients. Fifty-four patients had ACPR while 18 patients were lost for follow-up of DHA–PPQ. One patient had LCF. The corresponding figures of the AS/SP are 51 ACPR, one ETF and 13 LPF; five patients were lost to follow-up (Table 2).
Table 2.
PCR uncorrected follow-up results of the patients
| Outcome | DHA–PPQ N (%) (95% CI) | AS/SP N (%) (95% CI) |
|---|---|---|
| ETF | 0 (0%) (0.0–6.5) | 1 (1.5%) (0.0–8.3) |
| LCF | 1 (1.8%) (0.0–9.7) | 0 (0%) (0.0–5.5) |
| LPF | 0 (0%) (0.0–6.5) | 13 (20%) (11.1–31.8) |
| ACPR | 54 (98.2%) (90.3–100) | 51 (78.5%) (66.5–87.7) |
| Total | 55 | 65 |
Gametocytes were found in seven patients (9.6%) in the DHA–PPQ centre and seven (10%) in the AS/SP centre. During follow-up no gametocytes were recorded in DHA–PPQ group while four (5.7%) patients from the AS/SP centre had gametocytes.
Table 3; Figs. 1 and 2 show the PCR-corrected respective numbers for DHA–PPQ and AS/SP. Kaplan–Meier survival curves in Figs. 1 and 2 are representation of the results in Table 3. The one patient denoted as LCF in DHA–PPQ at day 35 was a recrudescence, while nine of the AS/SP group were recrudescence, two were re-infection, and two samples were lost.
Table 3.
PCR corrected follow-up results of the patients
| Outcome | DHA–PPQ (N%) (95% CI) | AS/SP (N%) (95% CI) |
|---|---|---|
| ETF | 0 (0%) (0.0–6.5) | 1 (1.6%) (0.0–8.8) |
| LCF | 1 (1.8%) (0.0–9.7) | 0 (0%) (0.0–5.9) |
| LPF | 0 (0%) (0.0–6.5) | 9 (14.8%) (7–26.2) |
| ACPR | 54 (98.2%) (90.3–100) | 51 (83.6%) (71.9–91.8) |
| Total | 55 | 61 |
Fig. 1.
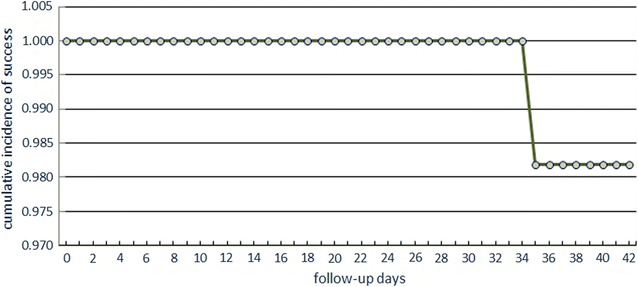
Kaplan–Meier survival curve for DHA–PPQ treatment (PCR corrected)
Fig. 2.
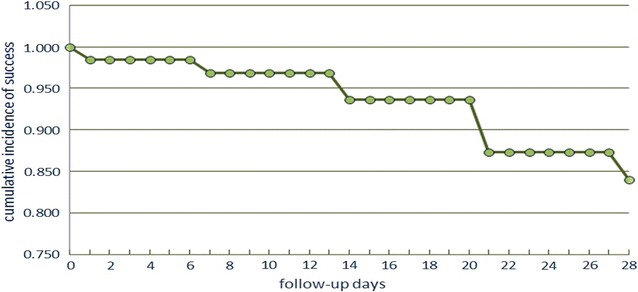
Kaplan–Meier survival curve for AS/SP treatment (PCR corrected)
Discussion
This efficacy study has shown that DHA–PPQ is effective in the treatment of uncomplicated falciparum malaria in an area of unstable transmission in Sudan. The single late parasitological failure on day 35 proved to be a recrudescence after verification by nPCR. The response to the treatment was dramatic and all slides were negative from day 1 and continued to be so to the end of follow-up. There were no complaints of adverse complications due to the treatment. There were no severe adverse events recorded during the follow up. As this is the first time the drug has been tried in Sudan there are no previous reports for comparison.
Other research in neighbouring countries has shown similar effectiveness [19, 20]. In Kenya, a study of efficacy of AL and DHA–PPQ in children under 5 years of age showed that DHA–PPQ is an effective and tolerable treatment for uncomplicated malaria. DHA–PQQ was adopted as a second-line treatment in Kenya in 2009 [19]. Equally, research in west, south and central Africa has shown similar results [20–22]. Dihydroartemisinin has been identified as a potent derivative of the parent drug artemether [4]. Its partner drug, piperaquine, has only been used as monotherapy for treatment of malaria in the 1970s in China and has never been used in Sudan or any other African country [12, 23]. However, the combination DHA–PPQ has only been used in Sudan lately in research and is being used in other African countries. This will make DHA–PPQ an ideal ACT for malaria in Sudan.
On the other hand, AS/SP has lost its effectiveness against falciparum malaria in Blue Nile region. Besides the single case ETF, 20% of the patients showed late parasitological failure and four patients had gametocytes on days 21 and 28 of the follow-up (Table 2). The corrected PCR results have shown that the failure rate was more than 14%, which is alarmingly high. This finding indicates that AS/SP has lost its position as an effective treatment for falciparum malaria in the Blue Nile region, similar to other reports from Kassala (15%) and Gedaref (14%) [13]. In fact, patients and medical personnel have started to lose confidence in this combination, and are using instead quinine and injectable artemether. The partner drug, SP was used before 2005 as a second-line treatment for falciparum malaria in Sudan [24]. It has been known to exhibit resistance before adoption of ACT in 2004 [8, 9, 24]. SP is also used alone as prophylaxis in pregnancy. AS/SP itself is not a fixed combination as it co-exists in separate compartments in the blister seal. All these factors led to an increase of resistance of the parasite to SP, which is responsible for ECF and LPF, especially LPF, as SP is responsible for long-term clearance of parasites [4, 11]. There were also remaining gametocytes in the late stages of follow-up in this study. Artesunate is known to have gametocidal effect [11]. However, those subjects with gametocytes remain infective to Anopheles mosquitoes and thus raise a question of potency of artesunate as a gametocidal therapy. The success of malaria control in Sudan largely depends on the use of AS/SP and AL in the treatment of cases beside other control measures. Failure of any of the control measures, especially treatment, will jeopardize that success. The health authority has acknowledged the failure of AS/SP and the need for change with DHA–PPQ.
Conclusion
Sudan is on its way to changing its first-line malaria treatment and the proposed DHA–PPQ is an ideal alternative. The policy makers at the Federal Ministry of Health have decided to choose AL as first-line malaria treatment and DHA–PPQ as a second-line malaria treatment. A decree has been issued to this effect by the Federal Minister of Health, Sudan on March 9th, 2017. This study has shown that DHA–PPQ would suitable as a first-line treatment in an area of unstable transmission in Sudan, but the Ministry of Health has chosen it as second-line. AS/SP has lost its place as first-line therapy for uncomplicated falciparum malaria and the results of this study justify its replacement.
Additional files
Additional file 1. DHA-PPQ treatment group.
Additional file 2. AS-SP treatment group.
Authors’ contributions
AOM, MAA, FAE, and EMM research and field design. AOM, OSM, MSE, and AS fieldwork, patient recruitment and sampling. OSM and MAA data entry and analysis. MMA molecular interpretation. AOM manuscript drafting. EMM and MMA critical review of the manuscripts. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Intisar Bakhit, Fawzya Kambal, Traique Musa, Hassan Karrar, Baha Aldin Alsamani, and Al-Amin Ibrahim from the malaria administration in Blue Nile State. Mr. Sayed Ali, expert microscopist, Awatif Salah and Omama Fath-Alrahman for the molecular work, Tarig Alfaki for procurements of all logistics, and Dr. Inaam Alsanosi, Director of Neelain Institute for her unfailing assistance. Thanks too to Amal Mohamed for her skilful personal assistance to AOM.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All the datasets generated or analysed during the current study are included in Additional files 1 and 2.
Funding
This research was funded from Neelain Institute for Medical Research- El-Neelain University and The Federal Ministry of Health, Sudan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACT
artemisinin-based combination therapy
- AS/SP
artesunate–sulfadoxine–pyrimethamine
- DHA–PPQ
dihydroartemisinin–piperaquine
- AL
artemether–lumefantrine
- SP
sulfadoxine–pyrimethamine
- WHO
World Health Organization
- nPCR
nested polymerase chain reaction
- ACPR
adequate clinical and parasitological response
- ETF
early treatment failure
- LPF
late parasitological failure
- LCF
late clinical failure
- W
withdrawal of patient
- LFU
lost for follow-up
- MSP
merozoite surface protein
- G mean
geometric mean
Contributor Information
Abdelrahim O. Mohamed, Email: abdelrahim_osman@yahoo.com, Email: abdelrahim_osman@uofk.edu
Muzamil M. Abdel Hamid, Email: mahdi@iend.org, Email: mahdi@uofk.edu
Omer S. Mohamed, Email: omory_4ever@hotmail.com
Nuha S. Elkando, Email: mkarimakasha@gmail.com
Abdelmaroof Suliman, Email: sabdelmaroof@yahoo.com.
Mariam A. Adam, Email: ansam_203@yahoo.com
Fahad Awad Ali Elnour, Email: fahadelnour2@gmail.com.
Elfatih M. Malik, Email: fatihmmalik2000@gmail.com
References
- 1.Malik EM, Hanafi K, Ali SH, Ahmed ES, Mohamed KA. Treatment-seeking behaviour for malaria in children under five years of age: implication for home management in rural areas with high seasonal transmission in Sudan. Malar J. 2006;5:60. doi: 10.1186/1475-2875-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO EMR. Malaria control and elimination. http://www.who.int/en/.
- 3.Musa MI, Shohaimi S, Hashim NR, Krishnarajah I. A climate distribution model of malaria transmission in Sudan. Geospat Health. 2012;7:27–36. doi: 10.4081/gh.2012.102. [DOI] [PubMed] [Google Scholar]
- 4.Davis TME, Karunajeewa HA, Ilett KF. Artemisinin-based combination therapies for uncomplicated malaria. Med J Aust. 2005;182:181–185. doi: 10.5694/j.1326-5377.2005.tb06650.x. [DOI] [PubMed] [Google Scholar]
- 5.Sridaran S, McClintock SK, Syphard LM, Herman KM, Barnwell JW, Udhayakumar V. Anti-folate drug resistance in Africa: meta-analysis of reported dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutant genotype frequencies in African Plasmodium falciparum parasite populations. Malar J. 2010;9:247. doi: 10.1186/1475-2875-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Certain LK, Briceño M, Kiara SM, Nzila AM, Watkins WM, Sibley CH. Characteristics of Plasmodium falciparum dhfr haplotypes that confer pyrimethamine resistance, Kilifi, Kenya, 1987–2006. J Infect Dis. 2008;197:1743–1751. doi: 10.1086/588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schunk M, Kumma WP, Miranda IB, Osman ME, Roewer S, Alano A. High prevalence of drug-resistance mutations in Plasmodium falciparum and Plasmodium vivax in southern Ethiopia. Malar J. 2006;5:54. doi: 10.1186/1475-2875-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam I, A-Elbasit IE, Idris SM, Malik EM, Elbashir MI. A comparison of the efficacy of artesunate plus sulfadoxine–pyrimethamine with that of sulfadoxine-pyrimethamine alone, in the treatment of uncomplicated, Plasmodium falciparum malaria in eastern Sudan. Ann Trop Med Parasitol. 2005;99:449–455. doi: 10.1179/136485905X36299. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed AO, Eltaib EH, Ahmed OA, Elamin SB, Malik EM. The efficacies of artesunate–sulfadoxine–pyrimethamine and artemether–lumefantrine in the treatment of uncomplicated, Plasmodium falciparum malaria, in an area of low transmission in central Sudan. Ann Trop Med Parasitol. 2006;100:5–10. doi: 10.1179/136485906X86239. [DOI] [PubMed] [Google Scholar]
- 10.Van Den Broek I, Amsalu R, Balasegaram M, Hepple P, Alemu E, Hussein EB, et al. Efficacy of two artemisinin combination therapies for uncomplicated falciparum malaria in children under 5 years, Malakal, Upper Nile, Sudan. Malar J. 2005;4:14. doi: 10.1186/1475-2875-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Guidelines for the treatment of malaria. Geneva: World Health Organization; 2015. pp. 71–88. [Google Scholar]
- 12.Basco LK, Ringwald P. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob Agents Chemother. 2003;47:1391–1394. doi: 10.1128/AAC.47.4.1391-1394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adeel AA, Elnour FAA, Elmardi KA, Abd-Elmajid MB, Elhelo MM, Ali MS, et al. High efficacy of artemether-lumefantrine and declining efficacy of artesunate + sulfadoxine–pyrimethamine against Plasmodium falciparum in Sudan (2010–2015): evidence from in vivo and molecular marker studies. Malar J. 2016;15:285. doi: 10.1186/s12936-016-1339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himeidan YE, Hamid EE, Thalib L, Elbashir MI, Adam I. Climatic variables and transmission of falciparum malaria in New Halfa, eastern Sudan. East Mediterr Health J. 2007;13:17–24. [PubMed] [Google Scholar]
- 15.WHO . Basic malaria microscopy:Part I. Learner’s guide. 2. Geneva: World Health Organization; 2010. [Google Scholar]
- 16.Hamid MMA, Elamin AF, Albsheer MMA, Abdalla AA, Mahgoub NS, Mustafa SO, et al. Multiplicity of infection and genetic diversity of Plasmodium falciparum isolates from patients with uncomplicated and severe malaria in Gezira State, Sudan. Parasit Vectors. 2016;9:362. doi: 10.1186/s13071-016-1641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . Methods and techniques for clinicla trials on antimalarial drug efficacy: genotyping to identify parasite population. Geneva: World Health Organization; 2007. [Google Scholar]
- 18.World Health Organization . Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization; 2009. [Google Scholar]
- 19.Ogutu BR, Onyango KO, Koskei N, Omondi EK, Ongecha JM, Otieno GA, et al. Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: results of an open-label, randomized, single-centre study. Malar J. 2014;13:33. doi: 10.1186/1475-2875-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plucinski MM, Talundzic E, Morton L, Dimbu PR, Macaia AP, Fortes F, et al. Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for treatment of uncomplicated malaria in children in Zaire and Uíge provinces, Angola. Antimicrob Agents Chemother. 2015;59:437–443. doi: 10.1128/AAC.04181-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adjei A, Narh-Bana S, Amu A, Kukula V, Nagai RA, Owusu-Agyei S, et al. Treatment outcomes in a safety observational study of dihydroartemisinin/piperaquine (Eurartesim(®)) in the treatment of uncomplicated malaria at public health facilities in four African countries. Malar J. 2016;15:43. doi: 10.1186/s12936-016-1099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nji AM, Ali IM, Moyeh MN, Ngongang E-O, Ekollo AM, Chedjou J-P, et al. Randomized non-inferiority and safety trial of dihydroartemisin–piperaquine and artesunate–amodiaquine versus artemether–lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroonian children. Malar J. 2015;14:27. doi: 10.1186/s12936-014-0521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis TME, Hung T-Y, Sim I-K, Karunajeewa HA, Ilett KF. Piperaquine: a resurgent antimalarial drug. Drugs. 2005;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Muhsin A-M, Mackinnon MJ, Ali E, Nassir E-K, Suleiman S, Ahmed S, et al. Evolution of drug-resistance genes in Plasmodium falciparum in an area of seasonal malaria transmission in Eastern Sudan. J Infect Dis. 2004;189:1239–1244. doi: 10.1086/382509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. DHA-PPQ treatment group.
Additional file 2. AS-SP treatment group.
Data Availability Statement
All the datasets generated or analysed during the current study are included in Additional files 1 and 2.


