Abstract
Background: Anabolic responsiveness to teriparatide can be blunted or delayed in patients previously treated with alendronate. The extent of this effect is different for other antiresorptives. This study evaluated the early anabolic effects of teriparatide in postmenopausal women with osteoporosis previously treated with alendronate or risedronate.
Methods: Patients treated for at least 24 months with alendronate or risedronate discontinued their bisphosphonate and received teriparatide for 12 months. The primary endpoint was a comparison of changes from baseline in N-terminal propeptide of type 1 collagen after 3 months between prior bisphosphonate groups. We also examined changes in other bone turnover markers, bone mineral density (BMD), and relationships between early changes in bone turnover markers and 12-month areal and volumetric BMD.
Results: In the prior risedronate group, the N-terminal propeptide of type 1 collagen increase was significantly greater after 3 months of teriparatide than in the prior alendronate group (mean ± se, 86.0 ± 5.6 vs. 61.2 ± 5.3 ng/ml, respectively; P < 0.001). Findings were similar for the other bone turnover markers. The changes in areal BMD and trabecular spine volumetric BMD were also greater in the prior risedronate group (P < 0.05). Early changes in bone turnover markers correlated with changes in trabecular spine volumetric BMD at 12 months (Spearman r = 0.45). Teriparatide was well tolerated.
Conclusion: This nonrandomized but prospective study suggests that there may be differences in anabolic responsiveness to teriparatide as a function of the type of prior bisphosphonate exposure.
Teriparatide [human PTH (1–34)] reduces the risk of new vertebral and nonvertebral fractures in postmenopausal women with osteoporosis (1). Because the use of teriparatide is recommended for only 18–24 months, patients who respond the earliest and greatest are likely to experience optimal benefit from this anabolic agent. Prior treatment with antiresorptive agents such as estrogen or raloxifene permits teriparatide to rapidly increase bone turnover markers and bone mineral density (BMD) (2, 3, 4), whereas treatment with alendronate before teriparatide, or in combination with teriparatide or PTH (1–84) may blunt or delay these responses (5, 6, 7, 8).
Combination and sequential studies with bisphosphonates and teriparatide have used alendronate almost exclusively. Therefore, it is not known if other bisphosphonates might blunt or delay the anabolic response to teriparatide, an effect that may relate to the extent to which the bisphosphonate reduces bone turnover and maintains this reduction after cessation of the drug.
Based on pharmacological differences between alendronate and risedronate, we hypothesized that prior treatment with risedronate would not impair subsequent effects of teriparatide to the same extent as prior treatment with alendronate. This study is clinically relevant because many patients who receive teriparatide have been treated with one of these bisphosphonates.
Subjects and Methods
Study design
The Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide was a prospective, multinational, parallel-group trial conducted in accordance with good clinical practice guidelines, the provisions of the Declaration of Helsinki, and the regulations of the United States, Canada, Belgium, United Kingdom, France, Germany, The Netherlands, and Australia. The study was approved by a central institutional review board or the institutional review board of the respective institutions of the investigators. Each subject provided written informed consent before participation.
Role of the sponsors
The study was designed by Steering Committee investigators and The Alliance for Better Bone Health (Procter & Gamble Pharmaceuticals and sanofi-aventis). Investigators in each country collected the data; statisticians at sanofi-aventis analyzed the data according to a prespecified investigator- and sponsor-approved plan. All of the authors had complete access to the primary data, wrote the manuscript, and vouch for the accuracy and completeness of the article.
Independent statistical review
An independent statistical review was conducted at Helen Hayes Hospital (West Haverstraw, NY) by M. Zion and J. Nieves under the guidance of the Steering Committee investigators. The findings of the independent statistical review were in accord with those of the original statistical analysis.
Study population
Postmenopausal women with osteoporosis documented to have received either alendronate (10 mg daily or 70 mg weekly, Fosamax; Merck & Co., Inc., Whitehouse Station, NJ) or risedronate (5 mg daily or 30–35 mg weekly, Actonel; Procter & Gamble Pharmaceuticals, Cincinnati, OH) for at least 24 months were eligible for enrollment. Investigators enrolled subjects from their existing or incoming patients, from referrals of suitable patients, or through public advertisements. Inclusion criteria were osteoporosis severe enough to warrant treatment with teriparatide [lumbar spine or total hip T score ≤−2.0 and ≥1 prevalent osteoporotic fracture, or lumbar spine or total hip T score ≤−2.5 (United States and Australia); lumbar spine or total hip T score <−2.5 and ≥1 prevalent osteoporotic fracture (Europe and Canada), based on regional labeling for teriparatide], a serum 25-hydroxyvitamin D level of 16 or more and 80 or less ng/ml, and urinary N telopeptide (NTX) less than or equal to 50 bone collagen equivalents (BCEs) nmol/mmol creatinine, to ensure adherence with prior bisphosphonate therapy.
Exclusion criteria were: impaired renal function (creatinine clearance <30 ml/min); treatment with antiresorptive agent other than risedronate or alendronate; hormone replacement therapy within 36 months; systemic glucocorticoid or anabolic steroid within 3 months or for 1 month or more within 6 months; calcitonin, calcitriol, or calcifediol within 1 month or for 1 month or more within 6 months; combination risedronate and alendronate within 60 months; or any antiresorptive agent in combination with risedronate or alendronate.
The completer population consisted of all enrolled and treated subjects with both a baseline and an analyzable 3-month N-terminal propeptide of type 1 collagen (P1NP) value. The modified intent-to-treat population included all enrolled and treated subjects with both a baseline and post-baseline P1NP value. Last observation carried forward methodology was used to account for missing values in the modified intent-to-treat population. The per protocol population consisted of all completer subjects who did not have a protocol deviation that would have affected the interpretation of the primary efficacy variable. The modified intent-to-treat and per protocol populations were used to corroborate the findings in the completer population. The safety population included all enrolled and treated subjects. In all countries except France, compliance with teriparatide was assessed by reviewing daily medication diaries and inspection of used teriparatide pens that were returned to the study center at each visit. In France the study center collected and visually inspected all used teriparatide pens at each visit.
Study protocol
Upon enrollment, patients discontinued their bisphosphonate and initiated daily teriparatide (20 μg sc, Forteo; Eli Lilly and Co., Indianapolis, IN) for 12 months. Patients were required to visit their study center 11 times: a screening visit approximately 6 wk before baseline; a baseline visit; and visits at months 0.5, 1–6, 9, and 12. There was, in general, not more than a 1- to 2-wk hiatus between stopping bisphosphonate therapy and beginning teriparatide. Because this was a nonrandomized, open-label trial, subjects were matched and stratified into 6-month bisphosphonate exposure intervals to ensure enrollment of a comparable number of patients receiving each bisphosphonate over similar bisphosphonate treatment periods. An interactive voice response system was used to facilitate stratification across all 50 study sites (United States and Australia, 39 sites; Europe and Canada, 11 sites).
Outcomes
The primary endpoint was the comparison of mean absolute change from baseline in N-terminal propeptide (P1NP) after 3 months of teriparatide treatment between prior risedronate and prior alendronate subjects in the completer population. The P1NP 3-month time point was selected based on previous reports of associations between early changes in bone turnover markers with improvements in 12-, 18-, or 24-month BMD (9, 10, 11, 12).
Secondary endpoints included comparisons of mean changes from and ratio to baseline for P1NP, bone-specific alkaline phosphatase activity (BAP), osteocalcin (OC), serum C telopeptide (CTX), and N-telopeptide to urine creatinine ratio (NTX) at 0.5, 1–6, and 12 months. Secondary endpoints also included comparisons of mean and percent changes in areal BMD by dual-energy x-ray absorptiometry (DXA) at 6 and 12 months in all subjects. Volumetric BMD by quantitative computed tomography (QCT) was assessed at baseline and 12 months by a central laboratory (University of California, San Francisco) using methods described previously (13, 14). We investigated correlations of early changes in bone turnover markers with 12-month changes in BMD. Safety data included adverse events, vital signs, and serum calcium. Serum calcium was measured at each study visit after the patient’s morning administration of teriparatide. Investigators were to use their own clinical judgment concerning the management or retesting of the single elevated calcium level.
Measurement of bone turnover markers
Blood was obtained for P1NP, OC, BAP, and CTX in the fasting state at approximately the same time of the morning throughout the study. Urine was obtained for NTX measurement in the fasting state on 2 consecutive days, using the second morning void between 0500 and 0800 h. Baseline NTX measurements were made on two consecutive mornings after the screening visit. Subsequent NTX assessments were collected the morning before and the morning of each visit. Both urine and serum samples for biochemical markers were analyzed in a specialty laboratory (Synarc SAS, Lyon, France). Individual urine samples were analyzed separately, and the two values were averaged for each visit.
Serum intact P1NP was measured with a two-site immunoassay based on monoclonal antibodies raised against purified intact human P1NP, and detecting both intact mono and trimeric forms, but not fragments (Elecsys; Roche Diagnostics, Mannheim, Germany). BAP was measured by an immunochemiluminescence assay using the Ostase reagent (Ostase, Access; Beckman Coulter, Inc., Fullerton, CA). OC was measured using a two-site immunoassay recognizing the intact and N-terminal mid-region fragment, to correct for potential degradation of intact OC (Elecsys). Serum CTX was measured using a two-site assay using monoclonal antibodies raised against an eight-amino acid sequence from the C telopeptide of human type 1 collagen (Elecsys). The N-telopeptide to urine creatinine ratio was measured by ELISA using the OSTEOMARK assay (Ostex Inc., Seattle, WA; Vitros ECi from Ortho-Clinical Diagnostics, Inc., Rochester, NY).
Reference ranges from healthy premenopausal women for the bone turnover markers used in this study are listed in Table 1 (15, 16). Premenopausal reference ranges for OC applicable to our study population and assay were not available at publication.
TABLE 1.
Baseline characteristics in the completer population
| Variable | Prior risedronate (n = 146) | Prior alendronate (n = 146)1 | P value2 |
|---|---|---|---|
| Age (yr) | |||
| Mean ± sd (range) | 69.3 ± 7.4 (53–84) | 67.7 ± 7.8 (51–85) | 0.11 |
| Race | |||
| No. of caucasians (%) | 143 (97.9) | 139 (95.2) | 0.12 |
| BMD (g/cm2) | |||
| Total spine | |||
| Mean ± sd (range) | 0.74 ± 0.08 (0.53–1.01) | 0.74 ± 0.10 (0.47–1.16) | 0.71 |
| Total hip | |||
| Mean ± sd (range) | 0.69 ± 0.10 (0.32–0.92) | 0.69 ± 0.10 (0.38–0.98) | 0.85 |
| Minimum BMD T score3 | |||
| Mean ± sd (range) | −3.1 ± 0.6 (−5.1 to −2.2) | −3.1 ± 0.6 (−5.3 to −2.1) | 0.71 |
| Weight (kg) | |||
| Mean ± sd (range) | 61.3 ± 11.8 (36–110) | 59.3 ± 11.4 (35–116) | 0.10 |
| Body mass index (kg/m2) | |||
| Mean ± sd (range) | 24.3 ± 4.4 (15–45) | 23.6 ± 4.4 (14–42) | 0.12 |
| No. of prevalent fragility fractures (%) | 101 (69.2) | 100 (68.5) | 0.85 |
| Types of fractures | |||
| No. of overall spinal fractures (%)4 | 17 (11.6) | 25 (17.1) | 0.24 |
| No. of wrist fractures (%) | 16 (11.0) | 18 (12.3) | 0.86 |
| No. of upper arm fractures (%) | 8 (5.5) | 10 (6.8) | 0.81 |
| No. of hip fractures (%) | 8 (5.5) | 6 (4.1) | 0.76 |
| Previous bisphosphonate frequency | |||
| No. of daily (%) | 7 (4.8) | 4 (2.7) | 0.48 |
| No. of weekly (%) | 139 (95.2) | 142 (97.3) | |
| Previous bisphosphonate duration | |||
| Mean ± sd (range) | 37.2 ± 10.3 months (24–73) | 38.0 ± 11.1 months (24–78) | 0.07 |
| No. ≥24, <30 months (%) | 38 (26.0) | 39 (26.7) | 0.31 |
| No. ≥30, <36 months (%) | 33 (22.6) | 33 (22.6) | |
| No. ≥36, <42 months (%) | 36 (24.7) | 27 (18.5) | |
| No. ≥42, <48 months (%) | 18 (12.3) | 25 (17.1) | |
| No. ≥48, <54 months (%) | 10 (6.8) | 7 (4.8) | |
| No. ≥54, <60 months (%) | 4 (2.7) | 5 (3.4) | |
| No. ≥60 months (%) | 7 (4.8) | 10 (6.8) | |
| Mean P1NP ± sd (ng/ml) | 32.17 ± 17.64 | 24.50 ± 13.61 | <0.01 |
| Percent difference, RIS > ALN | 31 | ||
| Mean OC ± sd (μg/liter) | 18.96 ± 6.55 | 17.20 ± 6.41 | 0.02 |
| Percent difference, RIS > ALN | 10 | ||
| Mean BAP ± sd (ng/ml) | 11.35 ± 3.55 | 9.85 ± 3.42 | <0.01 |
| Percent difference, RIS > ALN | 15 | ||
| Mean NTX ± sd (nmol BCE/mmol) creatinine | 27.10 ± 9.48 | 23.70 ± 8.34 | 0.01 |
| Percent difference, RIS > ALN | 14 | ||
| Mean CTX ± sd (ng/ml) | 0.26 ± 0.15 | 0.19 ± 0.11 | <0.01 |
| Percent difference, RIS > ALN | 35 | ||
| Mean premenopausal bone turnover levels (95% CI of lower limit), (95% CI of upper limit)5 | |||
| P1NP (ng/ml) (15 ) | 31.4, (14.8–17.7), (55.5–66.7) | ||
| OC (μg/liter) | Not available | ||
| BAP (ng/ml) (16 ) | 8.8, (4.2–4.8), (14.6–16.9) | ||
| NTX (nmol BCE/mmol) creatinine (15 ) | 27.9, (12.6–15.3), (50.8–61.6) | ||
| CTX (ng/ml) (15 ) | 0.25, (0.09–0.12), (0.55–0.70) |
BAP (n = 145).
P values for continuous variables are based on an ANOVA containing prior treatment and pooled site, and for categorical variables Cochran-Mantel-Haenszel procedure controlling for pooled site.
Minimum T score was defined as the lesser of lumbar spine or total hip T scores.
Overall spinal fracture included those characterized as a spinal fracture, thoracic vertebral fracture, spinal compression fracture, lumbar vertebral fracture, or compression fracture.
Bone turnover marker ranges for healthy premenopausal women are provided for the reader’s reference.
Statistical analysis
A sample size of 290 patients was calculated based on a within-group sd of 80 ng/ml and estimated to have at least 85% power to detect a mean difference of 29 ng/ml in P1NP change from baseline to month 3 between the two prior bisphosphonate groups. This calculation assumed that 138 of the 145 patients (95%) that needed to be recruited per group would have evaluable P1NP data at both baseline and month 3.
Absolute mean and percent changes from baseline were analyzed using analysis of covariance (ANCOVA) with the prior bisphosphonate group, stratum for duration of previous bisphosphonate treatment, and pooled site as class effects, and corresponding baseline values as covariates. Ratios to baseline were also analyzed using the same ANCOVA model. The ratios to (or, n-fold changes from) baseline for bone turnover markers used the same statistical model after a logarithmic transformation of the data (17). Descriptive statistics, ranked data using ANCOVA, area under the curve, and repeated measures analyses were also conducted. Analytical methods other than the one used to analyze the absolute mean changes from baseline were used to validate, and investigate the robustness of, the primary model. To investigate the consistency of effect across levels of baseline variables, separate ANCOVAs were performed using the model described previously that included the baseline variable and the baseline variable by previous bisphosphonate treatment interaction. Correlations of bone turnover marker changes from baseline with 12-month volumetric BMD findings were investigated using the Spearman rank test. To investigate further the possible influence of baseline bone turnover marker levels on the primary and secondary findings, we performed a standard normal deviate transformation analysis, in which changes from baseline were expressed as sd values rather than ratios.
Results
Study population
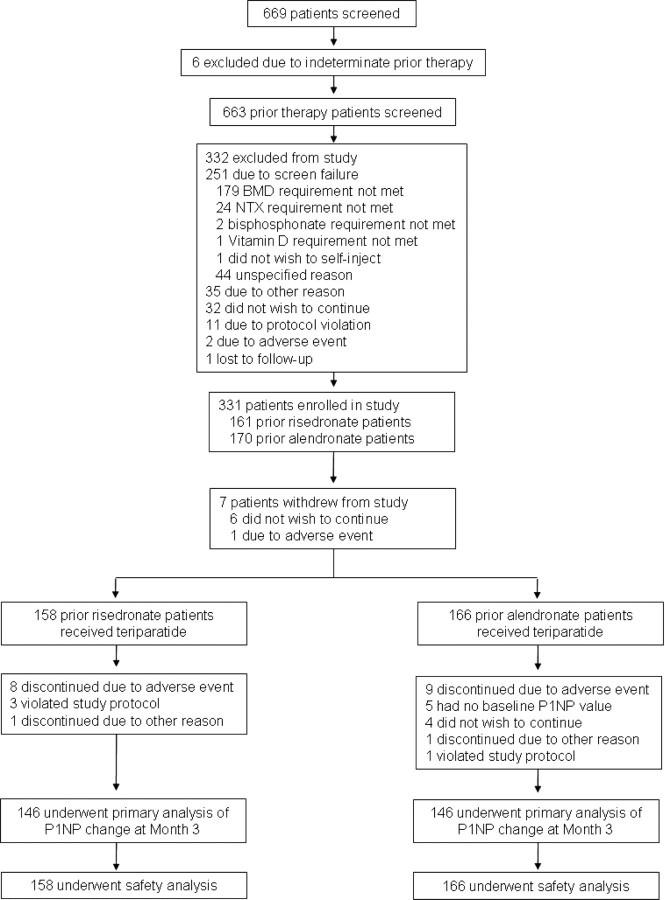
Between March 15, 2004, and March 31, 2006, 669 subjects were screened, 331 enrolled, and 324 treated with teriparatide (of whom 158 had been previously treated with risedronate and 166 with alendronate; Fig. 1). The modified intent-to-treat population consisted of 317 subjects (157 prior risedronate subjects and 160 prior alendronate subjects), and the completer population was 292 subjects (146 in each group).
Fig. 1.
Screening, enrollment, and analysis populations.
Baseline bone turnover markers were higher in subjects previously treated with risedronate (range 10–35%; P < 0.05; Table 1). Other baseline characteristics were similar between groups, including duration of prior bisphosphonate therapy, BMD, T scores, prevalent fracture incidence, and distribution across subgroup strata. Subjects had been taking their bisphosphonates for a mean of 37.6 months (prior risedronate, 37.2 months; prior alendronate, 38.0 months), with 95% or more subjects having used weekly formulations. Of subjects, 79% started teriparatide therapy within 7 d of discontinuing their prior bisphosphonate, with 98% starting teriparatide within 14 d of bisphosphonate discontinuation. Median times to initiation of teriparatide were similar between groups (prior risedronate, 4 d; prior alendronate, 5 d).
Primary endpoint
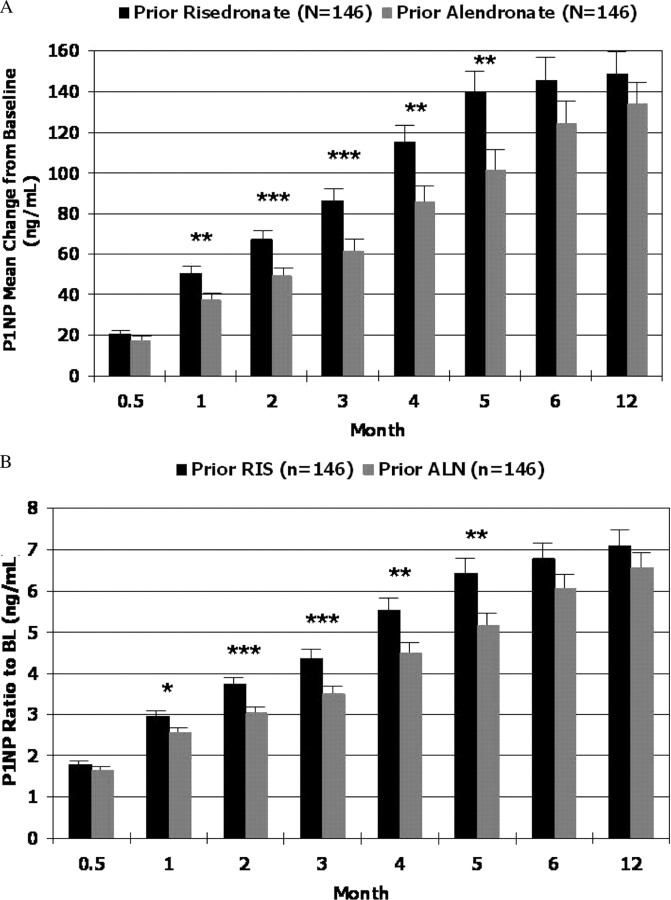
In the completer population, P1NP mean changes from baseline at month 3 were greater in the prior risedronate group compared with the prior alendronate group [mean ± se, 86.0 ± 5.6 vs. 61.2 ± 5.3 ng/ml; absolute difference, 24.8 ng/ml; 95% confidence interval (CI) 10.9–38.7 ng/ml; P < 0.001; Fig. 2]. Results from the modified intent-to-treat and per protocol populations were consistent with those of the completer population (P < 0.01; data not shown).
Fig. 2.
Comparison of bone turnover marker changes from baseline (completer population). A, P1NP mean change from baseline, controlling for prior bisphosphonate group, stratum for duration of prior therapy, and pooled site. B, P1NP ratio to baseline, controlling for prior bisphosphonate group, stratum for duration of prior therapy, and pooled site. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ALN, Alendronate; RIS, risedronate.
Ratios to baseline were analyzed to account for baseline differences between groups, with similar results demonstrating a greater than 4-fold increase from baseline in P1NP for the prior risedronate group compared with a greater than 3-fold increase for the prior alendronate group (P < 0.001; Fig. 2). Results from additional analyses, including ranked data using ANCOVA, area under the curve, and repeated measures analyses, all supported the findings from the primary model (P < 0.05; data not shown).
Secondary endpoints
Bone turnover markers
With teriparatide, all bone turnover markers increased from baseline for both prior therapy groups at every time point (P < 0.0001; data not shown). Greater increases in both absolute and ratio P1NP changes from baseline were observed in the prior risedronate group at months 1–5 across all study populations compared with the prior alendronate group (P < 0.05; data not shown). Patients previously treated with risedronate also experienced greater increases from baseline in OC, BAP, CTX, and NTX at most time points (P < 0.05; data not shown). Differences between prior therapy groups were apparent as early as 2 wk with CTX (prior risedronate, 153% vs. prior alendronate, 132%; P < 0.01), but not until 2 months with NTX (prior risedronate, 175% vs. prior alendronate, 141%; P < 0.001), and appeared to dissipate for all bone markers by month 12.
We considered the possibility that baseline bone turnover markers could have been determinants in the subsequent response to teriparatide, i.e. the lower the bone turnover markers after bisphosphonate therapy, the more sluggish the response to teriparatide. Therefore we matched the two patient groups by quintiles of baseline bone turnover levels and compared them with regard to the 3-month time point for all bone turnover markers. Even when bone turnover quintiles were matched, greater changes were still observed when teriparatide followed risedronate compared with alendronate (significance of interaction between baseline percentiles and month 3 P1NP differences between groups: P1NP, P = 0.55; CTX, P = 0.17). In the standard normal deviate transformation analysis, the greater bone turnover marker responses to teriparatide after risedronate compared with alendronate were again seen and appeared to be independent of baseline differences (data not shown). We also considered the possibility that duration of bisphosphonate therapy might influence subsequent responsiveness to teriparatide. For the 3-month bone turnover markers, no significant influence of duration of prior bisphosphonate therapy (2, 3, or ≥4 yr) on differential responsiveness to teriparatide was observed (P1NP, P = 0.48; BAP, P = 0.61; OC, P = 0.53; CTX, P = 0.98; NTX, P = 0.70).
Areal BMD
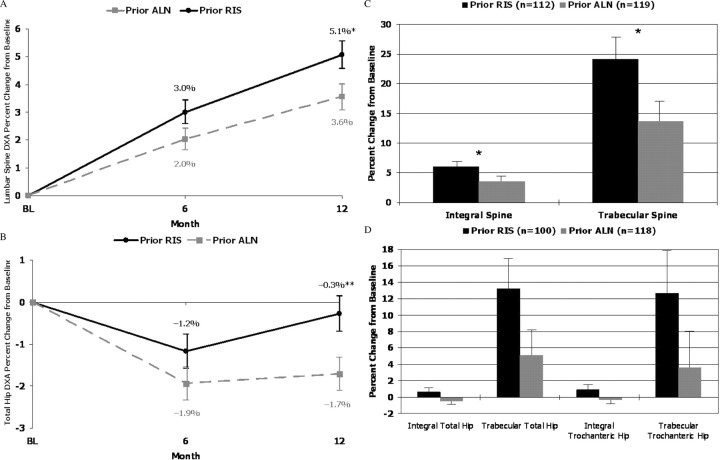
Areal BMD of the lumbar spine increased from baseline in both groups (P < 0.01), but the prior risedronate group demonstrated a greater 12-month increase than the prior alendronate group [0.037 ± 0.004 g/cm2 (5.1%) vs. 0.026 ± 0.003 g/cm2 (3.6%), respectively; P < 0.01 for actual BMD differences and P < 0.05 for percent differences; Fig. 3]. Total hip BMD decreased at 6 months in both prior bisphosphonate groups (prior risedronate, −0.009 ± 0.003 g/cm2; prior alendronate, −0.012 ± 0.002 g/cm2; P = 0.07) but returned to near baseline levels at 12 months in the prior risedronate group, while remaining reduced in the prior alendronate group [−0.003 ± 0.003 g/cm2 (−0.3%) vs. −0.012 ± 0.003 g/cm2 (−1.2%), respectively; P < 0.01 for both actual and percent differences; Fig. 3].
Fig. 3.
Comparison of BMD changes from baseline (BL) (completer population). A, DXA of the lumbar spine, percent change from baseline. B, DXA of the total hip, percent change from baseline. C, QCT of the integral and trabecular spine, percent change from baseline. D, QCT of the integral and trabecular total and trochanteric hip, percent change from baseline. *, P < 0.05; **, P < 0.01. ALN, Alendronate; RIS, risedronate.
Areal BMD was analyzed by baseline bone turnover markers and baseline BMD to determine whether differences in 12-month gains were a function of baseline levels. Twelve-month findings were found to be independent of baseline levels (lumbar spine, interaction P = 0.36; total hip, interaction P = 0.46). Areal BMD results did not correlate substantially with 3-month bone turnover findings.
Volumetric BMD
Volumetric BMD of the spine and hip was measured in a subset of patients (prior risedronate, n = 112; prior alendronate, n = 119). This subgroup was similar to the total cohort in all respects. After risedronate therapy, the 12-month change in trabecular spine volumetric BMD was nearly double that observed with the alendronate group (24.1 vs. 13.7%, respectively; P < 0.05), with a trend toward a significant difference at the hip (trabecular total hip, P = 0.06; Fig. 3). The 1-yr changes from baseline in volumetric BMD correlated significantly with month 3 changes in P1NP (Spearman r = 0.45).
Safety
Treatment with teriparatide was well tolerated in both groups. The most frequently reported adverse events were hypercalcemia, muscle spasms (this formal term captured “muscle cramps” as the more common adverse event in this category than true spasm), nausea, dizziness, and arthralgia (Table 2). During the 12-month teriparatide treatment period, 14 subjects (4.3%) experienced fractures, with no difference in fractures between the two groups. One of these patients, who experienced multiple compression vertebral fractures, was diagnosed shortly thereafter with multiple myeloma. There were 46 patients (23 in each group; 14.6% prior risedronate, 13.9% prior alendronate) who developed increased calcium levels (serum calcium increase ≥0.3 mmol/liter) on samples drawn soon after teriparatide dosing. Few of these required confirmation by repeat analysis according to investigator clinical judgment.
TABLE 2.
Adverse events during teriparatide therapy (safety population)
| Prior risedronate (n = 158) | Prior alendronate (n = 166) | |
|---|---|---|
| Possibly related treatment-emergent adverse events | ||
| No. with overall (%) | 69 (43.7) | 70 (42.2) |
| No. with hypercalcemia (%) | 23 (14.6) | 23 (13.9) |
| No. with muscle spasms (%)1 | 14 (8.9) | 18 (10.8) |
| No. with nausea (%) | 11 (7.0) | 14 (8.4) |
| No. with dizziness (%) | 10 (6.3) | 10 (6.0) |
| No. with arthralgia (%) | 2 (1.3) | 4 (2.4) |
| Fractures during teriparatide therapy | ||
| No. of subjects with any fracture (%) | 9 (5.7) | 5 (3.0) |
| No. of subjects with nonvertebral fracture (%)2 | 4 (2.5) | 3 (1.8) |
Greater than 95% of reported muscle spasms were labeled clinically as ″leg cramps.″
Nonvertebral fractures were assessed in a blinded fashion by anatomical site (hip, wrist, pelvis, clavicle, humerus, leg).
Discussion
Patients treated for at least 2 yr with risedronate demonstrated a greater response to 1 yr teriparatide than those previously treated with alendronate as measured by bone turnover markers, particularly the month 3 P1NP primary endpoint of the study. We found greater increases in the bone formation and resorption markers as early as 2 wk after teriparatide and lasting through as much as 6 months across all markers for patients previously treated with risedronate, though this effect dissipated by month 12. Because the responses became similar in the prior therapy groups by 12 months, it is not known whether the observed differences at the earlier time points have long-term significance; particularly, to what extent they may affect fracture risk.
Although bone turnover markers were higher at baseline for patients previously treated with risedronate, the observed differences in responsiveness to teriparatide could not be attributed to baseline bone turnover differences as measured by serum levels of biochemical markers of bone turnover. Duration of prior bisphosphonate therapy also did not appear to contribute to differences in responsiveness to teriparatide.
The greater responsiveness to subsequent teriparatide for prior risedronate subjects was also seen in a comparison of changes in areal and volumetric BMD. Of interest, areal BMD and T scores were similar between the two groups at baseline but greater in the prior risedronate group after 12 months of teriparatide.
The observed early bone turnover marker increases correlated with 12-month changes in volumetric BMD, but not with areal BMD. The correlation of 3-month changes in P1NP with the 12-month changes in areal BMD was observed by Dobnig et al. (11) in predominantly treatment-naive subjects. Ettinger et al. (5) studied relationships between bone turnover markers and changes in areal BMD (DXA of the hip and spine) only (no volumetric studies were made), and found statistically significant correlations between bone turnover markers and BMD for prior raloxifene users but not for previous alendronate users. The data with regard to volumetric BMD of the vertebral trabecular bone are of particular relevance to the specific anabolic actions of teriparatide at trabecular sites. How this finding might relate to greater bone strength is being pursued by finite element modeling of the QCT images. A recent study has also shown such correlations with teriparatide (18).
Our findings are consistent with previous reports that anabolic responsiveness to teriparatide differs based on prior antiresorptive therapy, and that the use of alendronate may blunt the magnitude of the bone turnover response to PTH in vivo in rats and human subjects (5, 7, 8, 19). Recently published data also suggest different responses to teriparatide as a function of the type of prior antiresorptive therapy (20).
Because neither baseline bone turnover markers nor duration of prior bisphosphonate therapy could account for the observations in this study, other differences between risedronate and alendronate may explain the findings. Potential explanations may include the differential actions of these two bisphosphonates on the enzyme they inhibit, namely farnesyl diphosphate synthase, differences in cellular effects on osteoclasts, osteoblasts and osteocytes, or differential uptake and binding affinity to bone mineral and to selectivity between formation and resorption sites (21, 22, 23, 24, 25, 26). Recent data regarding the offset characteristics of antiresorptives from bone might also help to explain our findings. Bone turnover markers have been found to remain reduced for up to 5 yr after discontinuation of alendronate, whereas Black (27) and Watts (28) et al. have found bone turnover markers to return to control group levels within 12 months of discontinuation of risedronate. Although these findings are not from a head-to-head comparison of these compounds, they may be clues to a fuller understanding of the differences in teriparatide responsiveness to prior antiresorptive therapy.
This study did not include a teriparatide-only treatment arm, so some blunting may have occurred in connection with both prior therapy groups. In addition, because patients in this study received 12 months of teriparatide, and treatment with teriparatide is often given for 18–24 months, the full relevance of early surrogate marker changes to the clinical setting may not be completely apparent. However, our findings support the importance of early responsiveness to teriparatide treatment so patients may receive the maximal effect of this anabolic agent. Our findings also support previous studies demonstrating meaningful effects of early bone turnover marker response on BMD and microarchitecture (11, 12, 29). Bauer et al. (30) have reported PTH (1–84)-treated patients who had not been on prior antiresorptive therapy to experience mean 3-month P1NP levels of 171.6 ng/ml, BAP of 29.0 ng/ml, and CTX of 0.7 ng/ml.
Subjects were not randomized, but patient selection and stratification measures implemented during the screening process effectively matched those taking risedronate or alendronate before teriparatide treatment with regard to demographics, T scores, prevalent fractures, calcium and vitamin D status, and duration and frequency of prior bisphosphonate therapy. We observed differences in baseline bone turnover markers between prior therapy groups but could not identify any influence of these baseline differences on subsequent responsiveness to teriparatide. Our study was not designed or powered to measure fracture incidence, and, thus, the data cannot be used to suggest or infer a conclusion along these lines. Fractures were captured only as adverse events, and there were no differences between the two groups.
This nonrandomized but prospective study suggests that there may be differences in anabolic responsiveness to teriparatide as a function of the type of prior bisphosphonate exposure. Additional studies are needed to elucidate further the mechanisms for these differences.
Acknowledgments
1 The participants in the Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide are: J.P.B., P.D.D., R.L., P.D.M., N.B.W., D. L. Cahall, A. Grauer, and S.M. (Steering Committee); D. L. Cahall, J. Stewart, S.M., J. Frimpter, E. Thomas, and M. Girard [sanofi-aventis, Bridgewater, New Jersey (cosponsor)]; D. Huber, B. Borah, H. Ebetino, G. Gross, and A. Grauer [Procter and Gamble, Mason, Ohio (cosponsor)]; J. Stewart and M. Girard, sanofi-aventis, Laval, Canada (statistical analysis); M. Zion and J. Nieves, Helen Hayes Hospital, West Haverstraw, New York (independent statistical review); P. Garnero, Synarc SAS, Lyon, France (analysis of bone turnover markers); T.F.L., University of California, San Francisco, California (analysis of quantitative computed tomography); and E.S. (Australia), S.B. (Belgium), J.A. (Canada), J. Brown (Canada), A. Hodsman (Canada), R. Josse (Canada), L.-G. Ste-Marie (Canada), R. Eastell (United Kingdom), P.D.D. (France), D. Felsenberg (Germany), C. Netelenbos (The Netherlands), S. Papapoulos (The Netherlands), J. Baker (United States), N. Binkley (United States), E. Boling (United States), M. A. Bolognese (United States), A. Chubick (United States), S. Cohen (United States), R. D. Emkey (United States), S.L.G. (United States), M. Greenwald (United States), S. L. Hall (United States), S. Hippler (United States), C. Johnston (United States), R. Kagan (United States), A. Kivitz (United States), K. D. Krohn (United States), E. M. Lewiecki (United States), R.L. (United States), M.L. (United States), R. Malamet (United States), M. R. McClung (United States), P.D.M. (United States), R. R. Recker (United States), C. Recknor (United States), T. Rooney (United States), C. J. Rosen (United States), J. Rosenstock (United States), K.G.S. (United States), M. A. Seltman (United States), S. L. Silverman (United States), R. E. Tamayo (United States), N.B.W. (United States), R. S. Weinstein (United States), and G. C. Woodson (United States) (clinical centers and investigators).
Postmenopausal women with osteoporosis previously treated for at least 2 years with risedronate have a greater response to 1 year of teriparatide than those previously treated with alendronate.
Footnotes
This work was supported by The Alliance for Better Bone Health (Procter & Gamble Pharmaceuticals and sanofi-aventis). The authors received editorial support in the preparation of this manuscript funded by The Alliance for Better Bone Health (Procter & Gamble Pharmaceuticals and sanofi-aventis). However, the authors were fully responsible for all content and editorial decisions, and received no financial support or other form of compensation related to the development of the paper.
ClinicalTrials.gov number, NCT00130403.
Disclosure Statement: The authors have nothing to disclose.
First Published Online August 5, 2008
For a list of members of the Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide Group, see Acknowledgments.
Abbreviations: ANCOVA, Analysis of covariance; BAP, bone-specific alkaline phosphatase activity; BCE, bone collagen equivalent; BMD, bone mineral density; CI, confidence interval; CTX, serum C telopeptide; DXA, dual-energy x-ray absorptiometry; NTX, urinary N telopeptide; OC, osteocalcin; P1NP, N-terminal propeptide of type 1 collagen; QCT, quantitative computed tomography.
References
- 1.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- 2.Deal C, Omizo M, Schwartz EN, Eriksen EF, Cantor P, Wang J, Glass EV, Myers SL, Krege JH 2005. Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 20:1905–1911 [DOI] [PubMed] [Google Scholar]
- 3.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F 1997. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350:550–555 [DOI] [PubMed] [Google Scholar]
- 4.Cosman F, Nieves JW, Zion M, Barbuto N, Lindsay R 2008. Effect of prior and ongoing raloxifene therapy on response to PTH and maintenance of BMD after PTH therapy. Osteoporos Int 19:529–535 [DOI] [PubMed] [Google Scholar]
- 5.Ettinger B, San Martin JA, Crans G, Pavo I 2004. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751 [DOI] [PubMed] [Google Scholar]
- 6.Neer R, Hayes A, Rao A, Finkelstein J 2002. Effects of parathyroid hormone, alendronate, or both on bone density in osteoporotic postmenopausal women. J Bone Miner Res 17(Suppl 1):S135 (Abstract 1039)
- 7.Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, Gibson K, Neer RM 2006. Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 91:2882–2887 [DOI] [PubMed] [Google Scholar]
- 8.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ, PaTH Study Investigators 2003. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215 [DOI] [PubMed] [Google Scholar]
- 9.Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD 2000. Short-term increases in bone turnover marker predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int 11:434–442 [DOI] [PubMed] [Google Scholar]
- 10.Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP 2000. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076 [DOI] [PubMed] [Google Scholar]
- 11.Dobnig H, Sipos A, Jiang Y, Fahrleitner-Pammer A, Ste-Marie LG, Gallagher JC, Pavo I, Wang J, Eriksen EF 2005. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab 90:3970–3977 [DOI] [PubMed] [Google Scholar]
- 12.Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB 2005. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res 20:962–970 [DOI] [PubMed] [Google Scholar]
- 13.Lang TF, Keyak JH, Heitz MW, Augat P, Lu Y, Mathur A, Genant HK 1997. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone 21:101–108 [DOI] [PubMed] [Google Scholar]
- 14.Lang TF, Li J, Harris ST, Genant HK 1999. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr 23:130–137 [DOI] [PubMed] [Google Scholar]
- 15.Glover SJ, Garnero P, Naylor K, Rogers A, Eastell R 2008. Establishing a reference range for bone turnover markers in young, healthy women. Bone 42:623–630 [DOI] [PubMed] [Google Scholar]
- 16.dePapp AE, Bone HG, Caulfield MP, Kagan R, Buinewicz A, Chen E, Rosenberg E, Reitz RE 2007. A cross-sectional study of bone turnover markers in healthy premenopausal women. Bone 40:1222–1230 [DOI] [PubMed] [Google Scholar]
- 17.Chen YH, Zhou XH 2006. Interval estimates for the ratio and difference of two lognormal means. Stat Med 25:4099–4113 [DOI] [PubMed] [Google Scholar]
- 18.Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA 2007. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res 22:149–157 [DOI] [PubMed] [Google Scholar]
- 19.Gasser JA, Kneissel M, Thomsen JS, Mosekilde L 2000. PTH and interactions with bisphosphonates. J Musculoskelet Neuronal Interact 1:53–56 [PubMed] [Google Scholar]
- 20.Graeff C, Timm W, Nickelsen TN, Farrerons J, Marín F, Barker C, Glüer CC, EUROFORS High Resolution Computed Tomography Substudy Group 2007. Monitoring teriparatide-associated changes in vertebral microstructure by high-resolution CT in vivo: results from the EUROFORS study. J Bone Miner Res 22:1426–1433 [DOI] [PubMed] [Google Scholar]
- 21.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ 2001. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 296:235–242 [PubMed] [Google Scholar]
- 22.Fromigué O, Body JJ 2002. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest 25:539–546 [DOI] [PubMed] [Google Scholar]
- 23.Plotkin LI, Manolagas SC, Bellido T 2006. Dissociation of the pro-apoptotic effects of bisphosphonates on osteoclasts from their anti-apoptotic effects on osteoblasts/osteocytes with novel analogs. Bone 39:443–452 [DOI] [PubMed] [Google Scholar]
- 24.Russell RGG, Watts NB, Ebetino FH, Rogers MJ 24 January 2008. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759 [DOI] [PubMed] [Google Scholar]
- 25.Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH 2006. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38 617–627 [DOI] [PubMed]
- 26.Masarachia P, Weinreb M, Balena R, Rodan GA 1996. Comparison of the distribution of 3H-alendronate and 3H-etidronate in rat and mouse bones. Bone 19:281–290 [DOI] [PubMed] [Google Scholar]
- 27.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group 2006. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938 [DOI] [PubMed] [Google Scholar]
- 28.Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, Grauer A 2008. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int 19:365–372 [DOI] [PubMed] [Google Scholar]
- 29.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF 2005. Opposite bone remodeling effects of Teriparatide and alendronate in increasing bone mass. Arch Intern Med [Erratum (2005) 165:2120] 165:1762–1768 [DOI] [PubMed] [Google Scholar]
- 30.Bauer DC, Garnero P, Bilezikian JP, Greenspan SL, Ensrud KE, Rosen CJ, Palermo L, Black DM 2006. Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 91:1370–1375 [DOI] [PubMed] [Google Scholar]