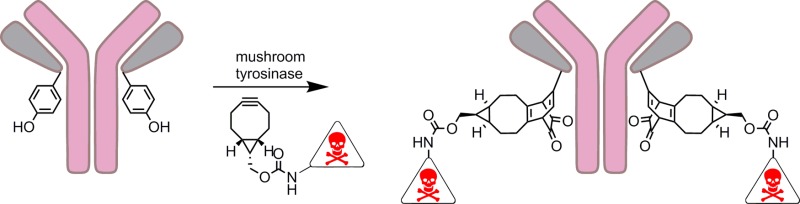
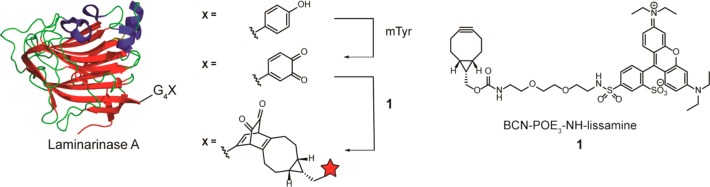
Abstract
Genetically encoded tyrosine (Y-tag) can be utilized as a latent anchor for inducible and site-selective conjugation. Upon oxidation of tyrosine with mushroom tyrosinase, strain-promoted cycloaddition (SPOCQ) of the resulting 1,2-quinone with various bicyclo[6.1.0]nonyne (BCN) derivatives led to efficient conjugation. The method was applied for fluorophore labeling of laminarinase A and for the site-specific preparation of an antibody–drug conjugate.
Introduction
Functional modification of proteins can be achieved in a wide variety of ways. Early generation approaches, entailing the reaction with amino or thiol groups of amino acid side chains,1−3 are effective and facile but in most cases lack selectivity due to relatively high natural abundance of lysines and cysteines in proteins.3 Other methods involving conjugation to tyrosine, histidine, or tryptophan side chains, or to the N-terminus of proteins have also been developed.2 As an alternative to native proteins, full control of regioselectivity can be achieved by introduction of a non-natural amino acid containing a functional handle such as an azide, a ketone or an ortho-aminophenol but often at the expense of protein expression yields.4−6 Site-specific conjugation can also be achieved through enzymatic means, as, for example, with sortase-mediated conjugation, formyl-generating enzyme (FGE), tubulin tyrosine ligase, or activation of dihydrotetrazines by oxidation for tetrazine–TCO ligation via horseradish peroxidase or a photocatalytic agent.7−10
Tyrosine residues show promise as selective conjugation sites, as the relative hydrophobicity of tyrosine combined with the tendency of π–π stacking of the aromatic rings results in the limited exposure of tyrosine residues on the periphery of proteins, resulting in generally low accessibility.11,12 As a consequence, reactive small molecules may conjugate to tyrosines for less-selective conjugation,13−15 while more-exposed tyrosines can allow for a wide variety of enzymatic reactions.12,16−20 For example, exposed tyrosine residues can be oxidized by mushroom tyrosinase to generate a 1,2-quinone, which can undergo nucleophilic attack by amines or thiols from the side chains of lysine, histidine, or cysteine.21−23
We recently showed that a 1,2-quinone undergoes fast strain-promoted oxidation-controlled quinone–alkyne cycloaddition (SPOCQ) with bicyclo[6.1.0]nonyne (BCN).24−26 Here, we report that SPOCQ finds useful application in protein labeling via in situ generation of a quinone by oxidation under the action of mushroom tyrosinase (mTyr). We demonstrate that fast and complete C-terminal labeling of proteins, including an enzyme and a monoclonal antibody, can be readily achieved. The potential usefulness of the SPOCQ labeling approach is exemplified by fully controlled, site-specific generation of an antibody-drug conjugate based on anti-influenza AT1002 and the highly potent tubulin binder monomethyl auristatin F (MMAF).
Results and Discussion
Laminarinase A
Given the inaccessibility of native tyrosine residues by mTyr,11,12 it was envisioned that an exposed tyrosine required installation in a protein of interest by means of a short spacer. Thus, tetra-glycyltyrosine (G4Y) was genetically fused to the C-terminus of a model protein laminarinase A (LamA), a hyperthermostable endo-β-1,3-glucanase from Pyrococcus furiosus, which contained an N-terminal His tag for purification.16,27 Modifications were achieved via site-directed mutagenesis and expression in Escherichia coli (see the Supporting Information section 7), resulting in a C-terminal G4Y fusion (LamA–G4Y, here referred to as Y-tag).
Purified LamA–G4Y was subjected to oxidation by catalytic mTyr (7.5 mol %) to generate the intermediate 1,2-quinone, anticipated to undergo in situ SPOCQ with BCN-modified lissamine 1, present in 4-fold excess (Figure 1). Gratifyingly, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis indicated the conversion of LamA–G4Y into the labeled product after incubation at 37 °C for 30 min with an apparent high conversion (Figure 2A). Negative controls indicated the specificity of conjugation: no fluorescently labeled LamA was detected in the absence of mTyr (lane C). Incubation with mTyr without 1 led solely to an unidentified band (lane B), most likely originating from aspecific intramolecular nucleophilic attack of an amino acid residue (e.g., Lys and His) to the generated quinone, causing it to appear at the expected position.22,23 No fluorescence was detected when SPOCQ was performed with wt-LamA (lane I), which implies that only the newly introduced C-terminal tyrosine is oxidized and undergoes SPOCQ. Mass spectrometry (MS) analysis of the SPOCQ reaction show a main product corresponding to the product resulting from oxidation and cycloaddition of 1 (Figure 2B,C), with a deviation of ±1 Da. Figure 2B displays MS profile of LamA–G4Y prior to oxidation, in agreement with the calculated mass, whereas Figure 2C exhibits LamA–G4Y after oxidation and SPOCQ. The desired product was identified as a peak with molecular weight 32647 Da, indicating an increase of 880 Da (calculated 879 Da) from oxidation of the tyrosine and subsequent conjugation with 1. The oxidized LamA, which underwent aspecific conjugation by a nucleophilic amino acid residue addition was also clearly detected on MS with a mass increase of approximately 13 Da. Further experiments showed that conjugation via SPOCQ could also be performed at 4 and 16 °C and ambient temperature with no observable difference in efficiency (Figure S2).
Figure 1.
SPOCQ labeling of G4Y-tagged laminarinase A by reaction of BCN-modified reagent 1 with in situ generated 1,2-quinone. Typical reaction conditions: LamA (1.0 mg/mL), mTyr (0.3 mg/mL), and 1 (4 equiv) in 50 mM potassium phosphate buffer pH 7.3, containing 135 mM NaCl and 10% DMSO as cosolvent.
Figure 2.
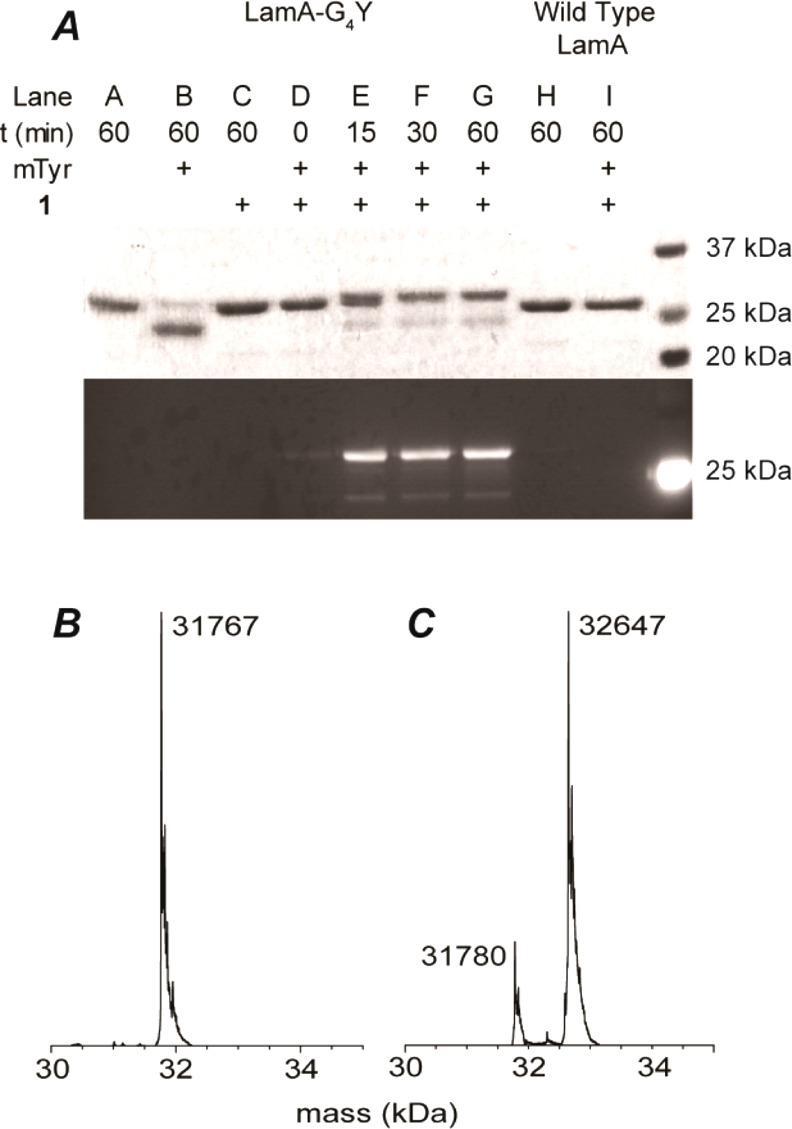
(A) SDS-PAGE analysis of SPOCQ on LamA–G4Y and wt-LamA. (B) MS profile of LamA–G4Y. (C) MS profile of LamA–G4Y after SPOCQ with 1.
Trastuzumab
Having successfully demonstrated the suitability of SPOCQ for C-terminal protein conjugation, its usefulness for site-specific modification of monoclonal antibodies was investigated next. Trastuzumab with genetically engineered tetra-glycyltyrosine on both light chains (Tras[LC]G4Y) was transiently expressed in CHO-K1 and purified by protein A affinity chromatography (Supporting Information section 8). Next, Tras[LC]G4Y was subjected to identical conditions for conjugation with 1 (5 equiv) by SPOCQ at 16 °C as described for LamA. As expected, SDS-PAGE analysis indicated exclusive conversion of Tras[LC]G4Y upon incubation with both 1 and mTyr (Figure S3A), while no reaction was detected on either chain for native trastuzumab under identical conditions. Mass spectrometric analysis confirmed successful addition of 1 via SPOCQ (Figure S3 B,C). It is worth pointing out that the reaction also proceeded at 4 °C, albeit much slower, but to our surprise, no fluorescent protein could be detected at higher temperature (37 °C), possibly via competing intramolecular reaction of the intermediate quinone with nearby lysine or histidine side chains (Figure S4).
AT1002
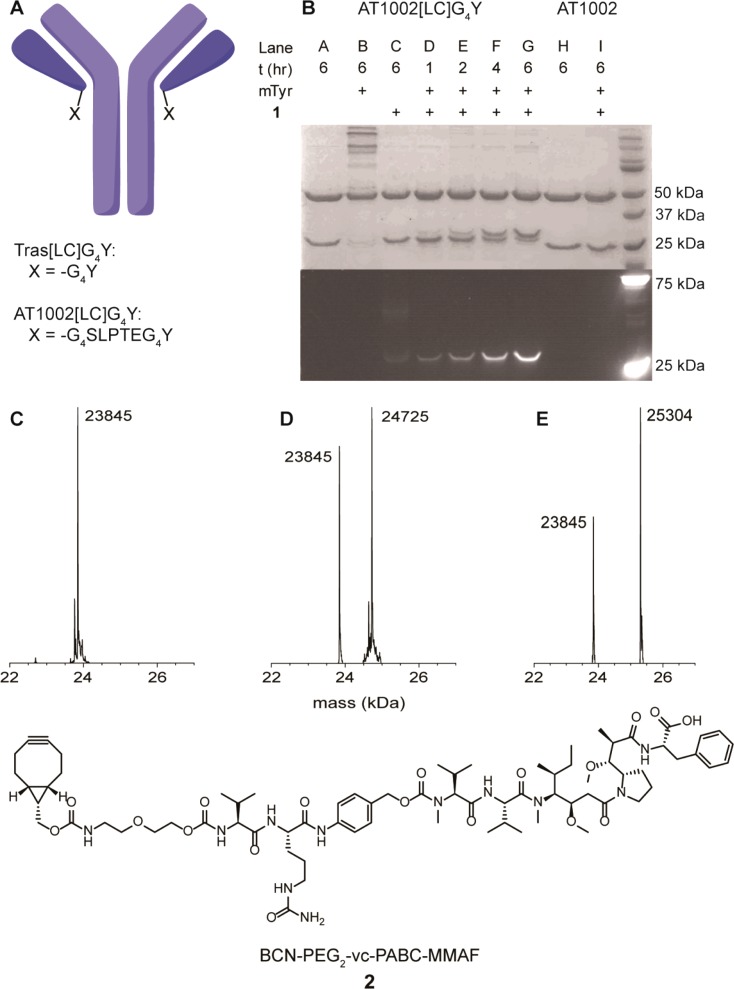
To further assess the applicability of SPOCQ for site-specific modification of monoclonal antibodies, we modified AT1002, a potent anti-influenza antibody, with a C-terminal G4Y tag (Supporting Information section 9).28 To this end, AT1002 with a sortase tag residing on the C-terminus of each light chain was employed to obtain a C-terminally fused G4Y (AT1002[LC]G4Y). Of relevance, the obtained AT1002[LC]G4Y possesses a longer C-terminally fused tag (-G4SLPETG4Y) compared to Tras[LC]G4Y, which was anticipated to contribute favorably with regard to accessibility of the tyrosine by mTyr.29 SPOCQ was attempted on AT1002[LC]G4Y under identical conditions compared to Tras[LC]G4Y, after which SDS-PAGE analysis demonstrated similar conjugation selectivity with high conversion: only fluorescence on the light chain was detected when reacted with mTyr and 1 (Figure 3B). Interestingly, fluorescence of the labeled AT1002 seems more intense than that of trastuzumab under identical condition, which was taken a confirmation that labeling to the light chain with a short spacer may be encumbered by less steric accessibility. Additionally, a protein band corresponding to the conjugated product was observed by SDS-PAGE analysis upon Coomassie staining, indicating a significantly higher conversion for AT1002[LC]G4Y. MS analysis confirmed SPOCQ on AT1002 (Figure 3C,D). As with trastuzumab, some nonlabeled material was still present, which may indicate that also in this case a fraction of the formed quinone reacts with lysine or histidine residues.
Figure 3.
(A) Schematic representation of G4Y-tagged antibodies. (B) SDS-PAGE analysis of SPOCQ on AT1002[LC]G4Y and wt-AT1002. (C) MS profile of AT1002[LC]G4Y (light chain only). (D) MS spectrum of AT1002[LC]G4Y after SPOCQ with 1. (E) MS profile of AT1002[LC]G4Y after SPOCQ with 2.
Our newly developed conjugation method was envisioned to be suitable as a site-specific approach to access antibody–drug conjugates (ADCs). These are a class of promising chemotherapeutics used for targeted treatment of tumors by combining the high cytotoxicity of a drug such as monomethyl auristatin E (MMAE) or maytansine with an antibody that has high binding affinity to the tumor cell of choice.30 The ability of ADCs to bring highly toxic compounds selectively to the tumor cells allows the treatment of cancers while reducing the effect on healthy tissue as with traditional chemotherapies, which has led to the recent market approval of Adcetris (for the treatment of non-Hodgkin lymphoma and anaplastic large-cell lymphoma) and Kadcyla (for treatment of HER2-positive breast cancer).31,32 To this end, AT1002[LC]G4Y was subjected to BCN-monomethyl auristatin F conjugate (BCN-MMAF, 2) after oxidation by mTyr under identical conditions as before, with the exception that DMF was used as cosolvent instead of DMSO. Much to our satisfaction, the desired ADC was seamlessly obtained, as indicated by the expected mass increase of 1459 Da (Figure 3E), which corresponds to oxidation followed by cycloaddition (SPOCQ) with 2.
Conclusions
We have successfully developed a site-specific bioconjugation method based on the in situ enzymatic oxidation of tyrosine by mushroom tyrosinase. Various BCN derivatives were successfully conjugated to C-terminal, oxidized tyrosine residues via SPOCQ. This cycloaddition occurs quickly, selectively, and under physiological conditions on a variety of proteins. Extensive optimization of reaction conditions is anticipated to allow fully selective and quantitative conversion. Research along those lines is currently ongoing in our laboratories. We envision that SPOCQ on tyrosine residues can be a valuable tool to create more unique, orthogonal conjugation possibilities for a wide variety of protein modifications, including the preparation of next-generation, site-specifically generated antibody–drug conjugates.
Acknowledgments
Remon van Geel and Peter van Galen are acknowledged for providing kind assistance with the mass spectrometry measurements. This work is funded by the NWO Gravity Program Institute for Chemical Immunology (ICI).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bioconjchem.7b00046.
Additional method and material details, SPOCQ and expression details, gene and protein sequences, a schematic view of the reaction and corresponding mass values, and MS data. (PDF)
The authors declare the following competing financial interest(s): K.W., L.B. and H.S. declare that they are employees of AIMM therapeutics. H.S. declares that he is also an employee of the department of experimental immunology at AMC, Amsterdam. F.L.V.D. declares that he is also an employee and share-holder of Synaffix BV.
Supplementary Material
References
- Foley T. L.; Burkart M. D. (2007) Site-specific protein modification: advances and applications. Curr. Opin. Chem. Biol. 11, 12–19. 10.1016/j.cbpa.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Jung S.; Kwon I. (2016) Expansion of bioorthogonal chemistries towards site-specific polymer-protein conjugation. Polym. Chem. 7, 4584–98. 10.1039/C6PY00856A. [DOI] [Google Scholar]
- Spicer C. D.; Davis B. G. (2014) Selective chemical protein modification. Nat. Commun. 5, 4740. 10.1038/ncomms5740. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Smith B. A.; Wang L.; Brock A.; Cho C.; Schultz P. G. (2003) A new strategy for the site-specific modification of proteins in vivo. Biochemistry 42, 6735–46. 10.1021/bi0300231. [DOI] [PubMed] [Google Scholar]
- Umeda A.; Thibodeaux G. N.; Zhu J.; Lee Y.; Zhang Z. J. (2009) Site-specific protein cross-linking with genetically incorporated 3,4-dihydroxy-L-phenylalanine. ChemBioChem 10, 1302–04. 10.1002/cbic.200900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdine L.; Gillette T. G.; Lin H. J.; Kodadek T. (2004) Periodate-triggered cross-linking of DOPA-containing peptide-protein complexes. J. Am. Chem. Soc. 126, 11442–3. 10.1021/ja045982c. [DOI] [PubMed] [Google Scholar]
- Mao H.; Hart S. A.; Schink A.; Pollok B. A. (2004) Sortase-mediated protein ligation: a new method for protein engineering. J. Am. Chem. Soc. 126, 2670–1. 10.1021/ja039915e. [DOI] [PubMed] [Google Scholar]
- Schumacher D.; Helma J.; Mann F. A.; Pichler G.; Natale F.; Krause E.; Cardoso M. C.; Hackenberger C. P. R.; Leonhardt H. (2015) Versatile and efficient site-specific protein functionalization by tubulin tyrosine ligase. Angew. Chem., Int. Ed. 54, 13787–91. 10.1002/anie.201505456. [DOI] [PubMed] [Google Scholar]
- Holder P. G.; Jones L. C.; Drake P. M.; Barfield R. M.; Banas S.; de Hart G. W.; Baker J.; Rabuka D. (2015) Reconstitution of formylglycine-generating enzyme with copper(II) for aldehyde tag conversion. J. Biol. Chem. 290, 15730–45. 10.1074/jbc.M115.652669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Trout W. S.; Liu S.; Andrade G. A.; Hudson D. A.; Scinto S. L.; Dicker K. T.; Li Y.; Lazouski N.; Rosenthal J.; et al. (2016) Rapid Bioorthogonal Chemistry Turn-on through Enzymatic or Long Wavelength Photocatalytic Activation of Tetrazine Ligation. J. Am. Chem. Soc. 138, 5978–5983. 10.1021/jacs.6b02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughey G. B.; Gagne M.; Rappe A. K. (1998) pi-Stacking interactions. Alive and well in proteins. J. Biol. Chem. 273, 15458–15463. 10.1074/jbc.273.25.15458. [DOI] [PubMed] [Google Scholar]
- Struck A. W.; Bennett M. R.; Shepherd S. A.; Law B. J.; Zhuo Y.; Wong L. S.; Micklefield J. (2016) An enzyme cascade for selective modification of tyrosine residues in structurally diverse peptides and proteins. J. Am. Chem. Soc. 138, 3038–45. 10.1021/jacs.5b10928. [DOI] [PubMed] [Google Scholar]
- Schlick T. L.; Ding Z.; Kovacs E. W.; Francis M. B. (2005) Dual-surface modification of the tobacco mosaic virus. J. Am. Chem. Soc. 127, 3718–23. 10.1021/ja046239n. [DOI] [PubMed] [Google Scholar]
- Ban H.; Gavrilyuk J.; Barbas C. F. 3rd (2010) Tyrosine bioconjugation through aqueous ene-type reactions: a click-like reaction for tyrosine. J. Am. Chem. Soc. 132, 1523–5. 10.1021/ja909062q. [DOI] [PubMed] [Google Scholar]
- Ban H.; Nagano M.; Gavrilyuk J.; Hakamata W.; Inokuma T.; Barbas C. F. 3rd (2013) Facile and stabile linkages through tyrosine: bioconjugation strategies with the tyrosine-click reaction. Bioconjugate Chem. 24, 520–32. 10.1021/bc300665t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamihata K.; Goto M.; Kamiya N. (2011) Site-specific protein cross-linking by peroxidase-catalyzed activation of a tyrosine-containing peptide tag. Bioconjugate Chem. 22, 74–81. 10.1021/bc1003982. [DOI] [PubMed] [Google Scholar]
- Tilley S. D.; Francis M. B. (2006) Tyrosine-selective protein alkylation using pi-allylpalladium complexes. J. Am. Chem. Soc. 128, 1080–81. 10.1021/ja057106k. [DOI] [PubMed] [Google Scholar]
- Romanini D. W.; Francis M. B. (2008) Attachment of peptide building blocks to proteins through tyrosine bioconjugation. Bioconjugate Chem. 19, 153–7. 10.1021/bc700231v. [DOI] [PubMed] [Google Scholar]
- Long M. J. C.; Hedstrom L. (2012) Mushroom tyrosinase oxidizes tyrosine-rich sequences to allow selective protein functionalization. ChemBioChem 13, 1818–25. 10.1002/cbic.201100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccio G.; Kampf M. M.; Piatti C.; Thony-Meyer L.; Richter M. (2014) Tyrosinase-catalyzed site-specific immobilization of engineered C-phycocyanin to surface. Sci. Rep. 4, 5370. 10.1038/srep05370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S.; Kato T.; Shinpo K.; Fujita K. (1984) Oxidation of tyrosine residues in proteins by tyrosinase. Formation of protein-bonded 3,4-dihydroxyphenylalanine and 5-S-cysteinyl-3,4-dihydroxyphenylalanine. Biochem. J. 222, 407–11. 10.1042/bj2220407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakovic K.; Abul-Hajj Y. J. (1994) Reaction of lysine with estrone 3,4-o-quinone. Chem. Res. Toxicol. 7, 696–701. 10.1021/tx00041a016. [DOI] [PubMed] [Google Scholar]
- Xu R.; Huang X.; Morgan T. D.; Prakash O.; Kramer K. J.; Hawley M. D. (1996) Characterization of products from the reactions of N-acetyldopamine quinone with N-acetylhistidine. Arch. Biochem. Biophys. 329, 56–64. 10.1006/abbi.1996.0191. [DOI] [PubMed] [Google Scholar]
- Borrmann A.; Fatunsin O.; Dommerholt J.; Jonker A. M.; Lowik D. W.; van Hest J. C.; van Delft F. L. (2015) Strain-promoted oxidation-controlled cyclooctyne-1,2-quinone cycloaddition (SPOCQ) for fast and activatable protein conjugation. Bioconjugate Chem. 26, 257–61. 10.1021/bc500534d. [DOI] [PubMed] [Google Scholar]
- Jonker A. M.; Borrmann A.; van Eck E. R.; van Delft F. L.; Lowik D. W.; van Hest J. C. (2015) A fast and activatable cross-linking strategy for hydrogel formation. Adv. Mater. 27, 1235–40. 10.1002/adma.201404448. [DOI] [PubMed] [Google Scholar]
- Dommerholt J.; Schmidt S.; Temming R.; Hendriks L. J.; Rutjes F. P.; van Hest J. C.; Lefeber D. J.; Friedl P.; van Delft F. L. (2010) Readily accessible bicyclononynes for bioorthogonal labeling and three-dimensional imaging of living cells. Angew. Chem., Int. Ed. 49, 9422–5. 10.1002/anie.201003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybysz A.; Volmer A. A.; Westphal A. H.; van Berkel W. J. (2014) Bifunctional immobilization of a hyperthermostable endo-beta-1,3-glucanase. Appl. Microbiol. Biotechnol. 98, 1155–63. 10.1007/s00253-013-4953-3. [DOI] [PubMed] [Google Scholar]
- Wagner K.; Kwakkenbos M. J.; Claassen Y. B.; Maijoor K.; Bohne M.; van der Sluijs K. F.; Witte M. D.; van Zoelen D. J.; Cornelissen L. A.; Beaumont T.; et al. (2014) Bispecific antibody generated with sortase and click chemistry has broad antiinfluenza virus activity. Proc. Natl. Acad. Sci. U. S. A. 111, 16820–5. 10.1073/pnas.1408605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorywalska M.; Strop P.; Melton-Witt J. A.; Hasa-Moreno A.; Farias S. E.; Galindo Casas M.; Delaria K.; Lui V.; Poulsen K.; Loo C.; et al. (2015) Effect of Attachment Site on Stability of Cleavable Antibody Drug Conjugates. Bioconjugate Chem. 26, 650–659. 10.1021/bc5005747. [DOI] [PubMed] [Google Scholar]
- Chari R. V.; Miller M. L.; Widdison W. C. (2014) Antibody-drug conjugates: an emerging concept in cancer therapy. Angew. Chem., Int. Ed. 53, 3796–827. 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- Younes A.; Bartlett N. L.; Leonard J. P.; Kennedy D. A.; Lynch C. M.; Sievers E. L.; Forero-Torres A. (2010) Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 363, 1812–21. 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- Verma S.; Miles D.; Gianni L.; Krop I. E.; Welslau M.; Baselga J.; Pegram M.; Oh D. Y.; Dieras V.; Guardino E.; et al. (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–91. 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.