Abstract
Context:
Genomic rearrangements at 15q21 have been shown to cause overexpression of CYP19A1 and resultant aromatase excess syndrome (AEXS). However, mutation spectrum, clinical consequences, and underlying mechanisms of these rearrangements remain to be elucidated.
Objective:
The aim of the study was to clarify such unsolved matters.
Design, Setting, and Methods:
We characterized six new rearrangements and investigated clinical outcome and local genomic environments of these rearrangements and of three previously reported duplications/deletions.
Results:
Novel rearrangements included simple duplication involving exons 1–10 of CYP19A1 and simple and complex rearrangements that presumably generated chimeric genes consisting of the coding region of CYP19A1 and promoter-associated exons of neighboring genes. Clinical severities were primarily determined by the copy number of CYP19A1 and the property of the fused promoters. Sequences at the fusion junctions suggested nonallelic homologous recombination, nonhomologous end-joining, and replication-based errors as the underlying mechanisms. The breakpoint-flanking regions were not enriched with GC content, palindromes, noncanonical DNA structures, or known rearrangement-associated motifs. The rearrangements resided in early-replicating segments.
Conclusions:
These results indicate that AEXS is caused by duplications involving CYP19A1 and simple and complex rearrangements that presumably lead to the usage of cryptic promoters of several neighboring genes. Our data support the notion that phenotypes depend on the dosage of CYP19A1 and the characteristics of the fused promoters. Furthermore, we show that the rearrangements in AEXS are generated by both recombination- and replication-mediated mechanisms, independent of the known rearrangement-inducing DNA features or late-replication timing. Thus, AEXS represents a unique model for human genomic disorders.
Aromatase excess syndrome (AEXS; MIM no. 139300) is a rare autosomal dominant disorder that causes prepubertal- or peripubertal-onset gynecomastia, hypogonadotropic hypogonadism, advanced bone age, and short adult height in male patients (1, 2). Female patients are usually asymptomatic, although macromastia, irregular menses, and short stature have been reported in a few individuals (2). AEXS results from excessive expression of the aromatase gene CYP19A1 on chromosome 15q21.2 (NM_000103) (1). CYP19A1 comprises 11 noncoding exons 1 that function as tissue-specific promoters (exons I.1, IIa, I.8, I.4, I.5, I.7, 1f, I.2, I.6, I.3, and PII), and nine coding exons (exons 2–10) (3, 4). We and other groups have identified various chromosomal rearrangements at 15q21 in patients with AEXS (1, 2, 5). These rearrangements included duplications that encompassed seven of the 11 noncoding exons 1 of CYP19A1 and deletions and inversions that generated chimeric genes consisting of coding exons of CYP19A1 and promoter-associated exons of neighboring genes. Genotype-phenotype analysis has indicated that clinical severities primarily depend on the functional properties of the fused promoters. These findings provide a novel example of gain-of-function mutations resulting from submicroscopic genomic rearrangements.
Rearrangements in the human genome are known to be generated by recombination-based mechanisms, namely, nonallelic homologous recombination and nonhomologous end-joining, and by replication-based mechanisms (6–9). Of these, nonallelic homologous recombination results from unequal crossover between two homologous sequences, usually on the same but sometimes on different chromosomes (10). Nonallelic homologous recombination accounts for most of the recurrent simple deletions and duplications in the human genome and represents the most common abnormality involved in human genomic disorders (9–11). Nonhomologous end-joining occurs as a result of double-strand DNA breakage and subsequent ligation of the two broken DNA ends (12). Nonhomologous end-joining often underlies nonrecurrent simple deletions associated with short nucleotide stretches at the fusion junctions (9–12). Replication-based mechanisms are caused by aberrant template switching during replication and can produce both simple and complex rearrangements that carry microhomologies at the fusion junctions (8, 9, 13). Previous studies have indicated that nonallelic homologous recombination, nonhomologous end-joining, and replication-based mechanisms are facilitated by various local DNA features including high GC content and palindromes (10, 14–16). Highly similar sequences widely spread in the genome (“repetitive elements”), such as Alu, LINE1, and MIR, can mediate the occurrence of genomic rearrangements (12). Non-B structures, ie, DNA conformations that differ from the canonical Watson-Crick right-handed double helix, and specific short sequence motifs and tri/tetranucleotides have also been suggested as local genomic stimulants (14–22). Furthermore, replication timing of each chromosomal region appears to determine the frequency of rearrangements; nonallelic homologous recombination preferentially occurs in DNA segments that replicate in early S phase (early-replicating segments), whereas nonhomologous end-joining and replication-based errors frequently appear in late-replicating segments (23).
At present, the underlying mechanisms of the AEXS-associated rearrangements remain largely unknown. Although sequence analysis of the fusion junctions has indicated that nonallelic homologous recombination and nonhomologous end-joining—and possibly replication-based mechanisms as well—are involved in the formation of simple duplications and deletions in AEXS (5), the molecular basis of inversions remains to be determined. Here, we characterized the fine genomic structures of six rearrangements involved in AEXS. Furthermore, we investigated clinical consequences and local genomic environments of the six rearrangements and of three previously reported duplications/deletions.
Patients and Methods
Patients
This study consisted of six cases (cases 1–6) ascertained by prepubertal- or peripubertal-onset gynecomastia. Clinical findings of cases 1–6 are summarized in Table 1. Cases 1–4 are hitherto unreported. Cases 5 and 6 have been described previously, although the genomic structure remains to be determined (1, 2). Cases 1–3 and 5–6 had a 46,XY karyotype, whereas case 4 had a 46,XY inv (9) karyotype that is known as a normal variant. Case 2 had a brother with prepubertal-onset gynecomastia, a sister with premature thelarche, and a father and several paternal relatives with advanced bone age and/or short stature. Case 6 had a son with prepubertal-onset gynecomastia. There was no family history of AEXS in the remaining cases. This study was approved by the Institutional Review Board Committee at the National Center for Child Health and Development. Written informed consent was obtained from the patients and/or their parents.
Table 1.
Phenotypic and Endocrine Findings of Cases 1–6
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Genomic rearrangement | Duplication | Deletion | Complex | Complex | Complex | Complex |
| Age at examination, y | 10 | 8 (18)a | 15 | 13 | 17 | 36 |
| Phenotypic findings | ||||||
| Gynecomastia (Tanner stage) | 2–3 | 3 | 4–5 | 3–4 | Severe | Severe |
| Onset of gynecomastia, y | 7 | Unknown | 8 | 11 | 7 | 5 |
| Mastectomy | No | Yes | Yes | No | Yes | Yes |
| Testis, mL | 6 | N.E. | 15 | 12 | Normal | Normal |
| Pubic hair (Tanner stage) | None | None | 3–4 | 4 | N.D. | Normal |
| Facial hair | None | None | None | Scarce | Scarce | None |
| Final height | Unknown | Unknown | −0.9 SD | Unknown | <1%ile | <1%ile |
| Bone age, yb | 13.0 | 13.5 | N.E. | 18.0 | N.E. | N.E. |
| Fertility (spermatogenesis) | Unknown | Unknown | Yes | Unknown | Unknown | Yes |
| Endocrine findingsc | ||||||
| At diagnosis | ||||||
| LH, mIU/mL | <0.1 (0.4–1.6) → 0.4 (10.9–20.6)d | 2.4 (1.6–3.5) | 1.3 (1.6–3.5) → 24.9 (21.7–39.5)d | 4.3 (1.4–9.2) | 1.7 (1.4–9.2) | |
| FSH, mIU/mL | 0.3 (1.7–4.2) → 1.6 (4.6–10.8)d | <1.0 (4.2–8.2) | 0.6 (4.2–8.2) → 2.1 (11.2–17.3)d | 2.7 (2.0–8.3) | 1.5 (4.2–8.2) | |
| T, ng/mL | 0.06 (0.4–1.1) → 3.6 (>2.0)e | 2.6 (2.8–7.0) | 0.7 (2.8–7.0) | 1.5 (2.8–7.0) | 2.3 (2.8–7.0) | 3.2 (2.8–7.0) |
| E1, pg/mL | 111 (14–50) | 556 (15–32) | 903 (15–32) | |||
| E2, pg/mL | 14 (<10) | 65 (10–35) | 406 (15–50) | 43 (2–30) | 392 (10–35) | 223 (10–35) |
| On AI treatment | ||||||
| LH, mIU/mL | 0.5 (0.4–1.6) → 7.3 (10.9–20.6)d | 44.8 (0.7–5.7)f | 4.7 (1.6–3.5) | 8.9 (1.4–9.2) | 2.9 (1.4–9.2) | |
| FSH, mIU/mL | 1.7 (1.7–4.2)→ 3.2 (4.6–10.8)d | 34.9 (2.0–8.3)f | 2.5 (4.2–8.2) | 5.6 (2.0–8.3) | 5.6 (4.2–8.2) | |
| T, ng/mL | 0.9 (0.4–1.1) | 8.6 (2.8–7.0) | 6.9 (2.8–7.0) | 5.3 (2.8–7.0) | 10.7 (2.8–7.0) | |
| E1, pg/mL | 89 (15–32) | 27 (15–32) | ||||
| E2, pg/mL | <10 (<10) | 6 (10–35) | 13 (15–50) | 59 (10–35) | 68 (10–35) | |
| Reference | Present study | Present study | Present study | Present study | Ref. 1 | Ref. 1 |
Abbreviations: AI, aromatase inhibitor; E1, estrone; E2, estradiol; N.D., not described; N.E., not examined. Abnormal clinical findings are boldfaced. Hormone values below the reference range (shown in parentheses) are underlined, and those above the reference range are boldfaced. Conversion factors to the SI unit: LH, 1.0 (IU/L); FSH, 1.0 (IU/L); E1, 3.699 (pmol/L); E2, 3.671 (pmol/L); and T, 3.467 (nmol/L).
Physical examination and endocrine studies were carried out at 8 and 18 years of age, respectively.
Assessed by the Tanner-Whitehouse 2 method standardized for Japanese or by the Greulich-Pyle method constructed for Caucasians.
Evaluated by age-matched male reference data.
GnRH stimulation tests (100 μg/m2, maximum 100 μg bolus iv; blood sampling at 0, 30, 60, 90, and 120 min).
Human chorionic gonadotropin stimulation tests (3000 IU/m2, maximum 5000 IU im for 3 consecutive days; blood sampling on d 1 and 4).
Increased levels of LH and FSH during AI treatment may be associated with low E2 levels (24).
Copy-number analyses
Leukocyte genomic DNA samples were obtained from cases 1–6, the parents and siblings of case 2, and the son of case 6. Genomic abnormalities involving CYP19A1 exons and/or its flanking regions were examined by comparative genomic hybridization (CGH) using a custom-made oligoarray or a catalog human array (4 × 180K format, ID 030700 or G4449A; Agilent Technologies). The procedures were performed as described previously (5).
Characterization of the genomic structures of rearrangements
Breakpoints of the rearrangements were determined by direct sequencing of the PCR-amplified DNA fragments harboring the fusion junctions. PCRs were carried out using a number of primer pairs for various genomic positions around CYP19A1. The sequences of the primers utilized in the present study are available upon request. To confirm the formation of a chimeric gene in a case with a complex rearrangement, we performed RT-PCR using leukocyte mRNA and primers annealing to exon 2 of CYP19A1 and exons of neighboring genes. The presence or absence of promoter-associated histone marks in the fused exons was analyzed using the UCSC genome browser (http://genome.ucsc.edu/).
Genotype-phenotype analysis
We performed genotype-phenotype analyses in cases 1–6 and in 18 patients identified in our previous study (5).
DNA sequences at the fusion junctions
To clarify the underlying mechanisms of the rearrangements, we examined the presence or absence of microhomologies and short nucleotide stretches at the fusion junctions. In addition, we searched for repeat elements around the breakpoints using Repeatmasker (http://www.repeatmasker.org).
Genomic environments around the breakpoints
We studied the frequencies of known rearrangement-inducing DNA features in the breakpoint-flanking regions. In silico analyses were carried out in the 300-bp regions at the proximal and distal sides of each breakpoint. We also examined control regions (n = 53) randomly selected at an interval of 1.5 Mb from the entire 15q (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). We calculated the average GC content using GEECEE (http://emboss.bioinformatics.nl/cgi-bin/emboss/geecee) and searched for palindromes using PALINDROME (http://mobyle.pasteur.fr/cgi-bin/portal.py#forms::palindrome) and Non-B structures using Non-B DB (http://nonb.abcc.ncifcrf.gov). Examined Non-B structures included direct repeats, inverted repeats (cruciforms), mirror repeats, A-phased repeats, G-quadruplex repeats, short tandem repeats, and Z-DNA motifs (17). The presence or absence of the 10 specific sequence motifs and two tri/tetranucleotides implicated in rearrangements in various chromosomal regions (14, 18–22) were analyzed using Fuzznuc (http://emboss.bioinformatics.nl/cgi-bin/emboss/fuzznuc).
Replication timing analysis
We analyzed whether the rearrangements at 15q21 have occurred at a specific timing of S phase (23). Replication timing profiles of the approximately 10-Mb genomic interval around CYP19A1 were evaluated using 92 datasets currently available in the ReplicationDomain database (http://www.replicationdomain.com/replication_timing.php).
Statistical analyses
Statistical significance of the average GC content between the breakpoint-flanking and control regions was analyzed by Student's t test. Differences in the frequencies of other rearrangement-inducing DNA features were examined by Fisher's exact probability test. P < .05 was considered significant.
Results
Copy-number alterations in cases 1–6
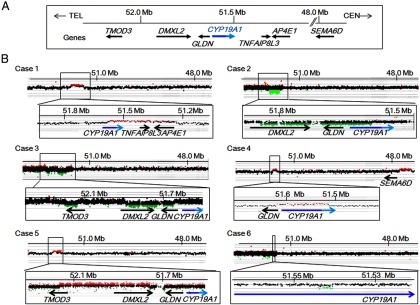
CGH analyses indicated heterozygous genomic rearrangements involving CYP19A1 and/or its neighboring genes; ie, an approximately 0.4-Mb duplication involving CYP19A1, TNFAIP8L3, and AP4E1 in case 1; an approximately 0.3-Mb deletion affecting DMXL2, CYP19A1, and GLDN in case 2; an approximately 80-kb deletion involving TMOD3 and an approximately 250-kb deletion involving DMXL2 and GLDN in case 3; an approximately 130-kb duplication involving GLDN and CYP19A1 and an approximately 340-kb duplication involving SEMA6D at a position of approximately 3.6 Mb distant from CYP19A1 in case 4; an approximately 370-kb duplication involving TMOD3, TMOD2, LYSMD2, SCG3, and DMXL2, and a 3- to 35-kb deletion between DMXL2 and GLDN in case 5; and an approximately 3.5-kb deletion in the promoter region of CYP19A1 in case 6 (Table 2 and Figure 1). The deletion in case 5 could not be narrowed down because of the absence of CGH probes around the breakpoints. The father and siblings of case 2 and the son of case 6 carried the same abnormalities as the probands.
Table 2.
Genomic Rearrangements in Cases 1–6
| Rearrangement | Genomic Abnormality | Affected Genesa | |
|---|---|---|---|
| Case 1 | Simple | Simple duplication | CYP19A1, TNFAIP8L3, AP4E1 |
| Case 2 | Simple | Simple deletion | CYP19A1, GLDN, DMXL2 |
| Case 3b | Complex | Multiple deletions? | TMOD3, GLDN, DMXL2? |
| Case 4 | Complex | Multiple duplications and inversion | CYP19A1, GLDN, SEMA6D |
| Case 5 | Complex | Multiple duplications, deletion, and inversion | TMOD3, DMXL2, TMOD2, LYSMD2, SCG3 |
| Case 6 | Complex | Multiple deletions and inversion | CGNL1, CYP19A1 |
Genes involved in the deletion or duplication. Genes affected by copy-number-neutral inversions are not shown.
Genomic structure of the rearrangement in case 3 remains to be characterized.
Figure 1.
Copy-number analyses in cases 1–6. A, Schematic representation of the normal genomic structure around CYP19A1. The arrows indicate genomic positions and transcriptional direction of genes (5′→3′). For CYP19A1, the dark and light blue lines denote the genomic regions for noncoding exons 1 and coding exons 2–10, respectively. Genomic positions refer to Human Genome Database (hg19, build 37). Only genes around the fusion junctions are shown. B, CGH analyses in the six cases. The black, red, and green dots denote signals indicative of the normal, increased (>+0.5) and decreased (<−1.0) copy-numbers, respectively.
Genomic structures of six rearrangements
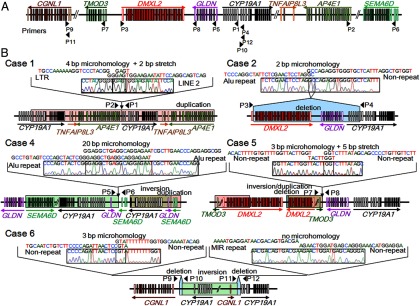
We were able to characterize all fusion junctions in cases 1, 2, and 6 and one of the multiple junctions in cases 4 and 5 (Table 3, Supplemental Table 2, and Figure 2). The remaining breakpoints could not be determined due to the low quality of the DNA samples, the presence of long repetitive sequences around the breakpoints, or the complex structures of the rearrangements. In case 1, we identified a 387 622-bp tandem duplication involving six of the 11 exons 1 (exons I.7, 1f, I.2, I.6, I.3, and PII) and all coding exons of CYP19A1, together with all exons of TNFAIP8L3 and AP4E1. In case 2, we detected a 303 624-bp deletion involving six of the CYP19A1 exons 1 (exons I.1, IIa, I.8, I.4, I.5, and I.7), all exons of GLDN, and DMXL2 exons 2–43. In case 4, we identified two duplications: an approximately 130-kb duplication encompassing all noncoding exons 1 and coding exons 2–3 of CYP19A1 and GLDN exon 1, and an approximately 340-kb duplication involving SEMA6D exons 1–3. PCR products were obtained with a primer pair for GLDN intron 1 and SEMA6D intron 3 (P5 and P6 in Figure 2A), indicating that the approximately 3.6-Mb genomic interval harboring GLDN exon 1, all noncoding and coding exons of CYP19A1, and SEMA6D exons 4–20 was inverted. In addition, we analyzed mRNA of case 4 and detected a chimeric clone composed of CYP19A1 exon 2 and SEMA6D noncoding exon 3 (Supplemental Figure 1). Thus, although we could not determine the fusion junctions of the duplication, these data imply that the rearrangement was caused by an inversion of an approximately 3.6-Mb region and a duplication of the telomeric part of the inverted DNA fragment. In case 5, we identified an approximately 370-kb duplication containing TMOD3 exon 1, DMXL2 exons 1–29, and all exons of TMOD2, LYSMD2, and SCG3. PCR products were obtained with a primer pair for TMOD3 intron 1 and the downstream region of GLDN (P7 and P8), indicating that the approximately 370-kb region was duplicated and inserted into the genome in a reverse direction. The small deletion between DMXL2 and GLDN detected by CGH could not be characterized because of the presence of long repetitive sequences around the breakpoints. In case 6, we identified a complex deletion–inversion–deletion rearrangement: a 202-bp deletion within CGNL1 intron 1, an approximately 6.1-Mb inversion encompassing CGNL1 exon 1, eight of the CYP19A1 exons 1 (exons I.1, IIa, I.8, I.4, I.5, I.7, 1f, and I.2), and ≥25 genes, and a 3476-bp deletion within CYP19A1 intron 1.
Table 3.
Fusion Junctions in Cases 1–6
| No. of Fusion Junctions | No. of Fusion Junctions Characterized in This Studya | Sequences at the Fusion Junctions |
Predicted Mechanism | ||
|---|---|---|---|---|---|
| Microhomology | Nucleotide Stretch | ||||
| Case 1 | 1 | 1 | Yes (4 bp) | Yes (2 bp) | RBM |
| Case 2 | 1 | 1 | Yes (2 bp) | No | RBM |
| Case 3b | 2? | 0 | Unknown | Unknown | RBM? |
| Case 4 | 5 | 1 | Yes (20 bp) | No | RBM |
| Case 5 | 3 | 1 | Yes (3 bp) | Yes (5 bp) | RBM |
| Case 6 | 2 | 2 | Yes (3 bp)c/No | No | RBM |
Abbreviation: RBM, replication-based mechanism.
Several breakpoints could not be determined due to low quality of the DNA samples, the presence of long repetitive sequences around the breakpoints, or the complex structures of the rearrangements.
Genomic structure of the rearrangement in case 3 remains to be characterized.
Microhomology was observed at the telomeric junction.
Figure 2.
Fine genomic structures of the rearrangements. A, Schematic representation of the normal genomic structure. Arrowheads indicate the positions and the directions (5′→3′) of PCR primers utilized in this study (P1–P12). The open and color-painted boxes denote noncoding and coding exons, respectively. The sizes of the exons, introns, and primers are not drawn to scale. B, Schematic representation of the rearrangements and the DNA sequences at the fusion junctions. The red, blue, and green areas indicate duplications, deletions, and inversions, respectively. P1–P12 indicate the same PCR primers as shown in panel A. The fusion junctions of case 3 were not characterized. For case 4, the precise genomic position of the duplication remains to be clarified.
Phenotypic consequences of the six new and three previously reported rearrangements
We studied genotype-phenotype correlation in cases 1–6 and 18 previously reported patients (four patients from families A–B with simple duplications involving the CYP19A1 promoter region, and 14 patients from families C–F with DMXL2-CYP19A1 chimeric genes) (5). The results are summarized in Table 4. First, clinical severities were relatively mild in case 1 and patients from families A–B with simple duplications, obviously severe in cases 5 and 6 with complex rearrangements, and moderate in the remaining cases/patients with simple deletions or complex rearrangements. Second, among cases/patients with simple duplications, case 1 showed earlier onset of gynecomastia and more severely advanced bone age than patients from families A–B. Third, among cases/patients with deletions, case 2 manifested milder gynecomastia than case 3 and patients from families C–F. Lastly, among cases/patients with deletions or complex rearrangements, cases 2–4 and patients from families C–F showed milder phenotypes than cases 5 and 6.
Table 4.
Genotype-Phenotype Correlation in Cases 1–6 and Families A–F
| Cases/Familiesa | Case 1 | Families A and B | Case 2 | Case 3b, Families C–F | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|---|
| Molecular defects | |||||||
| Predicted mechanism for CYP19A1 overexpression | Duplication of CYP19A1 coding exons | Duplication of CYP19A1 promoters | Chimeric gene formation | Chimeric gene formation | Chimeric gene formation | Chimeric gene formation | Chimeric gene formation |
| Genes involved in chimeric gene formation | None | None | DMXL2 | DMXL2 | SEMA6D | TMOD3 | CGNL1 |
| Copy-number of the CYP19A1 exon 1.4c | Normal | Increased | Decreased | Normal | Increasedd | Normal | Decreased |
| Clinical findings | |||||||
| Onset of gynecomastia, y | 7 | 10–13 | Unknown | 7–12 | 11 | 7 | 5 |
| Gynecomastia (Tanner stage) | 2–3 | 2–3 | 1–3e | 3–5 | 3–4 | Severe | Severe |
| Advanced bone age | Mild | Subtle | Moderate | Mild/ moderate | Severe | N.E. | N.E. |
Abbreviation: N.E., not examined.
Cases 1–6 were present cases, whereas families A–F were reported previously (5).
Fine genomic structure of case 3 remains to be characterized.
Exon 1.4 functions as the major promoter in extragonadal tissues.
Duplicated exon 1.4 has been disconnected from the coding exons of CYP19A1.
The patient and his father had gynecomastia of Tanner stages 3 and 1, respectively.
DNA sequences at the fusion junctions
We characterized fusion junctions of the rearrangements in cases 1, 2, and 4–6 and in patients from families A–F (Table 3, Supplemental Table 2, and Figure 2). The results indicated the following: 1) nonallelic homologous recombination for the recurrent simple deletions in patients from families D–F that took place between two homologous sequences; 2) nonhomologous end-joining for the nonrecurrent simple deletions in patients from family C that were associated with short nucleotide stretches at the fusion junction; and 3) replication-based mechanisms for the simple and complex aberrations in cases 1, 2, and 4–6, and in patients from families A–B that were accompanied by microhomologies at the fusion junction. Nine of the 18 breakpoints resided within repetitive elements, such as LINE 1, LINE 2, AluJo, AluY, and AluSx3.
Genomic environments around the breakpoints
The average GC content was similar between the breakpoint-flanking and control regions (Supplemental Tables 2 and 3). Furthermore, the frequencies of known rearrangement-inducing DNA features (12, 14, 18–22) did not significantly differ between the breakpoint-flanking and control regions, except for some non-B structures enriched around the breakpoints of the deletions in patients from families D–F (Supplemental Tables 2 and 3).
Replication timing of the 15q21 region
Replication timing analysis indicated that in most cell lines examined, the genomic region around CYP19A1 is replicated during early S phase (Supplemental Figure 2).
Discussion
We characterized six genomic rearrangements in patients with AEXS (Supplemental Figure 3). In case 1, the tandem duplication seems to have enhanced the transcriptional efficiency of CYP19A1 in native CYP19A1-expressing tissues by increasing the number of transcription start sites. In cases 2–6, the rearrangements are predicted to have created chimeric genes consisting of coding exons of CYP19A1 and promoter-associated exons of neighboring genes. Actually, the deletions in cases 2 and 3 appear to have permitted splicing between DMXL2 exon 1 and CYP19A1 exon 2, as has been shown in the patients with similar deletions (5). Furthermore, the inversion in case 4 was found to produce a chimeric gene consisting of exon 3 of SEMA6D and exon 2 of CYP19A1 (Supplemental Figure 1), and the inversions in cases 5 and 6 have previously been shown to form TMOD3- and CGNL1-CYP19A1 chimeric genes, respectively (2). In this regard, the rearrangements in cases 2–6 have brought not only exons 1 of other genes, but also their flanking regions of >10 kb, to lie near the coding region of CYP19A1. Because these flanking regions harbor several enhancer- and promoter-associated histone marks (H3K4Me1 and H3K4Me3) (Supplemental Figure 4), they appear to contain most, if not all, components of cis-regulatory elements. Thus, although we can not examine the actual expression pattern of the chimeric genes, these genes seem to be expressed in a wide range of tissues where the original genes are expressed. These results argue for a broad mutation spectrum of AEXS.
Such diverse genetic basis of AEXS would be relevant to phenotypic variations (Table 4). First, cases/patients with copy-number gains of CYP19A1 showed milder phenotypes than those with chimeric genes. This is consistent with the limited tissue expression pattern of CYP19A1 and broad expression patterns of other genes involved in the chimeric gene formation (5, 25). Second, among cases/patients with simple duplications, case 1 showed a more severe phenotype than patients from families A–B. This suggests that tandem duplications encompassing the transcriptional unit, ie, the promoter region plus the coding exons, permit more efficient aromatase protein production than tandem duplications encompassing the promoter region only. Third, among cases/patients with the same DMXL2-CYP19A1 chimeric gene, case 2 manifested milder phenotypes than case 4 and patients from families C–F. These results can be explained by the difference in the number of CYP19A1 exons 1, because six of CYP19A1 exons 1 were deleted in case 2 and all exons 1 were preserved in the remaining cases/patients (Supplemental Figure 5). Fourth, case 4 with a SEMA6D-CYP19A1 chimeric gene showed a milder phenotype than cases 5 and 6 with a TMOD3- and CGNL1-CYP19A1 chimeric gene, respectively. This is consistent with a tissue expression pattern being broader in TMOD3 and CGNL1 than in SEMA6D (5, 25). Lastly, cases/patients with DMXL2-CYP19A1 chimeric genes manifested milder phenotype than cases with a TMOD3- or CGNL1-CYP19A1 chimeric gene. This would primarily be ascribed to the presence or absence of a translational start codon on the fused promoter-associated exons (Supplemental Figure 6). It is likely that DMXL2-CYP19A1 chimeric mRNAs transcribed by the DMXL2 promoter preferentially recognize the natural start codon on DMXL2 exon 1 and undergo nonsense-mediated mRNA decay, and rather exceptional chimeric mRNAs utilize the start codon on CYP19A1 exon 2 and produce the aromatase protein (5). Such a phenomenon would not be postulated for the TMOD3- and CGNL1-CYP19A1 chimeric mRNAs because of the absence of a translation start codon on exons 1 of TMOD3 and CGNL1. Taken together, the present study suggests that phenotypic severity is primarily determined by the copy-number of CYP19A1 and by the expression patterns and structural properties of the fused promoters. It should be pointed out, however, that this conclusion is based on the observation of only a limited number of patients. Phenotypic variation of the patients may be due to low penetrance of the clinical features.
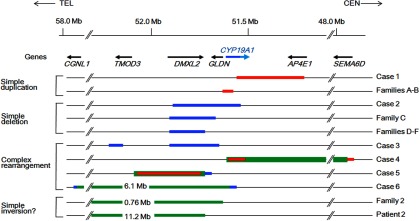
To date, 11 genomic rearrangements have been identified in patients with AEXS (Figure 3). The 11 rearrangements are widely distributed on an approximately 9-Mb region and include simple duplications, deletions, and inversions, as well as complex rearrangements. Of these, the rearrangements in cases 1, 2, and 4–6 and in patients from families A–B are predicted to be replication-based errors (Supplemental Table 2 and Figure 2). Although the short nucleotide stretches at the fusion junctions in cases 1 and 5 may represent “information scars” characteristic of nonhomologous end-joining (9), the complex structures of the rearrangements would be consistent with replication-based mechanisms rather than end-joining (8). However, these rearrangements may result from microhomology-mediated end-joining (26). In contrast, the simple deletions in patients from family C and those in patients from families D–F are compatible with nonhomologous end-joining and nonallelic homologous recombination, respectively (Supplemental Table 2 and Figure 2). These results imply that the genomic region at 15q21 is vulnerable to both recombination- and replication-mediated errors.
Figure 3.
Schematic representation of the 11 rearrangements. Cases 1, 2, and 4–6 are from the present study, and patients from families A–F, patient 2, and patients from family 2 have been reported previously (1, 2, 5). The genomic abnormalities of case 3 were not characterized. The arrows indicate the positions and transcriptional direction of CYP19A1 and its neighboring genes (5′→3′). Only genes around the fusion junctions are shown. The red, blue, and green lines indicate duplications, deletions, and inversions, respectively. For CYP19A1, the dark and light blue lines denote the genomic regions for the noncoding and coding exons, respectively. The inversions of family 2 and patient 2 may be complex rearrangements because copy-number analyses have not been performed in these cases.
In silico analyses revealed that deletions in families D–F due to nonallelic homologous recombination were associated with non-B structures and were located within an early-replicating segment of the genome, whereas the breakpoint-flanking regions of other rearrangements were independent of known rearrangement-inducing DNA features or late-replication timing. These data indicate that there are hitherto unidentified factors that facilitate nonhomologous end-joining and replication-based errors at 15q21. In this regard, it is noteworthy that nine of the 18 breakpoints resided within repetitive elements, and frequencies of Alus (16%) and LINEs (22%) in the breakpoint-flanking regions were slightly higher than expected from the draft human genome (Alu, 9.9%; and LINE, 16.1%) (27). An increased number of repetitive sequences was found around the breakpoints of various rearrangements (14, 18, 19, 21), and Boone et al (28) have reported that a high concentration of Alu elements may predispose replication-based errors. The presence of various Alu family members (AluJo, AluY, and AluSx3) at the fusion junction of our cases supports the notion that moderate sequence similarity between Alu elements would be sufficient to provide substrates for replication-based errors (28). Further studies are necessary to clarify the role of repetitive sequences in the formation of rearrangements.
In summary, the present study implies a broad mutation spectrum of AEXS and supports the previously proposed notion that clinical severities of AEXS are determined by the dosage of the promoter and coding regions of CYP19A1 and by characters of the fused promoters. We show that rearrangements involved in AEXS can be attributed to nonallelic homologous recombination that is induced by repeats and/or by early-replication timing, and to nonhomologous end-joining and replication-based mechanisms that occur independently of known rearrangement-inducing DNA features or a late-replicating timing. Thus, AEXS represents a unique model for human genomic disorders.
Acknowledgments
This work was supported by the Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology; by the Grant-in-Aid for Scientific Research and for Challenging Exploratory Research from the Japan Society for the Promotion of Science; by the Grant for Research on Intractable Diseases from the Ministry of Health, Labor, and Welfare; by grants from the National Center for Child Health and Development; and by grants from the Takeda Foundation and the Daiichi-Sankyo Foundation of Life Science.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology; by the Grant-in-Aid for Scientific Research and for Challenging Exploratory Research from the Japan Society for the Promotion of Science; by the Grant for Research on Intractable Diseases from the Ministry of Health, Labor, and Welfare; by grants from the National Center for Child Health and Development; and by grants from the Takeda Foundation and the Daiichi-Sankyo Foundation of Life Science.
For editorial see page 4676
- AEXS
- aromatase excess syndrome
- CGH
- comparative genomic hybridization.
References
- 1. Shozu M, Sebastian S, Takayama K, et al. . Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N Engl J Med. 2003;348:1855–1865. [DOI] [PubMed] [Google Scholar]
- 2. Demura M, Martin RM, Shozu M, et al. . Regional rearrangements in chromosome 15q21 cause formation of cryptic promoters for the CYP19 (aromatase) gene. Hum Mol Genet. 2007;16:2529–2541. [DOI] [PubMed] [Google Scholar]
- 3. Bulun SE, Takayama K, Suzuki T, Sasano H, Yilmaz B, Sebastian S. Organization of the human aromatase p450 (CYP19) gene. Semin Reprod Med. 2004;22:5–9. [DOI] [PubMed] [Google Scholar]
- 4. Demura M, Reierstad S, Innes JE, Bulun SE. Novel promoter I.8 and promoter usage in the CYP19 (aromatase) gene. Reprod Sci. 2008;15:1044–1053. [DOI] [PubMed] [Google Scholar]
- 5. Fukami M, Shozu M, Soneda S, et al. . Aromatase excess syndrome: identification of cryptic duplications and deletions leading to gain of function of CYP19A1 and assessment of phenotypic determinants. J Clin Endocrinol Metab. 2011;96:E1035–E1043. [DOI] [PubMed] [Google Scholar]
- 6. Lee C, Iafrate AJ, Brothman AR. Copy number variations and clinical cytogenetic diagnosis of constitutional disorders. Nat Genet. 2007;39:S48–S54. [DOI] [PubMed] [Google Scholar]
- 7. Lupski JR, Stankiewicz P. Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet. 2005;1:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hastings PJ, Ira G, Lupski JR. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet. 2009;5:e1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gu W, Zhang F, Lupski JR. Mechanisms for human genomic rearrangements. Pathogenetics. 2008;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaw CJ, Lupski JR. Implications of human genome architecture for rearrangement-based disorders: the genomic basis of disease. Hum Mol Genet. 2004;13:R57–R64. [DOI] [PubMed] [Google Scholar]
- 11. Conrad DF, Bird C, Blackburne B, et al. . Mutation spectrum revealed by breakpoint sequencing of human germline CNVs. Nat Genet. 2010;42:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JM, Cooper DN, Férec C, Kehrer-Sawatzki H, Patrinos GP. Genomic rearrangements in inherited disease and cancer. Semin Cancer Biol. 2010;20:222–233. [DOI] [PubMed] [Google Scholar]
- 13. Colnaghi R, Carpenter G, Volker M, O'Driscoll M. The consequences of structural genomic alterations in humans: genomic disorders, genomic instability and cancer. Semin Cell Dev Biol. 2011;22:875–885. [DOI] [PubMed] [Google Scholar]
- 14. Froyen G, Belet S, Martinez F, et al. . Copy-number gains of HUWE1 due to replication- and recombination-based rearrangements. Am J Hum Genet. 2012;91:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang G, Zhao J, Vasquez KM. Methods to determine DNA structural alterations and genetic instability. Methods. 2009;48:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurahashi H, Inagaki H, Ohye T, Kogo H, Kato T, Emanuel BS. Palindrome-mediated chromosomal translocations in humans. DNA Repair (Amst). 2006;5:1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cer RZ, Donohue DE, Mudunuri US, et al. . Non-B DB v2.0: a database of predicted non-B DNA-forming motifs and its associated tools. Nucl Acids Res. 2013;41:D94–D100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verdin H, D'haene B, Beysen D, et al. . Microhomology-mediated mechanisms underlie non-recurrent disease-causing microdeletions of the FOXL2 gene or its regulatory domain. PLoS Genet. 2013;9:e1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carvalho CM, Zhang F, Liu P, et al. . Complex rearrangements in patients with duplications of MECP2 can occur by fork stalling and template switching. Hum Mol Genet. 2009;18:2188–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kornreich R, Bishop DF, Desnick RJ. α-Galactosidase A gene rearrangements causing Fabry disease. Identification of short direct repeats at breakpoints in an Alu-rich gene. J Biol Chem. 1990;265:9319–9326. [PubMed] [Google Scholar]
- 21. Vissers LE, Bhatt SS, Janssen IM, et al. . Rare pathogenic microdeletions and tandem duplications are microhomology-mediated and stimulated by local genomic architecture. Hum Mol Genet. 2009;18:3579–3593. [DOI] [PubMed] [Google Scholar]
- 22. Liu P, Carvalho CM, Hastings PJ, Lupski JR. Mechanisms for recurrent and complex human genomic rearrangements. Curr Opin Genet Dev. 2012;22:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koren A, Polak P, Nemesh J, et al. . Differential relationship of DNA replication timing to different forms of human mutation and variation. Am J Hum Genet. 2012;91:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patry G, Jarvi K, Grober ED, Lo KC. Use of the aromatase inhibitor letrozole to treat male infertility. Fertil Steril. 2009;92:829.e1–e2. [DOI] [PubMed] [Google Scholar]
- 25. Nagase T, Kikuno R, Ishikawa K, Hirosawa M, Ohara O. Prediction of the coding sequences of unidentified human genes. XVII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000;7:143–150. [DOI] [PubMed] [Google Scholar]
- 26. Lieber MR. The mechanism of human nonhomologous DNA end joining. J Biol Chem. 2008;283:1–5. [DOI] [PubMed] [Google Scholar]
- 27. Venter JC, Adams MD, Myers EW, et al. . The sequence of the human genome. Science. 2001;291:1304–1351. [DOI] [PubMed] [Google Scholar]
- 28. Boone PM, Liu P, Zhang F, et al. . Alu-specific microhomology-mediated deletion of the final exon of SPAST in three unrelated subjects with hereditary spastic paraplegia. Genet Med. 2011;13:582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pope BD, Tsumagari K, Battaglia D, et al. . DNA replication timing is maintained genome-wide in primary human myoblasts independent of D4Z4 contraction in FSH muscular dystrophy. PLoS One. 2011;6:e27413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ryba T, Battaglia D, Chang BH, et al. . Abnormal developmental control of replication-timing domains in pediatric acute lymphoblastic leukemia. Genome Res. 2012;22:1833–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pope BD, Chandra T, Buckley Q, et al. . Replication-timing boundaries facilitate cell-type and species-specific regulation of a rearranged human chromosome in mouse. Hum Mol Genet. 2012;21:4162–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]