Abstract
Context:
Cellular characteristics of fat quality have been associated with cardiometabolic risk and can be estimated by computed tomography (CT) attenuation.
Objective:
The aim was to determine the association between CT attenuation (measured in Hounsfield units [HU]) and clinical outcomes.
Methods:
This was a prospective community-based cohort study using data from the Framingham Heart Study (n = 3324, 48% women, mean age 51 years) and Cox proportional hazard models.
Main Outcomes:
The primary outcomes of interest were incident cardiovascular disease (CVD) and all-cause mortality. The secondary outcomes of interest were incident cancer, non-CVD death, and cancer death.
Results:
There were 111 incident CVD events, 137 incident cancers, 85 deaths including 69 non-CVD deaths, and 45 cancer deaths in up to 23 047 person-years of follow-up. A 1-SD increment in visceral adipose tissue (VAT) HU was inversely associated with incident CVD in the age- and sex-adjusted model (hazard ratio [HR] 0.78, P = .02) but not after multivariable adjustment (HR 0.83, P = .11). VAT HU was directly associated with all-cause mortality (multivariable HR 1.40, P = .003), which maintained significance after additional adjustment for body mass index (HR 1.53, P < .001) and VAT volume (HR 1.99, P < .001). Non-CVD death remained significant in all 3 models, including after adjustment for VAT volume (HR 1.97, P < .001). VAT HU was also associated with cancer mortality (HR 1.93, P = .002). Similar results were obtained for sc adipose tissue HU.
Conclusions:
Fat quality, as estimated by CT attenuation, is associated with all-cause mortality, non-CVD death, and cancer death. These associations highlight how indirect indices of fat quality can potentially add to a better understanding of obesity-related complications.
The prevalence of obesity in the United States continues to increase and has reached epidemic proportions (1). Obesity is associated with an increased risk of multiple metabolic risk factors, including insulin resistance and diabetes (2–4), and is associated with increased overall mortality (5), cardiovascular disease (CVD) (6), and obesity-related cancers (6). However, not all individuals with obesity experience the same metabolic complications (7), and obesity has been shown to be a heterogeneous condition with individual variability in fat depot deposition (8) and differential metabolic risk (2, 4, 8).
Visceral adipose tissue (VAT) and sc adipose tissue (SAT) are considered to be unique pathogenic fat depots (2, 8) that can be imaged using computed tomography (CT). CT imaging uses Hounsfield units (HU) based on radiographic pixels to differentiate tissue subtype and quantify radiodensity. Adipose tissue with relatively lower lipid content and higher vascularity has a less negative (ie, higher) HU (9, 10). In addition, adipose tissue with higher HU has been associated with smaller adipocytes (11), suggesting that relative levels of adipose tissue extracellular matrix may also be contributing to CT attenuation of adipose tissue. Thus, the HU provides a noninvasive and indirect measure of fat composition and quality. We have recently shown that higher adipose tissue HU levels are associated with less adverse cardiometabolic risk factor profiles, suggesting that this modality may capture unique metabolic aspects of fat quality (12).
Given this framework, we sought to examine whether this measure of fat quality is associated with clinical outcomes to better understand how abdominal fat depot composition contributes to obesity-related CVD, cancer, and mortality. Given our previous findings showing that higher HU levels are associated with less adverse CVD risk factor levels, we hypothesized that adipose tissue characterized by higher HU would be associated with a lower incidence of CVD and lower all-cause mortality.
Subjects and Methods
Study participants
Data for the present study were obtained from offspring and third-generation cohort participants in the Framingham Heart Study who had previously undergone multidetector CT (MDCT) from 2002 to 2005 (2). Inclusion criteria included age >35 years for men and >40 years for nonpregnant women and weight <160 kg. A total of 3529 participants (1418 from offspring and 2111 from the third generation) underwent MDCT scanning, resulting in a final sample size of 3324 after excluding participants with missing data including missing covariates, SAT or VAT volumes, or SAT or VAT HU. The mean length of follow-up was 6.9 years; the maximum length of follow-up was 8.5 years.
Measurement of SAT and VAT
Participants had previously undergone supine MDCT scanning of the abdomen, obtaining 25 contiguous 5-mm slices. SAT and VAT volumes were acquired by manually outlining the visceral and sc fat depots. An image display window of −195 to −45 HU was applied. The mean HU of the window was recorded. This method has been previously documented and has shown excellent interreader correlations (r = 0.99 for both VAT and SAT) (12).
Measurement of covariates
Body mass index (BMI) was calculated by weight (kilograms) divided by the square of height (meters). Blood pressure (BP) was measured twice at rest with the average of the two measurements used in analysis; hypertension was defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or the use of hypertensive medications. Diabetes was classified as fasting plasma glucose ≥126 mg/dL or treatment with an oral hypoglycemic agent or insulin. Total and high-density lipoprotein (HDL) cholesterol were measured on the fasting plasma sample. Current smoking was defined as having smoked at least 1 cigarette per day during the previous year.
Measurement of angiogenic growth factors and systemic markers of fibrosis
Measurement of the biomarkers were determined from the fasting blood draw. Blood samples were centrifuged immediately and stored at −80°C until biomarkers were measured. Serum concentrations of angiogenic growth factors were assayed using commercial kits (R&D Systems Inc) as previously reported (13, 14). Angiogenic growth factors measured were vascular endothelial growth factor, the soluble vascular endothelial growth factor receptor (soluble fms-like tyrosine kinase-1 [sFlt-1]), hepatocyte growth factor, angiopoietin-2 (Ang-2) and its soluble receptor (soluble tyrosine kinase with Ig-like and epidermal growth factor-like domains 2). Urinary connective tissue growth factor (CTGF) was used as a biomarker of fibrosis. CTGF was measured using the human kidney Tox 1 assay (Rules-Based Medicine, Inc; www.rulesbasedmedicine.com), a panel that uses antigen-specific antibodies and a Luminex 100 Analyzer (Luminex Corp; www.luminexcorp.com) to measure secreted CTGF protein (15).
Ascertainment of CVD, cancer, and all-cause mortality
All participants in the Framingham Heart Study undergo continuous surveillance for incident cardiovascular events, cancer diagnoses, and death. Incident CVD is assessed according to previously reported standardized criteria (including coronary heart disease (recognized or unrecognized myocardial infarction, angina pectoris, coronary insufficiency, or coronary heart disease death), cerebrovascular disease (stroke or transient ischemic attack), or congestive heart failure). Outcome events are adjudicated by a panel of 3 physicians after review of all available information, hospitalization records, and physician charts. Cancer cases were identified at routine examinations and health updates, through surveillance of admissions at local Framingham hospitals, or from death records. The cases were confirmed by pathology reports, and 2 independent investigators reviewed the medical records. Nonmalignant neoplasms and nonmelanoma skin cancers were not included in cancer cases. A cause of death was obtained from death certificates, hospital admission records, medical records, and family members. All deaths were adjudicated by a panel of 3 investigators.
Statistical analysis
The primary outcomes of interest were incident CVD and all-cause mortality. Cox proportional hazards models were used to determine the longitudinal association between VAT HU and SAT HU and incident CVD and all-cause mortality. For incident CVD, we excluded all individuals with prevalent CVD at baseline. The secondary outcomes of interest were incident cancer, non-CVD mortality, and cancer mortality. Cox proportional hazards models were used to determine the longitudinal association between VAT HU and SAT HU and these secondary outcomes of interest. For incident cancer, we excluded all individuals with a diagnosis of malignant cancer, other than nonmelanoma skin cancer, at baseline. Non-CVD mortality included all causes of mortality except for CVD. Cancer mortality included all individuals identified with cancer and in whom cancer was identified as the primary cause of death. Subjects who died, not due to the reason of interest (ie, non-CVD or cancer), were censored at the time of death.
Four models were constructed. First, we examined the age- and sex-adjusted model. Second, we constructed a multivariable model adjusted for age, sex, smoking status, systolic BP, hypertension treatment, total cholesterol, HDL cholesterol, and diabetes mellitus. For all-cause mortality, we additionally adjusted for prevalent CVD and prevalent cancer at the time of the baseline examination; for CVD mortality, we adjusted for prevalent CVD; and for cancer mortality, we adjusted for prevalent cancer. The third model additionally adjusted the multivariable model for BMI. The fourth model additionally adjusted the multivariable model for fat volume (VAT or SAT depot volume, respectively).
Secondary analyses
Additional analyses investigated interactions between mean HU and SAT or VAT volumes within each outcome (incident CVD, incident cancer, all-cause mortality, non-CVD mortality, and cancer mortality). The interaction of sex with HU variables was also assessed for each outcome.
In a subgroup analysis, Cox proportional hazards models were used to evaluate the relationship of fat variables to all-cause mortality within clinical ranges of BMI: normal weight (BMI 18–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥30 kg/m2). The model was adjusted for age, sex, systolic BP, hypertension treatment, diabetes, total cholesterol, HDL cholesterol, smoking status, and VAT or SAT volume, respectively.
All analyses were performed using SAS version 9.3 (SAS Institute). Hazard ratios (HR) are presented for a 1-SD increase in the fat variable. A P value <.05 was considered statistically significant for all analyses with the exception of the primary outcomes. Because there were 2 primary outcomes, a P value <.025 (.05/2) was used for the primary analysis. Secondary outcomes including incident cancer, non-CVD mortality, and cancer mortality were exploratory, and therefore, a P value <.05 was considered statistically significant.
Results
Characteristics of the 3324 study participants are presented in Table 1. The sample was nearly half women, with a mean age of 52.2 years in women and 49.7 years in men. Women had a mean VAT volume of 1365 cm3 and VAT HU of −92.5 (range −105.7 to −80.5). Men had a mean VAT volume of 2237 cm3 and VAT HU of −95.2 (range −104.5 to −79.0). The SD of VAT HU was 4.4 for women and 4.5 for men. The SD of SAT HU was 5.1 for women and 4.4 for men. Based on our previous work, the correlation between VAT volume and VAT HU was −0.75 for women and −0.72 for men in this study sample (12). The correlation between SAT volume and SAT HU was −0.49 for women and −0.56 for men (12).
Table 1.
Study Sample Characteristics
| Women (n = 1603) | Men (n = 1721) | |
|---|---|---|
| Continuous characteristics, mean (SD) | ||
| Age, y | 52.2 (9.9) | 49.7 (10.6) |
| BMI, kg/m2 | 27.1 (5.8) | 28.4 (4.5) |
| VAT, cm3 | 1365 (834) | 2237 (1017) |
| SAT, cm3 | 3150 (1515) | 2637 (1204) |
| VAT HU | −92.5 (4.4) | −95.2 (4.5) |
| SAT HU | −102.3 (5.1) | −99.6 (4.4) |
| Systolic BP, mm Hg | 120 (18) | 123 (15) |
| Diastolic BP, mm Hg | 74 (9) | 78 (9) |
| Total cholesterol, mg/dL | 198 (37) | 195 (34) |
| HDL cholesterol, mg/dL | 61 (17) | 46 (12) |
| Categorical characteristics, n (%) | ||
| Hypertension | 427 (26.7) | 547 (31.8) |
| Diabetes | 88 (5.5) | 126 (7.3) |
| Hypertension treatment | 300 (18.7) | 341 (19.8) |
| Current smoker | 199 (12.4) | 232 (13.5) |
Incident CVD events
There were a total of 111 incident CVD events in 18 033 person-years of follow-up (Table 2 and Figure 1). As hypothesized, a 1-SD increment increase in VAT HU was associated with lower incident CVD in the age- and sex-adjusted model (HR 0.78, 95% confidence interval [CI] 0.64–0.98, P = .02). Upon multivariable adjustment, the association between VAT HU and incident CVD was attenuated and no longer statistically significant (HR 0.83, 95% CI 0.67–1.04, P = .11). Additional adjustment for BMI (HR 0.88, 95% CI 0.70–1.11, P = .29) and VAT volume (HR 1.05, 95% CI 0.79–1.40, P = .75) did not materially change these results. Results for SAT HU were not statistically significant.
Table 2.
Multivariable Cox Proportional Hazard Models of VAT and SAT Attenuation and Incident CVD (Death Plus Events)a
| Adjusted For | Incident CVDb (111 Events/18 033 Person-Years Follow-up) |
Incident Cancerc (137 Events/20 870 Person-Years Follow-up) |
||||||
|---|---|---|---|---|---|---|---|---|
| VAT HU |
SAT HU |
VAT HU |
SAT HU |
|||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age and sex | 0.78 (0.64–0.98) | .02 | 0.92 (0.75–1.14) | .46 | 0.96 (0.80–1.14) | .63 | 0.96 (0.79–1.16) | .67 |
| Multivariabled | 0.83 (0.67–1.04) | .11 | 0.95 (0.76–1.19) | .66 | 0.93 (0.77–1.13) | .48 | 0.95 (0.78–1.15) | .59 |
| Multivariable and BMI | 0.88 (0.70–1.11) | .29 | 0.99 (0.79–1.24) | .95 | 0.93 (0.57–1.14) | .46 | 0.95 (0.78–1.16) | .59 |
| Multivariable and fat depot volumee,f,g | 1.05 (0.79–1.40) | .75 | 1.04 (0.81–1.32) | .78 | 1.16 (0.89–1.50) | .27 | 0.92 (0.73–1.14) | .44 |
Data are presented as HR (95% CI) per 1-SD increase in HU.
Sex interaction for incident CVD in the age- and sex-adjusted model: VAT HU, P = .65; SAT HU, P = .60.
Sex interaction for incident cancer in the age- and sex-adjusted model: VAT HU, P = .03; SAT HU, P = .03.
Adjusted for age, sex, systolic BP, hypertension treatment, diabetes, total cholesterol, HDL cholesterol, and smoking.
Multivariable-adjusted P value for interaction between VAT HU and VAT volume: P = .09.
Multivariable-adjusted P value for interaction between SAT HU and SAT volume: P = .25.
Adjusted for VAT or SAT volume.
Figure 1. Event rates for all-cause mortality, cancer mortality, and incident CVD by quartiles of VAT HU and SAT HU.
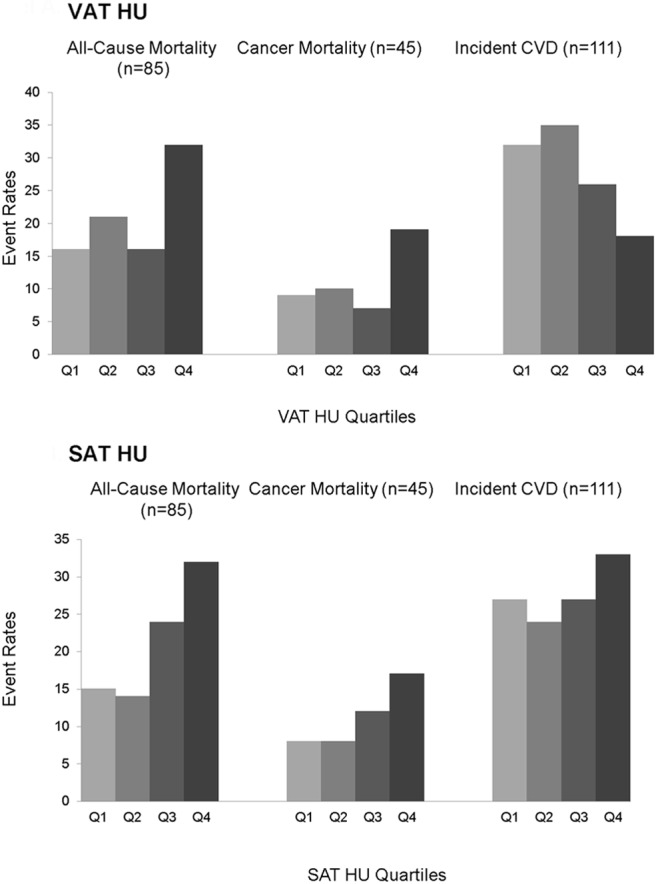
Quartile 1 (Q1) indicates the most negative HU quartile, whereas Q4 indicates the least negative HU quartile.
All-cause mortality
There were a total of 85 deaths in 23 047 person-years of follow-up (Table 3 and Figure 1). Contrary to our hypothesis, per 1-SD increase in VAT HU, there was a 40% increase in the multivariable-adjusted risk of all-cause mortality (HR 1.4, 95% CI 1.12–1.75, P = .003). This association was similar after additional adjustment for BMI (HR1.53, 95% CI 1.21–1.93, P < .001) and VAT volume (HR 1.99, 95% CI 1.47–2.69, P < .001).
Table 3.
Multivariable Cox Proportional Hazard Models of VAT and SAT Attenuation and Mortalitya
| Adjusted For | All-Cause Mortalityb (85 Events/23 047 Person-Years Follow-up) |
Non-CVD Mortalityc (69 Events/23 047 Person-Years Follow-up) |
Cancer Mortalityd (45 Events/23 047 Person-Years Follow-up) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAT | P Value | SAT | P Value | VAT | P Value | SAT | P Value | VAT | P Value | SAT | P Value | |
| Age and Sex | 1.30 (1.05–1.60) | .02 | 1.31 (1.09–1.57) | .004 | 1.42 (1.13–1.78) | .003 | 1.34 (1.10–1.63) | .004 | 1.38 (1.04–1.84) | .03 | 1.29 (1.01–1.65) | .04 |
| Multivariablee | 1.40 (1.12–1.75) | .003 | 1.27 (1.05–1.54) | .01 | 1.49 (1.17–1.90) | .001 | 1.29 (1.05–1.58) | .01 | 1.50 (1.10–2.03) | .01 | 1.29 (1.00–1.66) | .05 |
| Multivariable and BMI | 1.53 (1.21–1.93) | <.001 | 1.32 (1.09–1.61) | .005 | 1.62 (1.25–2.10) | <.001 | 1.34 (1.08–1.65) | .007 | 1.73 (1.25–2.40 | .001 | 1.39 (1.07–1.81) | .01 |
| Multivariable and fat depot volumef,g,h | 1.99 (1.47–2.69) | <.001 | 1.36 (1.08–1.70) | .008 | 1.97 (1.42–2.75) | <.001 | 1.36 (1.06–1.73) | .01 | 1.93 (1.27–2.94) | .002 | 1.45 (1.08–1.97) | .01 |
Data are presented as HR (95% CI) per 1-SD increase in HU.
Sex interaction for all-cause mortality in the age- and sex-adjusted model: VAT HU, P = .13; SAT HU, P = .60.
Sex interaction for non-CVD mortality in the age- and sex-adjusted model: VAT HU, P = .14; SAT HU, P = .10.
Sex interaction for cancer mortality in the age- and sex-adjusted model: VAT HU, P = .15; SAT HU, P = .09.
Adjusted for age, sex, systolic BP, hypertension treatment, diabetes, total cholesterol, HDL cholesterol, and smoking. All-cause mortality was adjusted for incident CVD, and cancer mortality was adjusted for incident cancer.
Multivariable-adjusted P value for interaction between VAT HU and VAT volume: all-cause mortality, P = .50; non-CVD mortality, P = .14; cancer death, P = .90.
Multivariable-adjusted P-value for interaction between SAT HU and SAT volume: all-cause mortality, P = .47; non-CVD mortality, P = .48; cancer death, P = .80.
Adjusted for VAT or SAT volume.
A 1-SD increment increase in SAT HU was associated with a 30% increase in the multivariable risk for all-cause mortality of 1.27 (95% CI 1.05–1.54, P = .01), which remained significant after additional adjustment for BMI (HR 1.32, 95% CI 1.09–1.61, P = .005) or SAT volume (HR 1.36, 95% CI 1.08–1.70, P = .008).
Within clinical categories of BMI, VAT HU was associated with all-cause mortality among normal-weight, overweight, and obese individuals (Figure 2).
Figure 2. Cox proportional hazards models of all-cause mortality stratified by clinical BMI categories: normal weight (BMI 18–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥30 kg/m2).The model was adjusted for age, sex, systolic BP, hypertension treatment, diabetes, total cholesterol, HDL cholesterol, smoking status, and VAT or SAT volume, respectively.
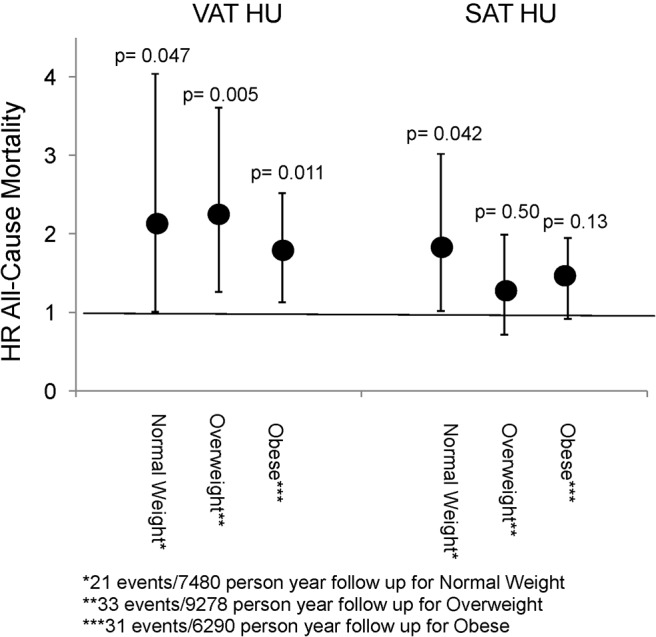
P values reflect the statistical significance of the HR within each category, and error bars reflect the 95% CI.
CVD mortality
There were 16 incident CVD deaths. A 1-SD increment increase in VAT was not associated with incident CVD death in either the age- and sex-adjusted model (HR 0.86, 95% CI 0.50–1.46, P = .6) or the model additionally adjusted for BMI or VAT volume. Similar negative results were obtained for SAT (data not shown).
Non-CVD mortality
The observation of an association between VAT and SAT HU and all-cause mortality, but not CVD mortality, raised the question of whether there is an association between HU and non-CVD mortality. There were 69 non-CVD deaths in 23 047 person-years of follow-up (Table 3). Indeed, a 1-SD increment increase in VAT HU was associated with a multivariable adjusted HR for non-CVD mortality of 1.49 (95% CI 1.17–1.90, P = .001). This association remained significant after additional adjustment for BMI (HR 1.62, 95% CI 1.25–2.10, P < .001) and VAT volume (HR 1.97, 95% CI 1.42–2.75, P < .001). Similar but somewhat less striking results were observed with SAT HU (Table 3).
Cancer mortality
To detail the association between adipose tissue attenuation and non-CVD mortality, a secondary analysis of cancer mortality was completed. There were a total of 45 cancer deaths in 23 047 person-years of follow-up (Figure 1). A 1-SD increment increase in VAT HU was associated with a multivariable adjusted HR for cancer mortality of 1.50 (95% CI 1.10–2.03, P = .01), which remained significant after additional adjustment for BMI (HR 1.73, 95% CI 1.25–2.40, P = .001) and VAT volume (HR 1.93, 95% CI 1.28–2.94, P = .002, Table 3). Similar results were seen with SAT HU (Table 3).
Secondary analyses
There were no major interactions between SAT or VAT HU and respective fat volumes (outlined in Tables 2 and 3, all P values >.05).
We further excluded individuals with cancer deaths within 2 years of follow-up. The number of cancer deaths decreased from 45 to 37. In this analysis, a 1-SD increment in VAT HU showed a slightly attenuated association for cancer mortality in the multivariable model (HR 1.24, 95% CI 0.88–1.75, P = .23) compared with the analysis not excluding cancer death within 2 years. Similar findings were seen in the BMI-adjusted model (HR 1.42, 95% CI 0.98–2.07, P = .07) and VAT volume-adjusted model (HR 1.64, 95% CI 1.03–2.63, P = .038). Similar results were also seen with SAT HU.
Additional multivariable linear regression models were constructed to determine the association between biomarkers of systemic fibrosis (CTGF), angiogenic growth factors, and SAT HU. A 1-SD increment increase in CTGF was associated with a 0.36 increase in SAT HU (95% CI 0.09–0.62, P = .0079) and a 1-SD increment increase in Ang-2 was associated with a 0.70 increase in SAT HU (95% CI 0.51–0.90, P < .0001). There was an inverse relationship with sTie-2 and positive correlation with sFlt-1 (outlined in Supplemental Table 1).
Discussion
Our main findings are 4-fold. First, as we hypothesized, higher CT HU was associated with lower incident CVD in the age- and sex-adjusted model. Second, contrary to our initial hypothesis, higher CT HU was associated with an increased risk of all-cause mortality. Third, the association between CT HU and all-cause mortality persisted even after additional adjustment for generalized adiposity and absolute adipose tissue volume. Finally, we observed an association between higher CT HU and non-CVD mortality, specifically cancer mortality, suggesting that fat quality characteristics of abdominal fat depots may be associated with obesity-related cancer mortality.
In the context of the current literature
Studies focusing on fat distribution have found central adiposity to be more closely associated with adverse cardiometabolic risk and increased CVD than generalized adiposity (16, 17). More specifically, studies using CT imaging to directly quantify abdominal fat have found that VAT volume in particular is associated with an adverse risk factor profile and all-cause mortality (2, 3, 8, 18). In addition, we have recently shown that VAT volume is associated with incident CVD and cancer above and beyond BMI and waist circumference (19).
The importance of fat quality has been increasingly recognized as an important correlate of cardiometabolic risk. Adipocyte hypertrophy has been associated with diabetes, independent of insulin resistance (20), and is a predictor of macrophage accumulation in adipocytes (21). Macrophage accumulation has in turn been associated with insulin resistance (22). Additionally, individuals with metabolic syndrome have increased levels of proinflammatory adipokines and macrophage accumulation in SAT than matched controls (23). Given the invasive techniques, these studies have been limited to small clinical samples and animal models.
Based on recent work that has shown that higher fat attenuation defines fat tissue with decreased lipid content and increased vascularity (9, 10), we exploited existing measurements of CT attenuation as a noninvasive measure of abdominal fat quality. Our study found an inverse association between fat quality estimated by CT attenuation and cardiometabolic risk (12). An increase in CT attenuation was associated with more favorable cardiometabolic risk factors (12). On the basis of this previous work, we hypothesized that higher fat attenuation would be associated with a lower risk of CVD. Indeed, we did find that incident CVD event rates were lower with increasing VAT HU. However, we observed this only in the age- and sex-adjusted model, and additional adjustment for CVD risk factors attenuated this relationship, suggesting that the association between VAT attenuation and incident CVD in the age- and sex-adjusted model may be due to shared CVD risk factors that are associated with both fat quality and CVD.
Contrary to our a priori hypothesis, we observed that the higher HU attenuation was associated with an increased risk of all-cause mortality, non-CVD mortality, and cancer mortality. This is similar to findings from a study completed in the Health ABC and AGES-Reykjavik cohorts in which they found that higher-density adipose tissue was associated with all-cause mortality (11). However, compared with that study, which specifically evaluated these outcomes in individuals 65 years and older, our study evaluated CT attenuation in all individuals in the Framingham Heart Study MDCT substudy (mean age = 52.2 years in women and 49.7 years in men). As individuals age, they typically lose weight with a preferential loss of SAT volume (24). Weight loss, changes in fat distribution, and other age-related fat quality changes distinguish these 2 study populations.
There are several potential physiologic mechanisms to explain our findings. Two leading possibilities may be adipose tissue fibrosis and vascularity. Invasive biopsy techniques in animal models and human studies have determined that adipose tissue from obese, insulin-resistant individuals is characterized by increased inflammatory macrophages, fibrosis, and increases in components of the extracellular matrix (25–27). Higher CT attenuation of fat depots would be concordant with extracellular matrix fibrosis in underlying fat tissue. In a secondary analysis, we did find that higher levels of CTGF, a marker of systemic fibrosis, was associated with an increase in SAT HU.
Furthermore, characteristics that promote fibrosis formation in adipose tissue, inflammation, and cytokine release (28, 29) are also microenvironment characteristics that promote tumor growth (30, 31). In sum, inflammation may be a shared pathway contributing to both adipose tissue fibrosis and cancer tumorigenesis. Higher CT attenuation of adipose tissue may reflect higher adipose tissue fibrosis, providing potential mechanistic insight into the observed association between CT attenuation and cancer mortality. Taken together with the findings of our current study, the association of higher HU and non-CVD mortality may in part be due to a higher level of extracellular collagen fibrosis.
Second, higher HU may also reflect an increase in adipose tissue vascularity. Highly vascularized tissue has a higher HU on CT studies due to the tissue properties of blood (32). Several recent studies have highlighted a role of decreased angiogenesis in association with dysfunctional adipose tissue (33, 34). In a secondary analysis, we found that increases in levels of Ang-2 and sFlt-1, angiogenic growth factors, were associated with an increase in SAT HU.
Adipose tissue vascularity also contributes to the cellular microenvironment in such a way as to make it more conducive to unregulated growth (35) as seen in cancer. This suggests a level of cross talk between adipose tissue and cancer cells such that surrounding adipose tissue may be altered to provide a supportive microenvironment in which cancer cells can take hold.
Implications for further research
Our findings suggest the importance of both fat distribution and fat quality studies in the study of obesity outcomes. Most previous studies on mortality outcomes have shown a positive association between obesity and mortality (5, 6, 36, 37). However, a small number of studies have also shown that overweight BMI has only a modest association with all-cause mortality (38–40). For example, a recent study found an association between all-cause mortality and BMI in the grade 2 and 3 obesity range (BMI ≥35 kg/m2) but not BMI in the overweight range (BMI 25 to <30 kg/m2) (39). Investigators have postulated that the variability in these findings may be due in part to the use of measurements of generalized adiposity rather than more specific measures of body fat distribution (16).
Imaging techniques can directly visualize fat depots and distinguish between pathogenic and nonpathogenic fat depots to clarify some of the concern regarding BMI. For example, VAT is more strongly associated with all-cause mortality than generalized adiposity (16) and SAT (18). However, if the cellular characteristics of SAT changes such as to limit expansion, as is seen in fibrosis of SAT, excess fat will be increasingly stored in VAT and other ectopic fat depots (26). Therefore, fat distribution studies may be further strengthened by the study of underlying fat composition as both fat quantity and fat quality studies may provide synergistic information regarding the metabolic consequences of obesity. In the present study, we found that within categories of BMI, the evaluation of CT HU provides additional information regarding all-cause mortality. Specifically, within normal-weight and overweight ranges, CT HU was associated with increased risk of all-cause mortality, suggesting that fat quality contributes to mortality risk above that of the BMI category alone. However, the underlying cellular mechanisms that link fat attenuation with incident CVD, all-cause mortality, and non-CVD death remain uncertain, and future mechanistic research is needed to further elucidate this association.
Strengths and limitations
There are several strengths of this study including a large, well-defined community-dwelling cohort with comprehensive phenotyping. We used a novel measure to estimate fat quality from CT scans. Some limitations warrant mention. The sample is predominately Caucasian, and generalizability to other populations is uncertain. These findings are derived from an observational study, and causality cannot be inferred. Finally, the mechanistic underpinning explaining the association between higher CT HU and mortality outcomes remains to be determined.
Conclusions
Fat quality estimated by CT attenuation is associated with age- and sex-adjusted incident CVD, all-cause mortality, non-CVD mortality, and cancer mortality. These findings highlight how indirect indices of fat quality can potentially add to our understanding of the complications of obesity.
Acknowledgments
This research was conducted in part using data and resources from the Framingham Heart Study (FHS) of the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health and Boston University School of Medicine. This work was supported by the NHLBI's FHS (Contract N01-HC-25195) and ROI-HL-077477 (to R.S.V.). K.J.R. is supported through funding from the Whitaker Cardiovascular Institute (T32 HL007224).
K.J.R. and CSF designed the study, led the statistical analysis, and wrote the manuscript. K.J.R. and C.S.F. are the guarantors of the study. J.M.Ma. and A.P. completed the statistical analyses and reviewed and edited the manuscript. M.T.L. contributed to the analysis. B.K., J.M.Mu., R.S.V., and U.H. contributed to the discussion and reviewed and edited the manuscript. U.H. provided expertise in imaging techniques, and R.S.V. provided expertise in serum biomarkers.
Disclosure Summary: A.P. is an employee of Merck. The rest of the authors have nothing to disclose.
Funding Statement
This research was conducted in part using data and resources from the Framingham Heart Study (FHS) of the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health and Boston University School of Medicine. This work was supported by the NHLBI's FHS (Contract N01-HC-25195) and ROI-HL-077477 (to R.S.V.). K.J.R. is supported through funding from the Whitaker Cardiovascular Institute (T32 HL007224).
Footnotes
- Ang-2
- angiopoietin-2
- BMI
- body mass index
- BP
- blood pressure
- CI
- confidence interval
- CT
- computed tomography
- CTGF
- connective tissue growth factor
- CVD
- cardiovascular disease
- HDL
- high-density lipoprotein
- HR
- hazard ratio
- HU
- Hounsfield unit
- MDCT
- multidetector CT
- sFlt-1
- soluble fms-like tyrosine kinase-1
- SAT
- sc adipose tissue
- VAT
- visceral adipose tissue.
References
- 1. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. [DOI] [PubMed] [Google Scholar]
- 2. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 3. Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165(7):777–783. [DOI] [PubMed] [Google Scholar]
- 4. McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167(7):642–648. [DOI] [PubMed] [Google Scholar]
- 5. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. [DOI] [PubMed] [Google Scholar]
- 6. Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299(3):E506–E515. [DOI] [PubMed] [Google Scholar]
- 8. McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96(11):E1756–E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nuclear Med. 2010;51(2):246–250. [DOI] [PubMed] [Google Scholar]
- 10. Hu HH, Chung SA, Nayak KS, Jackson HA, Gilsanz V. Differential computed tomographic attenuation of metabolically active and inactive adipose tissues: preliminary findings. J Comput Assist Tomogr. 2011;35:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murphy RA, Register TC, Shively CA, et al. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenquist KJ, Pedley A, Massaro JM, et al. Visceral and subcutaneous fat quality is associated with cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6(7):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lieb W, Safa R, Benjamin EJ, et al. Vascular endothelial growth factor, its soluble receptor, and hepatocyte growth factor: clinical and genetic correlates and association with vascular function. Eur Heart J. 2009;30(9):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lieb W, Zachariah JP, Xanthakis V, et al. Clinical and genetic correlates of circulating angiopoietin-2 and soluble Tie-2 in the community. Circ Cardiovasc Genet. 2010;3(3):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Seaghdha CM, Hwang SJ, Bhavsar NA, et al. Lower urinary connective tissue growth factor levels and incident CKD stage 3 in the general population. Am J Kidney Dis. 2011;57(6):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10(4):497–511. [DOI] [PubMed] [Google Scholar]
- 17. Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52(1):172–179. [DOI] [PubMed] [Google Scholar]
- 18. Katzmarzyk PT, Mire E, Bouchard C. Abdominal obesity and mortality: The Pennington Center Longitudinal Study. Nutr Diabetes. 2012;2:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts Type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–1506. [DOI] [PubMed] [Google Scholar]
- 21. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28(9):1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bremer AA, Jialal I. Adipose tissue dysfunction in nascent metabolic syndrome. J Obes. 2013;2013:393192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen W, Punyanitya M, Silva AM, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond). 2009;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spencer M, Unal R, Zhu B, et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96(12):E1990–E1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Divoux A, Tordjman J, Lacasa D, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010; 59(11):2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pasarica M, Gowronska-Kozak B, Burk D, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94(12):5155–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Divoux A, Clément K. Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev. 2011;12(5):e494–e503. [DOI] [PubMed] [Google Scholar]
- 29. Duncan MR, Berman B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J Invest Dermatol. 1991;97(4):686–692. [DOI] [PubMed] [Google Scholar]
- 30. Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831(10):1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dirat B, Bochet L, Dabek M, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455–2465. [DOI] [PubMed] [Google Scholar]
- 32. Furlan A, Fakhran S, Federle MP. Spontaneous abdominal hemorrhage: causes, CT findings, and clinical implications. AJR Am J Roentgenol. 2009;193(4):1077–1087. [DOI] [PubMed] [Google Scholar]
- 33. Gealekman O, Guseva N, Hartigan C, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123(2):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. [DOI] [PubMed] [Google Scholar]
- 36. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. [DOI] [PubMed] [Google Scholar]
- 37. Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–2037. [DOI] [PubMed] [Google Scholar]
- 39. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orpana HM, Berthelot JM, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: results from a national longitudinal study of Canadian adults. Obesity (Silver Spring). 2010;18(1):214–218. [DOI] [PubMed] [Google Scholar]


