Abstract
Context:
N6-methyladenosine (m6A) modification plays a fundamental role in the epigenetic regulation of the mammalian transcriptome. m6A can be demethylated by fat mass- and obesity-associated (FTO) protein and α-ketoglutarate-dependent dioxygenase alkB homolog 5 (ALKBH5) protein. However, the importance of m6A alteration in type 2 diabetes mellitus (T2DM) has not been explored.
Objective:
The objective of the study was to investigate whether m6A content was reduced in T2DM patients and whether m6A content was correlated with the mRNA expression levels of the FTO and ALKBH5 genes.
Methods:
In this case-control study, peripheral blood samples were obtained from 88 T2DM patients and 92 healthy controls. For the diabetic animal model experiment, blood samples were obtained from seven diabetic and eight nondiabetic rats. A sensitive liquid chromatography-electrospray ionization-tandem mass spectrometry method was developed for the determination of the m6A content in RNA, quantitative real-time PCR was used to examine the mRNA expression levels of the FTO and ALKBH5 genes, and high-resolution melting and DNA sequencing were used to detect FTO single-nucleotide polymorphisms.
Results:
Our results showed that the m6A contents in the RNA from T2DM patients and diabetic rats were significantly lower compared with the control groups (P = 2.6 × 10−24 for T2DM patients; P = .001 for diabetic rats, respectively), and T2DM can be characterized by the content of m6A. The mRNA expression level of FTO was significantly higher in T2DM patients than that of the controls (P = .0007) and was associated with the risk of T2DM (odds ratio 2.797, 95% confidence interval 1.452–5.389, P = .002). Moreover, the m6A contents were correlated with FTO mRNA expression.
Conclusions:
These data suggest that the increased mRNA expression of FTO could be responsible for the reduction of m6A in T2DM, which may further increase the risk of complications of T2DM. Low m6A should be investigated further as a novel potential biomarker of T2DM.
The incidence of type 2 diabetes mellitus (T2DM) has reached epidemic proportions in the world. The estimated prevalence of diabetes among Chinese adults was 11.6% and the prevalence of prediabetes was 50.1% (1). Recent reports reveal that epigenetic changes in response to environmental stimuli may play an important role in the development of T2DM (2). Given the rich layers of epigenetic regulation that results from modifications of DNA and histones, reversible RNA modification has been proposed to represent another realm for biological regulation in the form of RNA epigenetics (3). Naturally occurring RNA molecules contain various chemically modified nucleosides derived from four standard nucleosides. To date, more than 100 structurally distinct modified nucleosides have been identified in all three kingdoms of life (4). Discovered in the 1970s, N6-methyladenosine (m6A) is one of the most prevalent and abundant modifications on RNA molecules present in eukaryotes (5, 6).
Recently several studies suggest that the dynamic regulation of m6A on RNA may play broad and critical roles in fundamental biological processes, including adipogenesis, spermatogenesis, development, carcinogenesis, circadian rhythm, and stem cell renewal (6–10). The transcriptome profile of m6A sites was revealed by an antibody-based affinity approach in combination with a next-generation sequencing technique (11, 12). Approximately 7000 of the mRNAs have been found to possess m6A modification in both human and mouse cells with the possible functions in transcription, translational regulation, and microRNA maturation (11, 12). In addition, strong evolutionary pressures are likely being maintained for m6A modification because the distributions of m6A show high evolutionary conservation (11, 12). Furthermore, the cell type- and cell state-dependent m6A patterns indicate that m6A modification is highly regulated, which elevates m6A modification to a new layer of biological regulation at RNA level on multifaceted life processes.
m6A modification plays important roles in the functional interplay among m6A methyltransferase and demethylases. Although the methyltransferase complex of m6A still has not been fully elucidated (7), significant advance has been achieved for the recent discovery of two m6A demethylases, fat mass- and obesity-associated (FTO) protein and α-ketoglutarate-dependent dioxygenase alkB homolog 5 (ALKBH5) protein, which catalyze m6A demethylation in an α-ketoglutarate- and Fe(II)-dependent manner (13–15) (online Supplemental Figure 1). In genome-wide association studies, polymorphisms in the FTO gene were found to be associated with an increased risk of obesity (16–18). Recent results obtained in knockout mice demonstrated that the loss of function of FTO is associated with reduced body weight and fat mass (19), clearly supporting a role of FTO in fat accumulation. In addition, mice globally overexpressing FTO are found to be hyperphagic and obese (20). In line with these data, human studies report that FTO alleles are associated with increased food intake (21, 22). The latest studies have shown that the single-nucleotide polymorphisms (SNPs) in the FTO gene linked to obesity are interacting with the transmembrane 18 gene (TMEM18) (23) and the homeobox gene IRX3 (24).
Although these studies revealed the polymorphisms in the FTO gene are associated with an increased risk of obesity, few studies have specifically investigated the association between FTO expression and the risk of T2DM. Because of the functions of FTO gene in energy metabolism and homeostasis, we hypothesize that the expression level of the obesity-predisposing FTO gene will increase in T2DM, which therefore induces the decrease of m6A content due to its demethylase activity. Because a previous report showed that m6A could strongly stimulate glucose oxidation in rat adipocytes (25), the dysregulation of m6A in RNA induced by the increased FTO expression in T2DM patients may further cause a series of complications of T2DM.
To this end, in the present study, we assessed the FTO and ALKBH5 gene expressions as well as examined the m6A contents in RNA from the peripheral blood of T2DM patients, healthy controls, and diabetic and nondiabetic rats and also genotyped four common SNPs in the FTO gene using human genomic DNA. Our results showed that the m6A contents in RNA from T2DM patients were significantly lower compared with the controls, and similar results were present in rats. In addition, the mRNA expression of the FTO gene was significantly higher in the T2DM patients than that of the controls. The correlation analysis showed that m6A was highly correlated with the risk of T2DM as well as the FTO expression, whereas no such correlation was found between m6A and ALKBH5 and FTO SNPs. In addition, the receiver-operating characteristic (ROC) analysis suggested that T2DM can be characterized by the content of m6A in RNA, which suggests that m6A may also serve as a novel potential biomarker of T2DM.
Materials and Methods
Chemicals and reagents
Cytidine (rC), guanosine (rG), adenosine (rA), uridine, 2′-deoxycytidine (dC), 2′-deoxyguanosine (dG), 2′-deoxyadenosine (dA), and thymidine (T) were purchased from Sigma-Aldrich. m6A was from Hanhong Chemical Co, Ltd. Phosphodiesterase I was purchased from Sigma-Aldrich. S1 nuclease and alkaline phosphatase were from Takara Biotechnology Co, Ltd. Chloroform and formic acid were purchased from Sinopharm Chemical Reagent Co, Ltd. Chromatographic grade methanol was purchased from Merck. The water used throughout the study was purified by a Milli-Q apparatus (Millipore). Stock solutions of the ribonucleosides and 2′-deoxynucleosides were prepared in Milli-Q water at a concentration of 5 mmol/L.
Oligonucleotides
10-mer RNA (5′-AUCUAUAUGC-3′) was purchased from Takara Biotechnology Co, Ltd. 15-mer m6A-containing oligonucleotide was synthesized according to the previously described method (13).
Subjects and clinical measurements
This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University and conducted in accordance with the Helsinki Declaration. The study included 88 T2DM patients and 92 participants randomly selected from healthy individuals who had no history of endocrine or chronic diseases (online Supplemental Table 1). Fasting blood glucose, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured by an automated chemistry analyzer (Abbott-AEROSET; Abbott Diagnostics). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared.
Diabetic animal model
All animals were treated in accordance with the guidelines and approved protocols of the Institutional Animal Care and Use Committee of Wuhan University. Male Sprague Dawley rats (4 wk old; 125–150 g; animal quality certificate number 42000500001278) were purchased from the Center for Animal Experiment/ABSL-3 Laboratory of Wuhan University and housed in specific pathogen-free conditions. The rats were fed either a chow diet or a high-fat Western diet (Test Lab product 5TJN; 40% kcal from fat, 44% kcal from carbohydrates, and 16% kcal from protein) for 16 weeks. Two weeks after the diet was implemented, animals fed a high-fat diet were fasted for 12 hours, anesthetized with isoflurane, and injected with streptozotocin (Sigma-Aldrich; 40 mg/kg body mass) by ip injection. The animals were then allowed to recover in a postoperative recovery area, in which they received an ip injection of 10 mL of a 0.9% saline-2.5% dextrose mixture immediately after arrival and again 12 hours after the injection. All animals were monitored for 24 hours and subsequently returned to the general housing. Fasting blood glucose levels were measured 72 hours after the streptozotocin injection using a glucose meter (OneTouch Ultra Mini) by tail vein puncture blood sampling. Rats that had blood glucose levels greater than 11.6 mmol/L were served as diabetic rats. A total of seven diabetic and eight nondiabetic rats were used for this study.
Total RNA extraction
Total RNA was isolated from whole blood (human and Sprague Dawley rats) using a commercially available TIANamp blood RNA kit (Tiangen) according to the manufacturer's protocol. RNA quality was quantified by a spectrophotometry (ND-2000; NanoDrop Inc). The purity and yield of the RNA was determined using an OD at 260 and 280 nm. RNA integrity was examined by electrophoresis on a 1.2% denaturing formaldehyde gel.
Enzymatic hydrolysis of RNA
The isolated total RNA was enzymatically digested according to previously described method (26). A detailed RNA hydrolysis procedure is available in the Supplemental Data.
Analysis of m6A by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS)
An analysis of nucleosides was performed on the LC-ESI-MS/MS system consisting of an AB 3200 QTRAP mass spectrometer (Applied Biosystems) with an electrospray ionization source (Turbo Ionspray) and a Shimadzu LC-20AD HPLC with two LC-20AD pumps, a SIL-20A autosampler, a CTO-20AC thermostatted column compartment, and a DGU-20A3 degasser. Data acquisition and processing were performed using AB SCIEX Analyst 1.5 software (Applied Biosystems). The HPLC separation was performed on a Hisep C18-T column (150 mm × 2.1 mm inner diameter, 5 μm; Weltech Co, Ltd) with a flow rate of 0.2 mL/min at 35°C. Formic acid in water [0.1%, (vol/vol), solvent A)] and a mixture of 0.1% formic acid in methanol [solvent B (vol/vol)] were used as the mobile phase. A gradient of 5 minutes 5% B, 10 minutes 5%–30% B, 5 minutes 30%–50% B, 3 minutes 50%–5% B, and 17 minutes 5% B was used.
The mass spectrometry detection was performed under the positive electrospray ionization mode. The target nucleosides were monitored by multiple reaction monitoring (MRM) mode using the mass transitions (precursor ions → product ions) of dC (228.4 → 112.2), T (243.3 → 127.2), dA (252.4 → 136.2), dG (268.4 → 152.4), rC (244.4 → 112.2), uridine (245.4 → 113.1), rA (268.4 → 136.2), rG (284.5 → 152.2), and m6A (282.2 → 150.1). The MRM parameters of all nucleosides were optimized to achieve maximal detection sensitivity.
The m6A content was calculated using the following expression:
where Mm6A is the molar quantity of m6A, and MrA is the molar quantity of adenosine determined in the RNA sample.
Analysis of FTO and ALKBH5 gene expression by quantitative real-time PCR
For the mRNA quantifications of ALKBH5 and FTO, cDNA was synthesized by reverse transcription (Tiangen) and quantitative real-time PCR was performed in triplicate using iTaq universal supermixes (Bio-Rad Laboratories) on a CFX96 Touch real-time PCR detection system (Bio-Rad Laboratories). PCR primers are listed in Supplemental Table 2. mRNA levels were normalized to the reference genes of glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) and β-actin, and the results were expressed as mean ± SE. The detailed quantitative real-time PCR procedure and the data processing are available in the online Supplemental Data, and Supplemtental Figures 2 and 3.
FTO SNP genotyping
Genomic DNA was isolated from human whole blood using a commercially available TIANamp blood DNA kits (Tiangen) according to the manufacturer's instructions. Four common SNPs in the FTO gene were genotyped with high-resolution melting of a small amplicon (rs1121980, rs7202116, and rs8050136) and with DNA sequencing (rs9939609). The genotyping results of high-resolution melting were further confirmed randomly by DNA sequencing. The detail primers are available in Supplemental Table 3.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 software (SPSS Inc). A Student's t test was used to assess the differences in means between groups. Pearson correlation coefficients were estimated for each pair of study covariates, including the correlation of m6A with FTO mRNA expression, ALKBH5 mRNA expression, age, gender, BMI, blood glucose, TG, TC, HDL-C, and LDL-C. A logistic regression analysis with an adjustment for gender and age was used to determine whether FTO and ALKBH5 mRNA levels as well as m6A contents associated with T2DM risk, and the results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). The receiver-operating characteristic (ROC) analysis was performed to evaluate the ability of m6A in discriminating T2DM from the controls. The Hardy-Weinberg equilibrium, genotype, and allele frequency distributions were analyzed by a web-based SNP analysis software (http://bioinfo.iconcologia.net/snpstats/start.htm). All the P values were two sided, and the values of P < .05 were considered to be statistically significant.
Results
Detection of m6A by LC-ESI-MS/MS
The MRM mode was used for the analysis of m6A. Supplemental Figure 4A displays the MRM chromatogram of nine standard nucleosides. The linearity of the method was investigated using 40 pmol rA standard supplemented with m6A at different amounts ranging from 20 fmol to 2000 fmol (Supplemental Table 4). The calibration curves were constructed by plotting the mean peak area ratio of m6A to rA vs the mean molar ratio of m6A to rA based on data obtained from triplicate measurements. The results showed linearity within the range of 0.05%–5% (molar ratio of m6A to rA) with a coefficient value (R2) higher than 0.9982 (Supplemental Table 4). Limit of detection (LOD) and limit of quantification (LOQ) for m6A were calculated as the amounts of the analytes at signal to noise ratios of 3 and 10, respectively. The LOD and LOQ were 1.2 fmol and 4.0 fmol for m6A, respectively (Supplemental Table 4), which are, to the best of our knowledge, the best sensitivity compared with other previously reported MS-based detection methods.
Validation of the method was accomplished using the synthesized m6A-containing oligonucleotide by comparing the measured m6A content with the theoretical m6A content (Supplemental Table 5). m6A was determined from an RNA hydrolysis product, with relative SDs being 0.4%–7.1% and relative errors being 0.2%–11.2% (Supplemental Table 6), indicating that the LC-ESI-MS/MS method was reliable for the determination of m6A. In addition, the accuracy and precision of the LC-ESI-MS/MS method were evaluated (Supplemental Table 6). The relative SDs and relative errors were less than 11.2% and 11.5%, respectively.
With the developed LC-ESI-MS/MS method, we further investigated the m6A contents in the RNA from peripheral blood of T2DM patients. Supplemental Figure 4B displays the MRM chromatograms of nine nucleosides from the hydrolysis product of 20 ng RNA from a T2DM patient. m6A was well separated from other nucleosides; therefore, the quantification of m6A is not interfered by the presence of the high contents of normal nucleosides.
m6A is lower in T2DM
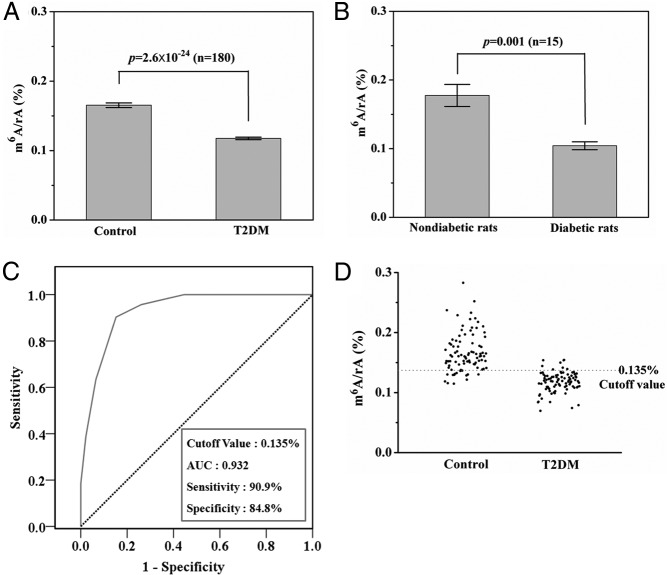
A total of 180 RNA samples (88 T2DM patients and 92 control subjects) were analyzed by LC-ESI-MS/MS system. The mean contents of m6A in RNA from T2DM patients and control subjects were 0.12% ± 0.02% and 0.17% ± 0.03% (n = 180), respectively (Figure 1A), which were significantly different based on an unpaired t test analysis (P = 2.6 × 10−24). We found that the m6A content was associated with a decreased risk of T2DM (OR 0.891, 95% CI 0.86–0.922, P = .000, Table 1). There was a 10.9% decrease in the odds of T2DM with a 1-unit increase in m6A content. In addition, the similar phenomena were present in rats, and the mean contents of m6A in RNA from diabetic and nondiabetic rats were 0.10% ± 0.02% and 0.18% ± 0.04% (n = 15, P = .001), respectively (Figure 1B).
Figure 1. Quantification and statistical analysis of m6A in RNA from whole blood.
A, The average contents of m6A in RNA from T2DM patients (n = 88) and healthy controls (n = 92). B, The average contents of m6A in RNA from diabetic (n = 7) and nondiabetic rats (n = 8). C, ROC curve for m6A score of T2DM patients and healthy controls. D, The distribution of m6A contents in RNA from T2DM patients and healthy controls. The dashed red line shows the cutoff value in ROC analysis.
Table 1.
Association of m6A Contents and the mRNA Levels of FTO and ALKBH5 Genes With T2DM Risk
| OR | 95% CI | P Value | |
|---|---|---|---|
| m6A | 0.891 | 0.860–0.922 | .000 |
| FTO | 2.797 | 1.452–5.389 | .002 |
| ALKBH5 | 0.664 | 0.345–1.276 | .219 |
It is well known that, in T2DM patients, BMI, blood glucose, and lipids often increase. Therefore, we performed the statistical analysis to evaluate the correlation of m6A in T2DM patients and control subjects with respect to age, BMI, blood glucose, TG, TC, HDL-C, and LDL-C. Based on the Pearson correlation coefficient, the m6A content was negatively correlated with BMI (r = −0.267, P = .003), blood glucose (r = −0.283, P = .0001), and TG (r = −0.301, P = .00004) and positively correlated with HDL-C (r = 0.208, P = .006) (Table 2). However, there was no correlation of m6A content with age (r = 0.028, P = .709), TC (r = 0.011, P = .888), and LDL-C (r = −0.070, P = .357) (Table 2).
Table 2.
The Pearson Correlations of m6A Content With FTO and ALKBH5 mRNA Expressions and Clinical Characteristics
| m6A |
||
|---|---|---|
| Pearson Correlation Coefficient | P Value | |
| Age | 0.028 | .709 |
| BMI | −0.267 | .003 |
| Fasting blood glucose | −0.283 | .0001 |
| TGs | −0.301 | .00004 |
| TC | 0.011 | .888 |
| HDL-C | 0.208 | .006 |
| LDL-C | −0.070 | .357 |
| FTO mRNA expression | −0.253 | .0006 |
| ALKBH5 mRNA expression | 0.064 | .392 |
We further evaluated the ability of m6A of predicting T2DM as a biomarker by performing the ROC analysis. The results showed that m6A was highly effective for the detection of T2DM, with the area under the curve being 0.932 and the cutoff value being 0.135% (Figure 1, C and D).
mRNA expression of FTO and ALKBH5 genes
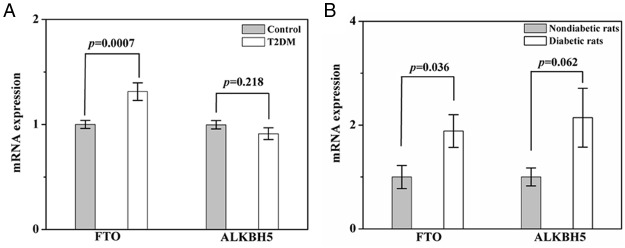
T2DM patients had significantly higher mRNA expression levels of FTO in peripheral blood compared with the controls (P = .0007) (Figure 2A). The mean mRNA expression levels of FTO in the T2DM patients and diabetic rats are 1.3- and 1.9-fold higher than that in the controls, respectively (Figure 2, A and B). Upon performing the Pearson correlation analysis, we found that the m6A contents in RNA are correlated with FTO mRNA levels (r = −0.253, P = .0006, Table 2). However, there was no significant difference in ALKBH5 mRNA levels between the T2DM patients or diabetic rats and the controls (P = .218 for T2DM patients; P = .062 for diabetic rats, respectively) (Figure 2). And m6A contents in RNA are not correlated with ALKBH5 mRNA levels (r = 0.064, P = .392, Table 2).
Figure 2. Quantitative real-time PCR analysis of FTO and ALKBH5 mRNA expression levels in whole blood.
A, Comparison of T2DM patients (n = 88) and healthy controls (n = 92). B, Comparison of diabetic (n = 7) and nondiabetic rats (n = 8).
It should be noted that the FTO mRNA level was associated with the risk of T2DM (OR 2.797, 95% CI 1.452–5.389, P = .002, Table 1). There was a 2.797-fold increased risk of T2DM with a 1-unit increase in the FTO mRNA level. Whereas the ALKBH5 mRNA level was not associated with the risk of T2DM (OR 0.664, 95% CI 0.345–1.276, P = .219, Table 1). There was a 33.6% decrease in the odds of T2DM with a 1-unit increase in the ALKBH5 mRNA level, but the P value was not less than the significance level. The results also demonstrate, for the first time, that not only the variants of the FTO gene are associated with an increased risk of obesity (14, 15), but also the expression of the FTO gene is associated with an increased risk of T2DM.
Association of FTO SNPs with FTO mRNA levels and m6A contents
The genotype distributions of the four SNPs (rs1121980, rs7202116, rs8050136, and rs9939609) were in Hardy-Weinberg equilibrium in the controls (P > .05). After adjustment for age and gender, the results of risk estimation based on genotype distribution, allele frequency, and genetic model by logistic regression analysis are shown in Supplemental Table 7. We found no significant difference in the distributions of the four SNPs between T2DM patients and the controls. It is unclear whether the four SNPs in the FTO gene affect the FTO mRNA levels and m6A contents, which is the substrate of FTO demethylase. We further compared FTO mRNA levels and m6A contents between different FTO SNP genotypes (Supplemental Figure 5). As a result, neither the FTO mRNA levels nor m6A contents differed significantly for the four SNPs (data not shown).
Discussion
The discovery and characterization of the two RNA demethylases, FTO and ALKBH5, indicate that, similar to the epigenetic regulatory role of DNA methylation, the reversible m6A modification on RNA may also serve as a novel epigenetic marker in fundamental biological processes (27, 28). Abnormal m6A modification may lead to the dysfunction of RNA and further cause diseases (6). T2DM is one of the most common human diseases. However, the importance of RNA epigenetic alterations in T2DM has not been explored. In this study, we investigated the m6A contents in peripheral blood RNA from T2DM patients by developing a sensitive LC-ESI-MS/MS detection method. Using this method, nine nucleosides, including five ribonucleosides (rA, rC, U, rG, and m6A) and four 2′-deoxyribonucleosides (dA, dC, dG, and T), were well separated, which effectively eliminated the interference from the normal high-abundant nucleosides in the detection of m6A. The highly sensitive method allowed for the determination of the low levels of m6A with only the 5-ng RNA sample, which was, to the best of our knowledge, the most sensitive method developed for the analysis of m6A in RNA.
With this method, we found that the contents of m6A decreased in RNA from T2DM patients, and the ROC analysis suggested that T2DM can be characterized by the change of m6A (Figure 1C). We then investigated the possible reasons that caused the decrease of m6A. In this respect, we examined the mRNA expression of the recently discovered demethylases of m6A, FTO and ALKBH5. Our data demonstrated a significant increase of the FTO mRNA expression in T2DM patients compared with the controls, but we did not observe the similar phenomena for ALKBH5. The correlation analysis showed that m6A was also correlated with FTO mRNA expression, whereas no such correlation was found for ALKBH5 (Table 2). In addition, our findings suggested that the genotypes of rs1121980, rs7202116, rs8050136, and rs9939609 in the FTO gene did not affect the mRNA expression levels of the FTO and m6A contents. A previous study showed that mice globally overexpressing FTO are found to be hyperphagic and obese (20); therefore, we surmise that the mRNA expression of the FTO gene may increase in T2DM patients. Our results confirmed that the increased mRNA expression of FTO gene in T2DM patients could contribute to the reduction of m6A.
An early study demonstrated that m6A strongly stimulated glucose oxidation in rat adipocytes (25), which suggested that the proper content of m6A may be required to maintain certain concentration of blood glucose. Consistent with previous reports, our data revealed that m6A content was negatively correlated with fasting blood glucose. The decreased content of m6A in T2DM could cause the accumulation and abnormal metabolism of glucose, which therefore may deteriorate the situation of T2DM. Due to the broad functions of m6A in the fundamental physiological process, the discovery that m6A levels were significantly reduced in T2DM suggests that the depletion of m6A may further increase the risk of complications of T2DM. The biological significance of the loss of m6A in T2DM remains to be elucidated; nevertheless, low m6A could be used as a novel potential biomarker for the early identification of susceptible individuals to T2DM.
Our experiment is limited by studies performed only in peripheral cells. Results in other tissues should be done to confirm these findings. On the other hand, the sample size is small for FTO SNP analysis; thus, these results should be repeated in larger populations and different ethnic groups.
In summary, we developed a sensitive method for the determination of m6A in RNA by the LC-ESI-MS/MS system. The measurement of m6A content in 180 RNA samples demonstrated a significant reduction of m6A level in T2DM patients compared with the controls. The content of m6A was highly associated with the risk of T2DM, and the increased mRNA expression of FTO may be responsible for the reduction of m6A, which can further cause the complications of T2DM.
Acknowledgments
We thank Professor Chunhong Wang (School of Public Health, Wuhan University) and Dr Richard Pang (Queen Mary Hospital, The University of Hong Kong and Adjunct Professor, School of Medicine, Wuhan University) for their advice on the revision of the manuscript.
Other e-mail addresses of correspondence include the following: Bi-Feng Yuan, bfyuan@whu.edu.cn; and Yu-Qi Feng, yqfeng@whu.edu.cn.
This work was supported by the National Natural Science Foundation of China (Grants 81271919 and 81472023) (to S.-M.L.); the National Basic Research Program of China (973 Program) (Grant 2012CB720605) (to S.-M.L.); the National Basic Research Program of China (973 Program) Grant 2012CB720603 (to B.-F.Y.); the National Natural Science Foundation of China (Grants 21205091 and 21228501) (to B.-F.Y.); the National Basic Research Program of China (973 Program) Grant 2012CB720601 (to Y.-Q.F.); and the National Natural Science Foundation of China (Grants 91017013 and 91217309) (to Y.-Q.F.).
Disclosure Summary: The authors have nothing to declare.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grants 81271919 and 81472023) (to S.-M.L.); the National Basic Research Program of China (973 Program) (Grant 2012CB720605) (to S.-M.L.); the National Basic Research Program of China (973 Program) Grant 2012CB720603 (to B.-F.Y.); the National Natural Science Foundation of China (Grants 21205091 and 21228501) (to B.-F.Y.); the National Basic Research Program of China (973 Program) Grant 2012CB720601 (to Y.-Q.F.); and the National Natural Science Foundation of China (Grants 91017013 and 91217309) (to Y.-Q.F.).
Footnotes
- ALKBH5
- α-ketoglutarate-dependent dioxygenase alkB homolog 5
- BMI
- body mass index
- CI
- confidence interval
- dA
- 2′-deoxyadenosine
- dC
- 2′-deoxycytidine
- dG
- 2′-deoxyguanosine
- FTO
- fat mass and obesity associated
- HDL-C
- high-density lipoprotein cholesterol
- LC-ESI-MS/MS
- liquid chromatography-electrospray ionization-tandem mass spectrometry
- LDL-C
- low-density lipoprotein cholesterol
- m6A
- N6-methyladenosine
- MRM
- multiple reaction monitoring
- OR
- odds ratio
- rA
- adenosine
- rC
- cytidine
- rG
- guanosine
- ROC
- receiver-operating characteristic
- SNP
- single-nucleotide polymorphism
- T
- thymidine
- TC
- total cholesterol
- T2DM
- type 2 diabetes mellitus
- TG
- triglyceride.
References
- 1. Xu Y, Wang L, He J, et al. . Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert ER, Liu D. Epigenetics: the missing link to understanding β-cell dysfunction in the pathogenesis of type 2 diabetes. Epigenetics. 2012;7:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He C. Grand challenge commentary: RNA epigenetics? Nat Chem Biol. 2010;6:863–865. [DOI] [PubMed] [Google Scholar]
- 4. Machnicka MA, Milanowska K, Osman Oglou O, et al. . MODOMICS: a database of RNA modification pathways—2013 update. Nucleic Acids Res. 2013;41:D262–D267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:3971–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jia G, Fu Y, He C. Reversible RNA adenosine methylation in biological regulation. Trends Genet. 2013;29:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niu Y, Zhao X, Wu YS, Li MM, Wang XJ, Yang YG. N6-methyl-adenosine (m6A) in RNA: an old modification with a novel epigenetic function. Genomics Proteomics Bioinformatics. 2013;11:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fustin JM, Doi M, Yamaguchi Y, et al. . RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Lu Z, Gomez A, et al. . N-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2013;505:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz S, Agarwala SD, Mumbach MR, et al. . High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. . Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. [DOI] [PubMed] [Google Scholar]
- 13. Jia G, Fu Y, Zhao X, et al. . N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng G, Dahl JA, Niu Y, et al. . ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerken T, Girard CA, Tung YC, et al. . The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dina C, Meyre D, Gallina S, et al. . Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. [DOI] [PubMed] [Google Scholar]
- 17. Frayling TM, Timpson NJ, Weedon MN, et al. . A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang J, Loos RJ, Powell JE, et al. . FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Bruning JC, Ruther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. [DOI] [PubMed] [Google Scholar]
- 20. Church C, Moir L, McMurray F, et al. . Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Do R, Bailey SD, Desbiens K, et al. . Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes. 2008;57:1147–1150. [DOI] [PubMed] [Google Scholar]
- 22. Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–2566. [DOI] [PubMed] [Google Scholar]
- 23. Kalnina I, Zaharenko L, Vaivade I, et al. . Polymorphisms in FTO and near TMEM18 associate with type 2 diabetes and predispose to younger age at diagnosis of diabetes. Gene. 2013;527:462–468. [DOI] [PubMed] [Google Scholar]
- 24. Smemo S, Tena JJ, Kim KH, et al. . Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Souness JE, Stouffer JE, Chagoya de Sanchez V. Effect of N6-methyladenosine on fat-cell glucose metabolism. Evidence for two modes of action. Biochem Pharmacol. 1982;31:3961–3971. [DOI] [PubMed] [Google Scholar]
- 26. Chen ML, Shen F, Huang W, et al. . Quantification of 5-methylcytosine and 5-hydroxymethylcytosine in genomic DNA from hepatocellular carcinoma tissues by capillary hydrophilic-interaction liquid chromatography/quadrupole TOF mass spectrometry. Clin Chem. 2013;59:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng G, Dahl JA, Niu Y, et al. . Sprouts of RNA epigenetics: the discovery of mammalian RNA demethylases. RNA Biol. 2013;10:915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saletore Y, Chen-Kiang S, Mason CE. Novel RNA regulatory mechanisms revealed in the epitranscriptome. RNA Biol. 2013;10:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]