Abstract
Context:
The endocannabinoid (eCB) system is involved in the regulation of food intake and of peripheral metabolism. Although the cross talk between energy metabolism and the circadian system is well documented, little is known about a potential circadian modulation of human eCB activity.
Objective:
The objective of the study was to define the 24-hour profile of circulating levels of the most abundant endogenous ligand of the CB1 receptor, 2-arachidonoylglycerol (2-AG), in healthy young nonobese adults studied under controlled bedtime, dietary, and activity conditions.
Methods:
Fourteen subjects participated in this 4-day laboratory study with fixed light-dark cycles, standardized meals, and bedtimes. Sleep was recorded each night. On the third day, blood sampling at 15- to 30-minute intervals began at 9:30 pm and continued for 24 hours. Cortisol, leptin, and ghrelin were assayed on all samples, whereas the levels of 2-AG and its structural analog, 2-oleoylglycerol (2-OG), were measured at 60-minute intervals.
Results:
All participants exhibited a large circadian variation of 2-AG serum concentrations with a nadir around midsleep, coincident with the middle of the overnight fast. Levels of 2-AG increased continually across the morning, peaking in the early to midafternoon. Peak values represented, on average, a nearly 3-fold increase above nocturnal nadir levels. Concentrations of 2-OG followed a similar pattern, although with a shorter morning increase and lower amplitude.
Conclusions:
The findings demonstrate that activity of the eCB system is profoundly modulated by circadian rhythmicity and suggest that its impact on the regulation of food intake is suppressed during sleep and is maximal during early to midafternoon.
Much attention has been paid in recent years to the ability of the endocannabinoid (eCB) system to control appetite, feeding, and peripheral metabolism, and this system has been the target of major efforts to develop anti-obesity drugs. The eCB system is involved in modulating not only homeostatic pathways but also hedonic and reward mechanisms that have powerful influences on food intake (1). The eCB system is comprised of two G protein-coupled receptors: CB1 and CB2, the endogenous ligands of these receptors, 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamine (AEA or anandamide), and the enzymes responsible for their biosynthesis and degradation (2). The CB1 receptor can be found in the brain as well as in peripheral organs involved in energy metabolism including adipose tissue, endocrine pancreas, muscle, and liver (3). Although ligands of the CB receptors can be found in peripheral tissues (4), the origin of circulating eCBs is not known. In humans, there is evidence that peripheral concentrations of these lipids correlate with emotional variables and may be elevated in obese compared with normal-weight individuals, although overall the findings remain inconclusive (5, 6).
Despite the recent recognition that the circadian system plays a major role in the control of energy balance (7, 8), there is limited information on the interactions between the eCB system and circadian rhythmicity. In rodent models, circadian variations have been shown for brain tissue content of both 2-AG and AEA (2, 9). The rhythm in content of AEA seems to vary across brain regions, and limited evidence indicates that the rhythm in 2-AG content may be in the opposite phase (9). There is also evidence for circadian modulation of the density of the CB1 receptors as well as of protein expression in at least some brain regions (10, 11). Based on this evidence, it was recently proposed that eCB ligands may represent internal signals linking the output of the central circadian pacemaker in the suprachiasmatic nucleus with physiological processes under circadian control (2). A corollary of this hypothesis is that circulating eCB ligands may mediate stable individual differences in meal timing (such as skipping breakfast and shifting most caloric intake to the later part of the day) as well as their metabolic consequences (12, 13). At present, there is a paucity of experimental data that have addressed this question. Indeed, most previous studies assayed human eCB levels at a single time point, generally in the morning, or at a very limited set of time points. A pilot study involving five normal volunteers who provided each three blood samples over an approximate 20-hour period suggested that serum levels of AEA are higher in the morning than in the late evening (2). There is no information on the daily variations of levels of 2-AG.
To date, not much is known about the role of the structural analogs of eCBs, synthesized from the same enzymes but do not bind the CB1 or CB2 receptors, in energy homeostasis and metabolic control. Recent studies have suggested that the activation of the structural analog of 2-AG, 2-oleoylglycerol (2-OG), a potent agonist of the G protein-coupled receptor 119 (GPR119) located primarily in pancreatic β-cells, stimulates the release of glucagon-like peptide-1 and incretin (14). The putative existence of a circadian modulation of circulating 2-AG levels should be associated with a similar variation in the activity of congeners, synthesized from the same enzymes.
The objective of the present study is to determine whether the activity of the human eCB system is modulated by circadian rhythmicity. To this effect, we assessed the 24-hour profile of circulating concentrations of the most abundant eCB, 2-AG, in nonobese healthy individuals who participated in a rigorously controlled laboratory study.
Subjects and Methods
Participants
For participation in this study, we recruited volunteers who were healthy, nonobese men and women between the ages of 18 to 30 years, with a body mass index (in kilograms per square meter) less than 28 for men and 27 for women. We also required self-reported habitual sleep duration of 7.5–8.5 hours for inclusion in this study. Exclusion criteria included an irregular sleep schedule; habitual daytime naps; shift work; travel across time zones in the last 4 weeks; a chronic medical condition; acute illness; the use of any prescription medications; the use of over-the-counter medications or supplements known to affect sleep or glucose metabolism; smoking; excessive alcohol (more than two drinks per day) or caffeine (>300 mg/d) consumption; a history of psychiatric disorders; or abnormal findings on a medical history, physical examination, or routine laboratory testing.
Volunteers were screened with an overnight laboratory polysomnography to exclude sleep disorders as well as a standard 75-g oral glucose tolerance test and fasting blood sample collection for routine laboratory analyses. A 12-lead electrocardiogram was also obtained. Healthy individuals who had no sleep disorders and normal glucose tolerance were included. Individuals were administered both the Center for Epidemiologic Studies Depression Scale (a cutoff of > 16 for clinical depression) and the Beck Inventory for Depression (a cutoff of > 10). Only those with both scores below the cutoff value were included. Only nonpregnant women were studied, and data collection was scheduled during the follicular phase of the menstrual cycle.
Study protocol
The protocol was approved by the Institutional Review Board of the University of Chicago. All research volunteers gave written informed consent and were paid for their participation. Study procedures took place in the University of Chicago Clinical Resource Center. During the week preceding each sleep condition, participants were instructed to maintain fixed bedtimes (11:00 pm to 7:30 am) and to not deviate from this schedule by more than 30 minutes. To verify adherence, sleep-wake cycles of the participants were continuously monitored by wrist activity (Actiwatch; Philips/Respironics).
Subjects spent 4 consecutive inpatient days with 8.5 hours in bed (11:00 pm to 7:30 am in total darkness) in the laboratory; the first night served to habituate the participants to the experimental environment and procedures. The three subsequent study nights were used to characterize sleep. Participants were housed in a private room with a window providing natural light. They had sedentary activities during waking hours. No naps were allowed. Research staff continuously monitored volunteers to ensure compliance. In the afternoon after the third night in the laboratory, an iv sterile heparin-lock catheter was inserted into a forearm vein. The line was kept patent with a slow drip of heparinized saline. Blood sampling at 15- to 30-minute intervals was initiated at 9:30 pm and continued for 24 hours. During bedtimes, the catheter was connected to plastic tubing that extended to an adjacent room to sample distally without disturbing the participant. Samples were collected at room temperature in tubes that did not contain inhibitors. Serum samples were frozen at −80°C until the assay. Cortisol, leptin, and ghrelin were assayed on all samples, whereas 2-AG and 2-OG were assayed at 60-minute intervals.
Controlled caloric intake
Caloric intake was strictly controlled throughout the laboratory study. The content of the diet was calculated to meet individual participant's caloric requirements for sedentary conditions (15). A registered dietitian from the University of Chicago Clinical Resource Center Metabolic Kitchen supervised the preparation of all meals. Participants were not allowed to consume any foods or beverages that were not provided by the metabolic kitchen. All meals were served at 9:00 am, 2:00 pm, and 7:00 pm. The participants were instructed to consume each meal in its entirety within 20 minutes. During the 24-hour period of blood sampling, participants ate three identical carbohydrate-rich meals (20% fat, 68% carbohydrate, and 12% protein).
Sleep recording
Sleep was recorded by polysomnography (Neurofax EEG-1100A; Nihon Kohden) each night. The recordings were visually scored in 30-second epochs as wake, rapid eye movement (REM), or non-REM sleep stages N1, N2, and N3 sleep according to standardized criteria (16). The following summary variables were calculated: sleep period time (ie, time interval separating sleep onset from morning awakening), sleep efficiency (ie, total sleep time/time in bed × 100), total sleep time (ie, sleep period − duration of intrasleep wake periods), duration of light non-REM sleep (ie, stages N1+N2), duration of deep non-REM sleep (ie, stage N3), and duration of REM sleep.
Assays
Serum concentrations of 2-AG, the endogenous ligand of the CB1 and CB2 receptors, and its structural analog 2-OG were extracted from serum using Bond Elut C18 solid-phase extraction columns (Varian Inc), as previously described (5). The two lipids were quantified in the lipid extracts by liquid chromatography-electrospray ionization-mass spectrometry (Agilent LC-MSD 1100 series) and quantified by isotope dilution as described previously (17).
The serum cortisol levels were measured by an immunochemiluminescent assay (Immulite). The serum leptin and total ghrelin levels were measured by a RIA (Linco Research).
Analysis of individual profiles of serum concentrations of 2-AG, 2-OG, cortisol, leptin, and ghrelin
Isolated 2-AG values that represented a relative change of more than 100% in comparison with both the preceding and following value and were not concomitant with a similar change in the 2-OG level were assumed to represent an assay error and were replaced by linear interpolation. Similar criteria were used to identify the outlying 2-OG values. In the present study, a total of 336 values were measured for both 2-AG and 2-OG. In total, only eight values (2.4%) were interpolated.
To quantify the 24-hour profiles of the serum levels of 2-AG, 2-OG, cortisol, and leptin, a best-fit curve was calculated for each individual profile using a robust locally weighted nonlinear regression procedure with a window of 2 hours for 2-AG and 2-OG and 4 hours for cortisol and leptin (18). The acrophase (peak) and the nadir were defined as the maximum and minimum of the regression curve, respectively. The amplitude was defined as half of the difference between the acrophase and the nadir.
The 24-hour variations of ghrelin levels were quantified as previously described (19). To quantify the nocturnal ghrelin elevation, the profiles were smoothed using a four-point moving average, and the amplitude of the nocturnal rise was calculated as the difference between the nadir after the evening meal and the maximum level attained between 11:00 pm and 5:00 am.
Statistical analysis
All group values are expressed as mean ± SEM. The present study is the first to test the hypothesis that the circulating levels of 2-AG and 2-OG undergo a consistent circadian variation with a nonzero amplitude and a reproducible timing of acrophase and nadir. For each eCB ligand, the mean amplitude was tested against the null hypothesis of zero using a t test. The reproducibility of the timings of the acrophase and nadir was tested using the Rao Spacing Test (20), a powerful statistic that examines the clustering of periodic data against the hypothesis of uniform distribution across the 24-hour cycle.
Results
Fourteen individuals, 11 men and three women, with a mean age of 23.4 ± 0.8 years and a mean body mass index of 23.9 ± 0.7 kg/m2 participated in this study. Body weight did not change during the experimental session (weight change was −0.03 kg, P = .92).
Sleep duration and sleep stages
Over the 3 study nights (bedtime 8.5 h), participants slept on average 455 ± 4 min/night with a sleep efficiency of nearly 90% (89.5% ± 1.1%). Wake time after sleep onset was limited to 25 ± 3 min/night and time spent in all sleep stages was normal, including 261 ± 6 minutes in non-REM stages N1 and N2, 74 ± 5 minutes in stage N3, and 120 ± 5 minutes in REM sleep.
Twenty-four-hour profiles of serum levels of 2-AG and 2-OG
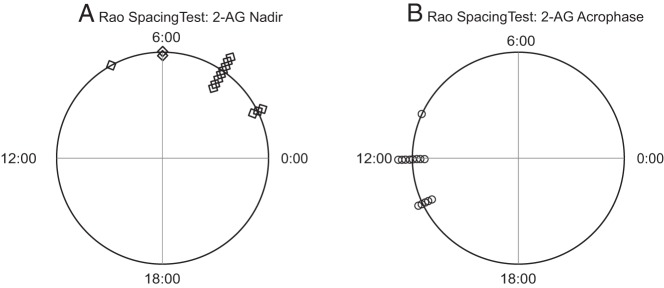
There was a wide inter-individual variability in the mean levels of both 2-AG and 2-OG. Thus, each individual profile was expressed as a percentage of the individual 24-hour mean concentration to illustrate the mean 24-hour profile for the group of 14 participants. As shown in Figure 1, A and B, the mean 24-hour profiles of both 2-AG and 2-OG displayed clear circadian rhythms. At approximately 4:00 am, when fasting conditions had been maintained for approximately 9 hours and subjects had been sleeping for nearly 5 hours, the serum concentrations of the eCB receptor ligand 2-AG were lowest (nadir). As illustrated on the polar diagram shown in Figure 2A, individual nadir timings were significantly clustered in the later part of the night (P < .001). Serum levels of 2-AG began to increase prior to morning awakening, and the rise extended throughout the morning. At approximately habitual lunchtime, in the early afternoon, concentrations of 2-AG were more than 3-fold higher than in the middle of the night (acrophase), on average (Table 1). The timings of the individual acrophases were significantly clustered (P < .001; Figure 2B). Overall, the mean amplitude of the 2-AG variation across the 24-hour cycle was highly significant (P = .0002). Subsequent to ingestion of the midday meal, the 2-AG levels began to slowly decline in the afternoon. We did not detect consistent periprandial changes in the 2-AG levels.
Figure 1. Mean 24-hour profiles of 2-AG (A), 2-OG (B), cortisol (C), leptin (D), and ghrelin (E).

Prior to calculating the mean profile, each individual profile was expressed as a percentage of the 24-hour mean (n = 14). Vertical bars at each time point represent the SEM. The sleep period in total darkness is denoted with the black bar (8.5 h). Nadir is denoted with gray arrow and acrophase with an open arrow. Black arrows represent the identical carbohydrate-rich meals, presented at 9:00 am, 2:00 pm, and 7:00 pm.
Figure 2. Polar diagrams representing the temporal distribution of the individual nadirs, denoted with diamonds (A), and acrophases, denoted with circles (B), of the 2-AG profiles.
Both the nadirs and the acrophases were significantly clustered (P < .001) of periodic data against the hypothesis of uniform distribution across the 24-hour cycle.
Table 1.
Quantitative characteristics of 2-AG, 2-OG, cortisol, leptin, and ghrelin profiles
| Characteristics | Values |
|---|---|
| 2-AG profile | |
| 24-hour mean, pmol/mL | 237 ± 61 |
| Amplitude, pmol/mL | 151 ± 30 |
| Value of acrophase, pmol/mL | 424 ± 98 |
| Time of acrophase, time ± min | 12 h 34 ± 20 min |
| Value of nadir, pmol/mL | 123 ± 40 |
| Time of nadir, time ± min | 04 h 09 ± 27 min |
| 2-OG profile | |
| 24-hour mean, pmol/mL | 2338 ± 313 |
| Amplitude, pmol/mL | 922 ± 131 |
| Value of acrophase, pmol/mL | 3424 ± 456 |
| Time of acrophase, time ± min | 11 h 43 ± 45 min |
| Value of nadir, pmol/mL | 1581 ± 218 |
| Time of nadir, time ± min | 2 h 43 ± 35 min |
| Cortisol profile | |
| 24-hour mean, μg/dL | 7.1 ± 0.3 |
| Amplitude, μg/dL | 7.8 ± 0.3 |
| Value of acrophase, μg/dL | 17.1 ± 0.7 |
| Time of acrophase, time ± min | 8 h 09 ± 11 min |
| Value of nadir, μg/dL | 1.5 ± 0.1 |
| Time of nadir, time ± min | 1 h 28 ± 18 min |
| Leptin profile | |
| 24-hour mean, ng/mL | 9.5 ± 2.4 |
| Amplitude, ng/mL | 3.6 ± 0.9 |
| Acrophase, ng/mL | 12.9 ± 3.3 |
| Time of acrophase, time ± min | 0 h 39 ± 15 min |
| Nadir, ng/mL | 5.7 ± 1.5 |
| Time of nadir, time ± min | 10 h 36 ± 23 min |
| Ghrelin profile | |
| 24-hour mean, pg/mL | 732 ± 81 |
| Amplitude, pg/mL | 250 ± 53 |
| Value of acrophase, pg/mL | 1043 ± 138 |
| Time of acrophase, time ± min | 1 h 39 ± 19 min |
| Value of presleep nadir, pg/mL | 543 ± 40 |
| Time of presleep nadir, time ± min | 21 h 43 ± 4 min |
Values reported for 2-AG and 2-OG (picomoles per milliliter), cortisol (micrograms per deciliter), and leptin (nanograms per milliliter) as well as ghrelin (picograms per milliliter) are mean ± SEM (n = 14) of summary measures extracted from the 24-hour profiles.
The profile of the structural analog 2-OG, which is synthesized and degraded by processes similar to those controlling the blood levels of 2-AG (21) but does not bind the CB receptors, was simultaneously measured. The changes in the 2-OG concentrations were approximately parallel to those of 2-AG, but the daytime increase in 2-OG was of shorter duration and of lesser magnitude, corresponding to an approximate doubling of its nocturnal concentrations (Table 1). The mean amplitude of the 2-OG profile was also significantly different from zero (P < .0001), and the individual nadirs and acrophases were both significantly clustered (P < .001 for both).
Twenty-four-hour profiles of serum cortisol, leptin, and ghrelin (Figure 1, C–E)
The well-documented circadian rhythm of plasma cortisol levels, with a nocturnal nadir and a morning acrophase, was present in all participants (Table 1). Small increases in cortisol levels were observed in response to the three high-carbohydrate meals at 9:00 am, 2:00 pm, and 7:00 pm, as previously reported (22). As in previous studies (23, 24), the 24-hour profile of the satiety hormone leptin displayed a circadian rhythm with an acrophase in the early part of the night and a morning nadir (Figure 1D). There were no detectable postprandial leptin excursions. In contrast, as expected (19, 25), the orexigenic factor ghrelin displayed postprandial dips and rebounds and a nocturnal acrophase in the early part of the sleep period, followed by a slow decline to return to morning values (Figure 1E).
Discussion
To our knowledge, this is the first study to examine concentrations of the most abundant endogenous ligand of the CB1 receptor, 2-AG, across the 24-hour period with rigorous control of energy intake, activity level, dark-light timing, and sleep-wake schedule in humans. The data reveal that the activity of the eCB system is profoundly modulated by circadian rhythmicity. We indeed observed a robust circadian rhythm of circulating concentrations of 2-AG, an endogenous ligand of the CB1 and CB2 receptors, with a nadir around the middle of the sleep/overnight fast and an extended period of maximal levels in the early to midafternoon. Levels of 2-AG increased, on average, more than 3-fold from nocturnal nadir to early afternoon acrophase. The return to lower nocturnal concentrations was not initiated until late afternoon or early evening.
The wave shape of this wide circadian rhythm suggests that the stimulation of appetite and food intake mediated by activation of the eCB system may increase substantially from morning to midafternoon. Similarly, the metabolic effects of the eCB system, including the modulation of insulin action at the level of the liver, muscle, and adipose tissue, are likely to be strongly dependent on time of day. Interestingly, a robust decrease of systemic insulin sensitivity from morning to evening has been well documented in healthy human volunteers (26, 27) and could be partly driven by the diurnal variation of eCB activity demonstrated in the present study. Importantly, our findings strongly suggest that the impact of the eCB system on food intake, metabolism, and neurobehavioral function needs to be assessed across the 24-hour cycle rather than during a narrow window of time and that controlling for circadian time in assessing the eCB system is crucial when attempting to discern potential differences between groups and/or conditions. In the context of the known effects of CB1 receptor activation to promote eating, the timings of the nadir and acrophase of 2-AG concentrations are consistent with the suppression of hunger during the overnight fast and maximal stimulation of the drive for feeding in the early afternoon. The circadian rhythm of 2-AG observed in the present study is qualitatively unique among endocrine rhythms because none of the hormones controlled by the hypothalamo-pituitary axis and none of the adipokines or gut hormones peak in the middle of the normal feeding period (28). Intake of carbohydrate-rich meals tailored to meet daily energy need did not elicit discernable changes in circulating 2-AG concentrations. It should be noted, however, that these standardized meals cannot be characterized as highly palatable, although they were not purposefully bland. Additionally, the hourly sampling rate might have been insufficient to detect the acute effects of meal ingestion.
In the present study, concentrations of 2-OG displayed a profile approximately similar to that observed for 2-AG over the 24-hour period, although blunted in comparison. 2-OG is a structural analog of 2-AG and is biosynthesized and degraded by the same enzymes as 2-AG (21). Thus, events that alter 2-AG metabolism (either stimulate or inactivate) would likely also modify the metabolism 2-OG. This is indeed the finding of this study and supports the conclusion that the alterations in 2-AG are the result of changes in the enzymatic activity in vivo. Recent studies suggest that 2-OG could act as an endogenous agonist of the orphan G protein-coupled receptor, GPR119, which is found in the gastrointestinal tract (14). With that noted, although the role of 2-OG in energy homeostasis is not fully understood, the simultaneous circadian modulation of 2-AG and 2-OG levels is not unexpected.
Food intake and energy expenditure are controlled via complex coordination of metabolic and behavioral systems including homeostatic and hedonic pathways (29). The drive to consume palatable foods, irrespective of energy need, constitutes the hedonic regulation of feeding and engages reward pathways (30). Components of the eCB system can be found in both homeostatic and hedonic pathways (31), interacting with both leptin and ghrelin signaling (32, 33). Most interestingly, activation of the eCB system affects hedonic (motivation and reward) circuits in the mesolimbic system, including the nucleus accumbens and ventral tegmental area. In rodent models, levels of eCB in the accumbens increase between meals or during fasting (34). CB1 receptors coexpress with dopamine D1 and D2 receptors and are present on dopamine neurons as well as on GABAergic and glutamatergic axon terminals that synapse on dopamine neurons (reviewed in reference 35). In this way, the eCB system may directly influence dopamine release, thus regulating the magnitude of reward value. Furthermore, data suggest that the eCB system interacts with the opioid system, which has a well-established role in mediating the hedonic value of food reward (36, 37). Activation of the eCB system within reward pathways elicits a preference for highly palatable rewarding food (34, 38). Intriguingly, in a recent clinical study, hedonic eating was associated with increased peripheral concentrations of 2-AG and ghrelin, 2 hours after feeding (39). Thus, the early to midafternoon peak in the eCB serum concentrations observed in the present study may suggest a higher drive for not only homeostatic but also hedonic eating during this time period.
The present study has several limitations, including the small number of study participants and the relatively low (ie, hourly) frequency of sampling of 2-AG, which may have precluded the observation of meal-related excursions. The use of identical high-carbohydrate meals ingested at 5-hour intervals was designed to assess diurnal variations in glucose tolerance but does not mimic usual dietary habits in real life. The meals were not highly palatable and had relatively low fat content. The absolute levels of 2-AG measured with our assay procedure are higher than those reported by other groups (40), but we analyzed the diurnal variations of 2-AG relative to the 24-hour mean level and also observed a consistent pattern of 2-OG levels. Despite these limitations, the findings are unequivocal and were obtained under highly controlled experimental conditions.
In conclusion, the present study shows that serum concentrations of the most abundant ligand of the CB1 receptor vary widely and reproducibly across the 24-hour period. The daily profile of 2-AG does not seem to be directly dictated by meals, as is the case of ghrelin. Moreover, the acrophase of the rhythm is almost 12 hours out of phase to that observed for leptin. The determinants of this circadian rhythm of serum 2-AG concentrations have yet to be elucidated, including the roles of meal composition and timing, activity levels, and the sleep-wake and light-dark cycles. Further understanding of the roles of peripheral eCB activity for the homeostatic and hedonic control of food intake and metabolism will require assessment across the 24-hour cycle. Finally, our data suggest that therapeutic approaches involving antagonism of peripheral eCB signaling will likely need to be tailored according to time of day.
Acknowledgments
The content contained in this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the Department of Defense, or the National Institutes of Health.
This work was supported by Grant KL2RR025000 from the National Center for Research Resources; Contract W81XWH-07-2-0071 from the Department of Defense Peer Reviewed Medical Research Program; The Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin (to K.L.S., E.D., and C.J.H.); and a pilot award from the University of Chicago Institute for Translational Medicine supported by Grant UL1 RR024999.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by Grant KL2RR025000 from the National Center for Research Resources; Contract W81XWH-07-2-0071 from the Department of Defense Peer Reviewed Medical Research Program; The Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin (to K.L.S., E.D., and C.J.H.); and a pilot award from the University of Chicago Institute for Translational Medicine supported by Grant UL1 RR024999.
Footnotes
- AEA
- N-arachidonylethanolamine
- 2-AG
- 2-arachidonoylglycerol
- eCB
- endocannabinoid
- 2-OG
- 2-oleoylglycerol
- REM
- rapid eye movement.
References
- 1. Di Marzo V, Ligresti A, Cristino L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int J Obes (Lond). 2009;233(suppl):S18–S24. [DOI] [PubMed] [Google Scholar]
- 2. Vaughn LK, Denning G, Stuhr KL, de Wit H, Hill MN, Hillard CJ. Endocannabinoid signalling: has it got rhythm? Br J Pharmacol. 2010;160:530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quarta C, Mazza R, Obici S, Pasquali R, Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med. 2011;17:518–526. [DOI] [PubMed] [Google Scholar]
- 4. DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci USA. 2011;108:12904–12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matias I, Gatta-Cherifi B, Cota D. Obesity and the endocannabinoid system: circulating endocannabinoids and obesity. Curr Obes Rep. 2012;1:229–235. [Google Scholar]
- 7. Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. [DOI] [PubMed] [Google Scholar]
- 8. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valenti M, Vigano D, Casico MG, et al. . Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci. 2004;61:945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinez-Vargas M, Murillo-Rodriguez E, Gonzalez-Rivera R, et al. . Sleep modulates cannabinoid receptor 1 expression in the pons of rats. Neuroscience. 2003;117:197–201. [DOI] [PubMed] [Google Scholar]
- 11. Rueda-Orozco PE, Soria-Gomez E, Montes-Rodriguez CJ, et al. . A potential function of endocannabinoids in the selection of a navigation strategy by rats. Psychopharmacology (Berl). 2008;198:565–576. [DOI] [PubMed] [Google Scholar]
- 12. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). 2009;17:2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garaulet M, Gomez-Abellan P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 14. Hansen HS, Rosenkilde MM, Holst JJ, Schwartz TW. GPR119 as a fat sensor. Trends Pharmacol Sci. 2012;33:374–381. [DOI] [PubMed] [Google Scholar]
- 15. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;1(suppl 39):5–41. [PubMed] [Google Scholar]
- 16. Iber C, Ancoli-Israel S, Chesson AJ, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 17. Patel S, Carrier EJ, Ho WS, et al. . The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. [DOI] [PubMed] [Google Scholar]
- 18. Cleveland W. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 19. Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Russell GS, Levitin DJ. An expanded table of probability values for rao's spacing test. Commun Stat Simul Comput. 1995;24:879–888. [Google Scholar]
- 21. Kleberg K, Hassing HA, Hansen HS. Classical endocannabinoid-like compounds and their regulation by nutrients. Biofactors. 2014;40(4):363–372. [DOI] [PubMed] [Google Scholar]
- 22. Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262:E467–E475. [DOI] [PubMed] [Google Scholar]
- 23. Mullington JM, Chan JL, Van Dongen HP, et al. . Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–854. [DOI] [PubMed] [Google Scholar]
- 24. Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. [DOI] [PubMed] [Google Scholar]
- 25. Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–E304. [DOI] [PubMed] [Google Scholar]
- 26. Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. [DOI] [PubMed] [Google Scholar]
- 27. Saad A, Dalla Man C, Nandy DK, et al. . Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61:2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Copinschi G, Turek FW, Van Cauter E. Endocrine rhythms, the sleep-wake cycle and biological clocks. In: Jameson LJ, DeGroot LJ, eds. Endocrinology. 6th ed Philadelphia: Elsevier-Saunders; 2010:199–229. [Google Scholar]
- 29. Chambers AP, Sandoval DA, Seeley RJ. Integration of satiety signals by the central nervous system. Curr Biol. 2013;23:R379–R388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bisogno T, Berrendero F, Ambrosino G, et al. . Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999;256:377–380. [DOI] [PubMed] [Google Scholar]
- 32. Di Marzo V, Goparaju SK, Wang L, et al. . Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. [DOI] [PubMed] [Google Scholar]
- 33. Kola B, Farkas I, Christ-Crain M, et al. . The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3:e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. [DOI] [PubMed] [Google Scholar]
- 36. Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Endocannabinoid system and alcohol addiction: pharmacological studies. Pharmacol Biochem Behav. 2005;81:369–380. [DOI] [PubMed] [Google Scholar]
- 37. Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms—a review of recent preclinical data. Psychopharmacology (Berl). 2003;169:115–134. [DOI] [PubMed] [Google Scholar]
- 38. De Luca MA, Solinas M, Bimpisidis Z, Goldberg SR, Di Chiara G. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology. 2011;63:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monteleone P, Piscitelli F, Scognamiglio P, et al. . Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab. 2012;97:E917–E924. [DOI] [PubMed] [Google Scholar]
- 40. Fanelli F, Garelli S, Mezzullo M, et al. . Circulating endocannabinoids are differentially modulated during the oral glucose tolerance test. Endocr Abstr. 2013;32:P756. [Google Scholar]