Abstract
Context:
Papillary thyroid cancer (PTC) patients <45 years old are considered to have an excellent prognosis; however, current guidelines recommend total thyroidectomy for PTC tumors >1.0 cm, regardless of age.
Objective:
Our objective was to examine the impact of extent of surgery on overall survival (OS) in patients <45 years old with stage I PTC of 1.1 to 4.0 cm.
Design, Setting, and Patients:
Adult patients <45 years of age undergoing surgery for stage I PTC were identified from the National Cancer Data Base (NCDB, 1998–2006) and the Surveillance, Epidemiology, and End Results dataset (SEER, 1988–2006).
Main Outcome Measure:
Multivariable modeling was used to compare OS for patients undergoing total thyroidectomy vs lobectomy.
Results:
In total, 29 522 patients in NCDB (3151 lobectomy, 26 371 total thyroidectomy) and 13 510 in SEER (1379 lobectomy, 12 131 total thyroidectomy) were included. Compared with patients undergoing lobectomy, patients having total thyroidectomy more often had extrathyroidal and lymph node disease. At 14 years, unadjusted OS was equivalent between total thyroidectomy and lobectomy in both databases. After adjustment, OS was similar for total thyroidectomy compared with lobectomy across all patients with tumors of 1.1 to 4.0 cm (NCDB: hazard ratio = 1.45 [confidence interval = 0.88–2.51], P = 0.19; SEER: 0.95 (0.70–1.29), P = 0.75) and when stratified by tumor size: 1.1 to 2.0 cm (NCDB: 1.12 [0.50–2.51], P = 0.78; SEER: 0.95 [0.56–1.62], P = 0.86) and 2.1 to 4.0 cm (NCDB: 1.93 [0.88–4.23], P = 0.10; SEER: 0.94 [0.60–1.49], P = 0.80).
Conclusions:
After adjusting for patient and clinical characteristics, total thyroidectomy compared with thyroid lobectomy was not associated with improved survival for patients <45 years of age with stage I PTC of 1.1 to 4.0 cm. Additional clinical and pathologic factors should be considered when choosing extent of resection.
Thyroid cancer is the most common endocrine malignancy, with an estimated 62 980 new cases in the United States in 2014 (1). Papillary thyroid cancer accounts for more than 90% of all cases (2). The long-term prognosis is generally excellent with appropriate treatment (3). The mainstay of treatment is surgical resection, with or without adjuvant radioactive iodine therapy.
Patient age is a well-recognized prognostic indicator in papillary thyroid cancer. Patients <45 years of age are generally considered to have low-risk disease and excellent survival, even when faced with advanced locoregional metastases (4, 5). The prognostic significance of patient age <45 years is recognized in the current American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid cancers, in that patients <45 years of age are classified as having only stage I or II disease, based on the absence or presence of distant metastases, respectively. AJCC staging does not include stage III or IV disease for these younger patients despite the size of their tumors or extent of locoregional disease (6).
Current American Thyroid Association (ATA) guidelines recommend total thyroidectomy for papillary thyroid cancers >1.0 cm, whereas thyroid lobectomy is reserved for tumors <1.0 cm (7). This recommendation was largely based on a population-level study from the National Cancer Data Base (NCDB) in which Bilimoria et al (8) demonstrated an overall survival benefit with total thyroidectomy compared with thyroid lobectomy for papillary thyroid cancers ≥1.0 cm in patients of all ages. Although patient age <45 years is a well-recognized prognostic marker for better survival, current ATA recommendations for extent of surgery in younger patients are similar to those for their older counterparts.
There is a paucity of data examining the impact of extent of surgery on overall survival in patients <45 years. We hypothesize that there is no survival advantage associated with undergoing total thyroidectomy compared with thyroid lobectomy for patients <45 years with AJCC stage I papillary thyroid cancer. This report examined the extent of surgery in young patients with papillary thyroid cancer from 2 of the largest oncology databases.
Materials and Methods
Study population
Adult patients <45 years with stage I papillary thyroid cancers 1.1 to 4.0 cm were included in the study. Patients with aggressive histologic variants such as columnar/tall cell, diffuse sclerosing, and insular histology were excluded whenever identified. We excluded patients with multiple cancer diagnoses, those with tumors >4.0 and ≤1.0 cm, and those with distant metastases. Due to conflicting published data from the NCDB and Surveillance, Epidemiology, and End Results (SEER) regarding the impact of extent of surgery on survival, we based our analyses on these 2 databases. The NCDB and SEER cohorts were analyzed separately. Both groups were characterized based on extent of surgical resection for their thyroid cancer: patients who underwent thyroid lobectomy and those who had total thyroidectomy. Patients were considered to have had thyroid lobectomy if they underwent removal of a thyroid lobe with or without an isthmusectomy. The total thyroidectomy group was defined as those who underwent total, near-total, or subtotal thyroid resection to permit comparison with previously published studies where this assignment was used (8). If a patient underwent a thyroid lobectomy followed by a completion thyroidectomy, extent of surgery was coded as a total thyroidectomy. Patients were excluded if they had removal of less than a lobe or when extent of surgical resection was unknown. Overall survival analyses were performed for all patients (tumors 1.1–4.0 cm) and for subgroups stratified by tumor size: 1.1 to 2.0 and 2.1 to 4.0 cm. Overall survival was defined from the time of diagnosis to time of death or last follow-up. The NCDB and SEER cohorts were restricted to patients who were diagnosed and treated for their thyroid cancer before 2006 to permit patients to have a minimum follow-up time of 5 years for evaluation of survival. Due to the deidentified nature of the datasets, the study was granted exempt status by the Duke University Health System Institutional Review Board.
Data sources
National Cancer Data Base
The NCDB is a joint program of the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons. The NCDB is a nationwide, facility-based, comprehensive clinical surveillance database that currently captures 70% of all incident malignancies in the United States. It contains more than 29 million cancer cases from more than 1500 CoC-accredited cancer institutions from all 50 states, Puerto Rico, and the District of Columbia. More than 85% of all newly diagnosed thyroid cancer cases in the United States are captured in the NCDB (9).
Data were coded according to the CoC Registry Operations and Data Standards Manual, the AJCC Manual for Staging of Cancer, and the International Classification of Diseases for Oncology. To reduce data errors and maintain the integrity of the database, all data were extracted from medical records by trained and certified database registrars. Data were validated at local and NCDB levels. Data were deidentified and submitted to the NCDB in accordance with the Health Insurance Portability and Accountability Act (10).
The NCDB Participant User File was used to identify all thyroid cancer cases that underwent thyroid surgery between 1998 and 2011. International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes 8050/3, 8260/3, 8340/3, 8341/3, 8342/3, and 8343/3 were used to identify patients with papillary thyroid cancer. The following demographic variables were obtained from the database: patient age at diagnosis, race, gender, annual income, insurance status, annual hospital case volume of papillary thyroid cancer surgeries, and year of diagnosis. Annual income was determined by the NCDB by linking the patient's ZIP code to the year 2000 U.S. Census data. Data on absence or presence of lymph node metastases and extrathyroidal extension, status of the surgical margins, and receipt of radioiodine treatment were extracted from the database.
SEER database
The SEER database collects data from 18 different registries (San Francisco-Oakland, Connecticut, Metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle-Puget Sound, Utah, Metropolitan Atlanta, Alaska, San Jose-Monterey, Los Angeles, rural Georgia,, Kentucky, Louisiana, New Jersey, greater Georgia excluding Atlanta and rural Georgia, and greater California excluding San Francisco, Los Angeles, and San Jose); collectively, these regions represent approximately 28% of the U.S. population. The SEER database was employed to identify patients diagnosed with papillary thyroid cancer (ICD-O-3 codes: 8050/3, 8260/3, 8340/3, 8341/3, 8342/3, and 8343/3) between 1988 and 2006. Demographic variables of interest included patient age at diagnosis, gender, race, survival status as of December 31, 2011, and year of diagnosis. Clinical data included extent of surgery and use of radioactive iodine ablation. Pathologic characteristics included tumor size, lymph node status, and extrathyroidal extension. The historic SEER stage was used to determine the presence of distant metastases at diagnosis.
Statistical analyses
Descriptive statistics (means, SDs, medians, and interquartile ranges for continuous variables and frequencies and percentages for categorical variables) were tabulated for baseline demographics and clinical characteristics by extent of surgery. Medians of continuous baseline variables were compared by extent of surgery using Wilcoxon rank-sum tests, whereas frequencies of baseline categorical variables were compared using Pearson's χ2 tests.
The unadjusted associations between overall survival and extent of surgery, overall and stratified by tumor size, were estimated using Kaplan-Meier survival curves and compared between groups using log-rank tests. Survival estimates and 95% confidence intervals were reported at 5, 10, and 14 years of follow-up.
Before multivariable modeling, missing data for baseline covariates were multiply imputed using chained equations (11). Because the data contained 32% observations with any missing covariates, we used 30 imputed datasets. The imputation model also included the event indicator variable and the Nelson-Aalen estimate of the baseline cumulative hazards to specify the effect of time (12). Descriptive statistics of the imputed variables were estimated by surgery group and compared with descriptive statistics before imputation. Similarity in the distributions across variables suggested adequacy of the imputation procedure.
Preliminary analyses, using a proportional hazards model to estimate the adjusted effect of surgery on survival for 2-year time intervals and graphical aids such as scaled Schoenfeld residual plots and score process plots based on Martingale residuals, showed that the relationship between extent of surgery and overall survival violated the proportional hazards assumption that is required for Cox proportional hazards modeling. As an alternative estimator, we employed a weighted Cox regression model (13). A weighted Cox regression model provided a parsimonious alternative to address the time-dependent hazards ratio (HR) of surgery by estimating an average HR over the entire follow-up period. This method also accounts for the built-in selection bias of the hazards by incorporating weights that reflect the relative importance of the log HR at different times (14). In our weighted Cox regression model, we used the weights recommended by Schemper et al (13), which are proportional to the Kaplan-Meier estimator of the survival function and inversely proportional to the Kaplan-Meier estimator of the censoring or potential follow-up distribution. Robust sandwich estimators were used to compute variance (15). The weighted Cox regression model was adjusted for patient age, race, gender, annual income, insurance status, annual hospital case volume, year of diagnosis, nodal status, margin status, radioactive iodine use, and presence of extrathyroidal extension, with continuous variables modeled as restricted cubic splines with 4 knots.
In the SEER cohort, we also estimated the association between overall survival and extent of surgery. It should be noted that income, insurance status, and margin status were not available in SEER for the specified time period (cases diagnosed before 2006). Based on previously mentioned graphical diagnostics (ie, residual and score process plots) for time-to-event models, extent of surgery met the proportional hazards assumption; radioactive iodine use did not. We constructed a stratified proportional hazards model to estimate the HR of surgery, adjusting for baseline covariates and stratifying on radioactive iodine use. Similar to the weighted Cox regression model, continuous variables were modeled as restricted cubic splines with 4 knots.
All analyses were conducted using SAS statistical software version 9.4. Two-sided statistical tests were specified in all analyses and P values < .05 were considered statistically significant.
Results
NCDB cohort
Among the 29 522 patients who underwent surgery for stage I papillary thyroid cancer, 3151 had thyroid lobectomy and 26 371 underwent total thyroidectomy between 1998 and 2006. Patient, clinical, and tumor characteristics are detailed in Table 1. Compared with the thyroid lobectomy group, the total thyroidectomy group included more patients with extrathyroidal extension (6% vs 15%, respectively) and lymph node metastases (8% vs 32%) (all P < .01). As expected, more patients with total thyroidectomy underwent radioactive iodine treatment than patients who had lobectomy (P < .01).
Table 1.
Characteristics of Patients by Extent of Thyroid Surgerya
| NCDB |
SEER |
|||||
|---|---|---|---|---|---|---|
| Thyroid Lobectomy (n = 3151) | Total Thyroidectomy (n = 26 371) | P Value | Thyroid Lobectomy (n = 1379) | Total Thyroidectomy (n = 12 131) | P Value | |
| Patient age, y (mean ± SD) | 34 ± 7 | 34 ± 7 | NS | 34 ± 7 | 34 ± 7 | NS |
| Female gender | 2701 (86%) | 21 748 (83%) | <.01 | 1182 (86%) | 9966 (82%) | <.01 |
| Race | NS | NS | ||||
| White | 2698 (87%) | 22 709 (88%) | 1136 (83%) | 10 003 (83%) | ||
| Black | 191 (6%) | 1324 (5%) | 69 (5%) | 514 (4%) | ||
| Other | 200 (7%) | 1747 (7%) | 165 (12%) | 1513 (13%) | ||
| Annual income | <.01 | |||||
| <$35 000 | 842 (28%) | 5846 (24%) | — | — | ||
| ≥$35 000 | 2120 (72%) | 18 955 (76%) | — | — | ||
| Insurance status | <.01 | |||||
| Not insured | 126 (4%) | 778 (3%) | — | — | ||
| Insured | 2933 (96%) | 24 785 (97%) | — | — | ||
| Tumor size, cm | NS | NS | ||||
| 1.1–2.0 | 1696 (54%) | 14 444 (55%) | 722 (52%) | 6494 (54%) | ||
| 2.1–4.0 | 1455 (46%) | 11 927 (45%) | 657 (48%) | 5637 (47%) | ||
| Extrathyroidal extension | <.01 | <.01 | ||||
| Absent | 2569 (94%) | 20 225 (85%) | 1275 (93%) | 10 056 (83%) | ||
| Present | 159 (6%) | 3684 (15%) | 103 (8%) | 2053 (17%) | ||
| Nodal disease | <.01 | <.01 | ||||
| Absentb | 2822 (92%) | 17 665 (68%) | 1256 (92%) | 8391 (69%) | ||
| Present | 240 (8%) | 8326 (32%) | 114 (8%) | 3711 (31%) | ||
| Margin status | <.01 | |||||
| Negative | 2720 (95%) | 20 994 (87%) | — | — | ||
| Positive | 156 (5%) | 3110 (13%) | — | — | ||
| RAI administration | 1048 (35%) | 16 553 (67%) | <.01 | 356 (27%) | 7847 (67%) | <.01 |
Abbreviation: RAI, radioactive iodine.
Values are presented as percentages of given sample size. Unknown and missing data were excluded from the statistical analyses. Dashes (—) indicate unavailable data. Insurance data were incomplete in the SEER dataset before 2012.
Percentage of patients whose lymph nodes were not examined and those without evidence of lymph node metastases upon pathological examination.
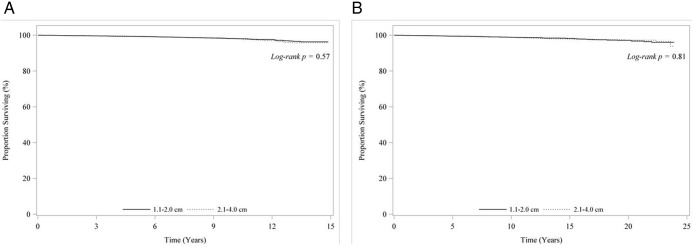
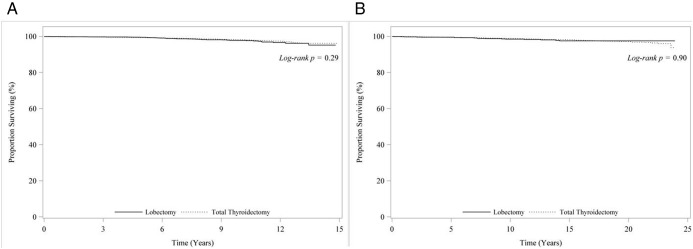
Median follow-up was 83 months in the NCDB. A total of 385 patients died between 1998 and 2006. Unadjusted overall survival estimates at 14 years were not different by tumor size: 96.2% for tumors 1.1 to 2.0 cm and 95.2% for tumors 2.1 to 4.0 cm (P = .57) (Figure 1A). There were no overall survival differences between total thyroidectomy compared with thyroid lobectomy at 5 years (99.3% vs 99.5%), 10 years (98.2% vs 97.8%), and 14 years (96.2% vs 95.2%), with a log-rank P = .29 (Figure 2A).
Figure 1. A, Unadjusted overall survival for patients <45 years with stage I papillary thyroid cancer by tumor size from the NCDB (1998–2006). B, Unadjusted overall survival for patients <45 years with stage I papillary thyroid cancer by tumor size from the SEER database (1988–2006).
Figure 2. A, Unadjusted overall survival for patients <45 years with stage I papillary thyroid cancers 1.1–4.0 cm by extent of surgery from the NCDB (1998–2006). B, Unadjusted overall survival for patients <45 years with stage I papillary thyroid cancers 1.1–4.0 cm by extent of surgery from the SEER database (1988–2006).
After adjustment for patient, clinical, and tumors factors, including administration of radioactive iodine, total thyroidectomy was associated with a similar overall survival compared with thyroid lobectomy in all patients with tumors 1.1 to 4.0 cm (HR = 1.45, 95% confidence interval [CI] = 0.84–2.51, P = .19) and when stratified by tumor size: 1.1 to 2.0 cm (HR = 1.12, 95% CI = 0.50–2.51, P = .78) and 2.1 to 4.0 cm (HR = 1.93, 95% CI = 0.88–4.23, P = .10) (Table 2). When we limited our adjustment variables in the multivariable weighted Cox regression model to those specified in the analysis of the SEER cohort, the overall and size-specific effects of surgery on overall survival (not shown) were similar to the above results.
Table 2.
Adjusted Overall Survival for Patients <45 Years of Age Undergoing Total Thyroidectomy Versus Thyroid Lobectomy for Stage I Papillary Thyroid Cancers of 1.1 to 4.0 cm in the NCDB and SEER Datasetsa
| Tumor size, cm | Total Thyroidectomy Versus Thyroid Lobectomy |
|||
|---|---|---|---|---|
| NCDB |
SEER |
|||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| 1.1–2.0 | 1.12 (0.50–2.51) | .78 | 0.95 (0.56–1.62) | .862 |
| 2.1–4.0 | 1.93 (0.88–4.23) | .10 | 0.94 (0.60–1.49) | .804 |
| 1.1–4.0 | 1.45 (0.84–2.51) | .19 | 0.95 (0.70–1.29) | .753 |
The model included patient age, gender, race, annual income, insurance status, hospital volume, nodal metastases, year of diagnosis, margin status, and radioiodine treatment.
SEER cohort
There were 13 510 patients who underwent surgery for stage I papillary thyroid cancer; 1379 had thyroid lobectomy, and 12 131 had total thyroidectomy between 1988 and 2006. Patient demographic and clinical characteristics were similar to those in the NCDB database (Table 1).
Median follow-up was 115 months. A total of 175 patients died between 1988 and 2006. At 14 years, unadjusted overall survival estimates were similar by tumor size: 97.9% vs 98.4% (P = .81) for tumors 1.1 to 2.0 cm vs 2.1 to 4.0 cm, respectively (Figure 1B). Similarly, overall survival was similar for total thyroidectomy and thyroid lobectomy at 5 years (99.5% vs 99.6%), 10 years (98.8% vs 98.8%), and 14 years (98.2% vs 97.8%) (P = .90) (Figure 2B).
After adjustment for patient, clinical, and tumors factors and radioactive iodine treatment, total thyroidectomy was associated with an equivalent overall survival compared with thyroid lobectomy in all patients with tumors 1.1 to 4.0 cm (HR = 0.95, 95% CI = 0.70–1.29, P = .75) and when stratified by tumor size: 1.1 to 2.0 cm (HR = 0.95, 95% CI = 0.56–1.62, P = .86) and 2.1 to 4.0 cm (HR = 0.94, 95% CI = 0.60–1.49, P = .80) (Table 2).
Discussion
To our knowledge, this is the first and largest study to date examining the association of extent of surgery with overall survival for patients <45 years of age with papillary thyroid cancer. Using 2 nationally representative oncology databases, we demonstrate that after adjustment for patient, clinical, and tumor factors and radioactive iodine treatment, there is no overall survival advantage associated with undergoing total thyroidectomy vs thyroid lobectomy for patients <45 years with stage I papillary thyroid cancers ≤4.0 cm and >1 cm. Furthermore, there is no survival advantage associated with using total thyroidectomy compared with thyroid lobectomy for patients with stage I papillary thyroid cancers even when the multivariable analysis is stratified by tumor size (1.1–2.0 and 2.1–4.0 cm). Despite current ATA guidelines, our findings call into question whether tumor size alone (up to 4.0 cm) should drive the decision to perform total thyroidectomy for patients <45 years with stage I papillary thyroid cancer.
There is considerable controversy surrounding what constitutes the optimal extent of surgery for papillary thyroid cancer, touching on issues pertaining to performing total thyroidectomy or thyroid lobectomy. Proponents of total thyroidectomy argue that the operation facilitates postoperative surveillance by reducing the thyroglobulin level, allows for the optimal use of postoperative radioactive iodine ablative therapy, and eliminates the possibility of leaving occult disease in the contralateral lobe (16–18). On the other hand, proponents of thyroid lobectomy argue that papillary thyroid cancer generally follows an indolent course with an outstanding prognosis, and total thyroidectomy, even in the hands of high-volume surgeons, is associated with higher rates of surgical complications, such as hypoparathyroidism and recurrent laryngeal nerve injury. In a recent population-based analysis of 62 722 thyroidectomies performed for benign and malignant disease, Hauch et al (19) demonstrated that total thyroidectomy, compared with thyroid lobectomy, is significantly associated with a higher risk of complications. After adjustment, total thyroidectomy compared with thyroid lobectomy was associated with increased risk of postoperative morbidity even in the hands of high-volume surgeons (odds ratio = 1.82, P < .0001). This increased morbidity, along with the need for lifelong thyroid hormone replacement, can have a long-lasting impact on quality of life for young patients (20). Patients <45 years are considered to have better prognosis compared with their counterparts who are ≥45 years, with lower risk for recurrence and mortality, bringing the issue of extent of surgery in this unique group of patients to the forefront of debate (21).
There have been no studies that specifically examined the issue of extent of surgery in papillary thyroid cancer patients <45 years. Previous reports have focused on patients with low-risk differentiated thyroid cancer. Shaha et al (22) analyzed data from 465 patients with low-risk differentiated thyroid cancer who underwent surgery at a single institution. Low-risk cancers were defined as tumor size <4.0 cm, low-grade histology, no distant metastases, no extrathyroidal extension, and patient age <45 years. At a median follow-up of 20 years, they found no difference in recurrence or survival between patients who had total thyroidectomy vs thyroid lobectomy for these low-risk cancers. Hay et al (23) studied the outcomes of 1656 patients with low-risk (age, metastases, extent, and size) differentiated thyroid cancers (women <51 years, men <41 years, no distant metastases, no extrathyroidal extension, and tumor size <5.0 cm) who were treated at the Mayo Clinic. After a median follow-up of 16 years, they found equivalent survival for patients undergoing total thyroidectomy compared with thyroid lobectomy, but recurrence rates were higher in the thyroid lobectomy group. These single-institution studies are in line with our finding that total thyroidectomy does not provide a survival advantage compared with thyroid lobectomy for young patients with low-risk papillary thyroid cancer.
The lack of association between extent of surgery and survival in patients <45 years may be explained by the indolent nature of papillary thyroid cancer in this young cohort. In a recent study of 2011 patients with differentiated thyroid cancer from Germany, Verburg et al reported that at 20 years of follow-up, patients <45 years had a life expectancy that is comparable to the general population, whereas life expectancy was significantly reduced for patients ≥45 years (4).
Current ATA guidelines recommend total thyroidectomy for papillary thyroid cancers >1.0 cm for all patients, largely based on a population-based study from the NCDB database demonstrating a survival benefit with total thyroidectomy (8); however, it is important to recognize that this analysis included patients of all ages. Therefore, conclusions from this study may not be applicable to patients <45 years. Our results support the National Comprehensive Cancer Network guidelines for management of papillary thyroid carcinoma, which suggest consideration of thyroid lobectomy for patients with low-risk disease (24).
There are several limitations to the current study, including those inherent to large administrative databases, such as the possibility of coding errors. Nevertheless, the NCDB and SEER use standardized abstraction and coding methods, and they are heavily audited at the reporting institution and national levels. Certain information was not captured in these 2 databases, including thyroid cancer recurrence data, serum thyroglobulin levels, preoperative sonographic findings of the thyroid gland, contralateral thyroid lobe and prevalence of goiters, and remedial therapeutic interventions. Consideration of risk of recurrence based on whether patients have low- or intermediate-/high-risk disease is potentially important; demographic, clinical, and pathologic features associated with these risk categories were built into all of our multivariable models predicting overall survival. Disease-specific survival from the SEER dataset was not examined due to the small number of events.
This is the first population-level study specifically targeting the issue of extent of surgery for patients <45 years with stage I papillary thyroid cancer. The large number of patients included in the NCDB and SEER datasets allowed us to adequately adjust for multiple demographic, clinical, and pathologic factors, a limitation for previous studies wherein the small number of death events, in these low-risk patients, prevented adequate multivariable adjustment. Additionally, we were able to externally validate our multivariable findings from NCDB in the SEER dataset.
Our study carries important implications; current ATA practice guidelines recommend total thyroidectomy based on a tumor size threshold >1 cm without distinguishing between papillary thyroid cancer patients <45 and ≥45 years of age. In the absence of a survival advantage justifying use of more extensive thyroid surgery, young patients with low-risk disease may unnecessarily be subjected to a higher risk for thyroidectomy-related complications such as hypoparathyroidism, recurrent laryngeal nerve injury, and bleeding. These events are known to correlate with extent of surgery even in the hands of experienced surgeons (19). However, it should be emphasized that determination of low-risk features should be based on final pathological examination of surgical specimens. Total thyroidectomy implies the need to take thyroid hormone replacement for life, creating a possible lifestyle and financial burden. Nevertheless, we believe that total thyroidectomy is indicated for patients with intermediate- and high-risk disease, such as those with extrathyroidal extension, lymph node metastases, and aggressive histologic variants and those requiring radioactive iodine therapy and/or aggressive surveillance strategies, regardless of tumor size. In addition, patient preference based on the relative risks and benefits of alternative surgical management strategies should play a prominent role in surgical decision-making. In particular, it would have relevance in patients who undergo diagnostic thyroid lobectomy for indeterminate thyroid nodules and who are found to have low-risk tumors on final surgical pathology; in these patients, completion thyroidectomy might not be warranted.
We believe that informing young papillary thyroid cancer patients who have low-risk disease that their survival may not be affected by extent of thyroid surgery is important so that they can make the best tradeoff decisions based on their unique situation and preference set. Long-term data regarding recurrence are necessary at the population level; potentially this will require multi-institutional collaboration or the creation of a prospective patient registry.
Acknowledgments
P.G. is supported by the Fondazione Italian per la Ricerca sul Cancro (Italian Foundation for Cancer Research).
A portion of these data were presented at the 35th Annual Meeting of the American Association of Endocrine Surgeons, April 27–29, 2014, Boston, MA.
Some of the data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigators.
Disclosure Summary: The authors report no financial conflict of interest.
Funding Statement
P.G. is supported by the Fondazione Italian per la Ricerca sul Cancro (Italian Foundation for Cancer Research).
Footnotes
- AJCC
- American Joint Committee on Cancer
- CI
- confidence interval
- CoC
- Commission on Cancer
- HR
- hazard ratio
- NCDB
- National Cancer Data Base
- SEER
- Surveillance, Epidemiology, and End Results.
References
- 1. American Cancer Society. Cancer Facts, Figures. 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf Accessed May 11, 2014
- 2. Siegel R, DeSantis C, Virgo K, et al. . Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. [DOI] [PubMed] [Google Scholar]
- 3. Davies L, Welch HG. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg. 2010;136:440–444. [DOI] [PubMed] [Google Scholar]
- 4. Verburg FA, Mäder U, Tanase K, et al. . Life expectancy is reduced in differentiated thyroid cancer patients ≥45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metab. 2013;98:172–180. [DOI] [PubMed] [Google Scholar]
- 5. Tubiana M, Schlumberger M, Rougier P, et al. . Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer. 1985;55:794–804. [DOI] [PubMed] [Google Scholar]
- 6. Hughes CJ, Shaha AR, Shah JP, Loree TR. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: a matched-pair analysis. Head Neck. 1996;18:127–132. [DOI] [PubMed] [Google Scholar]
- 7. merican Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, et al. . Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 8. Bilimoria KY, Bentrem DJ, Ko CY, et al. . Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–381; discussion 381–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–490. [DOI] [PubMed] [Google Scholar]
- 10. Phillips JK, Stewart AK, eds. Facility Oncology Data Standards. Chicago, IL: Commission on Cancer; 2006. [Google Scholar]
- 11. Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–96. [Google Scholar]
- 12. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schemper M, Wakounig S, Heinze G. The estimation of average hazard ratios by weighted Cox regression. Stat Med. 2009;28:2473–2489. [DOI] [PubMed] [Google Scholar]
- 14. Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 16. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–424. [DOI] [PubMed] [Google Scholar]
- 17. Kebebew E, Clark OH. Differentiated thyroid cancer: “complete” rational approach. World J Surg. 2000;24:942–951. [DOI] [PubMed] [Google Scholar]
- 18. Pasieka JL, Thompson NW, McLeod MK, Burney RE, Macha M. The incidence of bilateral well-differentiated thyroid cancer found at completion thyroidectomy. World J Surg. 1992;16:711–716; discussion 716–717. [DOI] [PubMed] [Google Scholar]
- 19. Hauch A, Al-Qurayshi Z, Randolph G, Kandil E. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol. 2014;21:3844–3852. [DOI] [PubMed] [Google Scholar]
- 20. Shaha AR. Extent of surgery for papillary thyroid carcinoma: the debate continues: comment on “surgery for papillary thyroid carcinoma”. Arch Otolaryngol Head Neck Surg. 2010;136:1061–1063. [DOI] [PubMed] [Google Scholar]
- 21. Grogan RH, Kaplan SP, Cao H, et al. . A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013;154:1436–1446; discussion 1446–1437. [DOI] [PubMed] [Google Scholar]
- 22. Shaha AR, Shah JP, Loree TR. Low-risk differentiated thyroid cancer: the need for selective treatment. Ann Surg Oncol. 1997;4:328–333. [DOI] [PubMed] [Google Scholar]
- 23. Hay ID, Grant CS, Bergstralh EJ, Thompson GB, van Heerden JA, Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998;124:958–964; discussion 964–956. [PubMed] [Google Scholar]
- 24. National Comprehensive Cancer Network (NCCN). NCCN Clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf Accessed July 27, 2014 [DOI] [PubMed]