Abstract
Context:
Rathke cleft cysts (RCCs) are benign embryonic remnants of the Rathke's pouch found in 13% to 33% of the general population. When symptomatic, they manifest themselves by compressing adjacent structures, causing pressure effects such as headache, visual disturbance, or pituitary hormone deficits. Most RCCs are asymptomatic, and their management remains controversial. Surgical resection has generally been indicated to treat symptomatic RCCs but carries the risk of complications.
Objective:
Our objective was to better characterize the outcomes for patients with presumed RCCs undergoing conservative management.
Design:
This was a retrospective cohort study.
Setting:
The setting was a pituitary program at a university medical center.
Participants:
The participants were 75 patients with radiographically diagnosed RCCs.
Methods:
All brain magnetic resonance imaging (MRI) scans performed at the University of Virginia from 2006 through 2013 were searched for the words “Rathke cleft cyst,” and pituitary clinic notes from 2007 to 2012 were reviewed for patients identified as probably having an RCC. Images for all patients were reviewed by the interpreting neuroradiologist, and those patients with at least 2 MRI scans were included. The dimensions of each cyst were assessed by the same neuroradiologist, and the volume of each cyst was analyzed as a function of the time from the first image obtained.
Results:
A total of 75 patients (4–76 years old) met our inclusion criteria. The length of follow-up was 1 to 126 months (median 24 months). In 43 patients (57%) no detectable change in the size of their cysts was seen, in 21 patients (28%) cysts increased in size, and in 11 patients (15%) cysts decreased in size. The predicted mean cyst growth rate was not significantly different from 0.
Conclusion:
The increasingly prevalent use of brain imaging modalities such as MRI has resulted in an increase in the incidental discovery of pituitary lesions. Our study demonstrates that the majority of radiologically diagnosed RCCs remain unchanged or decrease in size over time. These results suggest that, in the absence of pressure symptoms, it is reasonable to manage patients with RCCs conservatively.
Rathke cleft cysts (RCCs) are benign remnants of the Rathke's pouch, the anlage of the anterior pituitary during embryogenesis. They are relatively common, being found in 13% to 33% of patients at autopsy and comprising about 1% of all intracranial lesions (1, 2). Most RCCs are also asymptomatic and are often incidental findings on imaging studies such as computed tomography or MRI (1, 3). When symptoms are present, they result from progressive enlargement of the cyst, leading to compression of the adjacent structures. Such symptoms most commonly include visual disturbance due to compression of the optic chiasm, headaches, and pituitary dysfunction such as hypogonadism, hyperprolactinemia, growth hormone deficits, hypocortisolism, hypothyroidism, and diabetes insipidus (DI) (1, 4).
The conventional therapy for symptomatic RCCs is surgical resection, usually via a transsphenoidal approach. The appropriate management for asymptomatic RCCs is less well defined. One reason for this uncertainty is that relatively little is known about the natural history of these lesions. One recent case report by Amhaz et al (2) found that as many as 31% of 29 radiologically diagnosed RCCs actually decreased in size over time with conservative management alone. This finding raises the question of how likely RCCs are to increase in size and cause symptoms.
Surgical resection also carries the risk of complications. Adverse events can include cerebrospinal fluid leak, DI, anterior pituitary deficits, chemical meningitis, and sinusitis (1, 3–6). Although in most surgical patients such outcomes are avoided, surgery can have real and lasting effects for some patients. Given the uncertainty regarding the natural course for RCCs, it becomes relevant to ask which RCC lesions justify the accompanying risks of surgery. This study addresses this issue by seeking to better characterize outcomes for patients with presumed RCCs who undergo conservative management.
Materials and Methods
The design of this study was reviewed and approved by the institutional review board at the University of Virginia. This study used a retrospective cohort design with patient data obtained from a chart review of patients seen at the University of Virginia. We used a word search program to search the radiology reports of all brain MRI scans obtained at University of Virginia from 2006 through 2013 for the words “Rathke cleft cyst.” We then reviewed the individual charts of each patient identified by this search and selected those patients in whom the interpreting radiologist had indicated that an RCC was a likely diagnosis. We also reviewed the charts of all patients seen in the pituitary clinic between 2007 and 2012 and selected those patients whom the endocrinologist or neurosurgeon felt were likely to have an RCC. From among the patients identified by these searches, we included in our analysis those patients who had undergone at least 2 separate MRI scans over time. We excluded any patients who had undergone surgical resection before having at least 2 MRI scans. For the remaining patients who had undergone surgery, only MRIs obtained before surgery were included in our analysis. A neuroradiologist (D.A.O.) then reviewed the MRI scans of each patient in the study and provided dimensions for each cyst at each point in time. At this stage, we also excluded any patients for whom the neuroradiologist felt that the images obtained were unlikely to represent an RCC.
We then conducted our analysis with the primary outcome measure being the change in the radiographic size of each cyst as a function of the time from the date of the first image of each cyst. Cyst volumes were approximated as one-half the product of the 3 dimensions (in cubic centimeters), and time was measured in months.
Statistical analysis
Data summarization
Categorical scaled data were summarized by frequencies and percentages, and continuous scaled data were summarized by the mean, SD, and range of the measurement distribution.
Cyst size vs surveillance time analyses
The relationship between cyst size (cubic centimeters) and surveillance time (months) was analyzed via random coefficient regression (RCR) (7) Two RCR models were constructed. One RCR model examined the relationship between the change in cyst size, from its initial size, and surveillance time, whereas the second RCR model examined the relationship between absolute cyst size and surveillance time. Each RCR model was specified as a random intercept and random slope RCR model, whereby patient-specific time profiles could be predicted in addition to a prediction of the marginal time profile (ie, average time profile). With regard to hypotheses testing, an F test was used to test the null hypothesis that there was no association between cyst size and surveillance time (ie, RCR slope parameter is equal to 0). A P ≤ .05 decision rule was implemented a priori with the null hypothesis rejection criterion.
Statistical software
The MIX procedure of SAS version 9.2 (SAS Institute Inc., Cary, NC) was used to conduct the statistical analyses.
Results
Our word search identified 242 patients with MRI scans that had been read as probably showing an RCC, whereas our chart review identified an additional 57 such patients not already uncovered by the word search. Of this group of 299 patients, 11 patients were excluded because the original MRI images were unavailable for review, and 40 were excluded because the study neuroradiologist felt that their scans might not represent an RCC. An additional 32 patients were excluded because they received surgery after only 1 MRI scan. Another 141 patients were excluded because they had had only 1 MRI scan. The remaining 75 patients were included in our final analysis.
The demographics of these 75 patients are shown in Table 1. The length of follow-up ranged from 1 to 126 months with a median follow up of 24 months. The number of MRI scans obtained ranged from 2 to 10 with a median of 4, and the median interval between scans was 12 months with a range of <1 to 111 months. Nineteen patients (25%) ultimately underwent surgery, whereas 56 (75%) did not receive operative management (Table 2) The pathology reports from the 19 patients who underwent surgery revealed that 12 (63%) were consistent with RCC, 1 (5%) was suggestive of RCC, 5 (26%) were consistent with cyst contents of some kind, and for 1 (5%) the wall of the cyst was anterior pituitary tissue.
Table 1.
Patient Demographics and Clinical Characteristics for the 75 Patients Included in the Analyses
| Characteristic | Summary |
|---|---|
| Female sex | 60 (80.0) |
| Age, y | 41.3 (18.2) [4.0, 76.0] |
| Visual field defect | 5 (6.7) |
| Pituitary hormone abnormality | |
| Normal | 57 (76.0) |
| DI | 2 (2.7) |
| Growth hormone | 1 (1.3) |
| Gonadotropin | 2 (2.7) |
| Panhypopituitary | 3 (4.0) |
| Prolactin | 4 (5.3) |
| Thyroid | 3 (4.0) |
| Gonadotropin plus thyroid | 1 (1.3) |
| Adrenal plus thyroid | 1 (1.3) |
| Unknown | 1 (1.3) |
Data are no. (%) or mean (SD) [range].
Table 2.
Surgical Characteristics of the 19 Patients Who Underwent Surgery
| Reason for Surgery | Summary |
|---|---|
| Suspected adenoma | 2 (10.5) |
| Elevation of optic chiasm | 3 (15.8) |
| Increase in cyst size | 2 (10.5) |
| Headache | 1 (5.3) |
| Patient request | 3 (15.8) |
| Pituitary dysfunction | 2 (10.5) |
| Rhinorrhea | 1 (5.3) |
| Visual field defect | 1 (5.3) |
| Chiasm and pituitary dysfunction | 1 (5.3) |
| No reason given | 3 (15.8) |
Data are no. (%).
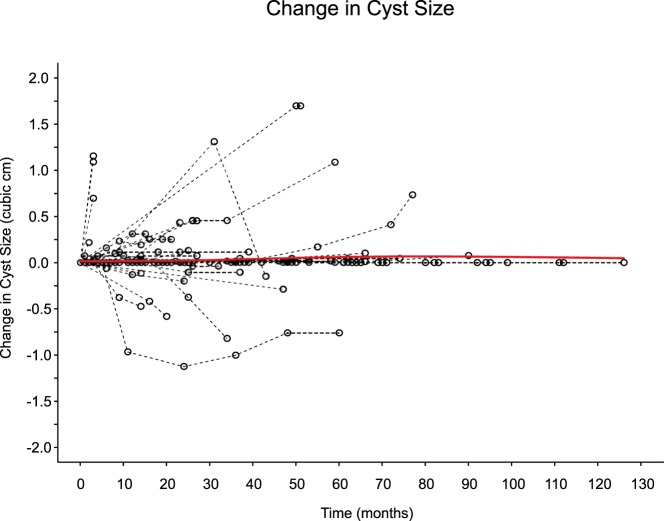
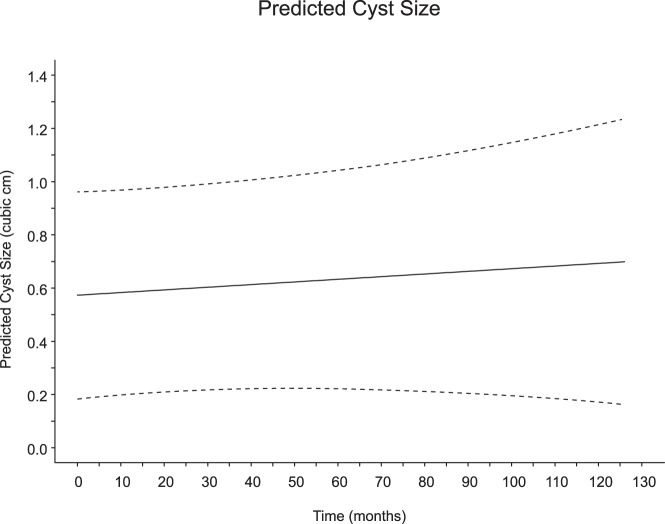
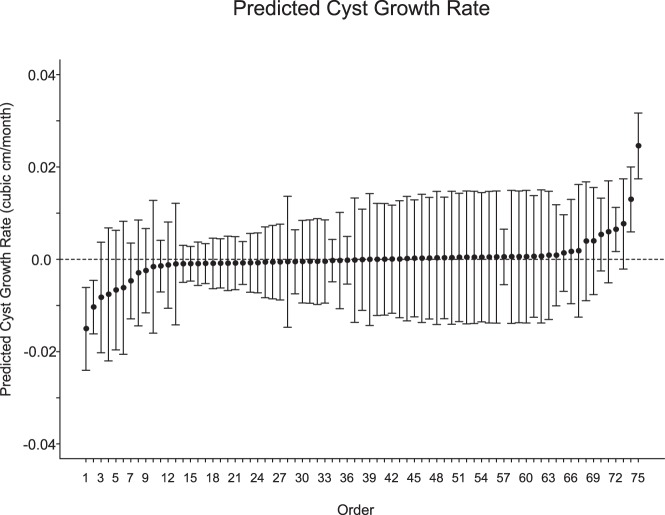
Of the 75 patients studied, 43 (57%) had no detectable change in the size of their cysts. Twenty-one (28%) of the cysts increased in size, whereas 11 (15%) decreased in size (the absolute size of each cyst as a function of time is shown in Supplemental Figure 1). The mean initial size of the cysts was 0.54 cm3 (SD, 1.14 cm3; for reference, the diameter of a sphere of 0.54 cm3 is 1.628 cm). Figure 1 shows the relative change in cyst size plotted for each patient in the study as a function of surveillance time. Overall, the predicted mean change in cyst size from these data was close to 0, and 0 could not be ruled out as a plausible value for the true rate of change. We calculated the predicted marginal (average) cyst size over time as shown in Figure 2. The slope of this line, ie, the mean cyst growth rate, was calculated as 0.0010 cm3/mo (95% confidence interval [CI], −0.0015 to 0.0035 cm3/mo; P = .427. We also calculated the predicted cyst growth rate for each individual cyst with 95% CIs (Figure 3). Only 3 of the patients in the study (4%) had a positive predicted rate of change at a P < .05 level of significance, whereas 2 patients had a negative predicted rate of change at a P < .05 level.
Figure 1. Subject profiles for change in cyst size (cubic centimeters) vs surveillance time (months).
The dashed lines identify the individual subject time profiles, and the solid red line identifies the predicted marginal profile time produced by a 2-degree gamma additive model spline function of time.
Figure 2. Random coefficient regression predicted cyst size (cubic centimeters) as a linear function of surveillance time (months).
The solid line identifies the predicted marginal (ie, average) cyst size, and the dashed lines denote the lower and upper limits of the simultaneous 95% CI. The slope of the regression (0.0010 cm3/mo; 95% CI, −0.0015 to 0.0035 cm3/mo; P = .427), represents the predicted mean cyst growth rate, and because the 95% CI includes 0, this implies that the null hypothesis that the mean cyst growth rate is equal to 0 cannot be rejected.
Figure 3. Subject-specific predicted cyst growth rate.
Points identify the predicted cyst growth rate. Vertical lines identify the 95% prediction interval. Vertical lines that include the value 0 indicate that the null hypothesis that the slope is equal to 0 cannot be rejected.
We further asked whether an increase in cyst size predicted whether patients ultimately required surgery. Of the 21 patients who had an increase in cyst size, only 9 (43%) underwent surgical resection. In addition, only 19 (25%) of the patients in the study underwent surgery. Of these patients, only 2 (10.5%) had an increase in cyst size reported as the reason for surgery.
To investigate whether bias was introduced through our use of both a chart review and a word search to identify patents, we examined each subgroup separately. Of the entire 75 patients in our analysis, 21 were identified by the chart review and 54 were discovered by the word search of the radiology reports. Among the 21 chart review patients, 10 (48%) had no detectable change in the size of their cysts, 6 (28%) had cysts that increased in size, and 5 (24%) had cysts that decreased in size. Of the 54 patients identified by the word search, 33 (61%) had cysts with no detectable change over time, 15 (28%) had increases in the size of their cysts, and 6 (11%) had decreases in the size of their cysts over time. As shown in Figure 3, only 5 total patients had cysts with a rate of change that was statistically significant (3 positive and 2 negative). None of the 21 patients identified by the chart review were among these patients with statistically significant rates of change in size.
Discussion
We demonstrate that most radiologically diagnosed RCCs remain unchanged, whereas a small minority increase or even decrease in size with time. Although a small number of RCCs did enlarge, only 3 cysts had a positive predicted rate of change that was statistically significant. Although RCCs have a prevalence as high as 13% to 33% on autopsy series, only a very small number produce symptoms (1, 3). The increasing use of advanced imaging techniques such as brain MRI has led to an increase in the incidental diagnosis of sellar lesions such as RCCs. Computed tomography and MRI are now frequently used in the setting of even minor head trauma or to investigate nonspecific symptoms such as headache. Reflecting this is the fact that whereas historically the most common presenting symptoms for RCC were endocrinopathies, headache is now the most prevalent complaint leading to the diagnosis of RCCs (1). The result is that more RCCs that might otherwise have gone undiscovered are now being diagnosed, and clinicians must then decide how best to manage them.
Further complicating these decisions is the fact that the natural history of RCCs has not been well understood. Most prior reports have been case series limited to relatively few subjects (2, 8, 9). Sanno et al (10) reported on more than 500 patients with incidentally discovered sellar lesions of which 94 were diagnosed radiologically to be RCCs. Of this subset, 75% had no change in size, whereas only 5.3% increased in size. However, this series excluded any patients who were found to have anterior pituitary dysfunction. Aho et al (6) also reported a group of 61 patients with an incidental diagnosis of RCCs, in whom 69% of cysts remained unchanged with follow-up of up to 9 years, but this included only RCCs of <1 cm in diameter, as any patient with an RCC of >1 cm underwent surgery (6). In general, many of these studies were also not designed to specifically study RCCs or their change in size with time. One additional limitation to these earlier reports is that the aforementioned increase in incidental diagnosis recently may also be changing the population of patients with RCCs who are now presenting to clinicians. Our current study therefore seeks to capture a fuller extent of the patient population with RCCs.
Operative management meanwhile carries significant risks. These include the potential for new pituitary dysfunction. In one series of 62 patients undergoing surgical management of RCC, 10% developed new anterior pituitary dysfunction and 6% developed DI (11). Recurrence is also common, with some authors reporting recurrences in 16% to 18% of surgical patients (6, 11). Further, more aggressive resection has not been shown to decrease the risk of recurrence and may be associated with a greater risk of anterior pituitary deficiencies and DI (11).
Surgery is also not equally effective for treating all of the adverse effects of RCCs. Although >90% of patients with visual impairment due to RCCs report improvement, anterior pituitary function generally improves in <30% of patients (6, 11). Interestingly, patients with headache often report improvement with transsphenoidal surgery for RCC (12), although headache is a subjective symptom, and it is difficult to prove that headache is related to RCC for a given patient. Only 1 patient in our series underwent surgery because of headache, and her headaches improved, consistent with these prior findings (12). However, this is only 1 patient and, given the very real potential for adverse outcomes, clinicians must balance their desire to provide treatment options to patients with their objective of doing no harm by carefully selecting the patients best suited for surgery.
One potential criticism of our study design is the concern that including patients who ultimately underwent surgery might have masked some patients who otherwise would have demonstrated a significant increase in the size of their cysts. However, analysis of our data shows that of the 21 patients with any detectable increase in the size of their RCC, only 9 (43%) were taken to surgery. Of the 19 patients in our series who had surgery, only 2 (10%) recorded an increase in cyst size as the reason for their surgery. There is also the potential issue that patients with the most severe symptoms or with visual field deficits were taken to surgery after only 1 MRI scan and therefore were excluded from our study. Although this is a valid concern, the purpose of this study was not to assess the best treatment for patients with a clear indication for surgery, but rather to determine outcomes in the vast majority of patients for whom the best management is uncertain. An additional 141 patients were also excluded because they had only 1 MRI scan and were essentially lost to follow-up. Although it could be argued that not knowing the outcomes for these patients introduces the possibility of bias, the fact that they are not known to have presented with complications from their cysts suggests an overall benign course consistent with our results.
There were also some limitations to our study, including the fact that our population consisted of considerably more women than men (80%), although prior literature reports have suggested a possible female predominance for RCC (1). This was also a retrospective study in which there was considerable variability in the length of follow-up, meaning that patients lost to follow-up could potentially have had later changes in cyst size not recorded by our study. Finally, others have observed that diagnosing RCCs by imaging alone can be difficult as there can be significant variability in the radiographic appearance (1, 13). In this study, we relied on the expert opinion of the neuroradiologist to determine whether a pituitary lesion seen on imaging probably represented an RCC. Thus, we cannot be certain of the radiographic diagnosis of RCC in all patients in our series, although it seems likely that a large majority had RCCs. It should be noted that the pathology reports from the patients who did undergo surgery also show that 94% had findings that were either consistent with or suggestive of RCC (68%) or had cysts of some kind although the exact nature could not be determined (26%). Regardless, the uncertainty of the radiologic diagnosis of RCC is nonetheless part of the very clinical quandary that we sought to address with the study.
An additional potential concern is the fact that we used both a word search and manual chart review to identify patients, raising the question of whether this might insert bias into our analysis of the entire group. However, the analysis of each subgroup shows that a similar proportion of patients in each group had an increase in the size of their cysts and thus suggests that each subgroup is similar to the larger analysis group.
There is also a need for further investigation after this study. There have been efforts to define the imaging characteristics of RCCs that predict pituitary deficits and anterior pituitary dysfunction as well as the risk of recurrence (5, 6). It has been shown that the size of an RCC does not necessarily predict symptoms, particularly pituitary dysfunction and headache (5). It nonetheless remains to be seen whether there are imaging features that may predict those cysts that are most likely to increase in size. Such information would have value as it may predict risk for vision loss and indicate patients most likely to benefit from surgery. Another issue concerns how frequently to obtain imaging studies in these patients. Although the interval of follow-up should always be individualized to account for changes in the patient's clinical presentation, a rapid increase in the size of these cysts appears to be rare. Therefore, we suggest that an initial MRI follow-up interval of 1 year is reasonable with the option of increasing this interval if the RCC remains stable in size. For now, we propose that in the absence of clear compressive effects on structures such as the optic chiasm and given the potential for adverse outcomes with surgery, the optimal management of most RCCs consists of surveillance by imaging at regular intervals.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- DI
- diabetes insipidus
- MRI
- magnetic resonance imaging
- RCC
- Rathke cleft cyst
- RCR
- random coefficient regression.
References
- 1. Kanter AS, Sansur CA, Jane JA Jr, Laws ER Jr. Rathke's cleft cysts. Front Horm Res. 2006;34:127–157. [DOI] [PubMed] [Google Scholar]
- 2. Amhaz HH, Chamoun RB, Waguespack SG, Shah K, McCutcheon IE. Spontaneous involution of Rathke cleft cysts: is it rare or just underreported? J Neurosurg. 2010;112:1327–1332. [DOI] [PubMed] [Google Scholar]
- 3. Trifanescu R, Ansorge O, Wass JA, Grossman AB, Karavitaki N. Rathke's cleft cysts. Clin Endocrinol (Oxf). 2012;76:151–160. [DOI] [PubMed] [Google Scholar]
- 4. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31:E1. [DOI] [PubMed] [Google Scholar]
- 5. Nishioka H, Haraoka J, Izawa H, Ikeda Y. Magnetic resonance imaging, clinical manifestations, and management of Rathke's cleft cyst. Clin Endocrinol (Oxf). 2006;64:184–188. [DOI] [PubMed] [Google Scholar]
- 6. Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH. Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg. 2005;102:189–193. [DOI] [PubMed] [Google Scholar]
- 7. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 8. Nishio S, Morioka T, Suzuki S, Fukui M. Spontaneous regression of a pituitary cyst: report of two cases. Clin Imaging. 2001;25:15–17. [DOI] [PubMed] [Google Scholar]
- 9. Simmons JD, Simmons LA. Spontaneous regression of a pituitary cyst. Neuroradiology. 1999;41:27–29. [DOI] [PubMed] [Google Scholar]
- 10. Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol. 2003;149:123–127. [DOI] [PubMed] [Google Scholar]
- 11. Benveniste RJ, King WA, Walsh J, Lee JS, Naidich TP, Post KD. Surgery for Rathke cleft cysts: technical considerations and outcomes. J Neurosurg. 2004;101:577–584. [DOI] [PubMed] [Google Scholar]
- 12. Fleseriu M, Yedinak C, Campbell C, Delashaw JB. Significant headache improvement after transsphenoidal surgery in patients with small sellar lesions. J Neurosurg. 2009;110:354–358. [DOI] [PubMed] [Google Scholar]
- 13. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke's cleft cysts: A radiological, surgical and pathological review. Pituitary. 2004;7:131–137. [DOI] [PubMed] [Google Scholar]