Abstract
Behavioral weight loss (BWL) treatments result in suboptimal weight losses for many individuals. Impulsivity appears to be a maintenance factor of obesity, yet few studies have examined impulsivity as a predictor of outcomes from BWL. We examined specific facets of impulsivity (inhibitory control and delay discounting) as moderators of outcome in BWL. Overweight adults (n=190) were randomized to standard behavioral treatment (SBT) or acceptance-based behavioral treatment (ABT). We hypothesized that impulsivity would be inversely associated with weight loss, and that the association between impulsivity and outcome would be attenuated in the ABT condition. Poorer general inhibitory control predicted lower percent weight lost at 12 months across conditions at the trend level (b=-0.003, p=.06). The negative impact of low inhibitory control on weight loss was attenuated by assignment to ABT versus SBT (b=0.004, p=.03). Treatment condition, at trend level, also moderated the impact of delay discounting (b=-0.011, p=.098) and food-specific inhibitory control (b=0.003, p=.06) on percent weight loss such that those with greater impulsivity benefitted most from ABT. Results reveal a potential pattern that impulsivity reduces benefit derived from SBT but not ABT. Further research on the moderating effect of impulsivity is necessary to inform the development of targeted treatments for clinically meaningful subtypes of patients.
Keywords: obesity, behavioral weight loss, acceptance-based treatment, impulsivity, delay discounting, inhibitory control
Introduction
Obesity (i.e., body mass index of greater than 30 kg/m2) represents a major public health problem (Yang & Colditz, 2015). While behavioral weight loss treatments robustly produce clinically significant weight losses averaging 5-8% at the end of a 12-month intervention (Butryn, Webb, & Wadden, 2011), participants generally lose substantially less weight than desired (Foster, Wadden, Vogt, & Brewer, 1997), and remain at increased risk for obesity-related medical issues, such as heart disease and cancer (Wilson, D'Agostino, Sullivan, Parise, & Kannel, 2002). Moreover, even within ‘successful’ interventions, substantial proportions of participants do not achieve clinically significant weight loss (i.e., > 5% of initial weight) (Butryn et al., 2011). Identification of individual factors that may contribute to suboptimal outcomes from behavioral weight loss interventions is thus warranted to provide direction for treatment development.
A large body of literature implicates impulsivity, i.e.,the tendency to engage in behavior with little regard for future consequences, as a potential risk and maintenance factor for obesity (Davis, Levitan, Muglia, Bewell, & Kennedy, 2004; Schag, Schönleber, Teufel, Zipfel, & Giel, 2013; Thamotharan, Lange, Zale, Huffhines, & Fields, 2013). In particular, inhibitory control (i.e., the ability to withhold a prepotent response) and delay discounting (i.e., the tendency to choose smaller, shorter-term rewards, over long-term rewards) have been identified as two domains of impulsivity that may be particularly important in the maintenance of obesity-related behaviors and potentially, differential response to behavioral treatment (Ames et al., 2014; Fields, Sabet, & Reynolds, 2013; Houben, 2011; Jasinska et al., 2012). Specifically, successful inhibitory control may allow an individual to withhold an already-initiated “automatic” response to approach-salient stimuli, such as palatable food. An individual with greater delay discounting would place a higher value on the short-term pleasure of consuming appetizing yet unhealthy food, compared to the longer-term benefit of weight loss. Thus, both of these constructs appear to be especially relevant to success in behavioral weight loss interventions, which requires continued adherence to specific dietary recommendations.
Cross-sectional research has robustly demonstrated that obese individuals evidence greater delay discounting than healthy weight controls (Davis, Patte, Curtis, & Reid, 2010; Weller, Cook, Avsar, & Cox, 2008), although some studies show differential effects depending on the type of stimuli (e.g., food versus money) used in the delay discounting task (Lawyer, Boomhower, & Rasmussen, 2015; Rasmussen, Lawyer, & Reilly, 2010). A body of research also shows that obese individuals display poorer inhibitory control than healthy weight controls as measured by go/no-go and stop-signal tasks (Batterink, Yokum, & Stice, 2010; Nederkoorn, Smulders, Havermans, Roefs, & Jansen, 2006). Individuals with obesity appear to also show even more pronounced deficits in inhibiting responses to food-specific stimuli, i.e., when the inhibitory control task asks participants to withhold responses to images of palatable food (e.g., pizza, ice cream) rather than neutral (e.g., hammers, staplers) stimuli (Houben, Nederkoorn, & Jansen, 2014). Furthermore, a prospective study showed that greater impulsivity predicted naturalistic weight gain over a one-year period in a sample of women (Nederkoorn, Houben, Hofmann, Roefs, & Jansen, 2010). These cross-sectional and prospective findings strongly suggest that impulsivity should be examined as a predictor of outcome from behavioral weight loss treatments.
Thus far, research examining impulsivity as a predictor of treatment outcome across eating and weight disorders is sparse. One behavioral weight loss trial demonstrated relatively poorer weight loss within highly impulsive, compared to less impulsive, obese children (Nederkoorn, Jansen, Mulkens, & Jansen, 2007), and a behavioral treatment for bulimia nervosa resulted in better treatment completion rates for less impulsive participants (Agras et al., 2000). More broadly, examinations of behavioral treatments for substance use have revealed impulsivity is associated with higher attrition, poorer compliance, and lower abstinence rates (Krishnan-Sarin et al., 2007; Patkar et al., 2004; Stanger et al., 2012). While these results suggest that impulsivity could represent a transdiagnostic predictor of treatment outcome, this variable has yet to be examined as a predictor of outcome from an adult behavioral weight loss intervention. Theoretically, impulsivity could be expected to weaken response to standard treatments through several mechanisms, including difficulty adhering to prescribed behavior changes (e.g., low calorie diets and physical activity) and poor in-the-moment decision making, resulting in dietary lapses. However, obese individuals with high impulsivity may especially benefit from behavioral treatments that address behavioral difficulties (e.g., lapsing from a diet) resulting from deficits in inhibitory control and impulsive decision-making.
“Acceptance-based” behavioral treatments (ABTs), which focus on decreasing avoidance and increasing tolerance of negative emotional and physical experiences, appear to result in increased weight loss among overweight and obese individuals (Forman et al., 2016) compared to gold standard behavioral treatments (SBTs; e.g., treatments such as those used in Look AHEAD that focus on behavioral changes to facilitate weight loss, such as self-monitoring of calorie intake, prescriptions for a balanced-deficit diet and physical activity, and stimulus control)(D. P. P. R. Group, 2002; L. A. R. Group, 2006). The benefit of ABT may be in its focus on clarification and awareness of one's long-term goals and values (e.g., to be an active and involved grandparent), tolerance of less pleasurable states (e.g., resisting an urge to eat), and slowing down decision-making processes to bring behaviors in line with these values (Forman & Butryn, 2015; Hayes, Luoma, Bond, Masuda, & Lillis, 2006). These strategies could be of particular benefit to individuals with a greater tendency to make decisions based on short-term comfort, and/or who have difficulties with inhibiting automatic responses. In fact, one study showed that an acceptance-based workshop decreased monetary discounting in obese adults (Morrison, Madden, Odum, Friedel, & Twohig, 2014), and another study showed that mindful eating (a tenant of many acceptance-based eating interventions) decreased food-specific discounting (Hendrickson & Rasmussen, 2013). However, despite this preliminary evidence, no studies to our knowledge have examined the impact of impulsivity on behavioral treatment outcomes in adults with obesity, and none have examined its effects within ABT-based interventions.
The current study aims to examine the potential moderating role of impulsivity (specifically, delay discounting, general inhibitory control, and food-specific inhibitory control) in behavioral weight loss treatment outcomes. To this end, we administered behavioral measures of impulsivity at baseline in a randomized controlled trial comparing ABT to SBT for overweight and obese adults. We hypothesized that impulsivity would negatively predict weight loss outcomes across treatment conditions at post-treatment (12 months), but that this relationship would be attenuated in the ABT condition (i.e., that poorer weight loss outcomes associated with greater baseline impulsivity would be less pronounced in the ABT versus SBT condition).
Methods
Participants
Participants (n = 190) had a body mass index [BMI] between 27-50 kg/m2 and were between 18-70 years of age. Participants were excluded if any of the following applied: had a medical or psychiatric condition which limited their ability to comply with the behavioral recommendations of the program or posed a risk to the participant during weight loss; began a course of or changed the dosage of medication within the previous three months that could cause significant change in weight; had lost more than 5% of their weight in the past 6 months.
Procedure
The current study was conducted as part of the Mind Your Health II randomized controlled trial; see Forman et al., 2016 for a complete description of recruitment methods and treatment procedures. Recruitment for the current study was completed in four waves of 38 – 45 participants. Potential participants were recruited through referrals from local primary care physicians and advertisements in newspapers and radio stations. Participants were randomly assigned to standard behavioral treatment (SBT; n = 90) or acceptance-based behavioral treatment (ABT; n = 100). Participants in both treatments attended 25 treatment sessions in 75-minute, small (10-14 participants), closed-group sessions. Treatments were manualized and groups were held weekly for the first 16 sessions, biweekly for the next 5 sessions, monthly for the next 2 sessions, and bi-monthly for the final 2 sessions. Session structure consisted of brief individual check-ins, skill presentation, and a skill building exercise. Interventionists were doctoral-level clinicians with previous experience delivering behavioral weight loss treatment, accompanied by a trainee co-leader. Drexel University's Institutional Research Board approved the study.
Treatment
Behavioral components of both treatments (i.e., self-monitoring of caloric intake, daily caloric and physical activity prescriptions, and stimulus control) were similar to those used in Look AHEAD and the Diabetes Prevention Program protocols (Look Ahead Protocol Review Committee, 2012; Diabetes Prevention Program Research D. P. P. R. Group, 2001).
Components of the SBT manual that were not included in ABT were introduction of the traditional cognitive model, which proposes that changing the content of one's thoughts can produce behavior change; cognitive restructuring; building self-efficacy and positive self-esteem; and distraction strategies.
The ABT manual synthesized traditional behavioral weight loss treatment components with those from several acceptance-based treatment models (Forman & Butryn, 2015), especially Acceptance and Commitment Therapy (Hayes et al., 2006). Participants identified personal life values (e.g., living a long and healthy life; being a present, loving, active grandparent) and chose weight-loss goals related to those values. Participants were also taught skills related to tolerating distress or discomfort related to pursuing these goals.
Measures
All measures were administered at a baseline assessment, prior to randomization. Measures were administered in a counterbalanced manner with other neuropsychological and behavioral tasks that were administered at the baseline1.
Inhibitory control
A modified version of a computerized Stop Signal Task (SST) was used to measure inhibitory control in response to both neutral and food-specific stimuli (see (Manasse et al., 2016) for more details). In this task, participants categorize various stimuli on a screen with a keyboard press. The task included two blocks: neutral (e.g., staplers) and food (e.g., pizza) stimuli. In a subset of categorization trials, a stop signal is presented after the stimulus presentation but before the participant response, which indicates to participants that they are to refrain from responding. As in previous studies, the outcome measure used for the current study was the stop signal reaction time (SSRT), which was calculated by subtracting the average reaction time on normal trials from the average stop signal delay (Verbruggen & Logan, 2008). Two outcome variables were derived form the SST: The SSRT was calculated for both neutral (i.e., SSRT-neutral, as a measure of general inhibitory control) and food stimuli (i.e., SSRT-food, as a measure of food-specific inhibitory control) for each subject. A smaller SSRT is indicative of greater inhibitory control whereas a larger SSRT reflects poorer inhibitory control. Because of an error in the task, the data from approximately half the sample (the first two waves of recruitment) was not usable.
Delay discounting
Delay discounting was assessed using the Delay discounting Task (DDT) (Robles & Vargas, 2007), a commonly used computerized monetary discounting task. Discounting is the extent to which the subjective value of an immediate reward is deemed greater than the subjective value of a future (“delayed”) reward, despite the future reward's higher objective value. Participants were asked over several trials to choose between a hypothetical variable monetary amount that could be received immediately and a larger amount to be received after varying time intervals. Area-under the-curve (AUC) was calculated from the points at which the subjective value of the delayed reward was equal to the amount of the immediate reward (Myerson, Green, & Warusawitharana, 2001). We chose to use AUC as the outcome variable of delay discounting given its prevalent use in the obesity literature (Appelhans et al., 2012; Rasmussen et al., 2010), and to not impose a specific distribution on the data. Greater AUC values indicate less discounting of delayed rewards.
Weight Loss Outcomes
Weight was measured at baseline, each of the 25 treatment sessions, and at post-treatment (12 months). Participants were measured in street clothes (without shoes) using a standardized Seca® scale accurate to 0.1 kg. Height was measured with a stadiometer to establish BMI (kg/m2).
Statistical Analyses
Consistent with a large body of literature on behavioral weight loss treatments, outcome was operationalized as percent of initial body weight lost (Franz et al., 2007; Tsai & Wadden, 2005; Womble et al., 2004). Weekly weight data were missing at random. Multiple imputation techniques were used for missing weight data (Buuren & Groothuis-Oudshoorn, 2011), allowing for an intent-to-treat analysis (Houck et al., 2004). Multiple imputation is an iterative form of stochastic imputation for improving power and reducing bias, which basically replaces missing values by generating several plausible numbers derived from distributions and relationships among observed variables in the data set (Li, Stuart, & Allison, 2015). We did not impute any missing data for the independent variables (impulsivity measures). Analyses were conducted in R (v.3.1.2). Multilevel modeling was selected to allow for increased power and the ability to detect nonlinear trends across multiple time points (i.e., weight taken at 25 sessions over 50 weeks). Multi-level models provide a useful approach for studying change over time, and have become increasingly popular for analyzing multi-wave longitudinal data (Kwok et al., 2008). Restricted maximum likelihood was used to estimate model parameters and to test the significance of random effects. For all models, observations of the outcome variables across time (Level 1) were nested within individual participants (Level 2). Linear and quadratic fixed effects of time were tested for percent weight loss.
To examine changes in percent weight loss over time (i.e., week) by treatment condition, cross-level interactions between time effects and treatment condition were included. Separate multilevel models were used to test the moderating effect of each of the three impulsivity variables. Impulsivity by time interaction terms were included in each of the three separate models (with SSRT-neutral, SSRT-food, or DDT as moderators) to examine the impact of impulsivity on percent weight loss over time. To examine the differential impact of impulsivity by treatment condition on the rate of change in percent weight loss, a three-way-interaction of time by condition by impulsivity variable was also included in the three separate models.
Results
Demographics
The sample was 79% female, and primarily White (71%, 23% African-American, 6% Hispanic). Treatment groups did not differ in gender (χ²=.52, df=1, p=.47), nor on any outcome and process measures at baseline (Table 1). The proportion of non-white participants did not differ significantly between groups (χ²=.03, df=1, p=.87).
Table 1. Baseline sample characteristics.
| ABT | SBT | Group differences | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variable | Mean | SD | Mean | SD | t | df | p |
| Age | 51.69 | 8.29 | 53.31 | 8.70 | 0.87 | 82 | .39 |
| Body mass index | 36.68 | 4.93 | 37.49 | 5.99 | 0.68 | 82 | .49 |
| SSRT-neutral | 226.27 | 122.48 | 228.25 | 78.42 | 0.08 | 82 | .93 |
| SSRT-food | 212.67 | 84.73 | 237.31 | 99.10 | 1.23 | 82 | .22 |
| DDT | 0.67 | 0.16 | 0.66 | 0.16 | -0.15 | 82 | .88 |
Note: SSRT-neutral = Stop-signal reaction task-neutral stimuli; SSRT-food = Stop-signal reaction task-food stimuli; DDT = Delay discounting task
Attendance and dropout
Treatment attendance (with inclusion of make-up sessions) was greater than 84% of expected sessions, and there were no differences between the two treatments in terms of the average number of sessions attended (MABT=21.26 ± 5.85, MSBT=20.88 ± 5.46; t(189)=-0.46, p=.65). There were no significant differences in dropout between ABT (n=21) and SBT (n=20) (χ²=.07, df=1, p=.79)
Main outcomes
As reported in Forman et al. (2016), results indicated a quadratic effect of percent weight loss over time, and a significant condition by time interaction. Specifically, the ABT condition yielded superior percent weight losses at post-treatment (i.e., 13.8% versus 9.8% in SBT). SBT showed a shallower trajectory of weight loss compared to ABT with upward deflection (weight regain) by 12-months, while ABT maintained weight losses through 12 months.
Moderator analyses
A general inhibitory control by time interaction approached significance (n = 96, b = -0.003, SE = 0.002 p = .06), indicating that higher SSRT-neutral sores (i.e., worse inhibitory control) at baseline predicted less weight loss over time, across participants. Unexpectedly, no support was obtained for either the food-specific inhibitory control (n = 90, b = -0.001, SE = 0.001, p = .26) or delay discounting (n = 190, b = -0.002, SE = 0.005, p = .65) interaction with time on weight loss.
Consistent with our hypothesis, the three-way general inhibitory control by condition by time interaction was statistically significant (b = 0.004, SE = 0.002, p = .03), such that the impact of general inhibitory control on weight loss was more pronounced in the SBT group (See Figure 1). Additionally, consistent with our hypothesis, the three-way inhibitory control/delay discounting by time by condition interaction approached significance for both food-specific inhibitory control (b = 0.003, SE = 0.002, p = .06) and delay discounting (b = -0.011, SE = 0.007, p =.096). For food-specific inhibitory control, the three-way-interaction was such that the relation between inhibitory control and percent weight loss was positive in the SBT group and negative in the ABT group (see Figure 2). For the three way interaction with delay discounting, the relation was such that those in the SBT group lost similar (lower amounts) of weight regardless of delay discounting level, whereas in the ABT group, those who tended to discount longer term in favor of shorter term options at baseline lost more weight at 12 months (see Figure 3).
Figure 1.
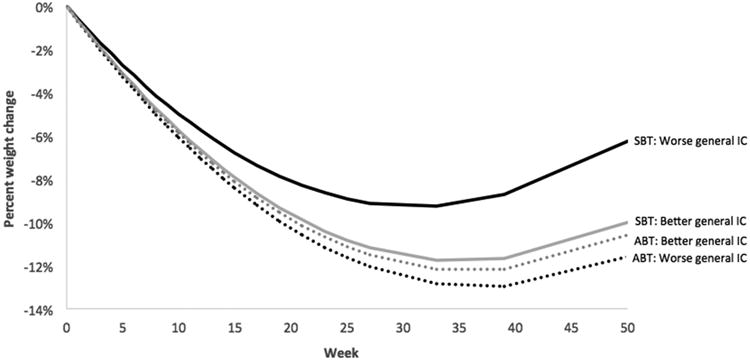
Percent weight change over time by treatment and general inhibitory control (IC), The time × condition × neutral IC was statistically significant (p = .03). Analyses were conducted with IC as a continuous variable, but IC was dichotomized above for graphical purposes. Better IC is defined as those who scored in the upper quartile of SSRT-neutral, and worse as those who scored in the lower quartile.
Figure 2.
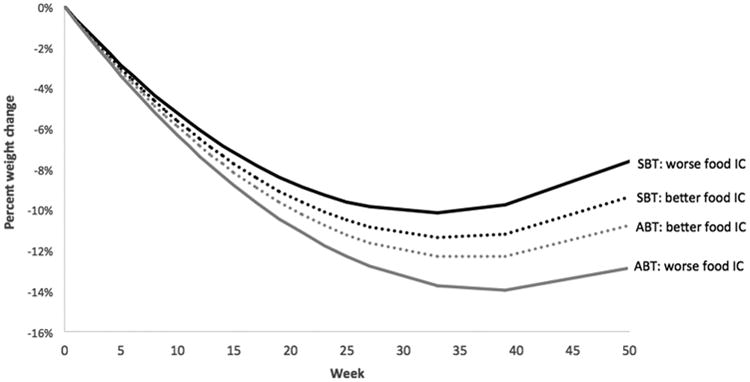
Percent weight change over time by treatment and food-specific inhibitory control (IC), The time × condition × neutral IC was marginally significant (p = .06). IC was dichotomized above for graphical purposes. Better IC is defined as those who scored in the upper quartile of SSRT-food, and worse as those who scored in the lower quartile. .
Figure 3.
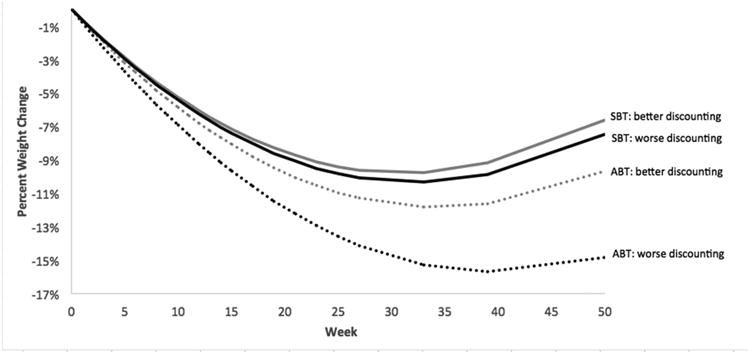
Percent weight change over time by treatment and delay discounting, The time × condition × delay discounting interaction was marginally significant (p < .10). Delay discounting was dichotomized above for graphical purposes. Better delay discounting (i.e., less discounting) is defined as those who scored in the upper quartile, and worse as those who scored in the lower quartile.
Discussion
This study is among the first to examine impulsivity as a predictor of behavioral treatment outcomes broadly, and the first to examine inhibitory control (both general and food-specific) and delay discounting as predictors and moderators of outcome from adult behavioral treatment for obesity. Our results indicated that poor general inhibitory control was associated with reduced weight loss at 12 months across treatment conditions, and generally, that assignment to acceptance-based treatment trended towards attenuating or even eliminating any negative impact of baseline poor inhibitory control and steeper monetary discounting on 12-month weight loss.
The finding of poorer general inhibitory control being associated with less weight loss is consistent with a literature linking inhibitory control deficits with obesity and naturalistic weight gain over time (Batterink et al., 2010; Thamotharan et al., 2013), but this study is the first to show that inhibitory control may impair weight loss outcomes in behavioral treatment in adults. Clinically, inhibitory control difficulties may make adherence to behavioral treatment recommendations difficult. For example, withholding automatic responses, especially to palatable, high-calorie food is a necessary component of lowering calorie intake to an extent that will induce weight loss.
In addition, consistent with hypotheses, treatment condition moderated the relation between inhibitory control and percent weight lost over time. These analyses demonstrated that there was relatively lower weight loss (and greater upward deflection of weight in the second half of treatment) in individuals with poorer inhibitory control in the SBT condition, but that there were greater weight losses in ABT, regardless of inhibitory control level. Stated otherwise, ABT attenuated the impact of general inhibitory control on weight loss. We speculate that ABT attempts to provide psychological strategies for slowing down momentary decision-making (e.g., by noticing internal and external cues that may be driving a desire to eat), which could potentially improve outcomes for those with poorer baseline inhibitory control.
The food-specific inhibitory control by time interaction did not emerge as statistically significant; however, a trend-level moderating effect of treatment condition qualified this non-effect, such that any negative impact of poor food-specific inhibitory control on weight loss was eliminated in the ABT condition. In fact, those with poorer baseline food-specific inhibitory control in the ABT group lost the most weight over time and those with poorer baseline food-specific inhibitory control in the SBT group lost the least amount of weight over time. That is, food-specific inhibitory control impacted weight loss in SBT in the expected direction, but impacted weight loss in ABT in an unexpected direction. While we did not predict that those with poorer inhibitory control in the ABT group would outperform others in terms of weight loss (rather, we predicted an attenuated relationship between impulsivity and outcome in the ABT group), it is possible that ABT is an especially good fit for those whose overeating is driven by food-specific inhibitory control deficits as opposed to other factors (e.g., lack of motivation, lack of nutritional knowledge), and that SBT is a particularly poor fit for these individuals. For example, we can speculate that the explicit focus in ABT on accepting and tolerating the decision to “ride out” (i.e., inhibit the behavioral response to) a craving, urge, or automatic impulse towards a palatable food may be an especially good fit for subtypes of individuals with poorer food-specific inhibitory control. The lack of psychological strategies targeting inhibitory decision-making processes in SBT may result in especially poor outcomes for this potential subtype of impulsive participants.
Delay discounting also did not significantly predict percent weight loss over time across conditions; however treatment condition qualified this lack of effect at the trend level, such that those in SBT, regardless of delay discounting lost similar (lesser) amounts of weight compared to the ABT condition, but those with greater discounting (i.e., greater tendency to prioritize short-term over long-term reward) in the ABT condition lost the most weight over time. While this finding (specifically, that those with steeper discounting in the ABT condition lost the most weight) is somewhat counterintuitive, the underlying premise of ABT is that individuals must be able to accept and tolerate making the behavioral decision associated with the less pleasurable short-term option (e.g., choosing fruit instead of ice cream) in the service of one's ultimate long-term values (e.g., being an active grandparent). Thus, it is possible that individuals whose obesity is driven by increased tendency to prioritize short-term reward responded especially well to an intervention designed to shift behavior in line with long-term rewards rather than short-term comfort or pleasure, although this finding is in need of replication.
In summary, it appears that impulsivity, as measured by inhibitory control and delay discounting, may be related to poorer weight outcomes, especially in SBT; however, impulsivity may have the inverse relation with weight outcomes in ABT, depending on the exact construct being measured. These findings preliminarily support the idea that ABT may be an especially good fit for improving weight outcomes for subtypes of people with poorer inhibitory control or greater tendency to discount longer-term rewards. Future research should aim to replicate these findings, and should aim to examine whether impulsivity changes throughout treatment and whether such changes mediate outcomes. Because ABT is delivered as a treatment package, future studies should examine which treatment components are most effective for improving outcomes for subtypes of individuals with greater levels of different facets of impulsivity. In addition, future research should examine whether impulsivity moderates weight loss maintenance in the follow-up period, and whether treatment matching enhances outcomes. Broadly, acceptance-based behavioral treatments may hold promise for improving outcomes for subtypes of individuals with higher levels of impulsivity across maladaptive behaviors (e.g., binge eating, addictions), but future research is necessary to test this hypothesis.
Findings from the current study must be interpreted in light of several limitations. First, we only measured outcome up to post-treatment rather than a follow-up period, which would provide information about weight loss maintenance, for which outcomes are generally poor (Cooper & Fairburn, 2001; Wing & Hill, 2001). We also only had SST data for 50% of the sample, which may have limited our power to detect effects. In addition, several of our findings were significant only at the trend level, so should be interpreted cautiously as preliminary findings. Our sample was predominantly white and female, limiting the generalizability of our findings. We also did not control for the time of day that the participants completed the inhibitory control and delay discounting tasks, or for level of hunger, which could have impacted performance on the tasks. Despite these limitations, this study featured several strengths. For example, we used behavioral measurement instead of self-report which likely helped to limit measurement bias. We also randomized participants to two types of behavioral weight loss treatments and thus were the first to be able to examine the differential impact of impulsivity on outcome between treatments, which is a first step towards developing more efficacious, tailored interventions.
In conclusion, inhibitory control and delay discounting may play unique roles in predicting and moderating weight outcomes from two types of behavioral treatments. Future research should continue to examine relevant maintenance and predictive factors to inform development of behavioral treatments and treatment components that can improve outcomes for subtypes of individuals with higher levels of impulsivity.
Acknowledgments
This study was funded by the National Institute for Diabetes and Digestive and Kidney Diseases (R01 DK095069, PI: Dr. Forman) and the National Institute of Mental Health (F31 MH108279, PI: Ms. Manasse).
Footnotes
Other administered neuropsychological and behavioral tasks included a set-shifting task (the Conditional Exclusion Task), a working memory task (the Letter N-Back), a planning task (the Dellis Kaplan Executive Functioning System [D-KEFS] Tower Task), a conflict monitoring task (the DKEFS color-word interference task) and a disinhibited eating lab paradigm.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agras WS, Crow SJ, Halmi KA, Mitchell JE, Wilson GT, Kraemer HC. Outcome predictors for the cognitive behavior treatment of bulimia nervosa: Data from a multisite study. American Journal of Psychiatry. 2000;157(8):1302–1308. doi: 10.1176/appi.ajp.157.8.1302. [DOI] [PubMed] [Google Scholar]
- Ames SL, Kisbu-Sakarya Y, Reynolds KD, Boyle S, Cappelli C, Cox MG, et al. Stacy AW. Inhibitory control effects in adolescent binge eating and consumption of sugar-sweetened beverages and snacks. Appetite. 2014;81:180–192. doi: 10.1016/j.appet.2014.06.013. doi: http://dx.doi.org/10.1016/j.appet.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, Waring ME, Schneider KL, Pagoto SL, DeBiasse MA, Whited MC, Lynch EB. Delay discounting and intake of ready-to-eat and away-from-home foods in overweight and obese women. Appetite. 2012;59(2):576–584. doi: 10.1016/j.appet.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. The Psychiatric clinics of North America. 2011;34(4):841. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. Journal of statistical software. 2011;45(3) [Google Scholar]
- Committee, L.A.P.R. Protocol Action for Health in Diabetes. 2012 Retrieved Febuary 20, 2016, from https://http://www.lookaheadtrial.org/public/LookAHEADProtocol.pdf.
- Cooper Z, Fairburn CG. A new cognitive behavioural approach to the treatment of obesity. Behaviour research and therapy. 2001;39(5):499–511. doi: 10.1016/s0005-7967(00)00065-6. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision-Making Deficits and Overeating: A Risk Model for Obesity. Obesity Research. 2004;12(6):929–935. doi: 10.1038/oby.2004.113. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54(1):208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Fields SA, Sabet M, Reynolds B. Dimensions of impulsive behavior in obese, overweight, and healthy-weight adolescents. Appetite. 2013;70:60–66. doi: 10.1016/j.appet.2013.06.089. doi: http://dx.doi.org/10.1016/j.appet.2013.06.089. [DOI] [PubMed] [Google Scholar]
- Forman EM, Butryn ML. A new look at the science of weight control: how acceptance and commitment strategies can address the challenge of self-regulation. Appetite. 2015;84:171–180. doi: 10.1016/j.appet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman EM, Butryn ML, Manasse SM, Crosby RD, Goldstein SP, Wyckoff EP, Thomas JG. Acceptance-based versus standard behavioral treatment for obesity: Results from the mind your health randomized controlled trial. Obesity. 2016;24(10):2050–2056. doi: 10.1002/oby.21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients' expectations and evaluations of obesity treatment outcomes. Journal of consulting and clinical psychology. 1997;65(1):79. doi: 10.1037//0022-006x.65.1.79. [DOI] [PubMed] [Google Scholar]
- Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Pronk NP. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. Journal of the American Dietetic Association. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. doi: http://dx.doi.org/10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Group, D.P.P.R. Diabetes Prevention Program. 2001 Retrieved Febuary 20, 2016, from https://dppos.bsc.gwu.edu/documents/1124073/1127212/DPPPROTOCOL.PDF/807eddd1-d9bf-497d-89f0-5de15fc43d79.
- Group DPPR. The Diabetes Prevention Program (DPP) description of lifestyle intervention. Diabetes care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group LAR. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring, Md) 2006;14(5):737. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: Model, processes and outcomes. Behaviour research and therapy. 2006;44(1):1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hendrickson KL, Rasmussen EB. Effects of mindful eating training on delay and probability discounting for food and money in obese and healthy-weight individuals. Behaviour research and therapy. 2013;51(7):399–409. doi: 10.1016/j.brat.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Houben K. Overcoming the urge to splurge: Influencing eating behavior by manipulating inhibitory control. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42(3):384–388. doi: 10.1016/j.jbtep.2011.02.008. doi: http://dx.doi.org/10.1016/j.jbtep.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, Jansen A. Eating on impulse: The relation between overweight and food-specific inhibitory control. Obesity. 2014;22(5):E6–E8. doi: 10.1002/oby.20670. [DOI] [PubMed] [Google Scholar]
- Houck PR, Mazumdar S, Koru-Sengul T, Tang G, Mulsant BH, Pollock BG, Reynolds CF., 3rd Estimating treatment effects from longitudinal clinical trial data with missing values: comparative analyses using different methods. Psychiatry Res. 2004;129(2):209–215. doi: 10.1016/j.psychres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Yasuda M, Burant CF, Gregor N, Khatri S, Sweet M, Falk EB. Impulsivity and inhibitory control deficits are associated with unhealthy eating in young adults. Appetite. 2012;59(3):738–747. doi: 10.1016/j.appet.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and alcohol dependence. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok OM, Underhill AT, Berry JW, Luo W, Elliott TR, Yoon M. Analyzing longitudinal data with multilevel models: An example with individuals living with lower extremity intra-articular fractures. Rehabilitation Psychology. 2008;53(3):370. doi: 10.1037/a0012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer SR, Boomhower SR, Rasmussen EB. Differential associations between obesity and behavioral measures of impulsivity. Appetite. 2015;95:375–382. doi: 10.1016/j.appet.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA. 2015;314(18):1966–1967. doi: 10.1001/jama.2015.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, Goldstein SP, Wyckoff E, Forman EM, Juarascio AS, Butryn ML, et al. Nederkoorn C. Slowing down and taking a second look: Inhibitory deficits associated with binge eating are not food-specific. Appetite. 2016;96:555–559. doi: 10.1016/j.appet.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KL, Madden GJ, Odum AL, Friedel JE, Twohig MP. Altering impulsive decision making with an acceptance-based procedure. Behavior therapy. 2014;45(5):630–639. doi: 10.1016/j.beth.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the experimental analysis of behavior. 2001;76(2):235. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Houben K, Hofmann W, Roefs A, Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychology. 2010;29(4):389. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behaviour research and therapy. 2007;45(5):1071–1075. doi: 10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FT, Havermans RC, Roefs A, Jansen A. Impulsivity in obese women. Appetite. 2006;47(2):253–256. doi: 10.1016/j.appet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre—Treatment Measures of Impulsivity, Aggression and Sensation Seeking Are Associated with Treatment Outcome for African—American Cocaine—Dependent Patients. Journal of addictive diseases. 2004;23(2):109–122. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, Lawyer SR, Reilly W. Percent body fat is related to delay and probability discounting for food in humans. Behavioural Processes. 2010;83(1):23–30. doi: 10.1016/j.beproc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Robles E, Vargas PA. Functional parameters of delay discounting assessment tasks: Order of presentation. Behavioural Processes. 2007;75(2):237–241. doi: 10.1016/j.beproc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Schag K, Schönleber J, Teufel M, Zipfel S, Giel K. Food-related impulsivity in obesity and Binge Eating Disorder – a systematic review. Obesity Reviews. 2013;14(6):477–495. doi: 10.1111/obr.12017. [DOI] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ. Delay discounting predicts adolescent substance abuse treatment outcome. Experimental and clinical psychopharmacology. 2012;20(3):205. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamotharan S, Lange K, Zale EL, Huffhines L, Fields S. The role of impulsivity in pediatric obesity and weight status: A meta-analytic review. Clinical Psychology Review. 2013;33(2):253–262. doi: 10.1016/j.cpr.2012.12.001. doi: http://dx.doi.org/10.1016/j.cpr.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Tsai AG, Wadden TA. Systematic Review: An Evaluation of Major Commercial Weight Loss Programs in the United States. Annals of Internal Medicine. 2005;142(1):56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in cognitive sciences. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RE, Cook EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51(3):563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Archives of internal medicine. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- Wing RR, Hill JO. Successful weight loss maintenance. Annual Review of Nutrition. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- Womble LG, Wadden TA, McGuckin BG, Sargent SL, Rothman RA, Krauthamer-Ewing ES. A Randomized Controlled Trial of a Commercial Internet Weight Loss Program. Obesity Research. 2004;12(6):1011–1018. doi: 10.1038/oby.2004.124. [DOI] [PubMed] [Google Scholar]
- Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA internal medicine. 2015;175(8):1412–1413. doi: 10.1001/jamainternmed.2015.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]


